Abstract
Increased mucus production in asthma is an important cause of airflow obstruction during severe exacerbations. To better understand the changes in airway epithelium that lead to increased mucus production, ovalbumin-sensitized and -challenged mice were used. The phenotype of the epithelium was dramatically altered, resulting in increased numbers of mucous cells, predominantly in the proximal airways. However, the total numbers of epithelial cells per unit area of basement membrane did not change. A 75% decrease in Clara cells and a 25% decrease in ciliated cells were completely compensated for by an increase in mucous cells. Consequently, by day 22, 70% of the total epithelial cell population in the proximal airways was mucous cells. Electron microscopy illustrated that Clara cells were undergoing metaplasia to mucous cells. Conversely, epithelial proliferation, detected with 5-chloro-2-deoxyuridine immunohistochemistry, was most marked in the distal airways. Using ethidium homodimer cell labeling to evaluate necrosis and terminal dUTP nick-end labeling immunohistochemistry to evaluate apoptosis, this proliferation was accompanied by negligible cell death. In conclusion, epithelial cell death did not appear to be the stimulus driving epithelial proliferation and the increase in mucous cell numbers was primarily a result of Clara cell metaplasia.
Asthma is a chronic inflammatory disease that manifests as periodic limitations to airflow. Some asthmatics experience a progressive decline in pulmonary function throughout time. In these patients persistence of chronic inflammation may initiate the progressive structural changes known as airway remodeling. 1-3 The features of airway remodeling include epithelial erosion, mucous gland hyperplasia 4 and hypertrophy, increased numbers of epithelial mucous cells, smooth muscle hyperplasia, and subepithelial fibrosis. 5,6
The classical view of the airway epithelium as a passive barrier and target for the inflammatory response has been replaced. In recent years, a more complex concept has emerged that epithelial cells can be active participants in the pathogenic processes critical to the development of the physiological manifestation of asthma, namely airflow obstruction and airway hyperresponsiveness. The epithelium can amplify the inflammatory response with the secretion of cytokines, chemokines, and other proinflammatory proteins and peptides 7-9 and the secretion of growth factors may orchestrate airway remodeling. 8,9 One of the underlying reasons why the epithelium of asthmatics maintains its state of activation with the release of proinflammatory mediators and growth factors may be fundamental differences in the response of the epithelium to stress and repair. 7 There is evidence that epithelial cells of asthmatics respond abnormally to environmental stimuli. 10 Furthermore, changes in the epithelial phenotype itself may play a role in the maintenance of asthma, particularly increased mucus production, which contributes to airflow obstruction. Mucus plugging has been identified at postmortem examination in patients who have died from asthma, even in the smallest airways, which lack mucus-secreting cells. 11,12
Mouse models are increasingly used in asthma research primarily because of the availability of a wide range of immunological tools, however these models lack the documentation of quantitative pathology and site-specific airway changes, which are crucial to our understanding of airway remodeling. The experiments described in this article enhance our understanding of the role that epithelium plays in asthma by documenting the changes occurring in the bronchial epithelium at different airway generations during the development of the asthmatic phenotype in allergic sensitized and challenged mice. We tested the hypothesis that epithelial cell death (necrosis, apoptosis) results in stem cell proliferation and replacement of the Clara and ciliated cell population with mucous cells.
Materials and Methods
Animals and Maintenance
A total of 132 male BALB/c BYJ mice (6 to 8 weeks old on arrival) were purchased from Jackson Laboratories, Bar Harbor, ME. The animals were housed in an Association for the Assessment of Laboratory Animal Care accredited facility under specific pathogen-free conditions. All studies were conducted in accordance with an approved protocol.
Model Validation
Eighteen 8-week-old BALB/c BYJ male mice were sensitized with 10 μg of ovalbumin (OVA) (Sigma, St. Louis, MO) and 1 mg of aluminum hydroxide (Intergen Co., Purchase, NY) in 0.1 ml vol of Dulbecco’s phosphate-buffered saline intraperitoneally on days 0 and 7. Eighteen control mice received 1 mg of aluminum hydroxide in 0.1 ml of Dulbecco’s phosphate-buffered saline. Starting on day 14, all mice received 20-minute OVA inhalation challenges in a custom-built pie acrylic exposure chamber that separated the mice from each other. Challenges were repeated on days 17 and 20. The aerosol was delivered using the PARI IS-2 nebulizer at 22 PSI containing 10 mg/ml OVA with 0.01% Tween-20 that produced a particle size of 2.75-μm mass median aerodynamic diameter with a span of 1.98 μm.
Airflow obstruction during antigen challenge was measured on day 22. Forty-eight hours after the third aerosol challenge, 10 sensitized mice and 10 control mice were anesthetized with pentobarbital (25 mg/kg) and urethane (1.8 g/kg), tracheotomized, and a saline-filled cannula was placed two-thirds of the way down the esophagus. Mice were then loaded into an acrylic pressure plethysmograph for measurement of thoracic expansion and esophageal pressure. Respiratory resistance and dynamic compliance were calculated from the simultaneous measurement of tidal volume, esophageal pressure, and gas flow rate in the respiratory system. Data were collected using an automated data acquisition program (Buxco Electronics Inc., Sharron, CT). A total of 3.5 ml of OVA (10 mg/ml) was aerosolized every other 2 minutes for 20 minutes (total exposure 10 minutes) using a PARI IS-2 nebulizer as described above. Data were expressed as a maximum percentage increase in respiratory resistance in which the baseline resistance was expressed as 100%.
Airway hyperresponsiveness was also measured on day 22 in different groups of similarly treated mice. These remaining eight sensitized mice and eight control mice were anesthetized and prepared for continuous measurement of breathing mechanics and ventilation as described above. Methacholine was aerosolized using a PARI IS-2 nebulizer for 1 minute and then stopped for a 5-minute period. This protocol was repeated six times as the dose of methacholine (0, 0.3, 1, 3, 10, and 30 mg/ml) was increased. Data were expressed as provocative concentration 50%, which is the dose of methacholine required to give a 50% increase in respiratory resistance.
