Abstract
During episodic recognition tests, meaningful stimuli such as words can engender both conscious retrieval (explicit memory) and facilitated access to meaning that is distinct from the awareness of remembering (conceptual implicit memory). Neuroimaging investigations of one type of memory are frequently subject to the confounding influence of the other type of memory, thus posing a serious impediment to theoretical advances in this area. We used minimalist visual shapes (squiggles) to attempt to overcome this problem. Subjective ratings of squiggle meaningfulness varied idiosyncratically, and behavioral indications of conceptual implicit memory were evident only for stimuli given higher ratings. These effects did not result from perceptual-based fluency or from explicit remembering. Distinct event-related brain potentials were associated with conceptual implicit memory and with explicit memory by virtue of contrasts based on meaningfulness ratings and memory judgments, respectively. Frontal potentials from 300 to 500 msec after the onset of repeated squiggles varied systematically with perceived meaningfulness. Explicit memory was held constant in this contrast, so these potentials were taken as neural correlates of conceptual implicit memory. Such potentials can contaminate putative neural correlates of explicit memory, in that they are frequently attributed to the expression of explicit memory known as familiarity. These findings provide the first neural dissociation of these two memory phenomena during recognition testing and underscore the necessity of taking both types of memory into account in order to obtain valid neural correlates of specific memory functions.
Encountering a stimulus for the second time triggers a variety of memory phenomena, presenting a pervasive challenge to experimental attempts to identify the neural events responsible for specific memory processes (Schacter and Buckner 1998; Henson 2003). Explicit memory (also referred to as declarative memory) is demonstrated when an individual recalls or recognizes prior events or facts; conceptual priming (a type of nondeclarative memory) is a behavioral manifestation of facilitated access to stimulus meaning due to prior experience with that stimulus or a conceptually related stimulus (Squire 1987; Gabrieli 1998). Conceptual priming is generally taken to go beyond priming attributable to processing of surface-level features of a stimulus (perceptual priming). In either case, priming need not be accompanied by explicit memory, and is therefore a form of implicit memory. However, questions remain about the possible interdependence of explicit and implicit forms of memory, as both may be brought to bear in an interactive manner in everyday experiences.
Explicit and implicit expressions of memory can be indexed separately in specialized tests. Nonetheless, the brain events that support explicit and implicit memory phenomena can occur regardless of whether a corresponding behavioral index is provided. Thus, presumptive neural measures of explicit memory can reflect not only the operation of explicit memory systems, but also the operation of implicit memory systems that are concurrently active. Contributions from perceptual priming to neuroimaging measures obtained during recognition testing have been well-characterized (Rugg et al. 1998; Schweinberger et al. 2002; Paller et al. 2003; Schott et al. 2005), but very little is known regarding the contribution from conceptual priming. We advocate the position that the repetition of meaningful stimuli, such as words or nameable pictures, can engender both the conscious experience of explicit memory as well as enhanced access to stimulus meaning that can be measured when tests of conceptual priming are used. Hence, neural activity supporting conceptual priming could contaminate neural measures obtained during explicit memory tests, particularly when those tests use meaningful stimuli such as words or nameable pictures.
An important step in understanding neural correlates of memory involves juxtaposing implicit and explicit memory. However, accomplishing this using implicit memory tests to yield neural correlates of implicit memory and explicit memory tests to yield neural correlates of explicit memory has serious drawbacks. The problem of contamination emerges in both directions, as priming can occur during explicit tests of memory and explicit retrieval can occur during some tests of implicit memory. In the functional magnetic resonance imaging (fMRI) literature, one argument used to circumvent this problem is that explicit memory often occurs with an increase in cortical activation, whereas implicit memory tends to occur with a decrease. However, this argument can rest on circular reasoning in accounting for observed results and is particularly problematic when both types of memory occur simultaneously. Moreover, notable exceptions challenge the generalization. For example, heightened rather than reduced cortical responses are sometimes associated with implicit memory (Henson 2003).
A method that might limit the influence of conceptual priming on neural correlates of explicit memory is to use novel visual shapes having little inherent meaning and, thus, a minimal capability to support conceptual priming (Curran et al. 2002; Groh-Bordin et al. 2006). Nevertheless, if conceptual priming can occur even under such circumstances, this method would be problematic. Indeed, subjects attempting to remember minimalist shapes, like patients taking the Rorschach test, may be able to manufacture meaning for purportedly meaningless stimuli.
Thus, a suitable way to make progress in this area is to study neural correlates of both conceptual priming and explicit memory during recognition testing, and to assess both types of memory. We adopted this approach using stimuli low in inherent meaning (Fig. 1). These minimalist squiggles, unlike highly meaningful stimuli, vary widely and idiosyncratically in perceived meaningfulness. We reasoned that evaluations of subjective meaningfulness could be obtained to provide an index of each squiggle’s ability to support conceptual priming in each individual. Indeed, only squiggles classified as relatively high in meaningfulness were found to support conceptual priming in two behavioral experiments. This finding provided critical leverage, which we took advantage of in an experiment using event-related brain potentials (ERPs) to dissociate neural correlates of conceptual priming and explicit memory.
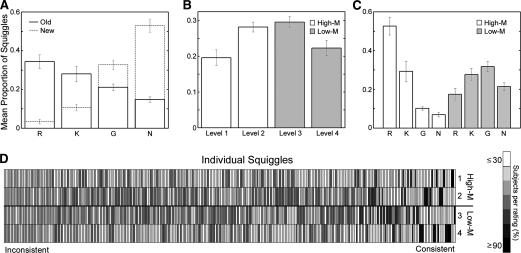
Figure 1.
Examples of squiggle stimuli. (A) A very small number of squiggle stimuli were given high and low meaningfulness ratings by a majority (>80%) of subjects (top and bottom two rows, respectively). (B) The majority of stimuli, such as these, were given inconsistent ratings across subjects. (Stimuli were from Groh-Bordin et al. 2006.)
We used analyses that are standard in the contemporary neuroimaging literature to produce neural correlates of explicit memory, and we observed two ERP effects that are commonly identified: late-onset posterior-maximum positive potentials and early-onset frontal-maximum positive potentials (Friedman and Johnson 2000; Mecklinger 2000; Paller 2000; Rugg and Allan 2000; Curran et al. 2006). Indices of stimulus meaningfulness allowed us to then isolate ERP correlates of conceptual priming. We hypothesized that frontal potentials in the N400 latency would thus be obtained given predictions from several recent studies (Olichney et al. 2000; Yovel and Paller 2004; Voss and Paller 2006). Substantiating this prediction would not only confirm that these frontal potentials can index conceptual priming, but it would also confirm that the neural processing that drives conceptual priming commonly occurs incidentally during recognition testing.
