Abstract
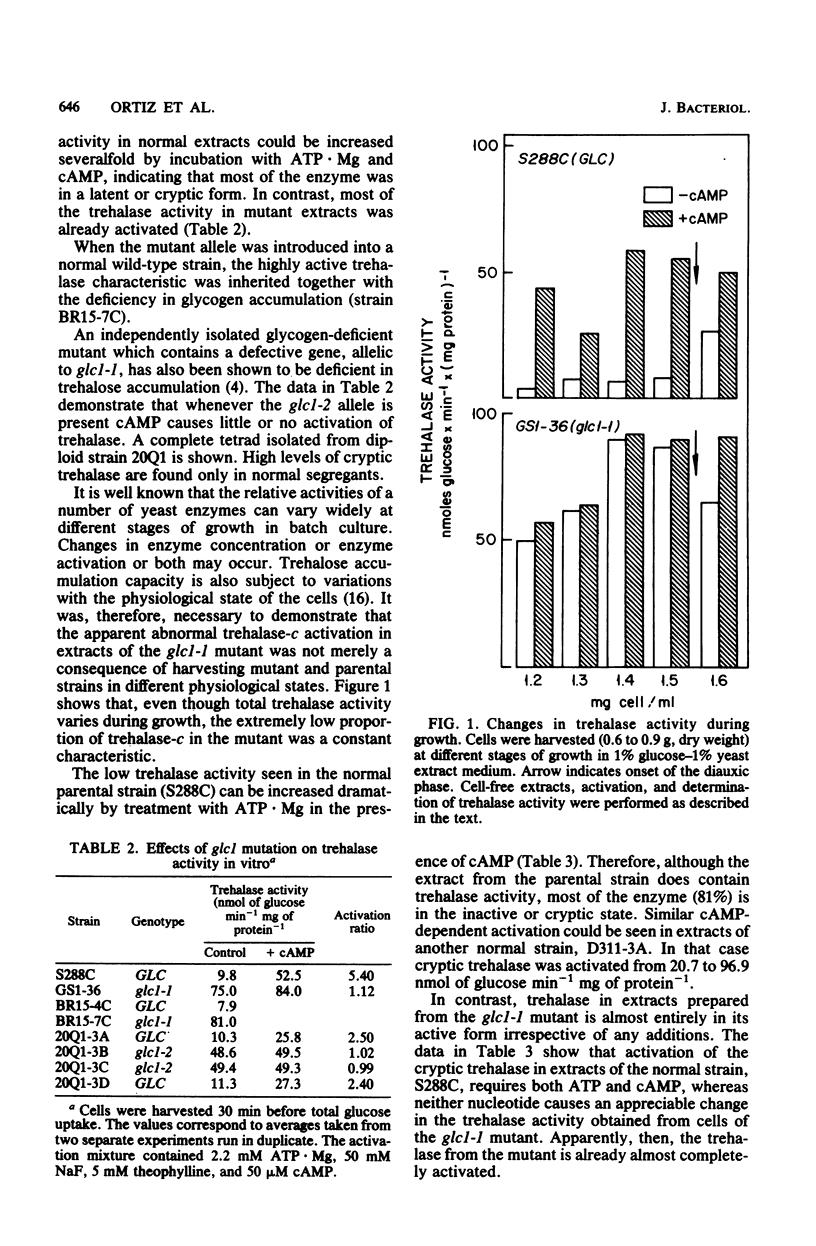
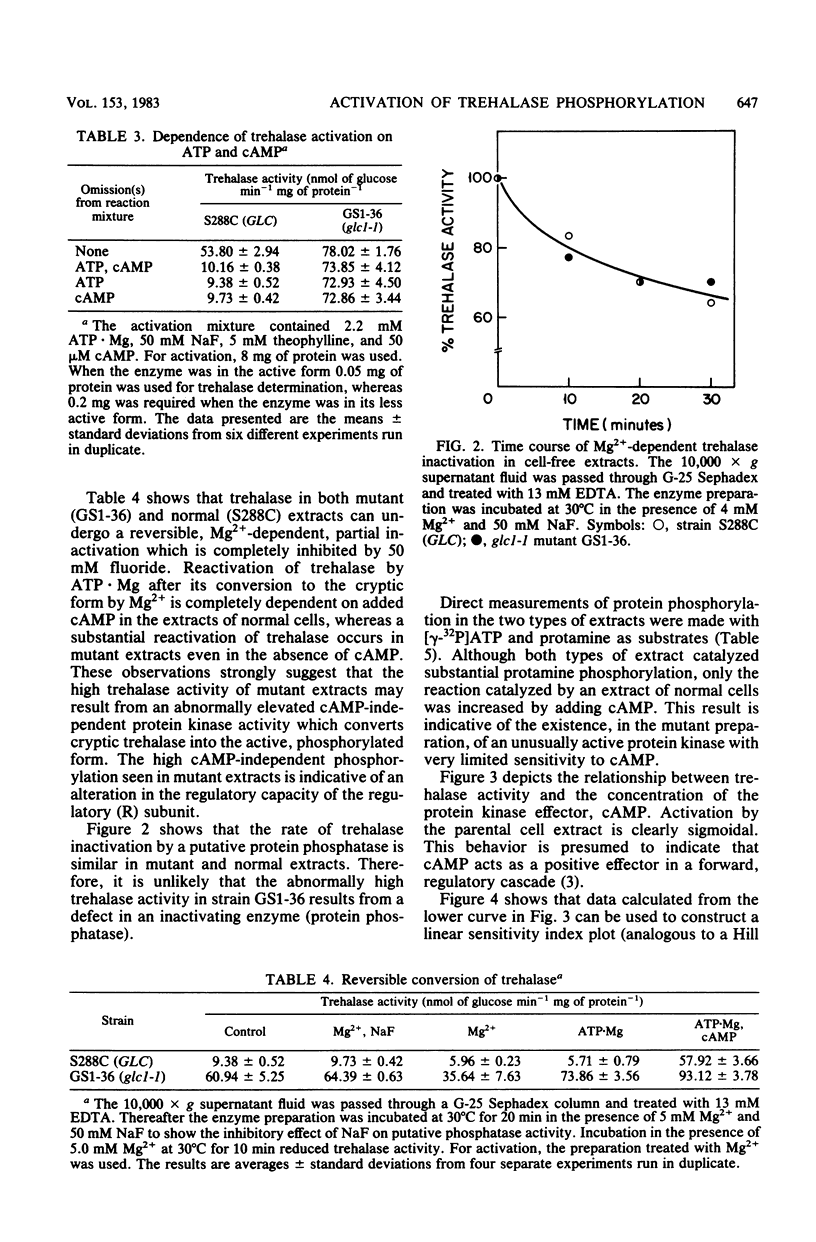
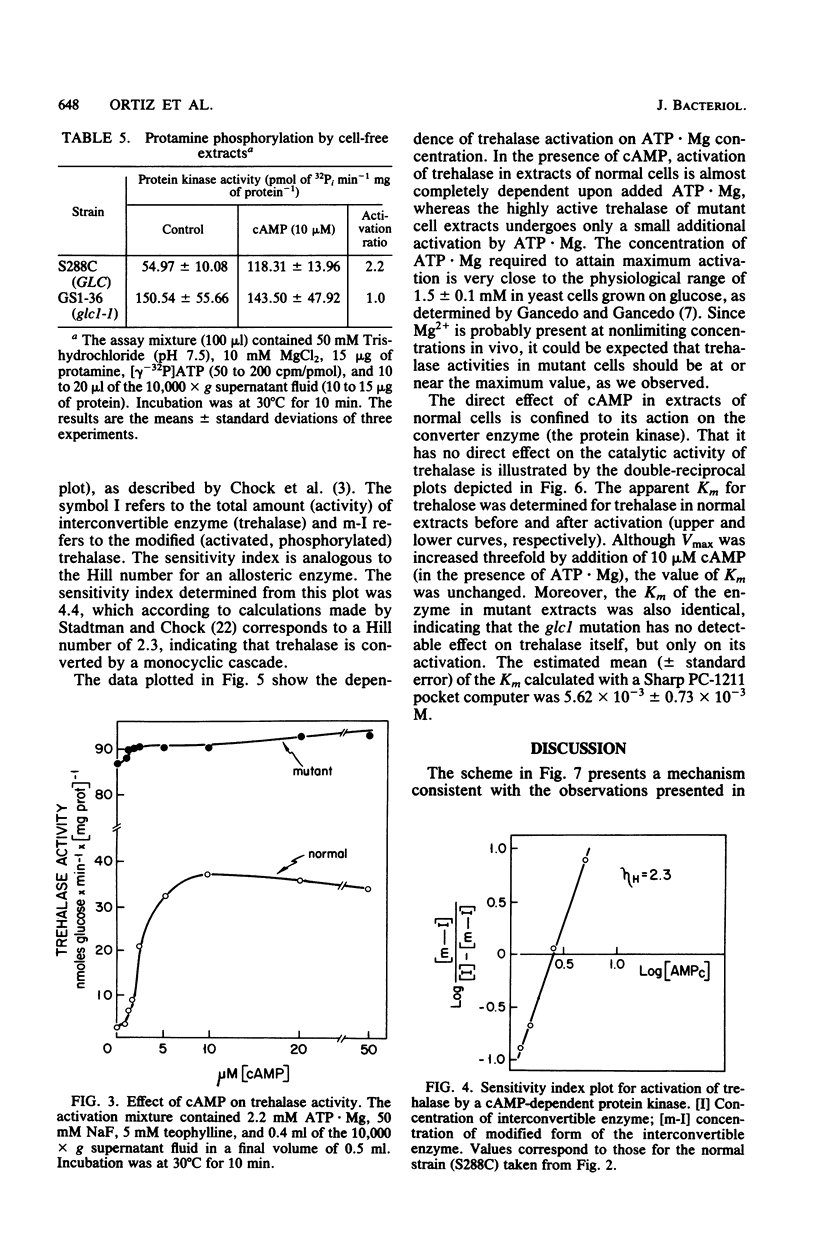
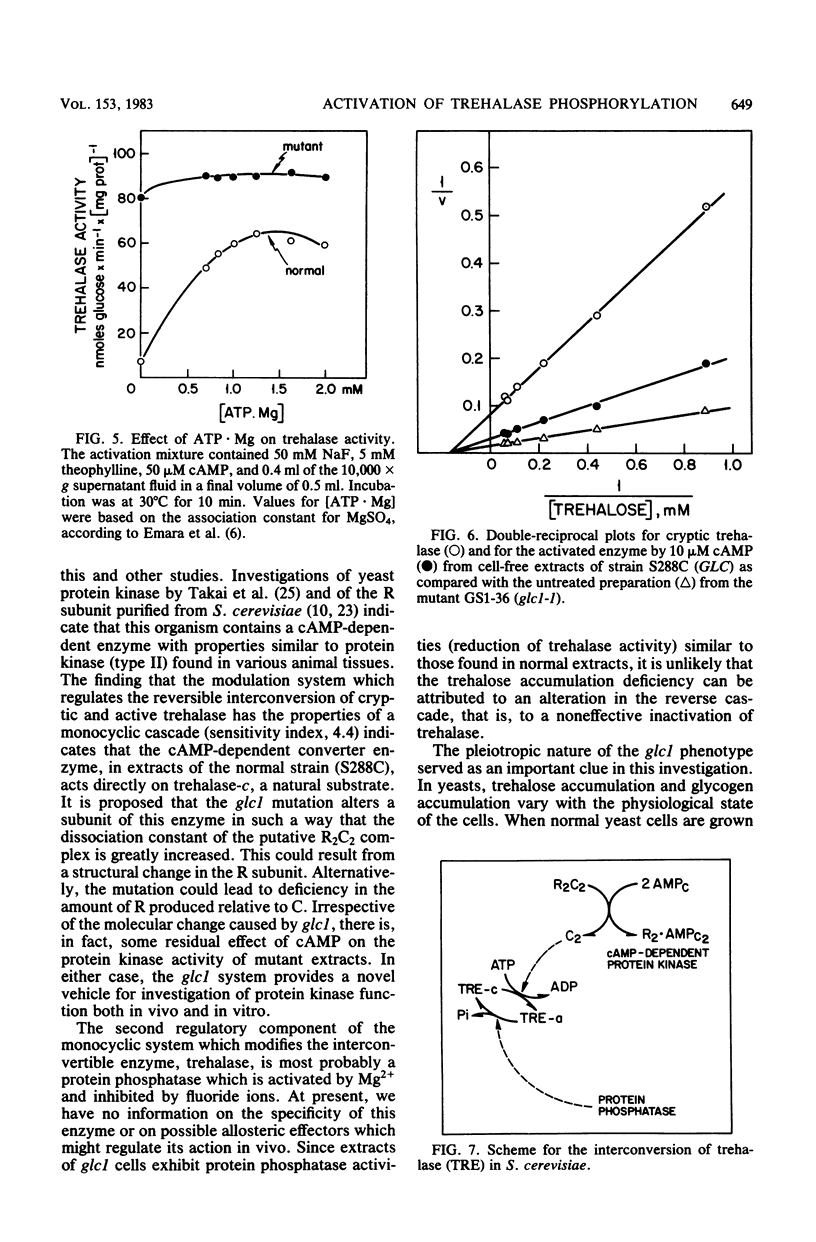
Mutation at the GLC1 locus in Saccharomyces cerevisiae resulted in simultaneous deficiencies in glycogen and trehalose accumulation. Extracts of yeast cells containing the glc1 mutation exhibited an abnormally high trehalase activity. This elevated activity was associated with a defective cyclic AMP (cAMP)-dependent monocyclic cascade which, in normal cells, regulates trehalase activity by means of protein phosphorylation and dephosphorylation. Trehalase in extracts of normal cells was largely in a cryptic form which could be activated in vitro by ATP . Mg in the presence of cAMP. Normal extracts also exhibited a correlated cAMP-dependent protein kinase which catalyzed incorporation of label from [gamma-32P]ATP into protamine. In contrast, cAMP had little or no additional activating effect on trehalase or on protamine phosphorylation in extracts of glc1 cells. Similar, unregulated activation of cryptic trehalase was also found in glycogen-deficient strains bearing a second, independently isolated mutant allele, glc1-2. Since trehalase activity was not directly