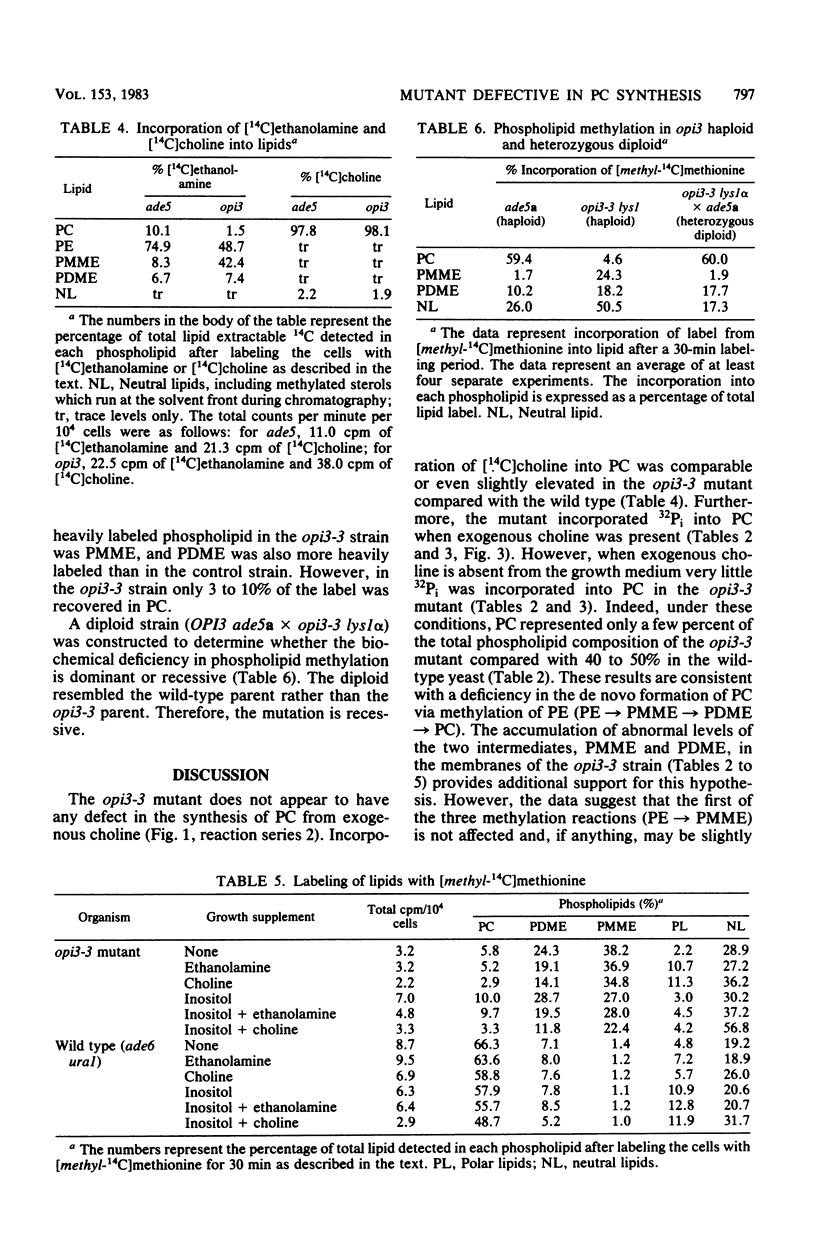
Abstract
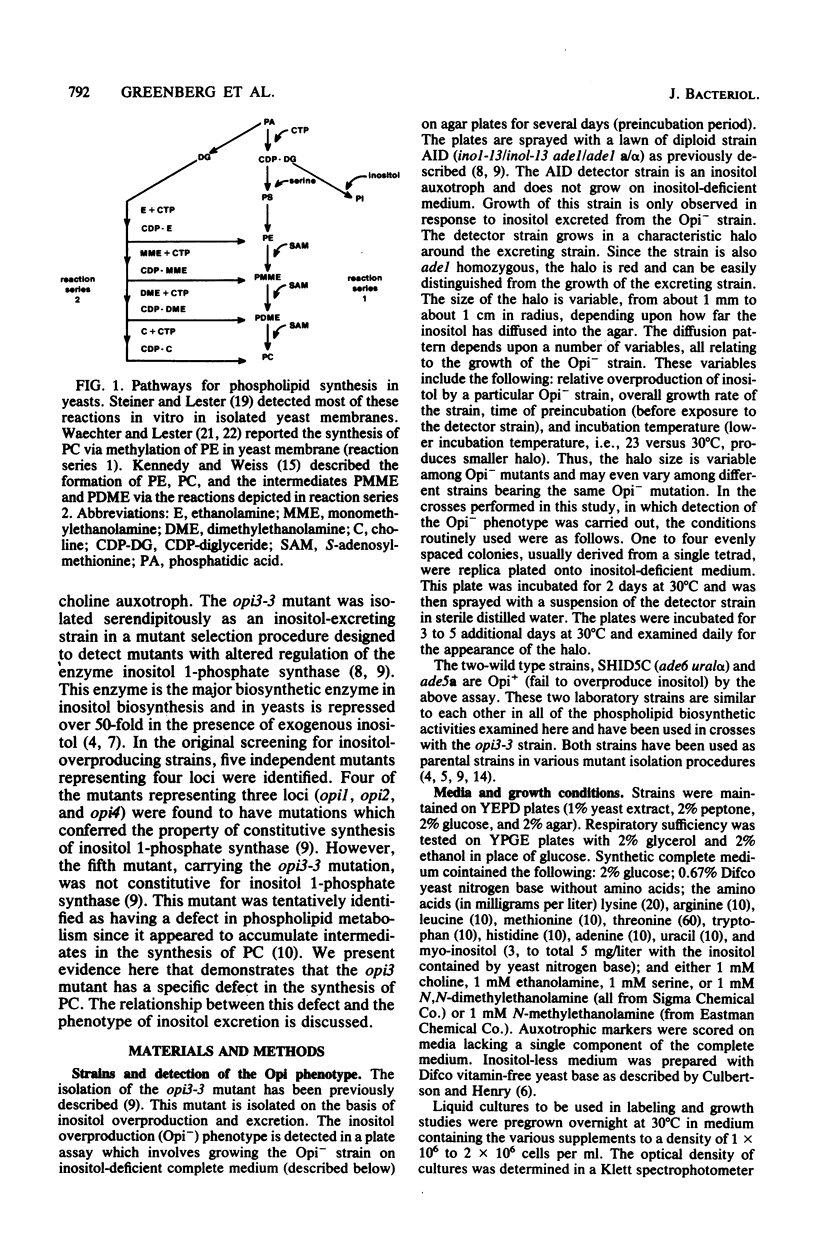
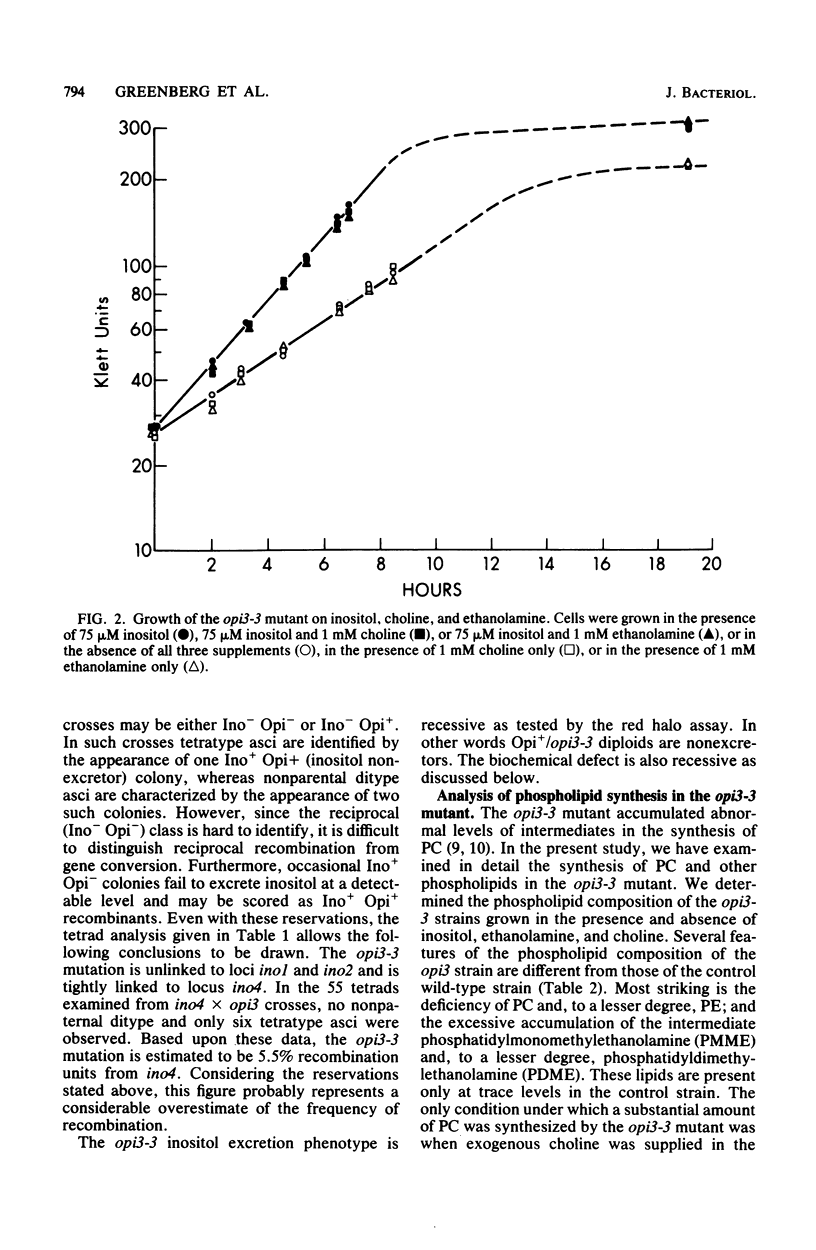
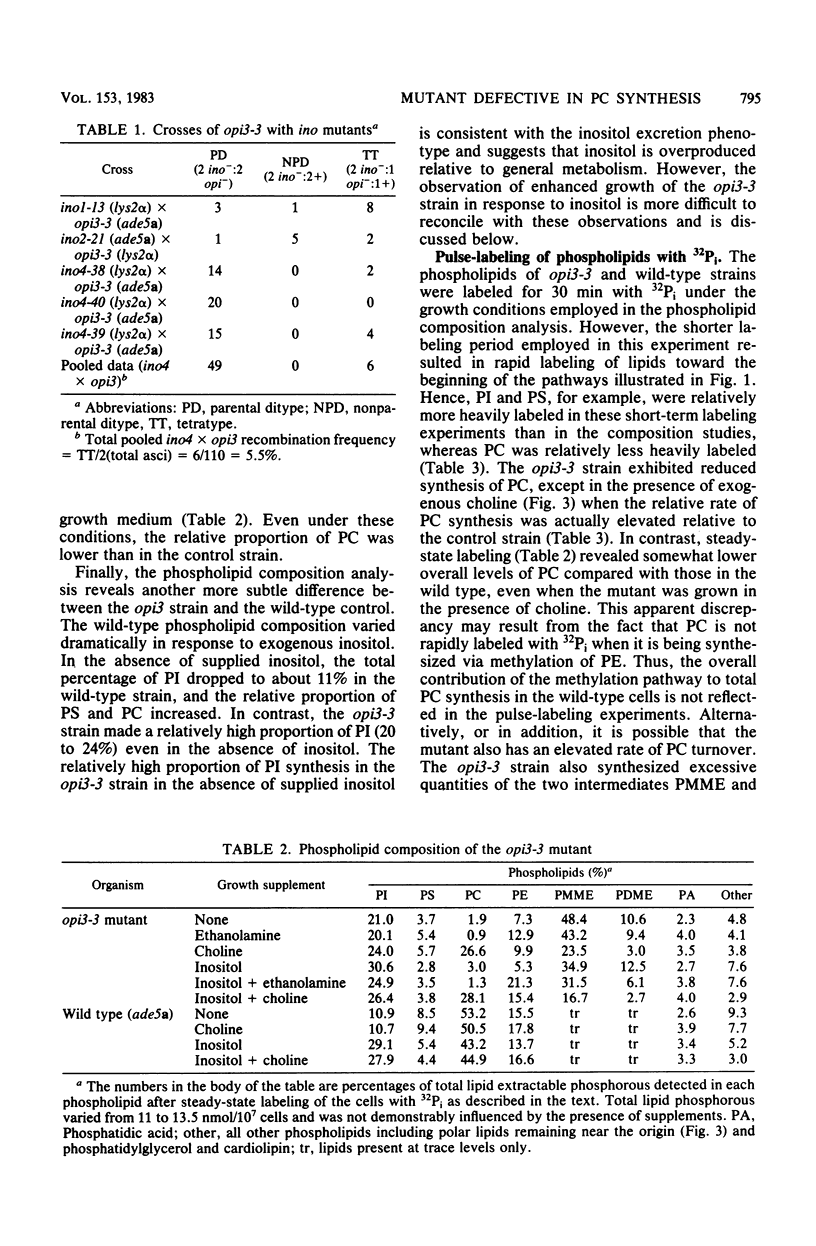
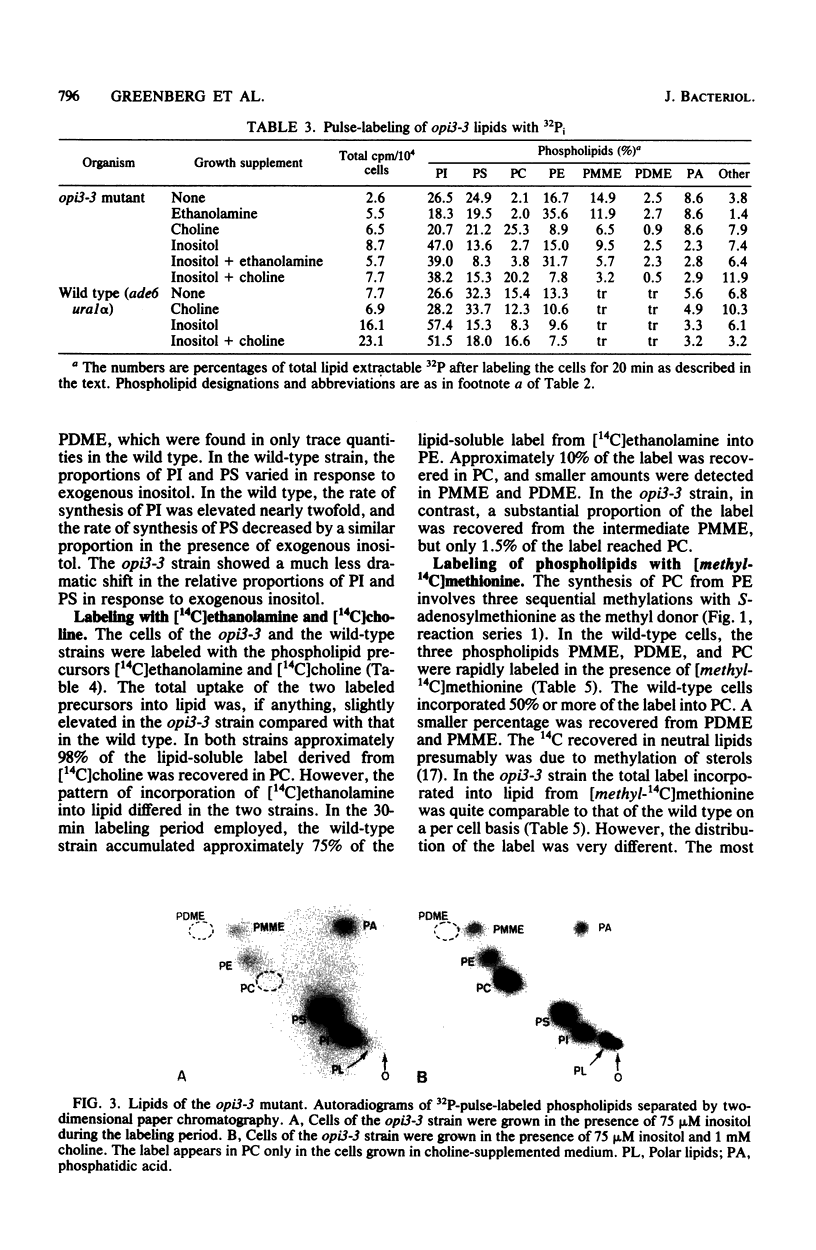
The Saccharomyces cerevisiae opi3-3 mutant was shown to be defective in the synthesis of phosphatidylcholine via methylation of phosphatidylethanolamine. The opi3-3 mutant was isolated on the basis of an inositol excretion phenotype and was not auxotrophic for choline. Inositol, but not choline, stimulated growth of the mutant. The opi3-3 mutation was recessive and was genetically linked to the ino4 locus. When grown in the absence of exogenous choline, the opi3-3 mutant had a phospholipid composition consisting of 2 to 3% phosphatidylcholine compared with 40 to 50% in wild-type strains. In addition, the mutant accumulated elevated amounts of two