Abstract
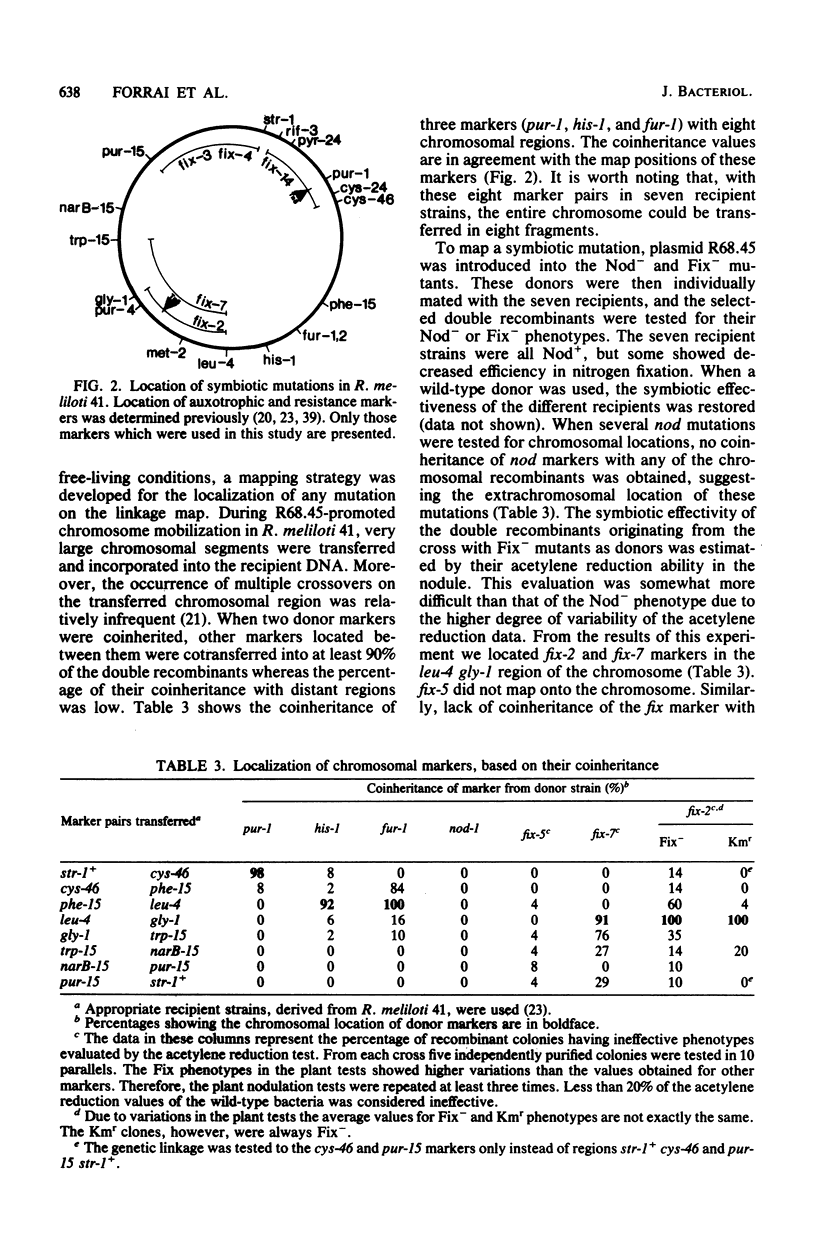
A total of 5 Nod- and 57 Fix- symbiotic mutants of Rhizobium meliloti strain 41 have been isolated after either nitrosoguanidine or Tn5 transposition mutagenesis. Chromosomal locations of mutations in 1 Nod- and 11 Fix- derivatives were ascertained by transferring the chromosome (mobilized by plasmid R68.45), in eight fragments, into symbiotically effective recipients and testing the recombinants for symbiotic phenotype. Alternatively, the kanamycin resistance marker of Tn5 was mapped. In five mutants the fix alleles were localized on different chromosomal regions, but six other fix mutations and one nod mutation tested did not map onto the chromosome. It was shown that the chromosome-mobilizing ability (Cma+) of R68.45 was not involved in the mobilization of genes located extrachromosomally. Moreover, Cma- derivatives of R68.45 could mobilize regions of the indigenous plasmid pRme41b but not chromosomal genes. Thus, mobilization of a marker by Cma- R68.45 indicates its extrachromosomal location. With a 32P-labeled DNA fragment carrying Tn5 as a hybridization probe, it was shown that in five extrachromosomally located Tn5-induced fix mutants and one nod mutant Tn5 was localized on plasmid pRme41b. This is in agreement with the genetic mapping data.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausubel F. M. Molecular genetics of symbiotic nitrogen fixation. Cell. 1982 May;29(1):1–2. doi: 10.1016/0092-8674(82)90082-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Kiss G. B., Dobo K., Dusha I., Breznovits A., Orosz L., Vincze E., Kondorosi A. Isolation and characterization of an R-prime plasmid from Rhizobium meliloti. J Bacteriol. 1980 Jan;141(1):121–128. doi: 10.1128/jb.141.1.121-128.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi A., Barabás I., Sváb Z., Orosz L., Sik T., Hotchkiss R. D. Evidence for common genetic determinants of nitrogenase and nitrate reductase in Rhizobium meliloti. Nat New Biol. 1973 Dec 5;246(153):153–154. doi: 10.1038/newbio246153a0. [DOI] [PubMed] [Google Scholar]
- Latreille J., Barlogie B., Dosik G., Johnston D. A., Drewinko B., Alexanian R. Cellular DNA content as a marker of human multiple myeloma. Blood. 1980 Mar;55(3):403–408. [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz L., Sváb Z., Kondorosi A., Sik T. Genetic studies on rhizobiophage 16-3. I. Genes and functions on the chromosome. Mol Gen Genet. 1973 Sep 27;125(4):341–350. [PubMed] [Google Scholar]
- Prakash R. K., Schilperoort R. A., Nuti M. P. Large plasmids of fast-growing rhizobia: homology studies and location of structural nitrogen fixation (nif) genes. J Bacteriol. 1981 Mar;145(3):1129–1136. doi: 10.1128/jb.145.3.1129-1136.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lund E., Smithies O., Blattner F. R. Hybridization of labeled RNA to DNA in agarose gels. Nucleic Acids Res. 1975 Oct;2(10):1911–1929. doi: 10.1093/nar/2.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. K., Singh H. N. Isolation and preliminary characterization of mutants of the cyanobacterium Nostoc muscorum resistant to growth inhibition by methylamine. Mol Gen Genet. 1981;184(2):334–336. doi: 10.1007/BF00272927. [DOI] [PubMed] [Google Scholar]
- Török I., Kondorosi A. Nucleotide sequence of the R.meliloti nitrogenase reductase (nifH) gene. Nucleic Acids Res. 1981 Nov 11;9(21):5711–5723. doi: 10.1093/nar/9.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Crowther C., Holloway B. W. The insertion sequence IS21 of R68.45 and the molecular basis for mobilization of the bacterial chromosome. Plasmid. 1981 Jul;6(1):30–52. doi: 10.1016/0147-619x(81)90052-4. [DOI] [PubMed] [Google Scholar]