affected by cAMP, the results indicate that the glc1 mutation results in an abnormally active protein kinase which has lost its normal dependence on cAMP. Trehalase in extracts of either normal or mutant cells underwent conversion to a cryptic form in an Mg2+-dependent, fluoride-sensitive reaction. Rates of this reversible reduction of activity were similar in extracts of mutant and normal cells. This same, unregulated protein kinase would act on glycogen synthase, maintaining it in the phosphorylated low-activity D-form. The glc1 mutants provide a novel model system for investigating the in vivo metabolic functions of a specific, cAMP-dependent protein kinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chock P. B., Rhee S. G., Stadtman E. R. Interconvertible enzyme cascades in cellular regulation. Annu Rev Biochem. 1980;49:813–843. doi: 10.1146/annurev.bi.49.070180.004121. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Granner D. K. Protein kinase: altered regulation in a hepatoma cell line deficient in adenosine 3',5'-cyclic monophosphate-binding protein. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1516–1522. doi: 10.1016/0006-291x(72)90779-6. [DOI] [PubMed] [Google Scholar]
- Hawthorne D C, Mortimer R K. Chromosome Mapping in Saccharomyces: Centromere-Linked Genes. Genetics. 1960 Aug;45(8):1085–1110. doi: 10.1093/genetics/45.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson C. S., Krebs E. G. Characterization of a cyclic AMP-binding protein from bakers' yeast. Identification as a regulatory subunit of cyclic AMP-dependent protein kinase. J Biol Chem. 1980 Mar 10;255(5):2137–2145. [PubMed] [Google Scholar]
- Inoue H., Shimoda C. Induction of trehalase activity on a nitrogen-free medium: a sporulation-specific event in the fission yeast, Schizosaccharomyces pombe. Mol Gen Genet. 1981;183(1):32–36. doi: 10.1007/BF00270134. [DOI] [PubMed] [Google Scholar]
- Padrão G. R., Malamud D. R., Panek A. D., Mattoon J. R. Regulation of energy metabolism in yeast. Inheritance of a pleiotropic mutation causing defects in metabolism of energy reserves, ethanol utilization and formation of cytochrome a.a3. Mol Gen Genet. 1982;185(2):255–261. doi: 10.1007/BF00330795. [DOI] [PubMed] [Google Scholar]
- Panek A. D. Adenosine triphosphate inhibition of yeast trehalase. J Bacteriol. 1969 Sep;99(3):904–905. doi: 10.1128/jb.99.3.904-905.