Abstract
Pavlovian fear conditioning is a robust and enduring form of emotional learning that provides an ideal model system for studying contextual regulation of memory retrieval. After extinction the expression of fear conditional responses (CRs) is context-specific: A conditional stimulus (CS) elicits greater conditional responding outside compared with inside the extinction context. Dorsal hippocampal inactivation with muscimol attenuates context-specific CR expression. We have previously shown that CS-elicited spike firing in the lateral nucleus of the amygdala is context-specific after extinction. The present study examines whether dorsal hippocampal inactivation with muscimol disrupts context-specific firing in the lateral amygdala. We conditioned rats to two separate auditory CSs and then extinguished each CS in separate and distinct contexts. Thereafter, single-unit activity and conditional freezing were tested to one CS in both extinction contexts after saline or muscimol infusion into the dorsal hippocampus. After saline infusion, rats froze more to the CS when it was presented outside of its extinction context, but froze equally in both contexts after muscimol infusion. In parallel with the behavior, lateral nucleus neurons exhibited context-dependent firing to extinguished CSs, and hippocampal inactivation disrupted this activity pattern. These data reveal a novel role for the hippocampus in regulating the context-specific firing of lateral amygdala neurons after fear memory extinction.
For decades scientists have known that memory retrieval is facilitated when the context in which information is learned corresponds to the context in which it is retrieved (Spear 1973; Tulving and Thompson 1973; Godden and Baddeley 1975). Accumulating evidence shows that contextual information exerts control over memory retrieval in Pavlovian fear conditioning paradigms as well (Bouton and Bolles 1979; Maren and Holt 2000). Pavlovian fear conditioning is an enduring form of emotional learning that occurs when a neutral conditional stimulus (CS), such as a tone, is paired with an aversive unconditional stimulus (US), such as a footshock. After even a single pairing of the CS and US, the presentation of the CS alone will elicit a variety of conditional responses (CRs), including freezing, or somatomotor immobility.
After fear conditioning, the ability of a CS to elicit a CR is not context-specific: Animals will show fear CRs in any context in which the CS is presented. The presentation of a CS in extinction, however, results in the context-specific expression of fear CRs. Importantly, extinction does not result in “unlearning” of the CS–US memory. Rather, extinction results in the formation of a “CS–no US” memory, which renders the meaning of the CS ambiguous: It predicts shock in one context, but not in another. Context resolves this ambiguity. Consequently, a CS will elicit greater conditional responding outside compared with inside the extinction context (Bouton and Bolles 1979; Harris et al. 2000; Corcoran and Maren 2001, 2004), a phenomenon that has been termed “renewal.”
Considerable evidence suggests that an interaction between the amygdala and the hippocampus mediates fear memory renewal. A large body of research suggests that the amygdala is a locus for fear memory acquisition (Davis and Whalen 2001; Maren 2001; Maren and Quirk 2004; Rodrigues et al. 2004). Moreover, fear memory extinction elicits changes in spike firing in the lateral nucleus of the amygdala (Quirk et al. 1995, 1997; Maren 2000; Pare and Collins 2000), and lateral nucleus neurons display context-specific spike firing after extinction (Hobin et al. 2003). Another line of work indicates that the dorsal hippocampus is involved in acquiring contextual representations (Anagnostaras et al. 1999; Sanders et al. 2003; Rudy et al. 2004) and using these representations to retrieve memories (Hirsh 1974; Good and Honey 1991; Maren and Holt 2000; Rudy and O’Reilly 2001; Kennedy and Shapiro 2004). Supporting a role for the hippocampus in the renewal of extinguished fear responses, we have found that either inactivation of the dorsal hippocampus with the GABAA agonist muscimol (Corcoran and Maren 2001, 2004) or dorsal hippocampal lesions (Ji and Maren 2005) disrupt the context specificity of extinction. Collectively, these data suggest that the dorsal hippocampus may influence context-specific fear responses after extinction by modulating firing of lateral nucleus neurons. In the present study, we examined this question by reversibly inactivating the hippocampus and recording spike firing in the lateral amygdala evoked by extinguished CSs presented either inside or outside of the context in which they were extinguished.
Results
Histology
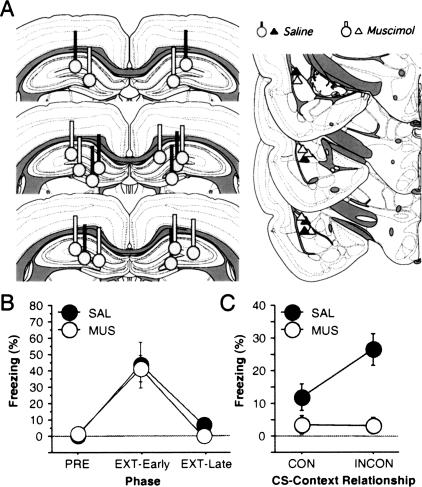
Nine rats (SAL, n = 4; MUS, n = 5) had cannulae implanted bilaterally in the dorsal hippocampus and recording electrodes in the lateral amygdala (Fig. 1A). Twenty-four rats were not included in the analysis because their electrodes and/or cannulae were not properly positioned, or because single units could not be reliably isolated from the recording electrodes.