Results
Conceptual priming for squiggles
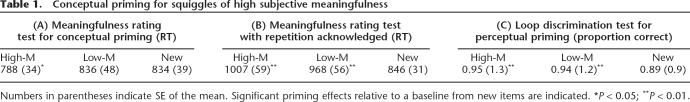
An analysis of response times collected during Experiment 1 confirmed that conceptual priming occurred preferentially for relatively meaningful squiggles (Table 1A). The implicit memory test required a rating of squiggle meaningfulness, and a measure of conceptual priming was obtained by comparing response times for repeated versus new squiggles. Responses were speeded by 48 msec for repeated squiggles that were initially given high meaningfulness ratings (High-M, 43% of stimuli on average, SE = 2.5), whereas for those given low meaningfulness ratings (Low-M) there was no speed-up (High-M priming, P = 0.03; Low-M priming, P = 0.88).
Table 1.
Conceptual priming for squiggles of high subjective meaningfulness
Numbers in parentheses indicate SE of the mean. Significant priming effects relative to a baseline from new items are indicated. *P < 0.05; **P < 0.01.
Conceptual priming was evident in reaction times, but did not influence the meaningfulness ratings assigned to stimuli. Overall, most stimuli garnered identical ratings at each presentation (mean = 89%, SE = 4.3). For those that were endorsed with a different rating during the test than during encoding, the likelihood of an increased rating was not significantly different from the likelihood of a decreased rating for either High-M (mean increase minus decrease difference = −4.1%; P = 0.57) or Low-M (mean increase minus decrease difference = 5.7%; P = 0.41) stimuli. Thus, High-M and Low-M stimuli were not perceived as being more or less meaningful with repetition.
Furthermore, this conceptual priming was tested under covert conditions in which subjects were instructed not to consider the initial encounter, and was disrupted when subjects were instead explicitly instructed to be alert to stimulus repetition while still responding only on the basis of the current stimulus (Table 1B). In this case, there was no response speed-up for either High-M or Low-M squiggles. Instead, repetition led to increased response latencies for both meaningfulness levels (High-M, P = 0.005; Low-M, P = 0.003), indicating that response facilitation under covert testing was due to conceptual priming instead of explicit remembering.
In contrast, equivalent perceptual priming was found for these categories using an implicit memory test that required squiggles to be discriminated on the basis of whether a loop was present (Table 1C). Discrimination was more accurate for both High-M (P = 0.006) and Low-M (P = 0.002) compared with new squiggles. Magnitude of priming (mean 5.3% accuracy improvement) did not differ significantly for High-M versus Low-M squiggles (P = 0.73). Priming was observed in accuracy, but not in reaction times, which did not differ between High-M, Low-M, and New conditions (513, 517, and 515 msec, respectively, all P’s >0.69).
Although the High-M/Low-M contrast is akin to a systematic manipulation of depth of processing, squiggles were categorized by subject ratings instead of counterbalanced assignment. Nonetheless, a high degree of rating variability for individual stimuli produced an intrinsic counterbalancing. Each squiggle was just about as likely to be assigned to one of the meaningfulness categories as to the other (across all three tasks, mean 47% chance of falling into the High-M category, SE = 3%). Priming effects thus cannot be readily attributed to nonspecific differences in stimuli comprising the two meaningfulness categories.
Similar conceptual priming effects were identified using a continuous presentation format in Experiment 2. Ratings were made increasingly faster with repetition for squiggles given high meaningfulness ratings (High-M) but not for those given low ratings (Low-M). On average, 44% (SE = 4%) of squiggles were given High-M ratings. Mean response time across the three repetitions was 949, 891, and 858 msec, respectively, for High-M ratings and 919, 920, and 923 msec for Low-M. Repeated-measures ANOVA with two factors, condition (High-M/Low-M) and repetition (first/second/third presentation), yielded a significant main effect of repetition (F(2,18) = 4.5, P = 0.03) and a significant interaction (F(1.9,17.3) = 6.17, P = 0.01). High-M response times decreased significantly with repetition (first vs. second P = 0.04; second vs. third P = 0.001), whereas Low-M response times did not (first vs. second t(9) = 0.06; second vs. third t(9) = 0.12).
The possibility that response priming (cf. Dobbins et al. 2004) contributed to conceptual priming effects is unlikely given results from subsets of stimuli rated inconsistently—stimuli that received ratings across the first two presentations that were either inconsistent High-M ratings (first 1, then 2, or first 2, then 1) or inconsistent Low-M ratings (first 3, then 4, or first 4, then 3). Thus, these stimuli were assigned to the same meaningfulness category on both encounters, but categorization responses were made using different fingers. Behavioral priming was observed for the inconsistently rated High-M squiggles (average decrease = 42 msec; P = 0.004), but not for the inconsistently rated Low-M squiggles (average increase = 23 msec; P = 0.22). Thus, response facilitation for High-M squiggles was not due merely to strengthened stimulus-response mapping.
The behavioral response speed-up attributed to conceptual priming in both Experiment 1 and Experiment 2 was not due merely to an influence of meaningfulness on rating speed, but rather reflected repetition-induced facilitated access to stimulus meaning. When squiggles were rated during encoding in Experiment 1, response times to High-M and Low-M stimuli were equivalent (1375 msec and 1364 msec on average, respectively, P = 0.64). Likewise, response times to High-M and Low-M stimuli were equivalent for the first presentation of each stimulus during Experiment 2 (949 msec and 919 msec on average, respectively, P = 0.49).
These behavioral results taken together demonstrate that the response facilitation for High-M squiggles cannot be readily attributed to perceptual priming, to explicit remembering of the initial encounter, to response priming, or to any confounding effects of stimulus factors. The priming effect for High-M squiggles thus belongs in the category of conceptual priming.
