Abstract
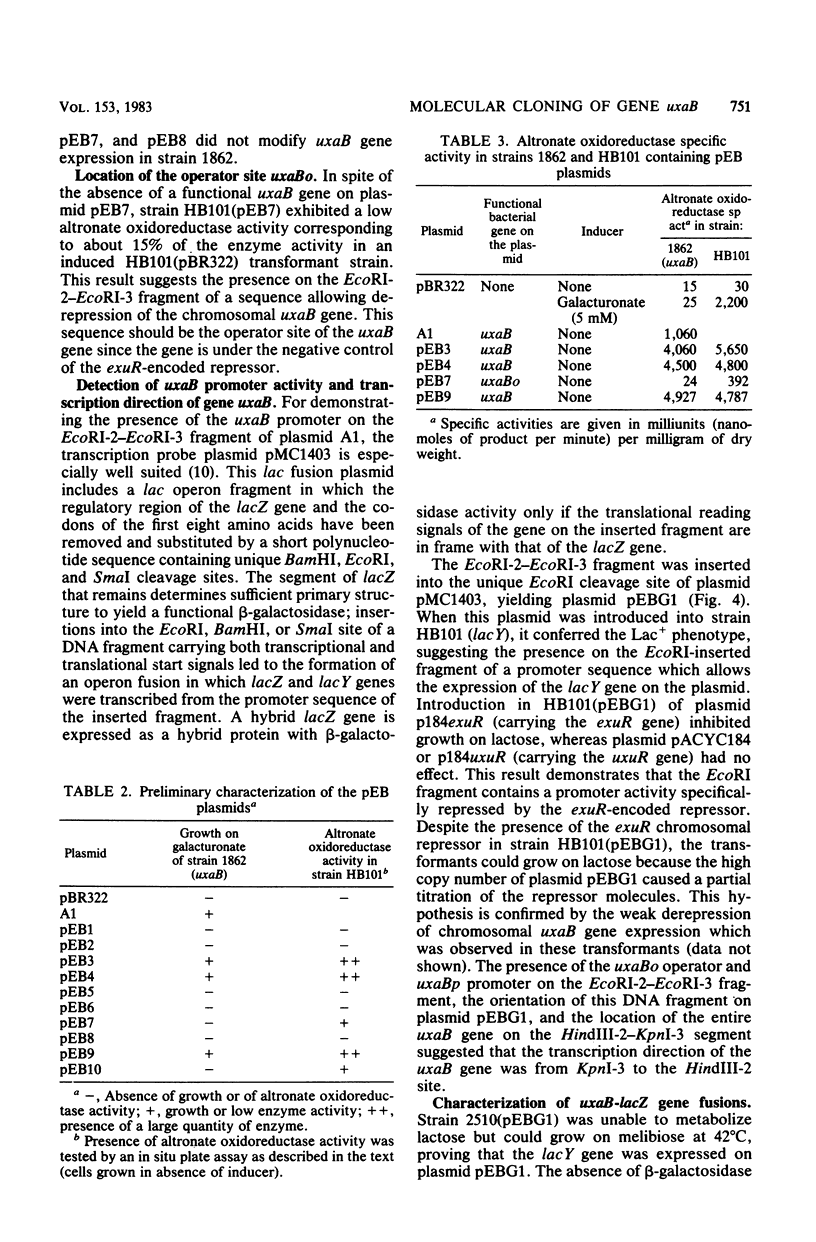
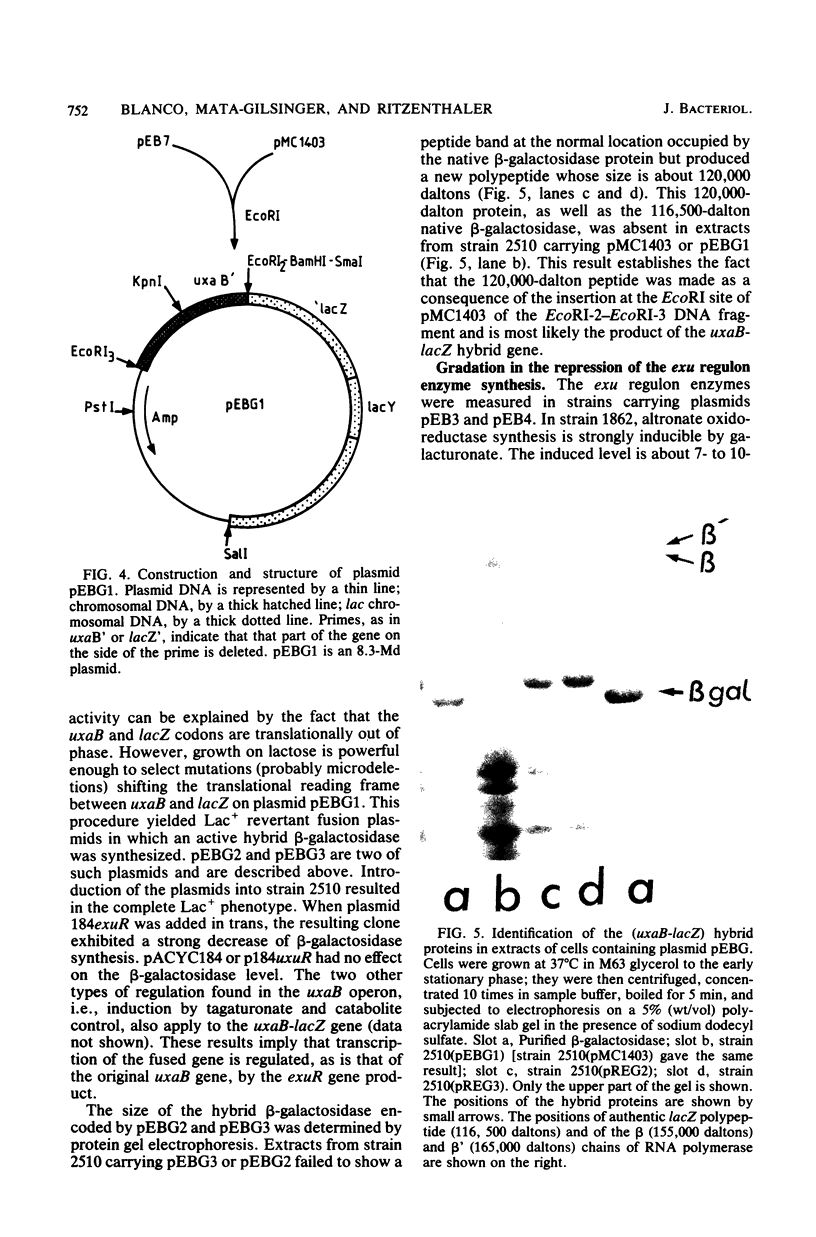
The uxaB gene of Escherichia coli, encoding for altronate oxidoreductase involved in the hexuronate degradative pathway, was isolated on a ColE1-uxaB hybrid plasmid from the Clarke and Carbon bank. The restriction map of this plasmid was established. The uxaB gene was mapped on a 1.5-megadalton HindIII-KpnI DNA fragment. Use of an in vitro gene fusion between uxaB and lacZ genes led to the determination that uxaB is transcribed from the KpnI towards the HindIII restriction sites. Gene amplification in cells containing various uxaB hybrid plasmids allowed us to show a gradation in the level of repression of exu operator sites by the exuR regulatory gene product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Jaoudé A., Lepelletier M., Ratouchniak J., Chippaux M., Pascal M. C. Nitrite reduction in Escherichia coli: genetic analysis of nir mutants. Mol Gen Genet. 1978 Nov 16;167(1):113–118. doi: 10.1007/BF00270327. [DOI] [PubMed] [Google Scholar]
- Betz J. L., Sadler J. R. Variants of a cloned synthetic lactose operator. II. Chloramphenicol-resistant revertants retaining a lactose operator in the CAT gene of plasmid pBR325. Gene. 1981 Nov;15(2-3):187–200. doi: 10.1016/0378-1119(81)90128-1. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Taylor A. L. Genetic analysis of diaminopimelic acid- and lysine-requiring mutants of Escherichia coli. J Bacteriol. 1971 Mar;105(3):844–854. doi: 10.1128/jb.105.3.844-854.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carothers A. M., McFall E., Palchaudhuri S. Physical mapping of the Escherichia coli D-serine deaminase region: contiguity of the dsd structural and regulatory genes. J Bacteriol. 1980 Apr;142(1):174–184. doi: 10.1128/jb.142.1.174-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. Overproduction of the Tn3 transposition protein and its role in DNA transposition. Cell. 1982 Feb;28(2):345–354. doi: 10.1016/0092-8674(82)90352-x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Dermody J. J., Robinson G. T., Sternglanz R. Conditional-lethal deoxyribonucleic acid ligase mutant of Escherichia coli. J Bacteriol. 