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek A. D., Mattoon J. R. Regulation of energy metabolism in Saccharomyces cerevisiae. Relationships between catabolite repression, trehalose synthesis, and mitochondrial development. Arch Biochem Biophys. 1977 Sep;183(1):306–316. doi: 10.1016/0003-9861(77)90444-1. [DOI] [PubMed] [Google Scholar]
- Panek A. D., Sampaio A. L., Braz G. C., Baker S. J., Mattoon J. R. Genetic and metabolic control of trehalose and glycogen synthesis. New relationships between energy reserves, catabolite repression and maltose utilization. Cell Mol Biol Incl Cyto Enzymol. 1979;25(5):345–354. [PubMed] [Google Scholar]
- RAABO E., TERKILDSEN T. C. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12(4):402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Glucose 6-phosphate dependent and independent forms of yeast glycogen synthetase. Their properties and interconversions. Biochemistry. 1971 Mar 30;10(7):1236–1242. doi: 10.1021/bi00783a021. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Two forms of yeast glycogen synthetase and their role in glycogen accumulation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):967–974. doi: 10.1073/pnas.66.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Chock P. B. Interconvertible enzyme cascades in metabolic regulation. Curr Top Cell Regul. 1978;13:53–95. doi: 10.1016/b978-0-12-152813-3.50007-0. [DOI] [PubMed] [Google Scholar]
- Sy J., Roselle M. Cyclic AMP-dependent protein kinase of yeast. FEBS Lett. 1981 Nov 30;135(1):93–96. doi: 10.1016/0014-5793(81)80951-9. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sakai K., Morishita Y., Yamamura H., Nishizuka Y. Functional similarity of yeast and mammalian adenosine 3',5'-monophosphate-dependent protein kinases. Biochem Biophys Res Commun. 1974 Jul 24;59(2):646–652. doi: 10.1016/s0006-291x(74)80028-8. [DOI] [PubMed] [Google Scholar]
- Takai Y., Yamamura H., Nishizuka Y. Adenosine 3':5'-monophosphate-dependent protein kinase from yeast. J Biol Chem. 1974 Jan 25;249(2):530–535. [PubMed] [Google Scholar]
- Zimmermann F. K., Eaton N. R. Genetics of induction and catabolite repression of Maltese synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1974;134(3):261–272. doi: 10.1007/BF00267720. [DOI] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem Biophys Res Commun. 1975 Feb 3;62(3):553–560. doi: 10.1016/0006-291x(75)90434-9. [DOI] [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3',5'-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem Biophys Res Commun. 1974 Feb 4;56(3):580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]