intermediates, phosphatidylmonomethylethanolamine and phosphatidyldimethylethanolamine. The incorporation of label from [methyl-14C]methionine into phosphatidylcholine was reduced 80 to 90% in the mutant compared with wild-type strains. However, label was recovered in the intermediates phosphatidylmonomethylethanolamine and phosphatidyldimethylethanolamine. The mutant is believed to be defective in the third and possibly the second methylation reaction in the formation of phosphatidylcholine from phosphatidylethanolamine. The first methylation reaction appeared to be occurring at normal or even elevated levels. Based upon incorporation of choline into phosphatidylcholine, it is concluded that the opi3-3 mutant has no defect in the synthesis of phosphatidylcholine from exogenous choline. Furthermore, phosphatidylcholine represents over 25% of the phospholipid composition of the mutant when it is grown in the presence of exogenous choline.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K. D., Jensen B., Kolat A. I., Storm E. M., Henry S. A., Fogel S. Yeast mutants auxotrophic for choline or ethanolamine. J Bacteriol. 1980 Feb;141(2):558–564. doi: 10.1128/jb.141.2.558-564.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- CROCKEN B. J., NYC J. F. PHOSPHOLIPID VARIATIONS IN MUTANT STRAINS OF NEUROSPORA CRASSA. J Biol Chem. 1964 Jun;239:1727–1730. [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae: properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J Bacteriol. 1976 Apr;126(1):232–242. doi: 10.1128/jb.126.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Donahue T. F., Henry S. A. Control of inositol biosynthesis in Saccharomyces cerevisiae; inositol-phosphate synthetase mutants. J Bacteriol. 1976 Apr;126(1):243–250. doi: 10.1128/jb.126.1.243-250.