Abstract
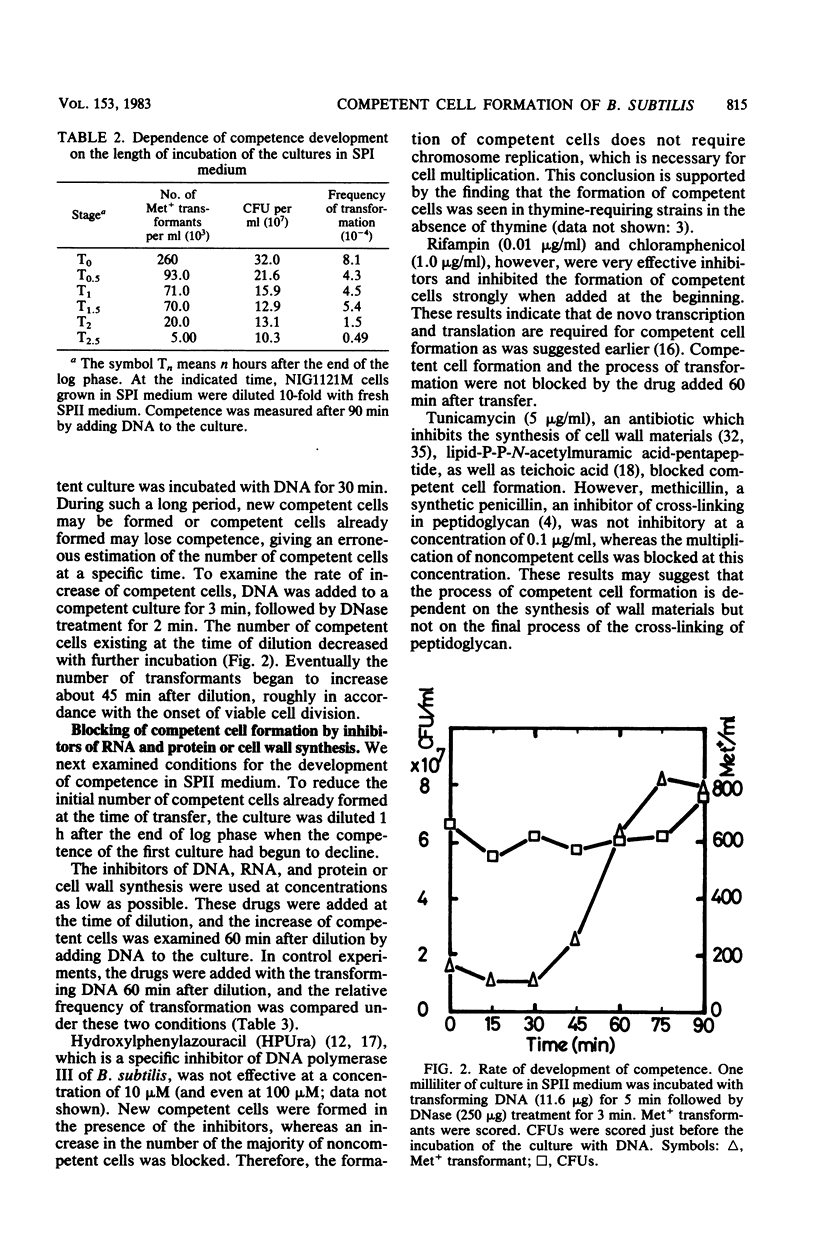
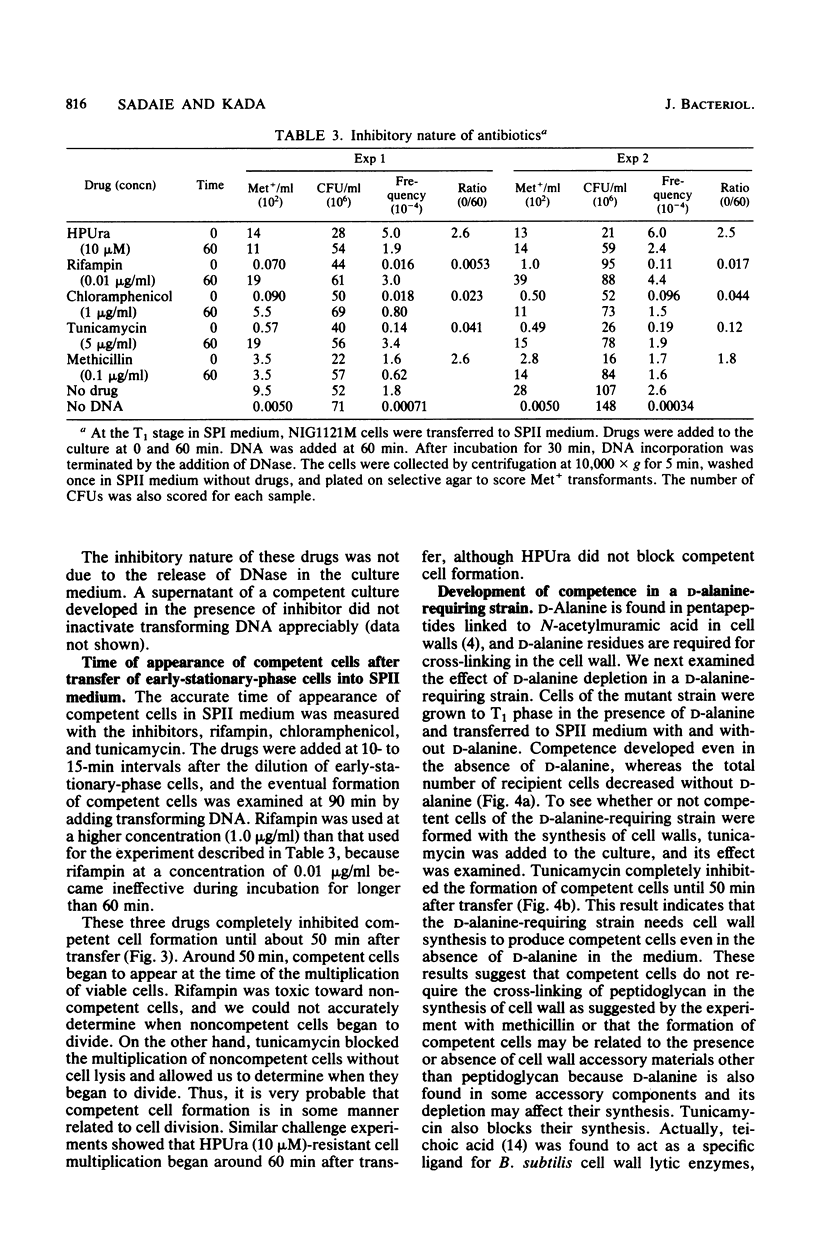
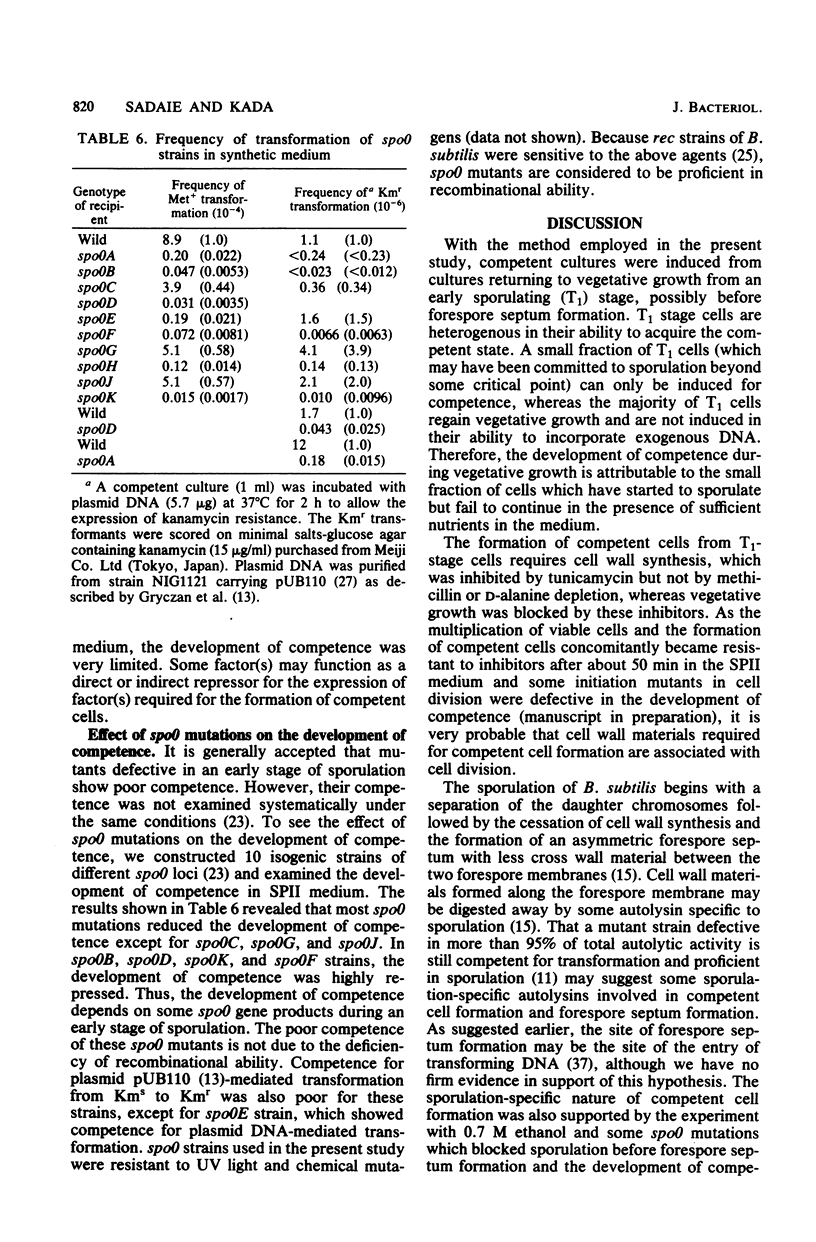
The process of competent cell formation for transformation has been studied with early-stationary-phase (T1) cells of Bacillus subtilis which had been grown in an enriched Spizizen minimal medium and transferred to a second synthetic medium. Rifampin, chloramphenicol, and tunicamycin were strong inhibitors of competent cell formation, as well as vegetative growth. After formation, competent cells were no longer sensitive to the above agents. Methicillin and an inhibitor of chromosomal replication, hydroxyphenylazouracil, did not inhibit the development of competence. A D-alanine-requiring mutant strain developed competence even in the absence of D-alanine in the second medium. A T1-stage culture showed the activity of extracellular serine protease which is necessary for sporulation. Competent cell formation was completely blocked by 0.7 M ethanol, which is a specific inhibitor of early events during sporulation, including forespore septum formation. Competent cells were formed even in media which supported sporulation. The development of competence was also studied with spo0 mutants at 10 different loci. Most spo0 mutations repressed the development of competence except for spo0C, spo0G, and spo0J. These results suggest that competent cells are formed from early sporulating cells with the synthesis of cell wall materials and by factors whose genes are activated by the supply of nutrients. It