Figure 1.
(A) Bilateral cannulae placements in dorsal hippocampus (left) and unilateral electrode placements in the lateral nucleus of the amygdala (right). Coronal sections were based on Swanson (1992). (B) Mean (±SEM) percentage of freezing for all rats before conditioning (PRE; average of 3-min period before first conditioning trial) and after the first (EXT-Early) and last (EXT-Late) five trials extinction. There were no differences between rats that were to be treated with either saline (SAL) or muscimol (MUS) during subsequent retention tests. (C) Mean (±SEM) percentage of freezing during the retention tests in rats treated with either saline (SAL) or muscimol (MUS). Rats in each group were tested to a single CS in both the context that the CS was extinguished (consistent or CON) and another context (inconsistent or INCON).
Behavior
As shown in Figure 1B, rats exhibited low levels of freezing prior to footshock (PRE), but exhibited high levels of freezing after the delivery of the tone and white noise conditioning trials (COND; the tone and white noise trials did not differ from one another and were collapsed). Conditional freezing, however, was greatly reduced in amplitude after the delivery of 30 extinction trials to each CS (EXT). This was indicated by a significant effect of training phase in the ANOVA (F(2,14) = 210, p < 0.0001). There were no differences in rats that were to be treated with SAL or MUS on the subsequent retention tests at this point in training (F’s < 2.7, p’s > 0.14). Moreover, there were no differences between the CSs or the particular CS–context combinations during either conditioning or extinction.
Consistent with previous reports (e.g., Bouton and Bolles 1979; Corcoran and Maren 2001), extinction was context-dependent. As shown in Figure 1C, rats receiving SAL infusions into the dorsal hippocampus prior to retention testing exhibited low levels of conditional freezing to an extinguished CS when it was presented in its extinction context (CON), but significantly higher levels of fear to the same CS when it was presented outside of its extinction context (INCON). This “renewal” of fear was prevented by muscimol infusions into the dorsal hippocampus (context × drug interaction: F(1,7) = 5.8, p < 0.05]. This replicates earlier results from our laboratory (Corcoran and Maren 2001, 2004) and confirms that the dorsal hippocampus has a critical role in the context-dependence of extinction.
Single-unit activity
We recorded a total of 60 single units from nine rats in the dorsal and ventral divisions of the lateral nucleus (SAL, n = 26; MUS, n = 34). In the SAL rats, we recorded seven cells across seven active wires from each of two of the animals, eight cells across seven wires in one animal, and four cells across four wires in one animal. In the MUS rats, we recorded nine cells across seven wires in one rat, five cells across five wires in a second rat, seven cells across seven wires from a third rat, six cells across five wires from a fourth rat, and seven cells across seven wires from a fifth rat. Of the 60 neurons we isolated, half of them (30 of 60) were CS-responsive (>1.5 SD above baseline in at least one of the recording sessions). Specifically, ∼40% of the cells in the SAL rats (10 of 26) and ∼60% of the cells in the MUS (20 of 34) rats significantly increased their firing within the first 100 msec of the auditory CS in one of the test sessions. The remaining neurons showed nonsignificant changes in spike firing to the CS, and none of the cells showed a significant (>1.5 SD) suppression of spike firing to the CS.
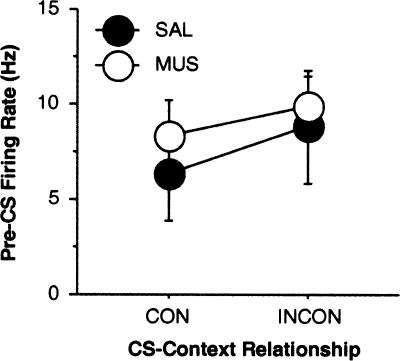
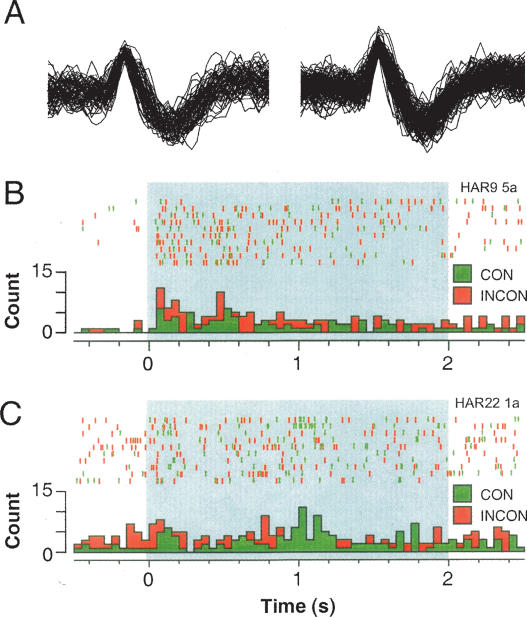
The firing rate of the neurons we sampled (calculated by averaging activity in the pre-CS periods over each test trial) was similar to that reported in other studies of lateral amygdala single-unit activity (Quirk et al. 1995; 1997; Maren 2000; Repa et al. 2001; Goosens et al. 2003; Hobin et al. 2003). The distribution of firing frequencies among these cells was unimodal with an average firing frequency (across sessions) of 7.9 ± 0.7 Hz and a modal firing frequency of 5.2 Hz. Classifying neuron types in the basolateral amygdala using firing frequency has proved difficult insofar as many putative projection neurons have firing rates within the range of interneuron firing rates (Likhtik et al. 2006). For this reason, we assume that we have recorded from a heterogeneous population of cells including both interneurons and projection neurons. As shown in Figure 2, there was no difference in baseline firing rate of CS-responsive neurons (in the 500-msec pre-CS period) in rats treated with saline or muscimol before the consistent or inconsistent test sessions (F’s < 1, p’s > 0.31). Moreover, the waveforms of neurons recorded across the consistent and inconsistent extinction test sessions were stable, as illustrated by a representative neuron shown in Figure 3A.