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Henry S. A. myo-Inositol-1-phosphate synthase. Characteristics of the enzyme and identification of its structural gene in yeast. J Biol Chem. 1981 Jul 10;256(13):7077–7085. [PubMed] [Google Scholar]
- Greenberg M. L., Goldwasser P., Henry S. A. Characterization of a yeast regulatory mutant constitutive for synthesis of inositol-1-phosphate synthase. Mol Gen Genet. 1982;186(2):157–163. doi: 10.1007/BF00331845. [DOI] [PubMed] [Google Scholar]
- Greenberg M. L., Reiner B., Henry S. A. Regulatory mutations of inositol biosynthesis in yeast: isolation of inositol-excreting mutants. Genetics. 1982 Jan;100(1):19–33. doi: 10.1093/genetics/100.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Kovác L., Gbelská I., Poliachová V., Subík J., Kovácová V. Membrane mutants: a yeast mutant with a lesion in phosphatidylserine biosynthesis. Eur J Biochem. 1980 Oct;111(2):491–501. doi: 10.1111/j.1432-1033.1980.tb04965.x. [DOI] [PubMed] [Google Scholar]
- Letts V. A., Dawes I. W. Mutations affecting lipid biosynthesis of Saccharomyces cerevisiae: isolation of ethanolamine auxotrophs [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):976–977. doi: 10.1042/bst0070976. [DOI] [PubMed] [Google Scholar]
- Parks L. W. Metabolism of sterols in yeast. CRC Crit Rev Microbiol. 1978;6(4):301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A., Nyc J. F. Methylation of ethanolamine phosphatides by microsomes from normal and mutant strains of Neurospora crassa. J Biol Chem. 1967 Jan 25;242(2):238–242. [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Regulation of phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):837–843. doi: 10.1128/jb.105.3.837-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]