Sensitized mice and nonsensitized controls were sacrificed after the pulmonary function tests at the end of the experiment. Blood was collected via the retro-orbital sinus. Using an aliquot of saline (0.03 ml × body weight in g) bronchoalveolar lavage (BAL) was collected by lavaging the lungs three times with the same aliquot. Lungs were removed and inflated with 10% formalin to 80% of total lung capacity before embedding and sectioning. Total cell counts and differential leukocyte counts were performed on the BAL samples. Stains performed on the tissues were hematoxylin and eosin and periodic-acid Schiff/alcian blue, pH 2.5 (PAS/AB). Serum samples were assayed for total IgE as previously described. 13
Epithelial Injury
Differential permeability to fluorescent dyes was used to identify epithelial necrosis. Thirty-six BALB/c BYJ mice were sensitized and challenged with OVA as described previously as were nine control mice. At 6, 12, and 24 hours after each of the three aerosol challenges, three sensitized mice and one control mouse per group were sacrificed and the lungs lavaged with 12 μmol/L of ethidium homodiemer-1 (EthD-1) (Molecular Probes Inc. Eugene, OR) in Gibco’s Ham’s F12 media (Invitrogen Corp., Carlsbad, CA) at a volume of 0.03 ml × body weight (g) at 37°C. After 20 minutes of incubation in the dark, the EthD-1 was gently withdrawn and the lungs were washed out with a similar volume of saline at 37°C. The combined lavage was reserved and cytospins prepared and examined under a fluorescent microscope for EthD-1-positive cells. The lungs were then fixed with an intratracheal infusion of Karnovsky’s fixative before removal from the animal. Fixed specimens were microdissected to expose the branching pattern of the lung lobe and counterstained with 2 μmol/L of Yo-Pro-1 Iodide (Molecular Probes Inc., Eugene, OR) to label the cell nuclei. Samples were then examined as whole wet mounts on a laser-scanning confocal microscope. Additional 5-μm tissue sections were used to detect the presence of apoptotic cells in the epithelium with a terminal dUTP nick-end labeling assay kit (Oncor’s Apoptag Kit) (Intergen, Purchase, NY).
Epithelial Proliferation and Mucous Cell Morphometry
Thirty sensitized and 30 nonsensitized mice were generated as previously described. On day 13, 24 hours before the first aerosol challenge, each mouse was anesthetized with isofluorane and osmotic pumps (Alzet pump model 2002; Durect Co., Cupertino, CA) loaded with 5-chloro-2-deoxyuridine (Cldu) (Sigma, St. Louis, MO) diluted in phosphate-buffered saline (75 mg/ml) were implanted subcutaneously between the scapulae. On days 16, 19, and 22, 48 hours after each of the aerosol challenges, 10 sensitized mice and 10 nonsensitized mice were sacrificed and their lungs were fixed in 10% formalin as previously described. A segment of duodenum was also collected in 10% formalin as a control for Cldu immunohistochemistry.
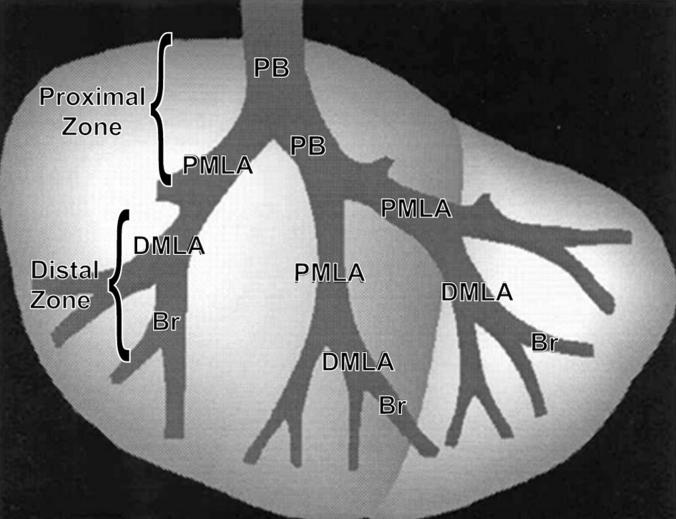
The right cranial lung lobe was microdissected along the main axial pathway of the airways 14 and embedded in paraffin, so that on sectioning the branching pattern of the airway tree was revealed (Figure 1) ▶ . Alternate serial sections 30-μm-thick and 5-μm-thick were cut. The 30-μm sections were used for immunohistochemistry to detect Cldu and the 5-μm sections were stained with PAS/AB. The incorporated Cldu was detected using a monoclonal antibody (DAKO Corp., Carpinteria, CA) and immunohistochemical techniques as outlined by Hsu and colleagues. 15
Figure 1.
Diagram of an airway microdissection of the right cranial lung lobe from a mouse. The primary bronchus (PB) is the airway entering the lung lobe and the proximal midlevel airway (PMLA), distal mid-level airway (DMLA) and bronchiole (Br) are the first, second, and third branches, respectively. Collectively the PB and PMLA are referred to as the proximal zone and the DMLA and Br are referred to as the distal zone.
Airways in 30-μm sections stained for Cldu were designated according to the branching pattern (Figure 1) ▶ . The primary bronchus (PB) was the bronchus as it first entered the cranial lung lobe; the proximal mid-level airway (PMLA) was the first branching, the distal mid-level airway (DMLA) the second branching, and the bronchiole (Br) the third branching, generally positioned just before the terminal bronchioles. The PB and PMLA were collectively termed the proximal zone and the DMLA and Br collectively the distal zone. The number of Cldu-positive cells and volume of mucus at each of the airway sites was calculated. An unbiased optical dissector technique was used to estimate the number of Cldu-positive cells per epithelial volume. 16 Briefly, using light microscopy and a ×100 oil immersion lens, cells labeled with Cldu in the top focal plane and two sides of the counting frame of the 30-μm section were not counted but all other labeled cells were counted (Ncl.epi). The area of the epithelium was calculated from point counts of the epithelium (Pepi) and because the height of the optical dissector was known, the volume of the epithelium was calculated (Vepi). Therefore the number of Cldu-positive cells per unit volume (Nv) was calculated using the formula:
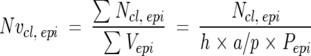 |
where h = height of the optical dissector and a/p = area per test point on the grid.