is suggested that common steps are involved both in forespore septation and in competent cell formation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Ayad S. R., Blamire J. Uptake of DNA by competent bacteria--a possible mechanism. J Theor Biol. 1969 Sep;24(3):266–272. doi: 10.1016/s0022-5193(69)80051-2. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer L. J., Landman O. E. Development of competence in thymine-starved Bacillus subtilis with chromosomes arrested at the terminus. J Bacteriol. 1969 Jan;97(1):166–173. doi: 10.1128/jb.97.1.166-173.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohin J. P., Rigomier D., Schaeffer P. Ethanol sensitivity of sporulation in Bacillus subtilis: a new tool for the analysis of the sporulation process. J Bacteriol. 1976 Aug;127(2):934–940. doi: 10.1128/jb.127.2.934-940.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARNEY J., FISHER W. P., HEGARTY C. P. Managanese as an essential element for sporulation in the genus Bacillus. J Bacteriol. 1951 Aug;62(2):145–148. doi: 10.1128/jb.62.2.145-148.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Criteria for categorizing early biochemical events occurring during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):411–415. doi: 10.1128/jb.121.2.411-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Interaction of N-acetylmuramic acid L-alanine amidase with cell wall polymers. J Biol Chem. 1975 Sep 25;250(18):7231–7238. [PubMed] [Google Scholar]