Explicit memory for squiggles
During Experiment 3, ERPs were recorded in a study-test structure identical to that used to assess conceptual priming in Experiment 1, except that recognition was assessed in the test phase. “Remember” and “know” responses were used by subjects to indicate phenomenological features of episodic memory retrieval, recollection, and familiarity, respectively (Yonelinas 2002). Although the validity of this approach has been questioned (Wixted 2007), here we only assume that remember and know responses made by subjects provide a good approximation to the corresponding self-rated subjective experiences of recollection and familiarity (not hypothetical processes also known as recollection and familiarity).
Recognition sensitivity (d′) was calculated separately for each response type to determine the extent to which the behavioral responses used to categorize ERPs reflected veridical memory. Subjects successfully distinguished old from new squiggles (Fig. 2A) using both remember and know judgments (mean remember d′ = 2.6, SE = 0.43; mean know d′ = 0.7, SE = 0.14). The frequency of guess responses, however, was similar for old and new squiggles (mean guess d′ = −0.4, SE = 0.04).
Figure 2.
Study- and test-phase behavioral results in Experiment 3. (A) Mean proportion of old and new squiggles given remember (R), know (K), guess (G), or new (N) responses during test phase in Experiment 2. (B) Mean proportion of squiggles (200 total) endorsed with each meaningfulness rating level during study phase. (C) Mean proportion of old squiggles given remember, know, guess, and new responses during test phase, subdivided by study-phase meaningfulness ratings (High-M or Low-M). Error bars indicate SE. (D) Across-subjects meaningfulness rating histogram for every squiggle (300 total), sorted by consistency. Light grays indicate that a low proportion of subjects endorsed the squiggle with the corresponding rating, whereas dark gray indicate a high proportion of consistent ratings (mean rating σ = 1.46).
To assess the influence of stimulus meaningfulness on memory, study-phase ratings were used to divide old squiggles into High-M and Low-M categories of approximately equal numbers (Fig. 2B). An analysis of test performance calculated separately for these categories (Fig. 2C) indicated superior memory for High-M than for Low-M squiggles. This improvement was due to more remember responses (t(14) = 9.6, P < 0.001) and fewer guess and new responses (t(14) = 8.7, P < 0.001 and t(14) = 7.5, P < 0.001, respectively). In contrast, the number of know responses was approximately equivalent for High-M and Low-M (P = 0.43). These findings show that higher meaningfulness led to stronger recollection. One might question whether familiarity per se was equivalent for High-M and Low-M squiggles in a general sense, given that familiarity also likely occurred for trials categorized by remember responses. However, if we take know responses as an indication of the behavioral phenomenon of pure familiarity without recollection, then these results simply imply that the High-M and Low-M conditions produced a very similar number of trials that engendered the experience of familiarity without recollection.
An analysis of study-phase rating variability highlighted the importance of assessing meaningfulness separately for each subject. Although some squiggles were endorsed by the majority of subjects as either High-M or Low-M (Fig. 1A), most squiggles were not given consistent ratings (Fig. 1B). Typically, squiggles were rated as High-M and Low-M, respectively, by approximately equal numbers of participants (Fig. 2D). Thus, relying on normative meaningfulness ratings (Groh-Bordin et al. 2006) rather than assessing stimulus meaning on an individual basis would not accurately characterize effects of inferred meaning on implicit memory or on neural correlates of memory.
Repetition ERPs
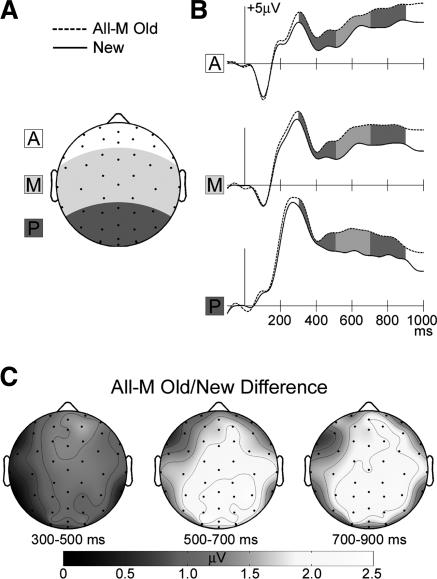
In order to facilitate comparison with prior studies that did not use meaningfulness ratings or remember/know judgments, we first identified neural correlates of explicit memory by averaging ERPs to all correctly endorsed old and new squiggles (Fig. 3). Because these comparisons were made between stimuli irrespective of phenomenological categorization of recognition experiences or of meaningfulness, neural correlates may have included responses related to recollection, familiarity, conceptual priming, and other mnemonic phenomena. ERPs to old squiggles were more positive compared with ERPs to new squiggles over most of the scalp starting at ∼300 msec post-stimulus. Inspection of scalp topographic maps suggested that ERP differences consisted of an early component, centered over the front of the head, and beginning at ∼300 msec, and a late component, centered over the rear of the head, and beginning at ∼400 msec.
Figure 3.
ERP correlates of episodic memory. (A) Anterior, middle, and posterior scalp regions, as seen on a schematic view of the head shown from above. ERPs in this and subsequent figures were computed by spatially averaging responses over these three regions (A, anterior, white; M, middle, light gray; P, posterior, dark gray). (B) Waveforms to correctly identified old squiggles of both meaningfulness levels (All-M) and to correctly rejected new squiggles averaged spatially by region. (C) Topographic maps of the All-M old vs. new ERP difference, averaged over three latency intervals (highlighted by shading on ERP waveforms: 300–500 msec, 500–700 msec, and 700–900 msec).
Statistical comparisons made between ERPs averaged for three regions and three latency intervals (Fig. 3A) yielded a significant repetition effect (F(1,14) = 19.6, P < 0.001) and 3-way interaction (old/new by region by interval, F(1.9,27.2) = 3.51, P < 0.05). Post-hoc comparisons for each of three latency intervals (300–500, 500–700, and 700–900 msec) and three scalp regions (anterior, middle, and posterior) indicated that old ERPs were reliably more positive than new ERPs only in the anterior region from 300 to 500 msec and in the middle and posterior regions from 500 to 700 msec and from 700 to 900 msec (all P’s < 0.006). As in many studies of recognition that emphasize such effects, it is difficult to make inferences about their functional significance, and we thus now turn to ERPs categorized based on “remember/know” recognition responses.