The 5-μm PAS/AB-stained vertical sections were used to calculate the volume of mucus per surface area of basal lamina using a staggered cycloid grid. 16,17 Images were captured at ×40 on a light microscope, using NIH image software (version 1.62; NIH, Bethesda, MD) and then imported into the program stereology toolbox (version 1.1; Morphometrix, Davis, CA) for analysis. The volume density of mucin in the airway epithelium was determined by a point count with the staggered cycloid grid. Points within the grid that fell on areas stained positive by the PAS/AB stain were counted as points on mucin (Pmuc) and points falling on epithelial cells of all types were counted as points on epithelium (Pepi). Lines within the grid crossing the basal lamina were counted as intersects of the basal lamina (Ibl). Then the volume of mucin per volume of epithelium was calculated as
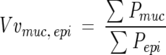 |
The surface of basal lamina per unit volume of epithelium (Svbl,epi) was calculated as
 |
Where l/p = length per test point on the cycloid grid. The volume of mucin per surface area of basement membrane (mm3/mm2) was calculated as
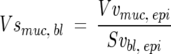 |
Further 5-μm sections from mice on day 22 were double-stained. Cldu immunohistochemistry was followed by a PAS/AB stain to allow the detection of epithelial cells that contained mucus and were in the process of proliferation.
Characterization and Morphometry of Epithelial Cell Types
Samples for transmission electron microscopy were selected from representative airways along the microdissected, major axial pathway (Figure 1) ▶ . Three sensitized mice were examined at day 22, 48 hours after the final aerosol challenge and two mice at each of the time points 6, 12, and 24 hours after the first aerosol challenge along with appropriate control mice. Samples were postfixed in 1% osmium tetroxide, dehydrated in a series of ethanol baths and embedded in Araldite. One-μm sections were cut from each airway so that the epithelium was cut perpendicular to the basal lamina and then stained with toluidine blue. Two blocks were selected by stratified sampling from each airway region (Figure 1) ▶ . Sections from these small blocks (1- to 2-mm face) were placed on grids and stained with uranyl acetate and lead citrate before viewing on a Zeiss EM-10A transmission electron microscope. A montage of micrographs was made from each airway for examination at ×2000 magnification.
At the final time point, day 22, additional sections (5 1-μm in series) processed and stained with toluidine blue as described above, were evaluated using the Physical Dissector component of the Computer-Assisted Stereology Toolbox software system (CAST-Grid; Olympus, International Stereology Center, Albertslund, Denmark) to determine the numbers of ciliated, Clara, and mucous cells per volume of epithelium (Nvcell,epi) in the proximal zone (PB and PMLA) (Figure 1) ▶ . A 5-μm physical dissector (sections 1 and 5 of the series) was used for the measurements using the following formula 16
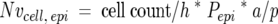 |
where cell count is the number of cell nuclear profiles appearing in the top section of the dissector, but not the bottom section of the dissector, h is the height of the dissector, Pepi are point hits on epithelium, and a/p is the area per point on the grid. The numbers of cells within the sampled airways were normalized to the surface area of the basal lamina, by dividing Nv by the surface of basal lamina in the same reference volume (Svbl, epi).
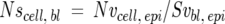 |
The arithmetic mean thickness of the epithelium was also measured using the following formula 18
 |
where l/p is the length per point on a cycloid grid, Pepi is the points that hit the epithelium, and Ibl are the number of intersections of the test line with the basal lamina.
Categorization of ciliated, mucous, and Clara cells was dependent on their morphological features and cells that did not meet the required criteria were categorized as other cells. Clara cells classically had a basal nucleus; the apical part of the cell projected into the lumen and was packed with well-organized arrays of smooth endoplasmic reticulum. In most cells classified as Clara cells, circular electron-dense membrane-bound secretory vesicles were located in the apical region of the cell and some cells had small microvilli at the cell surface. Ciliated cells were cubiodal or columnar with a large basal nucleus, a cytoplasm that was more electron lucent than the Clara cell and the presence of either cilia or basal bodies aligned adjacent to the apical surface. Any cell that contained identifiable mucus, namely membrane-bound vacuoles containing homogenous to fibrillar electron-lucent material in the cytoplasm was identified as a mucous cell. Often these cells had features of Clara cells although when they became heavily laden with mucus these features were no longer identifiable. Cells classified as other cells often had a small basal nucleus, were triangular with minimal cytoplasm, and did not appear to be in contact with the lumen. Many of these cells were probably basal cells.
Statistical Analysis
The data were analyzed using Minitab version 13.1 software and all data were reported as means plus or minus SE from the mean. Analysis of variance tests were run. The morphometric data were analyzed by analysis of variance and the Bonferroni test for multiple pairwise comparisons. (SYSTAT 10; SPSS Inc., Chicago, IL). P values <0.05 were accepted as a significant result.
Results
Model Validation
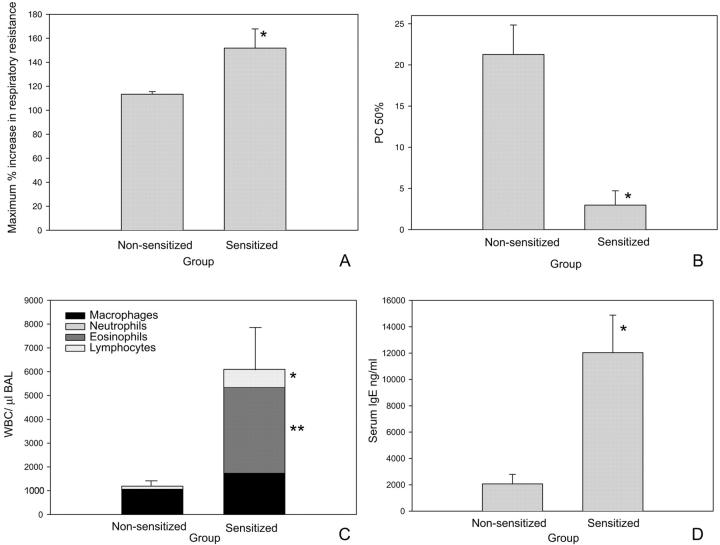
Before the characterization of the epithelial lesions, the asthma model was validated on day 22. At this time, a significant increase in airway hyperresponsiveness to aerosolized methacholine (P < 0.001) (Figure 2) ▶ and airflow obstruction to aerosolized OVA (P = 0.016) (Figure 2) ▶ were seen in sensitized mice. This was accompanied by increased numbers of white blood cells in the BAL (P = 0.007) (Figure 2) ▶ , which was predominantly because of increases in the influx of lymphocytes (P = 0.001) and eosinophils (P = 0.005). Serum IgE (P = 0.001) (Figure 2) ▶ was also significantly increased in the sensitized mice compared to the controls. Evaluation of histopathology demonstrated that the lungs of sensitized mice had marked perivascular and peribronchiolar eosinophilic and lymphocytic inflammation. Additionally, increased numbers of mucous cells were observed in the epithelium, which became progressively more numerous as the number of aerosol challenges increased. The data from each of these end points validate that allergen sensitization followed by aerosolized allergen challenge results in mice that demonstrate characteristics of asthma, including airflow obstruction, airway hyperresponsiveness, and an eosinophilic and lymphocytic inflammatory infiltrate.