Figure 2.
Mean (± SEM) spontaneous (pre-CS) firing rate among lateral nucleus neurons after saline (SAL) or muscimol (MUS) infusion into the dorsal hippocampus during the consistent (CON) and inconsistent (INCON) retention tests.
Figure 3.
(A) Representative single-unit waveforms in a lateral nucleus neuron recorded during consistent (left) and inconsistent (right) test session in a rat treated with saline. (B,C) Panels show peristimulus time histograms (summed over 10 CS trials) and associated spike rasters for representative single units recorded in saline- (B, for the neuron in A) or muscimol-treated (C) rats. Each rat was tested to a single CS both inside the extinction context (consistent or CON) and outside the extinction context (inconsistent or INCON).
As we have previously reported (Hobin et al. 2003), the majority of single units in rats receiving saline infusions exhibited context-dependent firing to the extinguished CS. A peristimulus time histogram from a representative neuron in the saline group is shown in Figure 3B. In this example, it is apparent that CS-evoked activity was greater in the INCON compared with the CON session. In fact, the majority of CS-responsive neurons in the SAL group (8 of 10) exhibited greater CS-elicited firing when the CS was presented outside the extinction context (INCON) relative to when the CS was presented inside the extinction context (CON); the remaining neurons did not exhibit different CS-elicited firing rates in the two sessions. Interestingly, muscimol infusion into the dorsal hippocampus prior to retention testing eliminated this context-dependent spike firing. As illustrated by the histogram from a representative neuron in the muscimol group (Fig. 3C), dorsal hippocampal inactivation retarded the context-dependence of CS-elicited activity after extinction. Indeed, <50% of the CS-responsive neurons recorded from MUS-treated rats exhibited greater firing to the extinguished CS when it was presented in the inconsistent context (8 of 20), and the majority of neurons (12 of 20) exhibited either less firing in the inconsistent context (6 of 20) or no change in firing (6 of 20) across the two tests.
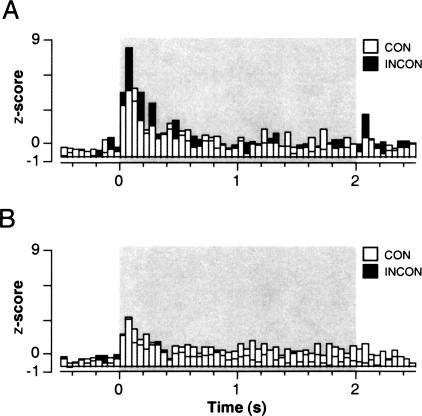
The population averages computed for all CS-responsive cells in the saline- and muscimol-treated groups are shown in Figure 4. As a population, neurons in both saline and muscimol-treated rats showed a short-latency (0- to 50-msec) response to the onset of the auditory CS in both the consistent and inconsistent sessions; this onset response peaked 50–100 msec after CS onset. However, only neurons in the saline group exhibited significant modulation of CS-elicited firing rate by context. Contextual modulation of lateral nucleus spike firing occurred in several post-CS onset bins, but was maximal 50–100 msec after CS onset. This adverse influence of dorsal hippocampal muscimol infusions on the context-dependence of lateral nucleus spike firing to an extinguished CS is summarized in Figure 5A. This figure displays normalized spike firing 50–100 msec after CS onset (which corresponds to the bin exhibiting the greatest contextual modulation as shown in the population histograms in Fig. 4) among CS-responsive neurons. Muscimol infusions into the dorsal hippocampus eliminated the “renewal” of spike firing normally observed to an extinguished CS when it is presented outside of its extinction context (context × drug interaction: F(1,28) = 5.9, p < 0.05). Spike firing was also greater in SAL compared with MUS-treated rats (F(1,28) = 5.3, p < 0.05), which was largely the consequence of the failure of neurons in MUS-treated rats to increment their CS-elicited firing in the INCON condition. This outcome was not related to the pre-CS (spontaneous) firing rates of the cells, which were not influenced by either drug treatment or the nature of the retention test (Fig. 2).
Figure 4.
Normalized neuronal activity (“population averages”) for all CS-responsive neurons recorded in the lateral amygdala of saline- (A) or muscimol-treated (B) rats. Spike firing was summed across the 10 CS trials in each retention test, and post-CS activity (binned in 50-msec intervals) was normalized to the pre-CS baseline (a total of 500 msec before CS onset). Standard scores (z-scores) were averaged across all units in the CON (open bars) and INCON (filled bars) tests. (Shaded rectangles) The 2-sec CS.