Recollection and familiarity ERPs
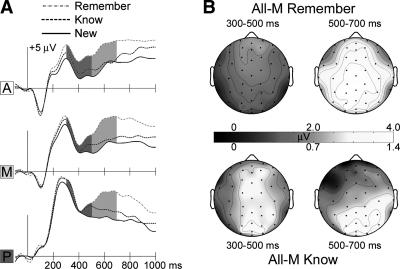
The overall old/new repetition effect described above was fractionated according to the putative behavioral indices of recollection and familiarity (i.e., remember and know responses, respectively). As is common practice in ERP studies of recognition, this analysis was conducted independent of stimulus meaningfulness—neural correlates of putative explicit memory may thus misleadingly include ERP correlates of conceptual priming. Compared with new squiggles, old squiggles in remember or know categories elicited more positive ERPs beginning at ∼250 msec, with overall greater amplitudes for remember (Fig. 4). Scalp topographic maps of the remember/new difference consisted of an early onset (∼300 msec) frontal-maximum positive difference and a late onset (∼500 msec) centroparietal-maximum positive difference, similar to the overall old/new difference. Know/new ERP differences also included a late-onset centroparietal-maximum positivity, but at early latencies differences appeared relatively diffuse.
Figure 4.
ERP correlates of episodic recollection and familiarity. (A) Spatially-averaged waveforms (see Fig. 3A) to All-M old squiggles correctly identified with remember and know responses, and to correctly rejected new squiggles. (B) Topographic maps of the old vs. new ERP difference averaged over two latency intervals separately for remember (top) and know (bottom) responses. ERPs to remember responses were much larger than to know responses, and so the color scales for amplitude were set differently to allow the topographic patterns of the two effects to be observed clearly.
Formal comparisons of these ERP differences substantiated this pattern of results. Comparisons were conducted over three regions and over the latency intervals from 300 to 500 msec and from 500 to 700 msec in order to distinguish early from late ERP effects. Old-new ERP effects for both remember and know conditions differed across regions and latency intervals (3-way interactions: F(1.3,17.5) = 14.6, P < 0.001; F(1.2,16.4) = 2.86, P = 0.08, respectively). Post-hoc comparisons were made for each latency interval and region. From 300 to 500 msec, know ERPs were more positive than new ERPs in the anterior region and remember ERPs were more positive than new ERPs in the anterior and middle regions; from 500 to 700 msec, both remember and know ERPs were more positive than new ERPs in the posterior region (all P’s < 0.008). Differences between remember and know ERPs depended on latency interval and region (3-way interaction: F(1.1,14.2) = 6.5, P = 0.02). Post-hoc comparisons for each latency interval and region indicated greater amplitudes for remember during the later interval in the posterior region (P < 0.001), but not in other regions or latency intervals.
Conceptual priming ERPs
Meaningfulness ratings were used to identify neural correlates of conceptual priming in contradistinction to those of explicit memory. Results could thus be used to infer the functional significance of the aforementioned ERP old/new effects obtained without reference to stimulus meaning or conceptual priming. This was accomplished by averaging ERPs to squiggles given know responses separately for High-M (conceptual priming present) and Low-M (negligible conceptual priming) categories. Remember responses were not included because too few trials were available in the Low-M-Remember category. Because know responses were given with identical response criteria and in similar numbers for both High-M and Low-M squiggles, this contrast identified ERP correlates of conceptual priming with explicit memory held constant. Four subjects were excluded from this analysis due to an inadequate number of High-M or Low-M trials (<20).
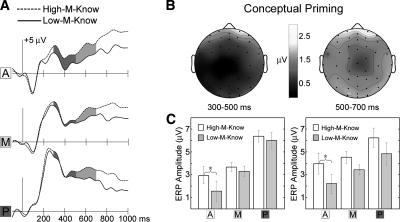
The ERP difference associated with conceptual priming (Fig. 5) was apparent over frontal electrodes at all latencies after ∼200 msec and over posterior electrodes starting at ∼400 msec. Based on our a priori hypotheses, mean ERP amplitude for High-M and Low-M know responses was compared over anterior, middle, and posterior regions for the 300–500- and 500–700-msec intervals. The conceptual priming difference was reliable in the anterior region during both intervals (P’s < 0.03) but not in middle and posterior regions during either interval (P’s > 0.10).
Figure 5.
ERP correlates of conceptual priming. (A) Spatially-averaged (see Fig. 3A) waveforms to squiggles given know responses made separately for High-M and Low-M conditions. (B) Topographic maps of the conceptual priming ERP difference (High-M-know minus Low-M-know) averaged over two latency intervals. (C) Mean High-M-know and Low-M-know ERP amplitudes at each latency interval and region. Asterisks mark statistically significant differences. Error bars indicate SE, after correcting for between-subject variability in mean values over all conditions.
The validity of this conceptual priming contrast depends on equivalent explicit memory strength for High-M-Know and Low-M-Know squiggles. Clearly, overall explicit memory was stronger for High-M compared with Low-M squiggles. However, by focusing on this subset of squiggles given know responses, which were equivalently prevalent across meaningfulness levels, explicit memory was equated. Moreover, subjects would have no reason to alter their criteria for registering a know response according to the level of meaningfulness attributed to the stimulus. Discrimination sensitivity (d′ calculated with a common new item baseline) did not differ for High-M-Know and Low-M-Know squiggles (mean = 0.75 and 0.63, respectively; t(10) = 0.46, P = 0.66). It is important to note that this experiment was not designed to assess the extent to which explicit memory and conceptual priming are functionally independent, which would be complicated by virtue of the difficulties associated with providing simultaneous behavioral estimates of both memory phenomena. Instead, we sought to categorize neural measures of these memory phenomena, for which some evidence indicates a degree of independence (see Discussion). Thus, we utilized a contrast aimed at isolating neural correlates of conceptual priming within a recognition test, even though a behavioral measure was not including during this test, and irrespective of the possibility that conceptual priming and explicit memory may have tended to go together for many stimuli.
It is possible that ERP correlates of conceptual priming reflected an influence of inferred meaning on ERPs rather than an effect of memory per se. To assess this possibility, ratings collected following ERP recordings were used to categorize new squiggles viewed during the test phase into High-M and Low-M categories. There were no systematic amplitude differences over the three regions and two intervals (all P’s > 0.23), and no striking differences were observed at any electrode for any latency. Thus, ERP correlates of conceptual priming were indeed repetition-related memory effects. Collectively, this pattern of results indicates that early-onset frontal effects identified in old/new contrasts at 300–500 msec reflect conceptual priming.
