Abstract
INTRODUCTION
The aim of this study was to evaluate the sensitivity of magnetic resonance imaging (MRI) in the detection of colorectal liver metastases.
PATIENTS AND METHODS
Pre-operative MRI scanning of the liver was performed by a single radiologist and the size and number of definite liver metastases were recorded. Patients then underwent hepatectomy with routine intra-operative ultrasonography (IOUS) and resected specimens were sent for histopathology. Pathology findings were compared with those of MRI scans to determine the sensitivity of this imaging modality. Exclusions were patients undergoing hepatic resection more than 4 weeks after the MRI scan, those undergoing chemotherapy at the time of the scan, and those with conglomerate unilobar metastases.
RESULTS
Complete data were available for 84 patients. There was total agreement between MRI, IOUS and histology in 79 patients (101 metastases). MRI missed 5 metastases in 5 patients that were found on IOUS (or palpation of superficial lesions) and subsequently confirmed by histological examination. These measured 5 mm or less (4 patients) and 7 mm (one patient). The sensitivity of MRI in the detection of colorectal liver metastases was thus 94% for all lesions and 100% for lesions 1 cm or larger in diameter.
CONCLUSIONS
MRI of the liver is a non-invasive technique with an extremely high degree of sensitivity in the detection of colorectal liver metastases and should be considered as the ‘gold standard’ in the pre-operative imaging of these patients.
Keywords: MRI, Liver metastases, Pre-operative imaging, Hepatectomy, Histopathology, Ultrasonography
Hepatic metastases from colorectal cancer are common. Approximately 15–25% of patients with primary colorectal cancer have synchronous metastases and a further 20% will develop metachronous lesions within 3 years of primary resection. Between 5–8% will be suitable for a curative resection and, with neo-adjuvant chemotherapy and ablative techniques, this proportion is likely to increase. The 5-year survival after resection for colorectal liver metastases varies between 25–40% in comparison to 0% when treated with palliative chemo-therapy.1–3 Accurate imaging of the liver is a crucial determinant in the decision to offer surgical resection and currently available imaging pre-operative modalities include transcutaneous ultrasound (TUS), computed tomography (CT), CT combined with arterio-portography (CTAP), magnetic resonance imaging (MRI) and 2-fluoro-2-deoxy-D-glucose-positron emission tomography (FDG-PET).
TUS may fail to image the whole liver due to anatomical inaccessibility and does not allow 3-dimensional planning of surgical resection.4 CT liver imaging offers increased sensitivity5,6 and may also able to assess extrahepatic disease but is inferior to MRI scanning in direct comparisons.7,8 CTAP is considered by many to be the ‘gold standard’ for hepatic imaging but it is an invasive technique with a high (up to 15%) false-positive rate.6 MRI provides a sensitive, non-invasive method of assessing liver lesions9 and direct comparison between CTAP and MRI shows that MRI is better at identifying and characterising liver lesions.10,11
Extrahepatic disease is probably best investigated by spiral CT of the chest and abdomen and, when appropriate, in combination with FDG-PET. Direct comparison of FDG-PET and MRI for liver lesions does not show a difference in sensitivity between these techniques with MRI having an added advantage of spatial resolution and lesion characterisation;12 however, this is a rapidly evolving area and improvements in PET-CT may be useful in planning surgical resections in the future.
The aim of this study was to evaluate retrospectively the sensitivity of MRI as an imaging modality for pre-operative staging of colorectal metastases in the liver by comparing pre-operative MRI findings with those of intra-operative ultrasound and pathological specimen analysis (Fig. 1).
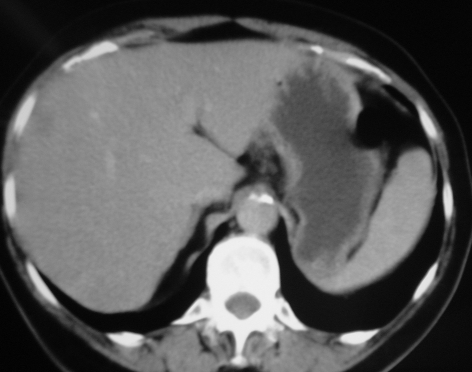
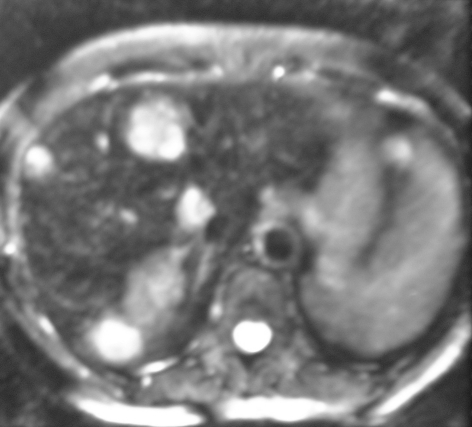
Figure 1.
(A) CT and (B) MRI from the same patient with colorectal cancer demonstrating the clarity of MRI in the detection of the liver metastases.
