Abstract
L-type Ca2+ channels select for Ca2+ over sodium Na+ by an affinity-based mechanism. The prevailing model of Ca2+ channel permeation describes a multi-ion pore that requires pore occupancy by at least two Ca2+ ions to generate a Ca2+ current. At [Ca2+] < 1 μM, Ca2+ channels conduct Na+. Due to the high affinity of the intrapore binding sites for Ca2+ relative to Na+, addition of μM concentrations of Ca2+ block Na+ conductance through the channel. There is little information, however, about the potential for interaction between Na+ and Ca2+ for the second binding site in a Ca2+ channel already occupied by one Ca2+. The two simplest possibilities, (a) that Na+ and Ca2+ compete for the second binding site or (b) that full time occupancy by one Ca2+ excludes Na+ from the pore altogether, would imply considerably different mechanisms of channel permeation. We are studying permeation mechanisms in N-type Ca2+ channels. Similar to L-type Ca2+ channels, N-type channels conduct Na+ well in the absence of external Ca2+. Addition of 10 μM Ca2+ inhibited Na+ conductance by 95%, and addition of 1 mM Mg2+ inhibited Na+ conductance by 80%. At divalent ion concentrations of 2 mM, 120 mM Na+ blocked both Ca2+ and Ba2+ currents. With 2 mM Ba2+, the IC50 for block of Ba2+ currents by Na+ was 119 mM. External Li+ also blocked Ba2+ currents in a concentration-dependent manner, with an IC50 of 97 mM. Na+ block of Ba2+ currents was dependent on [Ba2+]; increasing [Ba2+] progressively reduced block with an IC50 of 2 mM. External Na+ had no effect on voltage-dependent activation or inactivation of the channel. These data suggest that at physiological concentrations, Na+ and Ca2+ compete for occupancy in a pore already occupied by a single Ca2+. Occupancy of the pore by Na+ reduced Ca2+ channel conductance, such that in physiological solutions, Ca2+ channel currents are between 50 and 70% of maximal.
Keywords: calcium channels, ion channel selectivity, sodium, permeation
introduction
Calcium (Ca2+) channels are highly selective for Ca2+ over sodium (Na+) and other monovalent cations. Their remarkable selectivity is accomplished by differential affinity of Ca2+ and monovalent cations for one or more intrapore binding sites (Almers et al., 1984; Hess and Tsien, 1984; Hess et al., 1986). In the absence of Ca2+, monovalent cations conduct well through Ca2+ channels (Kostyuk et al., 1983; Almers et al., 1984; Fukushima and Hagiwara, 1985; Hess et al., 1986; Matsuda, 1986). Addition of μM [Ca2+] completely blocks (K d = ∼0.7 μM) the monovalent cation conductance but does not result in the generation of Ca2+ currents. At much higher [Ca2+], Ca2+ currents are generated. In L-type Ca2+ channels, Ca2+ current saturates with a K d for Ca2+ of ∼14 mM (Hess et al., 1986). This concentration difference required for block of Na+ conductance and generation of Ca2+ currents is one of the signature characteristics of a multi-ion pore (Almers et al., 1984; Hess and Tsien, 1984; Hille, 1992).
The classical two-site model of the Ca2+ permeation pathway describes the pore as containing two identical high affinity cation binding sites in the permeation pathway (Almers et al., 1984; Hess and Tsien, 1984). Due to the high affinity of Ca2+ for these sites, the first available site is bound by Ca2+ at low concentrations. At higher concentrations, a second Ca2+ enters simultaneously, and the electrostatic repulsion between these two tightly packed divalent ions lowers the apparent K d for the sites and results in Ca2+ current. In such a model, the 14 mM K d for Ca2+ current saturation can be interpreted as representing the affinity of Ca2+ for the second binding site once the first binding site is bound by Ca2+ (Tsien et al., 1987).
Alternative models that have a single high affinity binding site have been proposed that equally well explain the Ca2+ channel permeation data (Armstrong and Neyton, 1991; Yang et al., 1993; Dang and McCleskey, 1996). All of these models share the concept, however, that at least two ions must occupy the pore to generate current.