Late posterior ERPs associated with familiarity-based recognition
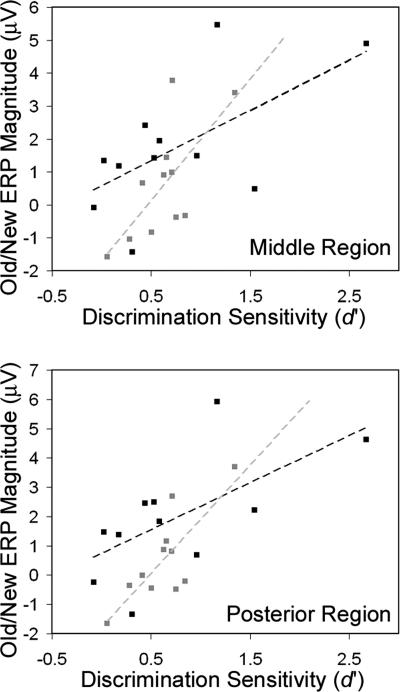
Relationships between familiarity-based recognition and ERP correlates were substantiated by additional correlational analyses. A close connection between familiarity and late posterior potentials (and not between familiarity and early anterior potentials) was supported by across-subject correlations between behavioral and ERP measures. To index memory strength for pure familiarity responses, d′ was calculated separately for High-M and Low-M squiggles recognized with know responses (using false-alarm rates derived from know responses to new stimuli). ERP differences were quantified for each subject, region, and latency interval for the contrast between squiggles recognized with know responses and new squiggles. In order to account for individual differences in the spatial focus of each ERP effect, a single electrode within each region was selected for each participant according to where the greatest between-condition difference was observed. For both High-M and Low-M squiggles, know response d′ was significantly correlated with know-versus-new ERP amplitude differences from 500 to 700 msec at middle (High-M r(9) = 0.62, P = 0.04; Low-M r(9) = 0.69, P = 0.02) and posterior (High-M r(9) = 0.64, P = 0.03; Low-M r(9) = 0.79, P = 0.004) regions (Fig. 6). No correlations involving the anterior region or the early latency interval reached statistical significance (P’s > 0.20).
Figure 6.
Correlations between behavioral estimates of familiarity-based recognition and ERPs. Across subjects, d′ for High-M-Know responses (black squares and lines) and Low-M-Know responses (gray squares and lines) correlated significantly with the corresponding ERP old/new effect for middle (top) and posterior (bottom) scalp regions from 500 to 700 msec. No correlations involving the anterior region or 300–500-msec latency interval reached statistical significance.
As indicated in Figure 6, memory performance as described by discrimination sensitivity was weak in many subjects and highly variable across subjects for High-M-Know and Low-M-Know stimuli. Accordingly, average ERP differences between High-M-Know and new stimuli and between Low-M-Know and new stimuli were nonsignificant over the regions and latency intervals used for primary analyses. Nonetheless, relationships between ERP old/new effects and memory performance were identified via the correlational analysis and supported a strong association between posterior-distributed old/new ERP effects and familiarity-based recognition, irrespective of inferred meaning.
Discussion
Using behavioral measures of multiple types of memory, we identified ERP correlates of explicit memory and of conceptual implicit memory during a recognition test. Remember/know judgments were used to index the phenomenological experiences of explicit recognition known in the literature as recollection and familiarity. When data were analyzed irrespective of any consideration of conceptual priming, as is a common practice in contemporary studies of neural correlates of recognition, two types of ERPs were associated with recognition, late-onset posterior maximum positive potentials (a “parietal old/new effect”) and early-onset frontal-maximum positive potentials (a “frontal N400 old/new effect”).
The present experiment provided the first evidence to link specific ERPs to conceptual priming for visual objects (although the results may correspond somewhat to those previously found with possible and impossible geometric objects using positron emission tomography; Uecker et al. 1997). Based on our prior experiments with other visual stimuli (Olichney et al. 2000; Yovel and Paller 2004; Voss and Paller 2006), we predicted that early frontal N400 old/new effects would be associated with conceptual priming. Of course, further studies are needed to understand differences due to different types of stimulus materials. Nevertheless, we attributed frontal effects observed here (Fig. 5) as correlates of conceptual implicit memory based on the following reasoning. First, behavioral results demonstrated conceptual priming selectively for the stimuli of highest perceived meaning. Although these squiggles were not very meaningful, subjects nevertheless were able to envision, on an idiosyncratic basis, some resemblance to meaningful visual objects. We excluded other possible explanations for the speed-up in responses to repeated squiggles, such that conceptual priming could be inferred. Second, we compared neural responses across conditions that differed selectively in conceptual priming. In an analysis of trials wherein old squiggles were recognized with pure familiarity, we contrasted ERPs to squiggles capable of engendering conceptual priming versus ERPs to squiggles subject to limited or no conceptual priming. This contrast thus constituted a manipulation of conceptual priming with explicit memory held constant.
Whereas early frontal ERPs were associated with conceptual priming, later parietal ERPs were associated with familiarity-based recognition. This association was marked first by virtue of the ERP contrast with old squiggles that gave rise to behavioral responses signaling pure familiarity (Fig. 4). This relationship was substantiated by a correlational analysis run across subjects, whereby a behavioral measure of familiarity sensitivity was systematically and selectively related to the magnitude of late parietal old/new ERP differences. As opposed to what might have been predicted based on the hypothesis that frontal ERPs index familiarity (Curran et al. 2006), the magnitude of behavioral familiarity was not correlated with frontal ERP differences. In sum, ERPs elicited during this recognition test were found to reflect both explicit memory and conceptual priming in the form of parietal old/new effects and frontal N400 old/new effects, respectively.