Figure 2.
Validation of a mouse model of allergic sensitization and challenge. A: Airflow obstruction expressed as maximum percent increase in respiratory resistance. Baseline resistance is 100%. On day 22 mice sensitized and challenged with OVA had significantly increased airflow obstruction compared to nonsensitized controls (*, P = 0.016). B: Airway responsiveness to aerosolized methacholine expressed as the dose of methacholine required to give a 50% increase in respiratory resistance above baseline (provocative concentration50%). On day 22, OVA-sensitized and -challenged mice had a significantly lower provocative concentration50% compared to nonsensitized control mice (*, P < 0.000), which is consistent with airway hyperresponsiveness. C: Bronchoalveolar lavage cells recovered on day 22. Differential cell counts were performed on cytospins of cells recovered from individual animals. Total white blood cells (P = 0.007), lymphocytes (*, P = 0.001), and eosinophils (**, P = 0.005) were all significantly elevated in sensitized mice compared to nonsensitized control mice. D: Serum IgE levels were significantly elevated on day 22 compared to nonsensitized controls (*, P = 0.001).
Epithelial Injury
EthD-1, a sensitive indicator of irreversible cell injury, is taken up by cells with increased cell membrane permeability (ie, cells undergoing necrosis). 19 Although several time points were examined, 6, 12, and 24 hours after each of the three aerosol challenges, there was no evidence of increased uptake of EthD-1 by epithelial cells in the sensitized mice, indicative of the lack of epithelial necrosis. Similarly, when a terminal dUTP nick-end labeling assay was performed on sections of tissue from the same time points, no evidence of any apoptotic cells in the epithelium was seen in either the sensitized or nonsensitized mice. Taken together these data suggest that there was no necrosis or apoptosis evident at any of the time points examined.
Epithelial Proliferation and Mucous Cell Morphometry
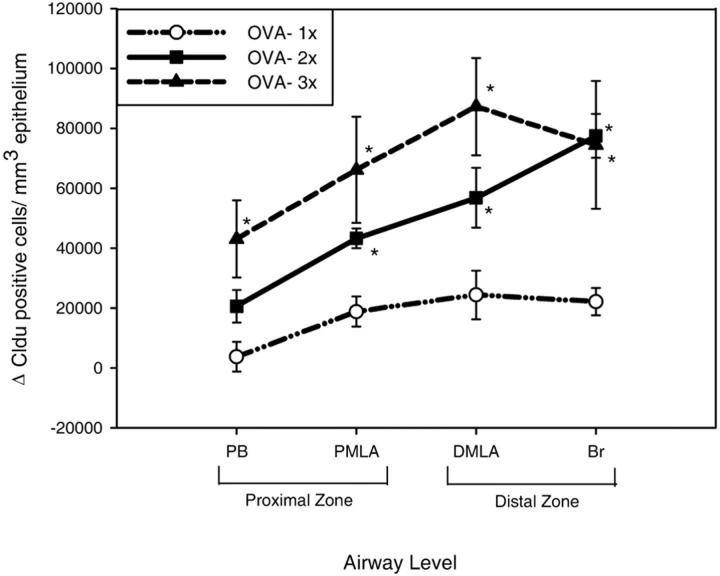
Cldu immunohistochemistry provided a cumulative index of epithelial proliferation in the airways, which increased in all airway levels with OVA sensitization and challenge (Figure 3) ▶ . At each time point epithelial proliferation was more marked in the distal zone (DMLA and Br) compared to the proximal zone (PB and PMLA) and this difference between the proliferation in the distal and proximal zones was amplified with increasing number of aerosol challenges (Figure 3) ▶ . By the second aerosol challenge, the numbers of proliferating cells in the epithelium were significantly increased at all airway levels compared to nonsensitized animals except in the PB (PMLA, P = 0.05; DMLA, P = 0.003; Br, P < 0.000). These proliferative events were accompanied by a marked increase in the number of mucus containing cells in the proximal zone but also included an increase in mucus-containing cells in more distal airways, especially as the number of aerosol challenges increased (Figure 4) ▶ . In the proximal zone of sensitized mice, the mucous cells had a significantly greater volume of stained mucosubstance per unit area of basement membrane compared to control mice, even after a single aerosol challenge (PB, P = 0.001; PMLA, P = 0.005). In the distal zone, after two aerosol challenges there was a significant increase in the volume of mucus in the DMLA (P = 0.002). Control mice had only rare mucous cells in the proximal airways and no mucous cells in the distal airways.
Figure 3.
The cumulative index of epithelial proliferation was calculated as the change in the number of Cldu-positive cells above baseline/mm3 of epithelium for each time point 48 hours after each of the three aerosol challenges. Epithelial proliferation was significantly increased in sensitized mice in the proximal mid-level airway (PMLA), distal mid-level airway (DMLA), and bronchiole (Br) after the second aerosol challenge and at all airway levels after the third aerosol challenge compared to controls (*, P < 0.05).
Figure 4.
The change in volume of mucus in the epithelium/unit area of basement membrane was calculated for each airway level 48 hours after each of the three aerosol challenges. The volume of stained mucosubstance in sensitized mice was significantly increased in the proximal zone compared to controls after a single aerosol challenge and significantly increased in the distal mid-level airway (DMLA) after the second aerosol challenge (*, P < 0.05).
When a combined Cldu immunohistochemistry and PAS/AB stain was performed on tissue sections from mice on day 22, a small number of the mucus-containing epithelial cells were also positive for Cldu (estimated as 1 in 5 to 10 Cldu to PAS/AB) (Figure 5) ▶ .
Figure 5.
Histology sections of the bronchial epithelium from nonsensitized mice (A) and sensitized mice (B) stained with PAS/AB and Cldu immunohistochemistry double stain to show cells that both contain mucus and are proliferating. Sensitized mice have an estimated 1:5 to 1:10 cells in the epithelium that are both Cldu- and PAS/AB-positive (asterisk). Other cells that contain mucus are Cldu-negative (arrows). Scale bar, 20 μm.