In the presence of μM [Ca2+], two observations suggest that these two sites in the Ca2+ channel pore can be simultaneously occupied by one Ca2+ and one monovalent cation (Kuo and Hess, 1993b ). First, in Ca2+ channels carrying outward Li+ currents, high external [Li+] decreases the outward exit rate of the blocking Ca2+ ion from the pore, producing a “lock in” type of effect in the channel. Second, elevation of external [Li+] decreases the on-rate of external Ca2+, which suggests that external Li+ interferes with access of Ca2+ to its high affinity site. When the channel is occupied by one Ca2+ or less, the affinity of Li+ for its binding site has an apparent K d of ∼100 mM (∼75 mM expressed as activity). The lock-in effect suggests that Li+ binds externally to Ca2+ within the pore.
The interaction between Ca2+ and monovalents in the pore is derived from studies in low [Ca2+] conditions, with Li+ as the monovalent charge carrier. There is little information, however, about the potential interaction between Ca2+ and Na+ in physiological solutions. Although Na+ appears to bind to the channel with somewhat lower affinity than Li+ (Hess et al., 1986), the studies in low [Ca2+] suggest that there is still less than a 10-fold difference in apparent affinity between Ca2+ and Na+ for the second binding site (∼14 vs. ∼100 mM). Consequently, if extrapolated to high [Ca2+] conditions, the model predicts that Ca2+ and Na+ would compete for the second binding site. If the competition predicted by the model occurs at physiological concentrations, the pore will be doubly occupied by Ca2+ less in the presence of Na+ than in the absence of Na+. Since a channel occupied by one Ca2+ and one monovalent cation does not conduct (Almers et al., 1984; Kuo and Hess, 1993a , b ), this large decrease in double occupancy by Ca2+ would be expected to result in Ca2+ current reduction.
An alternative possibility is that Na+ is excluded from the pore at physiological [Ca2+] and [Na+]. This possibility is supported by data from guinea pig ventricular cells and mouse neoplastic B lymphocytes, in which Ca2+ currents were unaffected when external Na+ was replaced by Tris or choline (Matsuda and Noma, 1984; Yamashita et al., 1990). Reconciliation of these conflicting possibilities is important for understanding Ca2+ channel permeation, since one suggests that permeation is strictly governed by competition for binding sites and the other suggests that higher [Ca2+] induces an allosteric change in the channel that prevents monovalent cations from binding.
Nearly all studies of Ca2+ channel permeation have used L-type Ca2+ channels. We demonstrate here that N-type Ca2+ channels in chick sensory neurons display permeation properties similar to those of L-type Ca2+ channels. In physiological [Na+] and [Ca2+], external Na+ blocks N-type Ca2+ channels in a concentration-dependent manner, and this block appears to result from a competitive interaction between Ca2+ and Na+ in the pore.
materials and methods
Cells
Dorsal root ganglion (DRG)1 neurons were acutely isolated from thoracic and lumbar level ganglia of 14-d-old white leghorn chick embryos (UCONN Poultry Farm, Storrs, CT). Ganglia were incubated in Tyrodes (in mM: 128 NaCl, 3 KCl, 1 MgCl2, 27 NaHCO3, 10 glucose, pH 7.3) containing 0.08% trypsin (#610-5050PG; Gibco Laboratories, Grand Island, NY) for 30 min at 37°C in a CO2 incubator (both 6 and 10% CO2 in air were used with no detectable difference). Cells were removed from the incubator and washed three times with Media 199 (#B-1202-AX plus 3.1 g/liter NaHCO3; Hyclone Laboratories, Inc., Logan, UT) plus 10% FBS (#A-1115-L; Hyclone Laboratories, Inc.). Cells were dissociated in Media 199 by trituration with a siliconized, fire-polished pasteur pipet and plated on polyornithine-coated 35-mm culture dishes. Cells were maintained in this media in a 37°C incubator and used in experiments 1–8 h after plating.
Patch Clamp Recording
Recordings were made with the standard whole cell patch clamp configuration (Hamill et al., 1981). Patch pipets were fabricated from N51A glass (Garner Glass Co., Claremont, CA), coated with Sylgard (#184; Dow Corning, Midland, MI), and fire-polished. Series resistance ranged from 0.8–3.0 MΩ (mean = 1.9 ± 0.3 MΩ, n = 221), and membrane capacitance ranged from 9.0 to 44.6 pF (mean = 24.5 ± 0.6 pF, n = 221). Capacitive transients were electronically neutralized and series resistance compensation was used, generally at ∼90% (3911A patch clamp amplifier; Dagan Corp., Minneapolis, MN). In all experiments except those described in Fig. 5, membrane currents were filtered at 2 kHz (internal patch clamp filter) and digitized at sample intervals of 100–400 μs/pt. Tail currents in Fig. 5 were filtered at 50 kHz and digitized at 3 μs/pt. Unless otherwise stated, the holding potential was −80 mV, and Ca2+ currents were evoked by a 100-ms depolarizing stimulus once every 5–10 s. Experiments were performed at room temperature (20–24°C). Data were acquired and measured with pClamp 6 (Axon Instruments, Foster City, CA).