These results have important implications for understanding memory functions and for interpreting neuroimaging evidence obtained using recognition tests. The identification of distinct neural correlates of episodic recollection in contradistinction to episodic familiarity has been taken as strong empirical support for dual-process accounts of recognition memory (Yonelinas 2002). Dual-process models assert that qualitatively distinct recollection and familiarity processes support recognition memory. In ERP experiments, recollection has been ubiquitously linked to parietal old/new effects (Senkfor and Van Petten 1998; Friedman and Johnson 2000; Mecklinger 2000; Paller 2000; Rugg and Allan 2000; Cycowicz et al. 2001). A large set of experimental reports have attributed frontal N400 old/new effects to familiarity (for recent review, see Curran et al. 2006). However, an alternate interpretation supported by the present and other findings (Olichney et al. 2000; Yovel and Paller 2004; Voss and Paller 2006) is that frontal N400 old/new effects instead reflect conceptual implicit memory elicited incidentally during episodic memory testing (for review, see Paller et al., in press). Although the published evidence on this point is mixed, discrepancies concerning these issues can be partly attributed to the failure of studies that used meaningful stimuli to adequately control for or measure conceptual priming. Given that interpretations of many neuroimaging results have failed to take conceptual priming into account, support from these studies for dual-process theories and for hypotheses about the neural substrates of familiarity, can thus be called into question.
Concern about adequately accounting for conceptual priming in studies of recognition is not limited to ERP research. Notably, fMRI has provided neuroanatomical support for dual-process models of recognition memory in that recollection and familiarity occur with activity in segregated brain regions (Eldridge et al. 2000; Yonelinas et al. 2001; Davachi et al. 2003; Henson et al. 2003; Ranganath et al. 2004; Yonelinas et al. 2005). Neuroanatomical correlates of recognition have been found not to include activity related to conceptual priming, as identified during specialized implicit memory tests (Donaldson et al. 2001). There is scant evidence, however, to show whether neural correlates of conceptual implicit memory differ when elicited during implicit memory testing versus during episodic memory testing. Future fMRI comparisons should address this concern by contrasting neural correlates of conceptual priming and explicit memory identified during the performance of a single task.
The results presented here provide a foundation for critically assessing the hypothesis that episodic familiarity is driven by conceptual implicit memory (Wagner et al. 1997; Verfaellie and Cermak 1999; Rajaram and Geraci 2000; Wolk et al. 2005). Some support for this hypothesis would be provided if neural correlates of conceptual implicit memory were found to precede those of episodic familiarity, and if both reliably occurred together during recognition. Effective connectivity analyses might also provide relevant evidence. However, compelling dissociations between conceptual priming and explicit memory have been provided by neuropsychological studies in amnesic patients (Graf et al. 1985; Vaidya et al. 1995; Keane et al. 1997; Levy et al. 2004). Research that might verify such dissociations in healthy individuals by directly examining putative functional relationships can now be pursued by making use of neural correlates of memory as characterized in the present study.
Our results also highlight the necessity of using behavioral measures that provide genuine reflections of the multiple memory processes co-occurring during memory testing. Accordingly, future investigations should include manipulations that differentially influence conceptual priming and explicit memory and provide behavioral measures of both memory phenomena under similar experimental circumstances (see Paller et al., in press). Conceptual priming may contaminate neural measures of explicit memory, even for the most unlikely stimuli. Neuroimaging can advance our understanding of memory and the brain only if it provides legitimate links between memory phenomena and neural activity.
Materials and Methods
Experiment 1
Subjects
Behavioral data were collected from 12 right-handed native English speakers (four males, ages 18–23 yr) recruited from the Northwestern University community.
Materials
Visual stimuli consisted of 270 squiggles (Fig. 1). Squiggles were presented on a computer monitor in black on a white background within a square subtending ∼5° of visual angle. Squiggles were taken from a total set of 300 squiggles from a recent study of explicit memory (Groh-Bordin et al. 2006) and were created via hand-deformation of a square, circle, or triangle.
Experimental design
The experiment consisted of nine study-test blocks, during which subjects viewed all 270 squiggles. Blocks were separated by a short break. Each block consisted of a study phase followed by one of three possible tests: loop discrimination, implicit rating, and explicit rating. Each test was administered three times in randomized order, and subjects were unaware of the total number of blocks, such that the test format could not be determined during the study phases.
Study phase
In each block, subjects viewed 20 squiggles, each for 2000 msec with a variable 1500–3000-msec interstimulus interval (ISI). Subjects rated each squiggle using the 4-point meaningfulness scale with 1 corresponding to “high meaningfulness” and 4 to “no meaningfulness.” Subjects were instructed to rate squiggles as 1 if the squiggle “looks like a nameable object, face, or animal” and as 2 if the squiggle “looks like a more abstract nameable object, face, or animal.” A rating of 3 indicated that the squiggle “does not look like anything nameable, but is in some way meaningful.” Subjects were provided the example that the squiggle “may be angled such that it appears to be angry.” A rating of 4 corresponded to “a random collection of lines that is in no way meaningful.” Subjects were instructed to distribute ratings across the four levels. We operationally defined squiggles given meaningfulness ratings of 1 or 2 as high in subjective meaningfulness (High-M) and those given ratings of 3 or 4 as low in subjective meaningfulness (Low-M). Subjects were made aware that memory for squiggles would be tested subsequently, and that the test format would vary randomly.
Test phase
The test phase followed the study phase in each block after a break of ∼45 sec, during which subjects counted backward aloud by threes from a designated integer for 20 sec and then were given test-phase instructions. Each test consisted of 20 squiggles repeated from the previous study phase (old) and 10 entirely novel squiggles (new), presented in randomized order, each for 1000 msec with a variable 1000–2000-msec ISI. The three possible test formats are described below.
Meaningfulness rating test
To index conceptual priming for squiggles, subjects rated the meaningfulness of each squiggle using the 4-point scale described above. Response speed was emphasized. Subjects were told that they had seen some of the stimuli previously, and that they should disregard this prior exposure as well as the rating made previously because attending to this information could slow responses. Responses were made using the right hand, with two assignments of meaningfulness rating to response finger counterbalanced across subjects. Adjacent fingers corresponded to adjacent ratings with either 1 pressed by the index finger and 4 by the little finger or vice versa.
Meaningfulness rating test with repetition acknowledged
To determine whether conceptual priming effects observed during the implicit rating test could be due to explicit remembering of study-phase stimuli or ratings, the implicit rating test was performed with the modification that subjects were not advised to disregard that some stimuli were repeated from the study phase. Rather, immediately after each test subjects provided a rough estimate of the approximate number of repeated and novel stimuli, but no feedback was provided. Rating response speed was emphasized, and, importantly, subjects were instructed to refrain from keeping a mental tally of old or new stimuli in order to provide their rough estimate at the end of the run, as this would slow them down.