Patients and Methods
Patients undergoing hepatic resection for colorectal metastases at a single institution underwent pre-operative MRI scanning of the liver. The MR imaging protocol was axial and coronal T2SSFSE, axial T1 in and out of phase and T2 FSE with fat sat before infusion of colloidal iron and axial and coronal T2SSFSE and T2 FSE with fat sat after infusion of iron. The size and number of all detected metastases were recorded. All patients underwent additional spiral CT imaging of the chest and abdomen/pelvis to rule out the presence of extrahepatic disease. At operation, the size and number of metastases visible on intra-operative ultrasonography (Aloka™ SSD-2200 60-mm T-shaped intra-operative ultrasound probe at 5MHz) was recorded and this guided the hepatectomy which was performed with curative intent in each case. After resection, the hepatectomy specimen was fixed in formalin and then sectioned at approximately 5-mm intervals to enable examination and description of the gross histological appearance. Samples were then set in paraffin wax and sliced 4-µm thick before being mounted and examined by a histopathologist. Findings of the pre-operative MRI scans were compared to intra-operative ultrasonography (IOUS) and histological outcomes on a lesion-by-lesion basis.
Patients undergoing repeat hepatic resections for recurrent liver metastases, patients undergoing concurrent chemotherapy at the time of resection and those with an interval between MRI scan and surgery more than 4 weeks were excluded.
Results
A total of 92 patients (54 male and 38 female) with a median age of 62 years (range, 35–86 years) were considered for the study. One patient was excluded due to concurrent chemotherapy and one was excluded due to the interval between scanning and surgery being more than 4 weeks. Six patients with multiple (conglomerate) unilobar metastases were also excluded because the exact number of metastases could not be counted by the reporting pathologist. Thus, 84 patients were considered eligible for inclusion the study.
There was complete agreement between pathological findings and MRI scanning in 79 patients (101 metastases). Histopathology identified a further 5 lesions in 5 patients which had not been seen on MRI. Diameters of the missed lesions were 2 mm (one lesion), 5 mm (three lesions) and 7 mm (one lesion). IOUS detected all lesions except the single 2 mm lesion. Thus, the sensitivity of MRI scanning in the detection of all metastases in this study is 94% and if sub-centimetre lesions are excluded, this rises to 100% (Table 1).
Table 1.
Sensitivity of MRI versus diameter of metastases
| Diameter of metastases (mm) | No. detected by MRI (true positive) | No missed by MRI (false negative) | Sensitivity of MRI according to diameter of metastases |
|---|---|---|---|
| 0–10 | 6 | 5 | 55% |
| 11–20 | 27 | 0 | 100% |
| 21–30 | 20 | 0 | 100% |
Each patient in this series has been on regular post-hepatectomy follow-up with ultrasonography, CEA measurement and chest radiography every 6 months for 2 years and then yearly for up to 5 years. Of the 84 patients, 49 (58%) have had recurrent disease; of these, 19 patients had extrahepatic recurrence, 18 had extrahepatic and hepatic recurrence and 12 had liver-only recurrence (none had recurrence at the resection margins) and of these, 5 patients have undergone repeat hepatic resections.
Discussion
This study is a direct comparison between histopathology and MRI and does not attempt to compare other imaging modalities (although all patients underwent CT and IOUS). We have shown that MRI is capable of a very high degree of sensitivity for detecting colorectal liver metastases and this imaging modality did not miss any lesions larger than 7 mm in diameter in this series. The specificity of this technique could not be assessed as patients with no metastases on MRI of the liver were not operated upon; neither is it possible to comment on the negative predictive value of this test. Of patients who were deemed inoperable, 54% had extrahepatic disease and 46% had extensive hepatic recurrence. These patients were not primarily operated upon but were referred for chemotherapy and only a small minority of the latter group were successfully down-staged. These patients were excluded from analysis for the purposes of this study due to the artefact introduced by the severe fatty change associated with chemotherapy. All the rest of patients in this group developed progressive disease which may serve as an indirect verification of the accuracy of diagnosis on MRI.
We have used a direct comparison with pathological examination rather than intra-operative ultrasound because we felt that pathology was likely to be highly accurate and much more objective as a reference standard. Intra-operative ultrasound was performed in each case and guided the hepatectomy but we have not used it as a benchmark due to the inherent subjectivity and possibility of operator error associated with the technique. The fact that IOUS did detect four lesions not seen on MRI underlines the importance for its use as an adjunct at the time of resection. A previous study examining the value of IOUS and MRI showed that IOUS provided additional information in 48% of patients and changed the surgical strategy in 18% of patients.13 We found IOUS valuable in identifying hepatic anatomy intra-operatively but the additional number of lesions detected was small.
We excluded patients undergoing repeat hepatic resections to obviate the effect of any imaging artefacts caused by previous hepatic surgery. Patients undergoing concurrent chemotherapy and those with an interval between MRI and surgery of more than 4 weeks were excluded to exclude the effect of tumour reduction or increase in size of the metastases.