1979 Aug;139(2):701–704. doi: 10.1128/jb.139.2.701-704.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Gorini L. A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol. 1969 Jan 14;39(1):73–87. doi: 10.1016/0022-2836(69)90334-9. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Marians K. J., Wu R., Stawinski J., Hozumi T., Narang S. A. Cloned synthetic lac operator DNA is biologically active. Nature. 1976 Oct 28;263(5580):744–748. doi: 10.1038/263744a0. [DOI] [PubMed] [Google Scholar]
- Mata M., Delstanche M., Robert-Baudouy J. Isolation of specialized transducing bacteriophages carrying the structural genes of the hexuronate system in Escherichia coli K-12: exu region. J Bacteriol. 1978 Feb;133(2):549–557. doi: 10.1128/jb.133.2.549-557.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoz G., Robert-Baudouy J., Stoeber F. Physiological and genetic regulation of the aldohexuronate transport system in Escherichia coli. J Bacteriol. 1976 Aug;127(2):706–718. doi: 10.1128/jb.127.2.706-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Stoeber F. R. Localisation génétique et caractérisation biochimique de mutations affectant le gène de structure de l'hydrolyase altronique chez Escherichia coli K 12. Mol Gen Genet. 1972;118(4):335–350. doi: 10.1007/BF00333569. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. Dosages colorimétriques des oxydoréductases aldoniques d'Escherichia coli K 12: applications. Biochim Biophys Acta. 1972 Nov 10;289(1):19–27. doi: 10.1016/0005-2744(72)90103-9. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Stoeber F. R. La D-altronate: NAD-oxydoréductase d'Escherichia coli K12. Purification, propriétés et individualité. Eur J Biochem. 1972 Mar 15;26(1):50–61. doi: 10.1111/j.1432-1033.1972.