Loop discrimination test
To index perceptual priming for squiggles, subjects indicated the presence or absence of a loop in the stimulus by pressing one of two buttons (50% of stimuli contained a loop, see Fig. 1). Response speed was emphasized.
Experiment 2
Subjects
Behavioral data were collected from 10 right-handed native English speakers (four males, ages 18–21 yrs) recruited from the Northwestern University community.
Materials
Stimuli were identical to those in Experiment 1.
Experimental design
A set of 150 squiggles (selected randomly for each subject from the total set) were shown for 1000 msec with a variable ISI of 1500–2500 msec. All squiggles were presented for a second time at a variable delay of 5–15 trials (average delay of 20 sec) from initial presentation. A randomly selected 75 squiggles were presented for a third time at a variable delay of 20–30 trials (average delay of 50 sec) from initial presentation.
Subjects rated squiggles using the 4-point meaningfulness scale described above. Subjects were informed that squiggles would repeat and were advised to make each rating irrespective of previous ratings. Response speed was emphasized. Responses were made using the right hand, with two assignments of meaningfulness rating to response finger counterbalanced across subjects. Adjacent fingers corresponded to adjacent ratings with either 1 pressed by the index finger and 4 by the little finger or vice versa.
Experiment 3
Subjects
Behavioral and ERP data were collected from 15 right-handed native English speakers (seven males, ages 18–35 yrs) recruited from the Northwestern University community.
Materials
Stimuli were identical to those in Experiment 1, except that a total of 300 squiggle stimuli were used. Three sets of 100 squiggles were created via random assignment for counterbalancing, such that each squiggle appeared as a new one for five subjects and as an old one for all other subjects.
Experimental design
The experiment consisted of 10 study-test blocks during which subjects viewed all 300 squiggles. Blocks were separated by a short break. Prior to experimental blocks, subjects completed an abbreviated practice block using an additional set of stimuli that were not included in the main experiment. Verbal instructions preceded every study and test phase throughout the experiment. Blocks were identical to blocks in Experiment 1 in stimulus timing parameters and study-test structure with one exception—each test phase was a recognition test instead of one of three priming tests. After conducting the experiment, considerations led to the collection of meaningfulness ratings for all squiggles, such that new test-phase stimuli could be divided into meaningfulness categories. All subjects returned and rated all stimuli after an average delay of 10 mo (range 8–11 mo). Despite the delay, ratings to stimuli that were rated during the initial experiment were highly consistent across the two sessions (91% of squiggles were assigned to the same meaningfulness category, SE = 2.3%).
ERP test phase
Subjects used four buttons to categorize each squiggle as old or new, with four response categories based on a modified “remember/know” paradigm (Tulving 1985; Gardiner and Java 1991). The categories (shown in Fig. 2) were: (1) high-confidence recollection of specific study-phase episodic detail (remember responses), (2) high-confidence recognition unsubstantiated by specific detail (know responses), (3) low-confidence guess responses, or (4) indication that the stimulus did not appear during the study-phase. The practice phase was used to ensure that subjects adopted appropriate criteria for each response category.
ERP data acquisition
Continuous electroencephalographic recordings were made during study and test phases from 59 evenly distributed scalp sites (Woldorff et al. 2002) using tin electrodes embedded in an elastic cap. Four additional channels were used for monitoring horizontal and vertical eye movements, and only artifact-free trials were included in ERP analyses (average of 89% of trials per subject, SE = 0.06%). Electrode impedance was ≤5 kΩ. EEG signals were amplified with a band pass of 0.05–200 Hz, sampled at a rate of 1000 Hz, and re-referenced offline to average mastoids. Each averaging epoch lasted 1100 msec, including 100 msec prior to stimulus onset. Baseline correction was performed by subtracting prestimulus mean amplitudes.
ERP analysis
Analyses focused on test-phase electroencephalographic responses. ERPs elicited by squiggles during the test phase were averaged separately for each response type (remember, know, guess, and new) and as a function of meaningfulness ratings made to old items when they had appeared in the study phase (High-M and Low-M). Trials were included in analyses if a correct response was given in the test phase. Statistical comparisons focused on amplitudes averaged over anterior, middle, and posterior regions (Fig. 3A). Visual inspection of ERPs from individual electrodes confirmed that spatially averaged data from the three scalp regions adequately characterize the experimental effects.
Formal comparisons of ERP amplitude were made using repeated-measures ANOVA (α = 0.05) with Huynh-Feldt corrections when necessary. Post-hoc pairwise comparisons were made between conditions for each region and latency interval and type-I error was controlled via Bonferroni correction. Only significant comparisons were reported. Waveforms were smoothed with a 10-Hz low-pass-zero-phase-shift Butterworth filter for presentation purposes only.