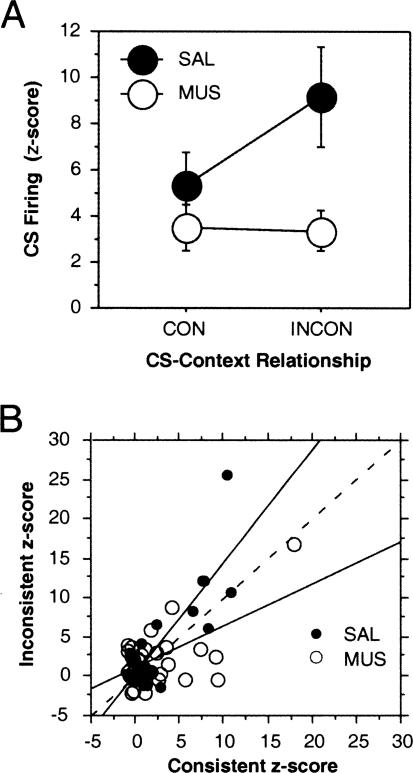
Figure 5.
(A) Mean (±SEM) CS-evoked firing (z-scores) in lateral amygdala neurons 50–100 msec after CS onset after saline (SAL) or muscimol (MUS) infusion into the dorsal hippocampus during the consistent (CON) and inconsistent (INCON) retention tests. (B) Scatterplot of normalized spike firing (50–100 msec after CS onset) for each neuron recorded under SAL (solid circles) or MUS (open circles) in the consistent and inconsistent test sessions. Regression lines indicate the deviation from a slope of 1 (dashed line) under the different drug conditions.
As we have demonstrated, the contextual modulation of CS-elicited spike firing and its disruption by muscimol infusions is robust in a subgroup of cells that we found to be highly CS-responsive. We were interested in whether there was a systematic relationship in CS-elicited spike firing across the two test sessions. Figure 5B plots normalized firing (50–100 msec after CS onset) for every neuron that we recorded during both the CON and INCON sessions (we also included in this analysis neurons that were not responsive to the CS by the criterion we used in our earlier analyses). As shown, there was a considerable range across the neurons in CS-responsiveness in the test sessions. Nonetheless, the normalized firing rates in the CON and INCON sessions were highly correlated in both the SAL (r = 0.84) and MUS (r = 0.60) conditions. Hence, neurons tended to maintain their CS responsiveness across the two test sessions. Importantly, the neurons in SAL-treated rats tend to exhibit greater firing in the INCON session as indicated by the slope of the regression line, which was >1 (the dashed line in Fig. 5B is equivalent to a slope = 1); neurons recorded in MUS rats tended to fire more in the CON session.
Discussion
We have previously reported that single units in the lateral amygdala exhibit context-dependent firing to extinguished CSs (Hobin et al. 2003). We now show that this post-extinction pattern of lateral nucleus neuronal firing is disrupted by infusion of the GABAA agonist muscimol into the dorsal hippocampus. Whereas the majority of CS-responsive neurons in SAL-treated rats exhibited greater firing to an extinguished CS when it was presented outside relative to inside its extinction context, only a minority of neurons exhibited this behavior in muscimol-treated rats. Moreover, at the population level, only neurons recorded from the lateral nucleus of saline-treated rats exhibited significant context-dependent firing to an extinguished CS. These data suggest that contextual control of fear behavior, at least after extinction, is mediated by an influence of the hippocampus on the activity of single lateral amygdala neurons. Hence, the suppression of fear behavior after extinction may be mediated, at least in part, by an inhibition of information processing at the sensory interface of the amygdaloid fear circuit.
One concern with the present study is the low number of CS-responsive neurons that we sampled (only 50% of the population for a total of 30 cells). Indeed, in previous work, we observed higher percentages of CS-responsive neurons. Our low yield was further complicated by difficulties in accurately targeting the lateral nucleus with a recording probe and the dorsal hippocampus with guide cannulae in the same animal. Nevertheless, despite the small sample size, we have replicated earlier results of the contextual modulation of spike firing that were reported in a significantly larger sample of lateral nucleus neurons (n = 59; Hobin et al. 2003). Moreover, we also had sufficient power in our sample of CS-responsive neurons to detect a robust influence of dorsal hippocampal muscimol infusions on the context-dependent firing in the lateral amygdala.
The present results are consistent with accumulating data that suggest an important role for the amygdala in encoding extinction memories (Davis 2002; Walker and Davis 2002; Maren and Quirk 2004). Extinction training reduces CS-evoked firing in many lateral nucleus neurons (Quirk et al. 1995; Repa et al. 2001), and pharmacological interventions that reduce amygdaloid synaptic plasticity impair the formation of extinction memories (Herry et al. 2006). For example, infusion of N-methyl-D-aspartate (NMDA) receptor antagonists (Falls et al. 1992; Lee and Kim 1998), mitogen-activated protein kinase (MAPK) inhibitors (Lu et al. 2001), or MAPK kinase inhibitor (Herry et al. 2006) into the basolateral amygdala blocks the extinction of conditioned fear-potentiated startle in rats. Conversely, NMDA receptor agonists facilitate the extinction of conditioned fear-potentiated startle in rats (Walker et al. 2002). Additionally, activation of gastrin-releasing peptide receptors (GRP) located on lateral nucleus inhibitory interneurons results in inhibition of principal neurons in the lateral amygdala, and GRP knockout mice show persistent tone fear expression compared with wild-type mice (Shumyatsky et al. 2002). Finally, we have shown that single units in the lateral nucleus of the amygdala represent CS memories after extinction (present work; Hobin et al. 2003). These data suggest that the basolateral complex of the amygdala, and the lateral nucleus in particular, may be essential for the acquisition and expression of extinction memories.