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Portalier R., Robert-Baudouy J., Stoeber F. Regulation of Escherichia coli K-12 hexuronate system genes: exu regulon. J Bacteriol. 1980 Sep;143(3):1095–1107. doi: 10.1128/jb.143.3.1095-1107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler P., Mata-Gilsinger M., Stoeber F. Molecular cloning of Escherichia coli K-12 hexuronate system genes: exu region. J Bacteriol. 1981 Jan;145(1):181–190. doi: 10.1128/jb.145.1.181-190.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler P., Mata-Gilsinger M. Use of in vitro gene fusions to study the uxuR regulatory gene in Escherichia coli K-12: direction of transcription and regulation of its expression. J Bacteriol. 1982 Jun;150(3):1040–1047. doi: 10.1128/jb.150.3.1040-1047.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C., Stoeber F. R. Régulation due métabolisme des hexuronates chez Escherichia coli K12. Modalités de l'induction des enzymes du système hexuronate. Eur J Biochem. 1974 Mar 15;43(1):1–15. doi: 10.1111/j.1432-1033.1974.tb03378.x. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. Mutants of Escherichia coli unable to make protein at 42 C. J Bacteriol. 1971 Nov;108(2):790–798. doi: 10.1128/jb.108.2.790-798.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Horn V., Yanofsky C. On the production of deletions in the chromosome of Escherichia coli. J Mol Biol. 1970 Oct 14;53(1):49–67. doi: 10.1016/0022-2836(70)90045-8. [DOI] [PubMed] [Google Scholar]
- Stoeber F., Lagarde A., Nemoz G., Novel G., Novel M., Portalier R., Pouyssegur J., Robert-Baudouy J. Le métabolisme des hexuronides et des hexuronates chez Escherichia coli K 12: aspects physiologiques et et génétiques de sa régulation. Biochimie. 1974;56(2):199–213. doi: 10.1016/s0300-9084(74)80379-2. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Nordström K. Microiodometric determination of beta-lactamase activity. Antimicrob Agents Chemother. 1972 Feb;1(2):94–99. doi: 10.1128/aac.1.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]