Characterization and Morphometry of Epithelial Cell Types
By day 22, after three aerosol challenges, 1-μm toluidine blue sections analyzed using the physical dissector procedure demonstrate that there was a dramatic shift in the phenotype of the epithelium in sensitized mice compared to nonsensitized controls, particularly in the proximal zone where mucous cells were replacing the normal epithelium. Although the number of proliferating cells per unit area of basement membrane in the proximal zone was significantly increased (P < 0.000), only 15% of the total number of cells had undergone proliferation by day 22 (Figure 6) ▶ . Mucus cells significantly increased (P = 0.01) after sensitization whereas Clara cells significantly decreased (P = 0.017) but even though there was a small decrease in the numbers of ciliated cells this was not significant (Figure 6) ▶ . Although there was no significant difference in the total number of cells per unit area of basement membrane in the proximal zone, the epithelial height was significantly increased (P = 0.001) in sensitized mice in the proximal zone (Figure 7) ▶ , indicating that the average cell volume was increasing. This suggests that smaller Clara cells were being replaced by larger mucous cells. In the lower airways there were no significant differences in the total number of cells per unit area, height of the epithelium, or between different cell types.
Figure 6.
The numbers of each cell population per surface area of basement membrane in the proximal zone (airways PB and PMLA) were calculated at a single time point 48 hours after the third aerosol challenge (day 22). In sensitized mice, the number of mucous cells significantly increased (*, P = 0.01) and the number of Clara cells decreased (**, P = 0.017) compared to nonsensitized controls. The total number of proliferating cells was significantly increased in sensitized mice (^, P < 0.000). The number of Cldu-positive cells that also contain mucus (hatched bar) was estimated from Cldu immunohistochemistry and PAS/AB double-labeled cells.
Figure 7.
The height of the epithelium was measured 48 hours after the third aerosol challenge in both proximal zone (airways PB and PMLA) and the distal zone (DMLA and Br). The height of the epithelium was only significantly increased in the proximal zone of sensitized mice (*, P = 0.001) compared to nonsensitized controls.
Electron microscopy also concurred that the predominant cell type in the upper airway by day 22 was the mucous cell. At this time, cells with the classical features of Clara cells, namely an apical cell projection, abundant smooth endoplasmic reticulum, and electron-dense secretory vesicles usually located in the apical region of the cell, became more difficult to find. Many of the epithelial cells were packed with mucus globules. After just a single antigen challenge epithelial cells containing mucus appeared within 12 hours. Micrographs demonstrated that mucus could be seen in cells with the features of Clara cells in the proximal airways adding further weight to the argument that Clara cells are differentiating into mucous cells (Figure 8) ▶ .
Figure 8.
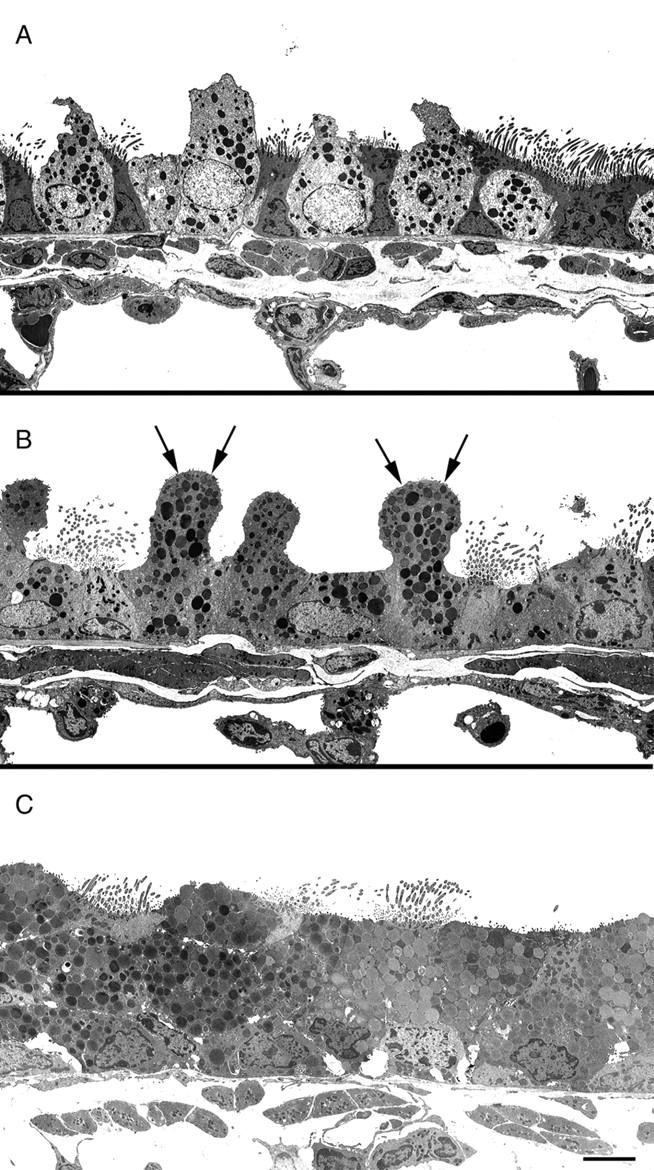
Electron micrographs from nonsensitized (A) and sensitized (B) mice 12 hours after the first OVA challenge and sensitized mice on protocol day 22 (C) (after the third OVA challenge). Mucus droplets can be seen within the apical projections of Clara cells at 12 hours (arrows) and by day 22 the epithelium was packed with mucus droplets. Scale bar, 5 μm.
Discussion
Using OVA-sensitized and -challenged mice, we tested the hypothesis that epithelial cell death results in stem cell proliferation and replacement of the Clara and ciliated cell population with mucous cells. A unique feature of our study was the quantification of the changes occurring within the epithelium at different airway generations using unbiased stereological techniques. The hallmark of the epithelial lesions was a dramatic increase in the numbers of mucous cells, which was initially confined to the proximal airways but progressed distally with increased numbers of antigen challenges. The increase in mucous cells was predominantly a result of Clara cell metaplasia rather than proliferation, which only played a minor role at later time points. Although we did see epithelial proliferation in our model this occurred at the greatest magnitude in the distal airways as opposed to the proximal airways, where the majority of the increase in mucous cells was seen. Interestingly, the proliferation was not driven by epithelial necrosis or apoptosis. This is the first study to show that there was no change in the total numbers of epithelial cells per surface area of basal lamina, even though there was a profound change in the epithelial cell population of the airways. After sensitization and challenge there was a 75% decrease in Clara cells and a 25% decrease in ciliated cells that was compensated by an increase in mucous cells, so that by day 22, 70% of the total epithelial cell population in the proximal zone were mucous cells.