In addition to local changes in the lateral amygdala that may encode extinction memories, there is also evidence that the medial prefrontal cortex is involved in the retrieval of extinction memories (Morgan et al. 1993; Morgan and LeDoux 1995; Milad and Quirk 2002; Lebron et al. 2004; Maren and Quirk 2004; Milad et al. 2004; cf., Garcia et al. 2006). Consistent with this, electrophysiological and anatomical studies indicate that the medial prefrontal cortex excites a population of inhibitory interneurons, the intercalated cell masses that are interposed between the lateral and the central nucleus of the amygdala (Quirk et al. 2003). Intercalated cells are well positioned to gate the flow of excitatory transmission between the lateral and central nuclei (Royer et al. 1999; Pare et al. 2004), which would limit the ability of the central nucleus to drive activity in downstream structures that produce fear responses. In addition to its influence on the central nucleus, stimulation of the prefrontal cortex also attenuates CS-evoked responses in lateral nucleus neurons during and after affective conditioning (Rosenkranz and Grace 2003). Hence, extinction may enable a network that suppresses conditional fear responses both at the input (lateral nucleus) and output (central nucleus) sides of the amygdaloid circuit. Our data suggest that modulation of the inhibitory network within the lateral nucleus may be essential to the renewal of extinction memories outside of the extinction context.
The data described above suggest how the amygdaloid fear circuit might come under inhibitory control after extinction, but they do not explain the contextual modulation of extinction. The hippocampus has extensive reciprocal projections with the amygdala (Pitkanen et al. 2000), and hippocampal projections to the amygdala exhibit synaptic plasticity (Maren and Fanselow 1995; Goosens and Maren 2002). Hence, the context-dependence of extinction may arise from an integration of contextual representations processed in the hippocampus and excitatory and inhibitory CS–US associations processed in the lateral amygdala. By this view, the context-dependence of fear extinction results from a direct interaction between the hippocampus and amygdala. Alternatively, the hippocampus may influence lateral nucleus activity indirectly via its projections to the prefrontal cortex (Thierry et al. 2000). Indeed, Ishikawa and Nakamura (2003) have shown that hippocampal and amygdalar inputs converge and interact in the prefrontal cortex, allowing the possibility that CS and contextual information converge there and are then relayed to the amygdala to engender context-dependent neuronal activity in the lateral amygdala. Whether a direct or indirect hippocampo–amygdala interaction underlies context-dependent extinction remains to be determined.
Regardless of the anatomical pathway by which the hippocampus influences the amygdala, we have shown that dorsal hippocampal inactivation results in a severe attenuation of context-dependent neuronal activity in the lateral amygdala. Considerable research has found that the dorsal hippocampus encodes representations of context (Anagnostaras et al. 1999; Rudy et al. 2002, 2004; Sanders et al. 2003), and it has been argued that the hippocampus uses contextual information to label and retrieve memories (Hirsh 1974; Good and Honey 1991; Maren and Holt 2000; O’Reilly and Rudy 2001; Rudy and O’Reilly 2001). Post-training dorsal hippocampal lesions disrupt context fear expression (Maren et al. 1997; Frankland et al. 1998), and dorsal hippocampal muscimol infusions prevent the formation and retrieval of contextual representations as well as the expression of contextual fear (Matus-Amat et al. 2004). Additionally, protein synthesis inhibition in the dorsal hippocampus prevents the formation of contextual representations (Barrientos et al. 2002). These data provide strong evidence that the dorsal hippocampus is involved in coding and storing a memory of context. Data from our laboratory are consistent with this view insofar as we have shown that dorsal hippocampal inactivation disrupts the acquisition (Corcoran and Maren 2005) and expression of context-specific fear memories (Holt and Maren 1999; Corcoran and Maren 2001, 2004; Goosens and Maren 2003; Ji and Maren 2005).
There is consensus from a large body of behavioral work that extinction results in the formation of an inhibitory memory that competes with the excitatory memory formed during conditioning (Bouton 1993). Contextual information is thought to gate the expression of the extinction memory, resulting in activation of that memory only in the extinction context. Because lateral amygdala neuronal firing typically correlates with fear memories and the expression of fear responses (Goosens et al. 2003), we anticipated that most lateral nucleus neurons would show greater CS-elicited firing outside compared with inside the extinction context. Consistent with this, we observed that the majority of lateral amygdala neurons fired more to a CS outside of the extinction context, which replicates our earlier report (Hobin et al. 2003). However, the lateral amygdala contains both glutamatergic projection neurons (McDonald et al. 1989) and GABAergic inhibitory neurons (McDonald 1985). Insofar as GABAergic neurons are believed to be involved in the retrieval of the extinction memory (Harris and Westbrook 1998; Bouton et al. 2006), one would actually expect this population of cells to fire more to a CS presented in the extinction context. We have observed this pattern of activity in a small number of cells in this and our earlier work (Hobin et al. 2003), yet have been unable to specify the neuronal populations that contribute to our recordings. Clearly, it is of considerable importance to identify the activity patterns of specific populations of amygdala neurons to better understand the network dynamics involved in the retrieval and expression of fear memories after extinction.