The issue of microscopic disease (not visible on conventional scans) can only be addressed by follow-up in these patients. The majority of patients who had recurrent disease in this study had extrahepatic recurrence and they presumably had microscopic disease in the liver and elsewhere. Only a small number had liver-only recurrence; all were away from the resection margin indicating that these were ‘true’ microscopic deposits and not residual disease present at the time of the resection.
Conclusions
These findings suggest that MRI is a highly sensitive method of pre-operative imaging of colorectal liver metastases and should be considered the ‘gold standard’ for this purpose, accepting that experienced interpretation is a pivotal factor in achieving such a high degree of sensitivity. Acceptance of MRI as the gold standard for liver-specific imaging will have economic and logistical implications, especially if its use is extended to the staging and follow-up of patients before and after resection of primary colorectal cancer. A recent Cochrane review demonstrated evidence that there is a survival benefit for patients undergoing liver imaging versus those that did not;14 however, these studies used liver CT or ultrasound. Whether the use of MRI in liver follow-up will improve this survival benefit needs to be addressed with randomised studies.
References
- 1.Scheele J, Altendorf-Hofmann A, Stangl R, Schmidt K. [Surgical resection of colorectal liver metastases: Gold standard for solitary and radically resectable lesions] Swiss Surg. 1996;(Suppl 4):4–17. [PubMed] [Google Scholar]
- 2.Hunt TM, Taylor I. The role of chemotherapy in the treatment and prophylaxis of colorectal liver metastases. Cancer Surv. 1989;8:71–90. [PubMed] [Google Scholar]
- 3.Beard SM, Holmes M, Price C, Majeed AW. Hepatic resection for colorectal liver metastases: a cost-effectiveness analysis. Ann Surg. 2000;232:763–76. doi: 10.1097/00000658-200012000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunven P, Makuuchi M, Takayasu K, Moriyama N, Yamasaki S, Hasegawa H. Preoperative imaging of liver metastases. Comparison of angiography, CT scan, and ultrasonography. Ann Surg. 1985;202:573–9. doi: 10.1097/00000658-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller DL, Simmons JT, Chang R, Ward BA, Shawker TH, Doppman JL, et al. Hepatic metastasis detection: comparison of three CT contrast enhancement methods. Radiology. 1987;165:785–90. doi: 10.1148/radiology.165.3.3685361. [DOI] [PubMed] [Google Scholar]
- 6.Soyer P, Levesque M, Elias D, Zeitoun G, Roche A. Detection of liver metas tases from colorectal cancer: comparison of intraoperative US and CT during arterial portography. Radiology. 1992;183:541–4. doi: 10.1148/radiology.183.2.1561365. [DOI] [PubMed] [Google Scholar]
- 7.Ward J, Naik KS, Guthrie JA, Wilson D, Robinson PJ. Hepatic lesion detection: comparison of MR imaging after the administration of superparamagnetic iron oxide with dual-phase CT by using alternative-free response receiver operating characteristic analysis. Radiology. 1999;210:459–66. doi: 10.1148/radiology.210.2.r99fe05459. [DOI] [PubMed] [Google Scholar]
- 8.Semelka RC, Shoenut JP, Ascher SM, Kroeker MA, Greenberg HM, Yaffe CS, et al. Solitary hepatic metastasis: comparison of dynamic contrast-enhanced CT and MR imaging with fat-suppressed T2-weighted, breath-hold T1-weighted FLASH, and dynamic gadolinium-enhanced FLASH sequences. J Magn Reson Imaging. 1994;4:319–23. doi: 10.1002/jmri.1880040316. [DOI] [PubMed] [Google Scholar]
- 9.Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471–8. doi: 10.1148/radiology.196.2.7617863. [DOI] [PubMed] [Google Scholar]
- 10.Semelka RC, Cance WG, Marcos HB, Mauro MA. Liver metastases: comparison of current MR techniques and spiral CT during arterial portography for detection in 20 surgically staged cases. Radiology. 1999;213:86–91. doi: 10.1148/radiology.213.1.r99oc3386. [DOI] [PubMed] [Google Scholar]
- 11.Seneterre E, Taourel P, Bouvier Y, Pradel J, Van Beers B, Daures JP, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200:785–92. doi: 10.1148/radiology.200.3.8756932. [DOI] [PubMed] [Google Scholar]
- 12.Yang M, Martin DR, Karabulut N, Frick MP. Comparison of MR and PET imaging for the evaluation of liver metastases. J Magn Reson Imaging. 2003;17:343–9. doi: 10.1002/jmri.10265. [DOI] [PubMed] [Google Scholar]
- 13.Conlon R, Jacobs M, Dasgupta D, Lodge JP. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003;16:211–6. doi: 10.1016/s0929-8266(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 14.Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database System Rev. 2006;(Issue 4) doi: 10.1002/14651858.CD002200. [DOI] [PubMed] [Google Scholar]