Our documentation of the lesions by time and airway generation is similar to other studies with lung toxicants that have highlighted the fact that different airway generations in the mouse can respond to the same insult in a different manner. 20 Additionally, recent studies in human asthmatics have shown that neither the epithelium nor the associated inflammatory response is uniform along the entire airway tree, but that significant differences exist between large and small airways. 21
Descriptions of asthma have classically been associated with epithelial disruption and desquamation as originally described by Laitinen and colleagues 22 Clinically, epithelial injury has been correlated with both patient symptoms and bronchial reactivity. 23,24 Damage to the bronchial epithelium has been observed in asthmatic airways at postmortem 25 or by bronchoscopy 24,26 and large numbers of epithelial cells in clumps have been seen in asthmatic sputum especially during exacerbations. 27 In a monkey asthma model epithelial denudation is seen early on, at ∼6 months after the initial sensitization, as detected by increased numbers of epithelial cells in the BAL. 28 Conversely, other studies concluded that there was no evidence of epithelial desquamation in mild 29 or even fatal asthma. 30 Although the majority of the evidence supports epithelial denudation as a feature of asthma, the lesions may vary depending on the stage or severity of disease and stability of the patient or response to treatment.
In asthmatic humans, some studies suggest that the ciliated cell undergoes preferential damage 22,31 but although the number of ciliated cells in the epithelium in our study were slightly decreased this was not a significant effect and was not associated with increased markers of apoptosis. Furthermore, we did not see any evidence of epithelial cell death (necrosis or apoptosis) at the time points we examined, which was similar to other studies in mice. 32-34 It is still possible that we missed the crucial time point because of the sample times used to detect these changes. In vivo, apoptotic cells are cleared within hours by macrophages and other cells. This may have occurred before DNA single-strand breaks and fragmentation, the marker detected by the terminal dUTP nick-end labeling assay. Even though eosinophils are present in the lung tissue and BAL, the lack of significant eosinophil degranulation in mouse asthma models 35-37 may explain the lack of epithelial necrosis. This may be a fundamental difference between the behavior of eosinophils in mouse asthma models compared to human asthmatics. 36 Ultrastructural studies in humans have demonstrated extensive eosinophil degranulation in airway tissue during active disease. 38-40
Despite the lack of any detectable necrosis or apoptosis, an increase in epithelial proliferation was detected with Cldu immunohistochemistry at all airway levels 2 days after the first aerosol challenge and it increased still further with the number of aerosol challenges. This increase in cell proliferation is consistent with other models of asthma in sensitized Brown-Norway rats, 41-43 guinea-pigs, 44 and mice. 34,45 For any given time point, Cldu-positive epithelial cells occurred more frequently in distal than proximal airway epithelium in sensitized mice and this difference became more pronounced with increasing airway challenges. Electron microscopy of the distal airways demonstrated that the cell type proliferating in the distal airways had the morphological characteristics of the Clara cell. Although epithelial proliferation was significantly increased in the proximal zone by day 22, still only 15% of the total numbers of cells had undergone proliferation. One potential explanation could be that some stem cells (Clara cells) were proliferating and undergoing metaplasia while other Clara/ciliated cells were sloughing, because we have shown that the majority of the mucous cells were not proliferating and we did not observe Clara/ciliated cells in apoptosis or necrosis. In a mouse OVA model, epithelial proliferation could be partially reversed by treatment with dexamethasone, which has potent anti-inflammatory properties. 34 Therefore proliferation may simply be the response to increased levels of growth factors and other mitogenic stimuli found in inflamed environments or it may play an integral role in amplifying the allergic response. To our knowledge, increased rates of epithelial proliferation have not been documented in untreated human asthmatics although asthmatics treated with steroids may have increased epithelial proliferation. 46 To the contrary, some evidence even suggests proliferation may be impaired in epithelial cells of untreated asthmatics despite extensive damage, revealing a potential failure in the epithelial injury-repair cycle. 47,48
A profound change in epithelial cell phenotype, most notably excessive production of airway mucus glycoproteins, is also one of the hallmarks of human asthma. Although mucous cell metaplasia in this model was primarily confined to the proximal airways, its distal progression with increasing numbers of aerosol challenges may be critical to the development of airflow obstruction in the mouse. 49 This generation-dependent distribution may be a result of cumulative allergen deposition in the lung or reflect regional differences in the response of the epithelium to antigen. The increase in mucous cells in the proximal airways developed after a single aerosol challenge as seen in electron micrographs and before evidence of epithelial proliferation. Furthermore, Cldu immunohistochemistry and PAS/AB double stains performed on tissue sections from mice given the three aerosol challenges revealed that only 10% of the mucus-containing cells had undergone proliferation. Together, this data indicates that metaplasia accounts for the majority of the increase in mucous cells with proliferation playing a minor role later on. Induction of the mucous cell phenotype as an early event after a single aerosol challenge concurs with observations made by other investigators. 32,33,50 The increase in the numbers of mucous cells in the proximal airways was accompanied by a compensatory decrease in Clara cell numbers and a small decrease in ciliated cell numbers. In normal mice the bronchi are lined by a simple cuboidal epithelium where ∼50% are Clara cells that are not PAS/AB-positive. 51 The remaining cells are predominantly ciliated cells with a few basal cells and there are rare mucus-secreting cells. We did observe the appearance of mucus in some Clara cells 12 hours after the first OVA challenge, which has also been previously documented, 33 and by 48 to 72 hours the entire supranuclear cytoplasm was packed with mucus granules. This observation, along with the previously described scarcity of double-staining PAS/AB and Cldu-positive cells and shifts in cell phenotype without a difference in cell numbers, is further evidence that Clara cells are undergoing metaplasia to mucous cells. Despite the pronounced shift in cell phenotypes in the proximal airways, there was no difference in the number of epithelial cells per surface area of basal lamina. However, the epithelium was significantly taller in asthmatic mice, which could be accounted for by an increase in the average cell volume as smaller Clara cells underwent metaplasia to larger mucous cells.