Considerable work has focused on the modulatory influence of the amygdala on hippocampal and neocortical function during the consolidation of memory (McGaugh 2004). Here, we show that modulation also operates in the opposite direction, in this case with hippocampal processes influencing amygdala neuronal activity during the retrieval of extinction memories. Indeed, our results demonstrate an important role for the dorsal hippocampus in gating CS-evoked spike firing in the lateral amygdala after a CS has acquired multiple meanings during the course of conditioning and extinction. These data are an important addition to the body of research aimed at elucidating the neural substrates of fear memory inhibition.
Materials and Methods
Subjects and surgery
Thirty-three male Long-Evans rats (Blue Spruce) provided by a commercial supplier (Harlan Sprague-Dawley) and weighing 275–500 g were used in this experiment. Rats were housed singly in hanging plastic cages, kept on a 14:10-h light:dark cycle (lights on at 7:00 A.M.), and provided with food and water ad libitum. They were handled for at least 3 d prior to surgery. On the day of surgery, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and administered atropine as needed. They were stereotaxically implanted with bilateral guide cannulae (26-gauge; Plastics One) aimed at the dorsal hippocampus (3.8 mm posterior to bregma; 2.5 mm lateral to bregma; 2.5 mm ventral to bregma). The cannulae were affixed to the skull using jewelers’ screws and dental acrylic. After the dental acrylic dried, a multiwire recording electrode assembly was aimed at the dorsal division of the lateral nucleus in the right hemisphere (3.3 mm posterior to bregma; 5.5 mm lateral to bregma; 6–6.4 mm ventral to dura). The electrode assembly was grounded via a skull screw implanted above the nasal sinus and was affixed to the skull with dental acrylic. Electrodes consisted of eight 25-μm tungsten wires housed in a 28-gauge steel cannula beyond which they extended ∼1 mm. The wires were cut to yield impedance between 100 and 250 kΩ at 1 kHz. Thereafter, dummy cannulae (33-gauge; Plastics One) were inserted into the dorsal hippocampal guide cannulae to prevent them from clogging. Animals were allowed to recover at least 4 d between surgery and testing. During this time, the dummy cannulae were changed at least once in order to keep the guide cannulae clear of debris and to habituate rats to handling. A dummy recording cable was plugged into the electrode to habituate rats to the handling that would later be associated with testing.
Behavioral apparatus
Fear conditioning was performed in standard rodent conditioning chambers (30 × 24 × 21 cm; Med-Associates). The chambers were constructed from aluminum (two side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and were situated in sound-attenuating chests located in an isolated room. The floor of each chamber consisted of 19 stainless steel rods (4-mm diameter) spaced 1.5 cm apart (center to center). The rods were wired to a shock source and solid-state grid scrambler (Med-Associates) for delivery of the footshock unconditional stimuli (US). For “context A” (used for fear conditioning), background noise (65 dB) was provided by ventilation fans built into the chests, house lights within the chambers and the fluorescent lights within the room provided illumination, the chest doors were left open, and the chambers were cleaned with a 1% ammonium hydroxide solution. Stainless steel pans containing a thin film of the cleaning solutions were placed underneath the grid floors before the animals were placed inside the boxes.
These chambers rested on a load-cell platform that was used to record chamber displacement in response to each rat’s motor activity. Load-cell amplifier output from each chamber was digitized at 5 Hz and acquired on-line using Threshold Activity software (Med-Associates, Inc.). Extinction and retention testing took place in two unique contexts (“B” and “C”). These sessions were conducted in the same room, which was separate from the fear conditioning room (context A), in standard rodent conditioning chambers modified to accommodate electrophysiological recording. For “context B” (used for extinction and retention testing), the illumination was provided by fluorescent red lights, the chest doors were closed, the ventilation fans were inactive, and the chambers were cleaned with a 1% acetic acid solution. For “context C,” (used for extinction and retention testing), the illumination was provided by a single house light, the ventilation fans were inactive, and the chambers were cleaned with a 70% ethanol solution. During these sessions, the load cell amplifier output was acquired on-line using DataWave software (DataWave Technologies). Electrophysiological recordings conducted during the retention tests were made via a recording cable containing an eight-channel FET head stage that passed high-impedance signals from the implanted electrode to a computer via a commutator. For each channel, signals were acquired in 3-sec epochs (0.5-sec pre-CS, 2-sec CS, 0.5-sec post-CS) for 10 test trials. All neuronal signals were amplified (gain = 10,000), filtered (600–9000 Hz), and acquired and digitized using Experimenter’s Workbench software (DataWave Technologies).
Infusions were made via 33-gauge internal cannulae (Plastics One). The internal cannulae were attached to 10-μL syringes via polyethylene tubing (PE-20; Small Parts, Inc.) that had been backed-filled with water. The syringes were mounted in infusion pumps (Harvard Apparatus) that were used to deliver the microinjections.