- Hitchins A. D. Polarity and topology of DNA segregation and septation in cells and sporangia of the bacilli. Can J Microbiol. 1978 Oct;24(10):1104–1134. [PubMed] [Google Scholar]
- Kammen H. O., Wojnar R. J., Canellakis E. S. Transformation in Bacillus subtilis. II. The development and maintenance of the competent state. Biochim Biophys Acta. 1966 Jul 20;123(1):56–65. [PubMed] [Google Scholar]
- Mackenzie J. M., Neville M. M., Wright G. E., Brown N. C. Hydroxyphenylazopyrimidines: characterization of the active forms and their inhibitory action on a DNA polymerase from Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):512–516. doi: 10.1073/pnas.70.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur H. A., Roberts F. M., Hancock I. C., Baddiley J. Lipid intermediates in the biosynthesis of the linkage unit between teichoic acids and peptidoglycan. FEBS Lett. 1978 Feb 15;86(2):193–200. doi: 10.1016/0014-5793(78)80561-4. [DOI] [PubMed] [Google Scholar]
- NESTER E. W. PENICILLIN RESISTANCE OF COMPETENT CELLS IN DEOXYRIBONUCLEIC ACID TRANSFORMATION OF BACILLUS SUBTILIS. J Bacteriol. 1964 Apr;87:867–875. doi: 10.1128/jb.87.4.867-875.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y. K., Freese E. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J Bacteriol. 1976 Aug;127(2):739–746. doi: 10.1128/jb.127.2.739-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigomier D., Bohin J. P., Lubochinsky B. Effects of ethanol and methanol on lipid metabolism in Bacillus subtilis. J Gen Microbiol. 1980 Nov;121(1):139–149. doi: 10.1099/00221287-121-1-139. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sadaie Y., Burtis K. C., Doi R. H. Purification and characterization of a kanamycin nucleotidyltransferase from plasmid pUB110-carrying cells of Bacillus subtilis. J Bacteriol. 1980 Mar;141(3):1178–1182. doi: 10.1128/jb.141.3.1178-1182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1976 Feb;125(2):489–500. doi: 10.1128/jb.125.2.489-500.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Narui K. Repair deficiency, mutator activity, and thermal prophage inducibility in dna-8132 strains of Bacillus subtilis. J Bacteriol. 1976 Jun;126(3):1037–1041. doi: 10.1128/jb.126.3.1037-1041.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J., Reilly B. E., Evans A. H. Microbial transformation and transfection. Annu Rev Microbiol. 1966;20:371–400. doi: 10.1146/annurev.mi.20.100166.002103. [DOI] [PubMed] [Google Scholar]
- Vasantha N., Freese E. The role of manganese in growth and sporulation of Bacillus subtilis. J Gen Microbiol. 1979 Jun;112(2):329–336. doi: 10.1099/00221287-112-2-329. [DOI] [PubMed] [Google Scholar]
- Ward J. B. Tunicamycin inhibition of bacterial wall polymer synthesis. FEBS Lett. 1977;78(1):151–154. doi: 10.1016/0014-5793(77)80294-9. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. II. Activation of the inducible system in competent bacteria. Mol Gen Genet. 1977 Jun 8;153(2):219–225. [PubMed] [Google Scholar]
- Young F. E. Competence in Bacillus subtilis transformation system. Nature. 1967 Feb 25;213(5078):773–775. doi: 10.1038/213773a0. [DOI] [PubMed] [Google Scholar]