The conclusions drawn from our mouse model cannot be readily extrapolated to the human because the epithelium lining the bronchi, where the majority of airway remodeling takes place in human asthmatics, is very different. The human bronchi are lined by a pseudostratified columnar epithelium primarily composed of ciliated and mucous cells with smaller numbers of basal cells and rare serous and neuroendocrine cells. Mucous glands are present in the submucosa and these glands are also associated with some serous cells. The numbers of Clara cells in the human airways do not increase until the level of the respiratory bronchioles. Therefore metaplasia of the Clara cell to a mucous cell is not going to play a major role in the increase in mucus seen in the bronchial airways of asthmatic humans. In a study looking at human tissue from asthmatics in which similar unbiased stereological techniques were used, the authors also concluded that stored mucin in the epithelium was three times higher in asthmatics compared to controls and was because of a doubling in mucous cell number rather than an increase in volume. 52 The authors could not address whether the increase in mucous cells was true mucous cell hyperplasia or a result of metaplasia. Therefore it is still possible that metaplasia of a cell type other than the Clara cell, such as the serous cell, may account in part, for the increased number of mucous cells observed in the airway epithelium and mucous glands of human asthmatics.
The data presented in this study confirms a dramatic shift in cell phenotype within the epithelium, which was primarily a result of Clara cell differentiation to mucous cells. Interestingly, the mechanism behind this change in epithelial phenotype did not follow our hypothesis. In our model, epithelial cell death does not appear to be the stimulus that drives epithelial proliferation. Moreover epithelial proliferation itself does not account for the majority of the increase in mucous cell numbers. As in human asthmatics, allergic airways in mice show similar increases in mucous cells, where chronic degranulation may represent a mechanism for airway obstruction in asthma, although the mechanisms behind this increase are likely to be different between the two species.
Acknowledgments
We thank Viviana Wong for the electron microscopy work, Frank Ventimiglia for the photography, Nancy Tyler for assistance in preparing the manuscript, and the pathology department at Genentech Inc. for processing tissue and lavage samples.
Footnotes
Address reprint requests to Dr. Dallas M. Hyde, California Regional Primate Research Center, University of California, One Shields Ave., Davis CA 95616. E-mail: dmhyde@ucdavis.edu.
Support by Genentech Inc. and the American Lung Association of California (grant RT 016-L).
References
- 1.Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D: Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest 1997, 111:852-857 [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM: Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000, 161:1720-1745 [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST: Inflammatory and structural changes in the airways of patients with asthma. Respir Med 2000, 94(Suppl D):S3-S6 [DOI] [PubMed] [Google Scholar]
- 4.Tanizaki Y, Kitani H, Okazaki M, Mifune T, Mitsunobu F, Kimura I: Mucus hypersecretion and eosinophils in bronchoalveolar lavage fluid in adult patients with bronchial asthma. J Asthma 1993, 30:257-262 [DOI] [PubMed] [Google Scholar]
- 5.Busse W, Elias J, Sheppard D, Banks-Schlegel S: Airway remodeling and repair. Am J Respir Crit Care Med 1999, 160:1035-1042 [DOI] [PubMed] [Google Scholar]
- 6.Wilson JW, Bamford TL: Assessing the evidence for remodelling of the airway in asthma. Pulm Pharmacol Ther 2001, 14:229-247 [DOI] [PubMed] [Google Scholar]
- 7.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF: The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 1999, 29:90-95 [DOI] [PubMed] [Google Scholar]
- 8.Holgate ST, Lackie P, Wilson S, Roche W, Davies D: Bronchial epithelium as a key regulator of airway allergen sensitization and remodeling in asthma. Am J Respir Crit Care Med 2000, 162:S113-S117 [DOI] [PubMed] [Google Scholar]
- 9.Hamilton LM, Davies DE, Wilson SJ, Kimber I, Dearman RJ, Holgate ST: The bronchial epithelium in asthma—much more than a passive barrier. Monaldi Arch Chest Dis 2001, 56:48-54 [PubMed] [Google Scholar]
- 10.Bayram H, Devalia JL, Abdelaziz MM, Khair OA, Sapsford RJ, Davies DE: Effects of ozone (O3) on release of pro-inflammatory mediators from bronchial epithelial cell cultures of atopic asthmatics and non-asthmatics. Clin Exp Allergy 1997, 27:1369 [Google Scholar]
- 11.Kraft M: The distal airways: are they important in asthma? Eur Respir J 1999, 14:1403-1417 [DOI] [PubMed] [Google Scholar]
- 12.Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM: Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis 1991, 143:138-143 [DOI] [PubMed] [Google Scholar]
- 13.Rudmann DG, Moore MW, Tepper JS, Aldrich MC, Pfeiffer JW, Hogenesch H, Tumas DB: Modulation of allergic inflammation in mice deficient in TNF receptors. Am J Physiol 2000, 279:L1047-L1057 [DOI] [PubMed] [Google Scholar]
- 14.Plopper CG, Mariassy AT, Lollini LO: Structure as revealed by airway dissection. A comparison of mammalian lungs. Am Rev Respir Dis 1983, 128:S4-S7 [DOI] [PubMed] [Google Scholar]
- 15.Hsu SM, Raine L, Fanger H: The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase techniques. Am J Clin Pathol 1981, 75:816-821 [DOI] [PubMed] [Google Scholar]
- 16.Bolender RP, Hyde DM, Dehoff RT: Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. Am J Physiol 1993, 265:L521-L548 [DOI] [PubMed] [Google Scholar]
- 17.Baddeley AJ, Gundersen HJ, Cruz-Orive LM: Estimation of surface area from vertical sections. J Microsc 1986, 142:259-276 [DOI] [PubMed] [Google Scholar]
- 18.Weibel ER: Stereological Methods: Practical Methods for Biological Morphometry. 1979:pp 204-206 Academic Press Inc., London
- 19.Postlethwait EM, Joad JP, Hyde DM, Schelegle ES, Bric JM, Weir AJ, Putney LF, Wong VJ, Velsor LW, Plopper CG: Three-dimensional mapping of ozone-induced acute cytotoxicity in tracheobronchial airways of isolated perfused rat lung. Am J Respir Cell Mol Biol 2000, 22:191-199 [DOI] [PubMed] [Google Scholar]
- 20.Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG: Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol 1995, 269:L800-L818 [DOI] [PubMed] [Google Scholar]