Procedure
All rats were fear conditioned in “context A” using 10 tone (80 dB; 2 kHz; 2 sec)-shock (1 mA; 0.5 sec) and 10 white-noise (80 dB; flat at 10–25 kHz; 2 sec)-shock pairings 3 min after placement into the fear conditioning chambers. Tone and white noise trials were presented alternately (62 sec ITI) and rats were returned to their home cages 1 min after the termination of the last CS. The following day, rats were extinguished to the tone CS in one of the extinction contexts and the white noise CS in the other extinction context. Tone and white noise extinction were conducted in separate sessions with at least 1 h between them. The order of extinction sessions and the cue-context combinations were counterbalanced. Hence, some rats received tone extinction in “context B” and white noise extinction in “context C,” while other rats received the opposite cue–context combination. Extinction consisted of 30 CS presentations (1 min ITI) beginning 1 min after placement in the conditioning chambers. Rats received two extinction sessions. Conditional freezing was recorded during the training and extinction sessions.
The day after the last extinction session, rats were transported to the infusion room in a bucket lined with bedding. Dummy cannulae were removed and internal cannulae were inserted into the guide cannulae. Once the internal cannulae were in place the infusion pumps were turned on and 0.5 μg of muscimol (MUS, 1.0 mg/mL) or saline (SAL) solution (0.9%) was infused into the dorsal hippocampus at a rate of 0.32 μL/min for 94 sec. The total infusion volume was 0.5 μL. The internal cannulae were left in place for 1 min after the infusion to allow for diffusion of the drug away from the cannulae tips. These parameters produce infusions of muscimol that are restricted to the dorsal hippocampus (Corcoran and Maren 2005). The microinjectors were removed, dummy cannulae were replaced, and rats were returned to their home cages.
Approximately 20 min later, rats were transported to the electrophysiological recording room for testing. About 1 min after attaching the recording cable to the implanted electrode assembly, the rats were tested to either the tone or white noise CS in both contexts B and C (the rats were tested only to a single CS). Each test session consisted of 10 CS presentations (1 min ITI). One minute after the last CS presentation, the rats were returned to their home cages. Approximately 1 h later, rats were transported back to the fear conditioning room for testing to the same CS in the other extinction context. Therefore, each rat was tested to a single CS in either its extinction context (consistent or CON) or in another context that had hosted extinction to a different CS (inconsistent or INCON). The order of testing (CON or INCON) and the individual CSs (tone or white noise) presented to each rat was counterbalanced within days (i.e., some rats heard tones first in the consistent context and then an hour later in the inconsistent context, while others heard white noises first in the inconsistent context and then an hour later in the consistent context and so forth). Conditional freezing and single-unit activity were recorded during every test session.
Histology
After retrieval testing, the animals were overdosed with sodium pentobarbital and a small lesion was made at the tip of one of the electrodes using anodal current (80 μA, 10 sec). Thereafter, animals were perfused across the heart using physiological saline solution followed by a 10% formalin solution. Brains were removed and placed in 10% formalin overnight. Before sectioning, all brains were placed in 10% formalin–30% sucrose for at least 4 h. Using a cryostat, brains were sectioned (40–45 μm), mounted on microscope slides, and stained with 0.25% thionin in order to verify electrode and cannulae placements.
Data analysis
Freezing was measured as previously described (Maren 1998). Average freezing was calculated for the pre-trial period on the conditioning day (PRE), the average of the first five post-CS periods after extinction commenced (EXT-Early), and for the final five CS trials on the extinction session (EXT-Late). Average freezing during the extinction session and on the retention tests (CON and INCON) after extinction was normalized to the pre-CS baseline for each session. This yielded an index of freezing to the CS that accounted for individual variability in the level of freezing prior to CS onset.
Neuronal analysis was performed off-line using Autocut (DataWave Technologies) and Neuroexplorer (Plexon, Inc.) software. Single units were isolated from multiple-unit records on each recording channel using standard spike sorting and clustering methods (see Maren 2000). For each unit, spikes were binned into 50-msec periods for each 3-sec CS, and summed across the 10 CS trials. A cell was classified as CS-responsive if average firing in the first two post-CS bins (0–100 msec) was at least 1.5 standard deviations (SD) above baseline firing in either of the retention tests. Single units that were CS-responsive were also classified according to whether they fired more (>1.5 SD) to the CS in the inconsistent or the consistent context by comparing the normalized neuronal response in the first 100 msec after CS onset in the INCON and CON test sessions.