Acknowledgments
This material is based on work supported by the National Science Foundation under grant number 0518800 and by the National Institutes of Health grants R01-NS34639, P30-AG13854, and T32-AG20506. We thank Christian Groh-Bordin for providing squiggle stimuli and for initial discussions of these issues.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.529807
References
- Curran T., Tanaka J.W., Weiskopf D.M. An electrophysiological comparison of visual categorization and recognition memory. Cogn. Affect. Behav. Neurosci. 2002;2:1–18. doi: 10.3758/cabn.2.1.1. [DOI] [PubMed] [Google Scholar]
- Curran T., Tepe K.L., Piatt C. ERP explorations of dual processes in recognition memory. In: Zimmer H.D., et al., editors. Handbook of binding and memory: Perspectives from cognitive neuroscience. Oxford University Press; Oxford, UK: 2006. pp. 467–492. [Google Scholar]
- Cycowicz Y.M., Friedman D., Snodgrass J.G. Remembering the color of objects: An ERP investigation of source memory. Cereb. Cortex. 2001;11:322–334. doi: 10.1093/cercor/11.4.322. [DOI] [PubMed] [Google Scholar]
- Davachi L., Mitchell J.P., Wagner A.D. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins I.G., Schnyer D.M., Verfaellie M., Schacter D.L. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Donaldson D.I., Petersen S.E., Buckner R.L. Dissociating memory retrieval processes using fMRI: Evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Eldridge L.L., Knowlton B.J., Furmanski C.S., Bookheimer S.Y., Engel S.A. Remembering episodes: A selective role for the hippocampus during retrieval. Nat. Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Friedman D., Johnson R., Jr. Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc. Res. Tech. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D. Cognitive neuroscience of human memory. Annu. Rev. Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gardiner J.M., Java R.I. Forgetting in recognition memory with and without recollective experience. Mem. Cognit. 1991;19:617–623. doi: 10.3758/bf03197157. [DOI] [PubMed] [Google Scholar]
- Graf P., Shimamura A.P., Squire L.R. Priming across modalities and priming across category levels: Extending the domain of preserved function in amnesia. J. Exp. Psychol. Learn. Mem. Cogn. 1985;11:386–396. doi: 10.1037//0278-7393.11.2.386. [DOI] [PubMed] [Google Scholar]
- Groh-Bordin C., Zimmer H.D., Ecker U.K. Has the butcher on the bus dyed his hair? When color changes modulate ERP correlates of familiarity and recollection. Neuroimage. 2006;32:1879–1890. doi: 10.1016/j.neuroimage.2006.04.215. [DOI] [PubMed] [Google Scholar]
- Henson R.N. Neuroimaging studies of priming. Prog. Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson R.N., Cansino S., Herron J.E., Robb W.G., Rugg M.D. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Keane M.M., Gabrieli J.D., Monti L.A., Fleischman D.A., Cantor J.M., Noland J.S. Intact and impaired conceptual memory processes in amnesia. Neuropsychology. 1997;11:59–69. doi: 10.1037//0894-4105.11.1.59. [DOI] [PubMed] [Google Scholar]
- Levy D.A., Stark C.E., Squire L.R. Intact conceptual priming in the absence of declarative memory. Psychol. Sci. 2004;15:680–686. doi: 10.1111/j.0956-7976.2004.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A. Interfacing mind and brain: A neurocognitive model of recognition memory. Psychophysiology. 2000;37:565–582. [PubMed] [Google Scholar]
- Olichney J.M., Van Petten C., Paller K.A., Salmon D.P., Iragui V.J., Kutas M. Word repetition in amnesia. Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Paller K.A. Neural measures of conscious and unconscious memory. Behav. Neurol. 2000;12:127–141. doi: 10.1155/2000/865250. [DOI] [PubMed] [Google Scholar]
- Paller K.A., Hutson C.A., Miller B.B., Boehm S.G. Neural manifestations of memory with and without awareness. Neuron. 2003;38:507–516. doi: 10.1016/s0896-6273(03)00198-3. [DOI] [PubMed] [Google Scholar]
- Paller K.A., Voss J.L., Boehm S.G. Validating neural correlates of familiarity. Trends Cogn. Sci. 2003 doi: 10.1016/j.tics.2007.04.002. (in press) [DOI] [PubMed] [Google Scholar]
- Rajaram S., Geraci L. Conceptual fluency selectively influences knowing. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:1070–1074. doi: 10.1037//0278-7393.26.4.1070. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Yonelinas A.P., Cohen M.X., Dy C.J., Tom S.M., D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Allan K. Event-related potential studies of memory. In: Tulving E., Craik F.I.M., editors. The Oxford handbook of memory. Oxford University Press; Oxford, UK: 2000. pp. 521–537. [Google Scholar]
- Rugg M.D., Mark R.E., Walla P., Schloerscheidt A.M., Birch C.S., Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Buckner R.L. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Schott B.H., Henson R.N., Richardson-Klavehn A., Becker C., Thoma V., Heinze H.J., Duzel E. Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. Proc. Natl. Acad. Sci. 2005;102:1257–1262. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinberger S.R., Pickering E.C., Burton A.M., Kaufmann J.M. Human brain potential correlates of repetition priming in face and name recognition. Neuropsychologia. 2002;40:2057–2073. doi: 10.1016/s0028-3932(02)00050-7. [DOI] [PubMed] [Google Scholar]
- Senkfor A.J., Van Petten C. Who said what? An event-related potential investigation of source and item memory. J. Exp. Psychol. Learn. Mem. Cogn. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and brain. Oxford University Press; New York: 1987. [Google Scholar]
- Tulving E. Memory and consciousness. Can. J. Psychol. 1985;26:1–12. [Google Scholar]
- Uecker A., Reiman E.M., Schacter D.L., Polster M.R., Cooper L.A., Yun L.S., Chen K. Neuroanatomical correlates of implicit and explicit memory for structurally possible and impossible visual objects. Learn. Mem. 1997;4:337–355. doi: 10.1101/lm.4.4.337. [DOI] [PubMed] [Google Scholar]
- Vaidya C.J., Gabrieli J.D., Keane M.M., Monti L.A. Perceptual and conceptual memory processes in global amnesia. Neuropsychology. 1995;9:580–591. doi: 10.1037//0894-4105.11.1.59. [DOI] [PubMed] [Google Scholar]
- Verfaellie M., Cermak L.S. Perceptual fluency as a cue for recognition judgments in amnesia. Neuropsychology. 1999;13:198–205. doi: 10.1037//0894-4105.13.2.198. [DOI] [PubMed] [Google Scholar]
- Voss J.L., Paller K.A. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. J. Neurosci. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Gabrieli J.D., Verfaellie M. Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. J. Exp. Psychol. Learn. Mem. Cogn. 1997;23:305–323. doi: 10.1037//0278-7393.23.2.305. [DOI] [PubMed] [Google Scholar]
- Wixted J.T. Dual-process theory and signal-detection theory of recognition memory. Psychol. Rev. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Woldorff M.G., Liotti M., Seabolt M., Busse L., Lancaster J.L., Fox P.T. The temporal dynamics of the effects in occipital cortex of visual-spatial selective attention. Brain Res. Cogn. Brain Res. 2002;15:1–15. doi: 10.1016/s0926-6410(02)00212-4. [DOI] [PubMed] [Google Scholar]
- Wolk D.A., Schacter D.L., Berman A.R., Holcomb P.J., Daffner K.R., Budson A.E. Patients with mild Alzheimer's disease attribute conceptual fluency to prior experience. Neuropsychologia. 2005;43:1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P. The nature of recollection and familiarity: A review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas A.P., Hopfinger J.B., Buonocore M.H., Kroll N.E., Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: An fMRI study. Neuroreport. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P., Otten L.J., Shaw K.N., Rugg M.D. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G., Paller K.A. The neural basis of the butcher-on-the-bus phenomenon: When a face seems familiar but is not remembered. Neuroimage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]