- 21.Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, Sugarbaker DJ, Doerschuk CM, Drazen JM: Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med 1998, 158:565-572 [DOI] [PubMed] [Google Scholar]
- 22.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T: Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 1985, 131:599-606 [DOI] [PubMed] [Google Scholar]
- 23.Beasley R, Roche WR, Roberts JA, Holgate ST: Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis 1989, 139:806-817 [DOI] [PubMed] [Google Scholar]
- 24.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB: Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis 1989, 140:1745-1753 [DOI] [PubMed] [Google Scholar]
- 25.James AL, Carroll N: The pathology of fatal asthma. Holgate ST Busse WW eds. Inflammatory Mechanisms in Asthma. 1998:pp 1-26 Marcel Dekker, New York
- 26.Montefort S, Roberts JA, Beasley R, Holgate ST, Roche WR: The site of disruption of the bronchial epithelium in asthmatic and non-asthmatic subjects. Thorax 1992, 47:499-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woltmann G, Ward RJ, Symon FA, Rew DA, Pavord ID, Wardlaw AJ: Objective quantitative analysis of eosinophils and bronchial epithelial cells in induced sputum by laser scanning cytometry. Thorax 1999, 54:124-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, Wong VJ, Joad JP, Pinkerton KB, Wu R, Evans MJ, Hyde DM, Plopper CG: Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae). Am J Pathol 2001, 158:333-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozewicz S, Wells C, Gomez E, Ferguson H, Richman P, Devalia J, Davies RJ: Morphological integrity of the bronchial epithelium in mild asthma. Thorax 1990, 45:12-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll N, Elliot J, Morton A, James A: The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993, 147:405-410 [DOI] [PubMed] [Google Scholar]
- 31.Montefort S, Djukanovic R, Holgate ST, Roche WR: Ciliated cell damage in the bronchial epithelium of asthmatics and non-asthmatics. Clin Exp Allergy 1993, 23:185-189 [DOI] [PubMed] [Google Scholar]
- 32.Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D: Lung inflammation and epithelial changes in a murine model of atopic asthma. Am J Respir Cell Mol Biol 1996, 14:425-438 [DOI] [PubMed] [Google Scholar]
- 33.Haile S, Lefort J, Joseph D, Gounon P, Huerre M, Vargaftig BB: Mucous cell metaplasia and inflammatory cell recruitment are dissociated in allergic mice after antibody- and drug-dependent cell depletion in a murine model of asthma. Am J Respir Cell Mol Biol 1999, 20:891-902 [DOI] [PubMed] [Google Scholar]
- 34.Trifilieff A, El Hashim A, Bertrand C: Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am J Physiol 2000, 279:L1120-L1128 [DOI] [PubMed] [Google Scholar]
- 35.Eum SY, Haile S, Lefort J, Huerre M, Vargaftig BB: Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc Natl Acad Sci USA 1995, 92:12290-12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malm-Erjefalt M, Persson CG, Erjefalt JS: Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation. Am J Respir Cell Mol Biol 2001, 24:352-359 [DOI] [PubMed] [Google Scholar]
- 37.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W, Hazen SL, Gleich GJ, Lee JJ, Lee NA: Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol 2001, 167:1672-1682 [DOI] [PubMed] [Google Scholar]
- 38.Erjefalt JS, Greiff L, Andersson M, Matsson E, Petersen H, Linden M, Ansari T, Jeffery PK, Persson CG: Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med 1999, 160:304-312 [DOI] [PubMed] [Google Scholar]
- 39.Erjefalt JS, Andersson M, Greiff L, Korsgren M, Gizycki M, Jeffery PK, Persson GA: Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J Allergy Clin Immunol 1998, 102:286-294 [DOI] [PubMed] [Google Scholar]
- 40.Gleich GJ: Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol 2000, 105:651-663 [DOI] [PubMed] [Google Scholar]
- 41.Salmon M, Walsh DA, Koto H, Barnes PJ, Chung KF: Repeated allergen exposure of sensitized Brown-Norway rats induces airway cell DNA synthesis and remodelling. Eur Respir J 1999, 14:633-641 [DOI] [PubMed] [Google Scholar]
- 42.Palmans E, Kips JC, Pauwels RA: Prolonged allergen exposure induces structural airway changes in sensitized rats. Am J Respir Crit Care Med 2000, 161:627-635 [DOI] [PubMed] [Google Scholar]
- 43.Panettieri RA, Jr, Murray RK, Eszterhas AJ, Bilgen G, Martin JG: Repeated allergen inhalations induce DNA synthesis in airway smooth muscle and epithelial cells in vivo. Am J Physiol 1998, 274:L417-L424 [DOI] [PubMed] [Google Scholar]
- 44.Wang ZL, Walker BA, Weir TD, Yarema MC, Roberts CR, Okazawa M, Pare PD, Bai TR: Effect of chronic antigen and beta 2 agonist exposure on airway remodeling in guinea pigs. Am J Respir Crit Care Med 1995, 152:2097-2104 [DOI] [PubMed] [Google Scholar]
- 45.Williams CM, Galli SJ: Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med 2000, 192:455-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Druilhe A, Wallaert B, Tsicopoulos A, Lape e Silva JR, Tillie-Leblond I, Tonnel AB, Pretolani M: Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol 1998, 19:747-757 [DOI] [PubMed] [Google Scholar]
- 47.Demoly P, Simony-Lafontaine J, Chanez P, Pujol JL, Lequeux N, Michel FB, Bousquet J: Cell proliferation in the bronchial mucosa of asthmatics and chronic bronchitis. Am J Respir Crit Care Med 1994, 150:214-217 [DOI] [PubMed] [Google Scholar]
- 48.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino AM, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, Chanez P: Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J Allergy Clin Immunol 2001, 108:738-746 [DOI] [PubMed] [Google Scholar]
- 49.Tepper JS, Yuan F, Pfeiffer JW, Aldrich MC: Airflow obstruction in murine allergic inflammation: the effectiveness of albuterol vs dexamethasone. Am J Respir Crit Care Med 1998, 157(Suppl 3):A822 [Google Scholar]
- 50.Blyth DI, Pedrick MS, Savage TJ, Bright H, Beesley JE, Sanjar S: Induction, duration, and resolution of airway goblet cell hyperplasia in a murine model of atopic asthma: effect of concurrent infection with respiratory syncytial virus and response to dexamethasone. Am J Respir Cell Mol Biol 1998, 19:38-54 [DOI] [PubMed] [Google Scholar]
- 51.Pack RJ, Al Ugaily LH, Morris G: The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat 1981, 132:71-84 [PMC free article] [PubMed] [Google Scholar]
- 52.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, Fahy JV: Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001, 163:517-523 [DOI] [PubMed] [Google Scholar]