Acknowledgments
This research was supported by a grant from the NIMH (R01MH065961) to S.M. and a DOD NDSEG fellowship to J.A.H. We thank Patricia Welsh-Droz and Andrea Lubaway for technical assistance.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.477007
References
- Anagnostaras S.G., Maren S., Fanselow M.S. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J. Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., O’Reilly R.C., Rudy J.W. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav. Brain Res. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., Bolles R.C. Contextual control of the extinction of conditioned fear. Learn. Motiv. 1979;10:445–466. [Google Scholar]
- Bouton M.E., Westbrook R.F., Corcoran K.A., Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol. Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn. Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran K.A., Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J. Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: Clinical implications for exposure therapy. Eur. J. Neurosci. 2002;16:395–398. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Falls W.A., Miserendino M.J., Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland P.W., Cestari V., Filipkowski R.K., McDonald R.J., Silva A.J. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Garcia R., Chang C.-h., Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn. Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden D.R., Baddeley A.D. Context-dependent memory in two natural environments: On land and underwater. Br. J. Psychol. 1975;66:325–331. [Google Scholar]
- Good M., Honey R.C. Conditioning and contextual retrieval in hippocampal rats. Behav. Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- Goosens K.A., Maren S. Long-term potentiation as a substrate for memory: Evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Goosens K.A., Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Goosens K.A., Hobin J.A., Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: Mnemonic code or fear bias? Neuron. 2003;40:1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- Harris J.A., Westbrook R.F. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Harris J.A., Jones M.L., Bailey G.K., Westbrook R.F. Contextual control over conditioned responding in an extinction paradigm. J. Exp. Psychol. Anim. Behav. Process. 2000;26:174–185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- Herry C., Trifilieff P., Micheau J., Luthi A., Mons N. Extinction of auditory fear condiitonign required MAPK/ERK activation in the basolateral amygdala. Eur. J. Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: A theory. Behav. Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Hobin J.A., Goosens K.A., Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W., Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J. Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A., Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J. Neurosci. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J., Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn. Mem. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P.J., Shapiro M.L. Retrieving memories via internal context requires the hippocampus. J. Neurosci. 2004;24:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron K., Milad M.R., Quirk G.J. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn. Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Lee H., Kim J.J. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J. Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E., Pelletier J.G., Popescu A.T., Pare D. Identification of basolateral amygdala projection cells and interneurons using extracellular recordings. J. Neurophysiol. 2006;96:3257–3265. doi: 10.1152/jn.00577.2006. [DOI] [PubMed] [Google Scholar]
- Lu K.T., Walker D.L., Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J. Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-16-j0005.2001. RC162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 2000;12:4047–4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S., Fanselow M.S. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav. Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S., Quirk G.J. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S., Aharonov G., Fanselow M.S. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P., Higgins E.A., Barrientos R.M., Rudy J.W. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J. Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A.J. Immunohistochemical identification of γ-aminobutyric acid-containing neurons in the rat basolateral amygdala. Neurosci. Lett. 1985;53:203–207. doi: 10.1016/0304-3940(85)90186-7. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Beitz A.J., Larson A.A., Kuriyama R., Sellitto C., Madl J.E. Co-localization of glutamate and tubulin in putative excitatory neurons of the hippocampus and amygdala: An immunohistochemical study using monoclonal antibodies. Neuroscience. 1989;30:405–421. doi: 10.1016/0306-4522(89)90261-3. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Vidal-Gonzalez I., Quirk G.J. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav. Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Morgan M.A., LeDoux J.E. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan M.A., Romanski L.M., LeDoux J.E. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- O’Reilly R.C., Rudy J.W. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychol. Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Pare D., Collins D.R. Neuronal correlates of fear in the lateral amygdala: Multiple extracellular recordings in conscious cats. J. Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D., Quirk G.J., Ledoux J.E. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pitkanen A., Pikkarainen M., Nurminen N., Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Repa C., LeDoux J.E. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Armony J.L., LeDoux J.E. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Likhtik E., Pelletier J.G., Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa J.C., Muller J., Apergis J., Desrochers T.M., Zhou Y., LeDoux J.E. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat. Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- Rodrigues S.M., Schafe G.E., LeDoux J.E. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J.A., Grace A.A. Affective conditioning in the basolateral amygdala of anesthetized rats is modulated by dopamine and prefrontal cortical inputs. Ann. N. Y. Acad. Sci. 2003;985:488–491. doi: 10.1111/j.1749-6632.2003.tb07107.x. [DOI] [PubMed] [Google Scholar]
- Royer S., Martina M., Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J. Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J.W., O’Reilly R.C. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn. Affect. Behav. Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Barrientos R.M., O’Reilly R.C. Hippocampal formation supports conditioning to memory of a context. Behav. Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Huff N.C., Matus-Amat P. Understanding contextual fear conditioning: Insights from a two-process model. Neurosci. Biobehav. Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sanders M.J., Wiltgen B.J., Fanselow M.S. The place of the hippocampus in fear conditioning. Eur. J. Pharmacol. 2003;463:217–223. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- Shumyatsky G.P., Tsvetkov E., Malleret G., Vronskaya S., Hatton M., Hampton L., Battey J.F., Dulac C., Kandel E.R., Bolshakov V.Y. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Spear N.E. Retrieval of memory in animals. Psychol. Rev. 1973;80:163–194. [Google Scholar]
- Swanson L.W. Brain maps: Structure of the rat brain. Elsevier; New York: 1992. [Google Scholar]
- Thierry A.M., Gioanni Y., Degenetais E., Glowinski J. Hippocampo-prefrontal cortex pathway: Anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tulving E., Thompson D.M. Encoding specificity and retrieval processes in episodic memory. Psychol. Rev. 1973;80:352–373. [Google Scholar]
- Walker D.L., Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol. Biochem. Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Ressler K.J., Lu K.T., Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]