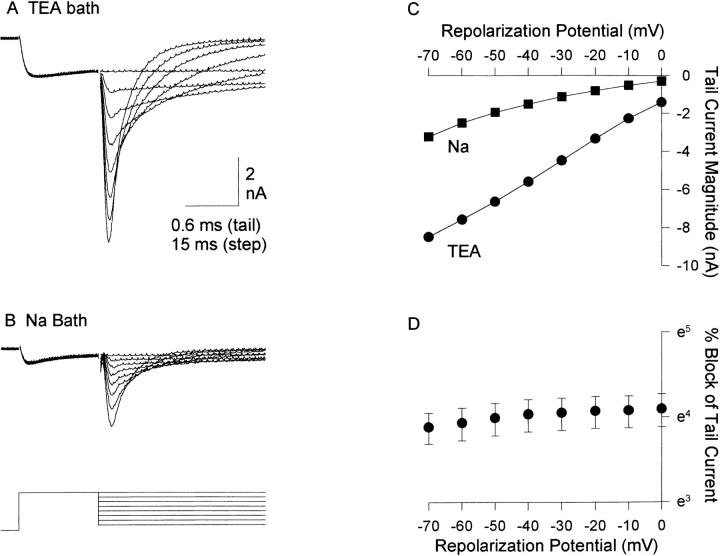
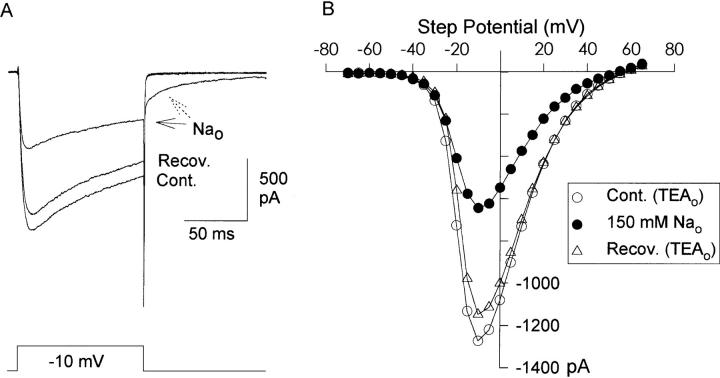
Figure 5.
Voltage dependence of Na+ block. Currents in TEA bath (A) and Na+ bath (B) were evoked by the protocol illustrated at the bottom of B. Repolarization potentials ranged from −70 to 0 mV. Currents were carried by 3 mM Ba2+, and 120 mM Na+ was substituted for TEA. 150 μs of data were blanked from each trace during the capacitive transient before and after the depolarizing voltage step. (C) I-V relationship, measured at the peak of the tail currents illustrated in A and B. (D) Percent block by Na+, plotted semilogarithmically. Data represent mean ± SEM of six cells.
Solutions
Recordings were made from cells plated in 35-mm tissue culture dishes that contained 1.5 ml of bathing solution. Both static and continuous flow baths were used. When a static bath was used, the external solution bathing the cells was changed by manually lowering a large bore pipet (N51A glass coated with Sigmacote) that contained the desired test solution near the cell under study. Application of test solution was terminated by removal of the large bore pipet from the bath. In general, solutions bathing the cells were exchanged within 5 s using this technique. In continuous flow experiments, a large bore quartz pipet that contained multiple solution inputs was placed near the cell before recording. Solutions flowed continuously over the cell under study and were changed by manually switching valves on 1 of 6 input lines. Except as stated otherwise, the control external solution bathing the cells contained (in mM): 150 TEA-Cl, 2–3 BaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.3 (TEAOH), osmolality = 320 ± 5 mosm/kg. Tetrodotoxin (TTX; 1 μM) was added to all external solutions. Except in the experiments described in Fig. 4, Na+ was applied externally by equimolar replacement of 120 mM TEA. The standard pipet solution contained (in mM): 90 NMG-Cl, 30 CsCl, 30 TEA-Cl, 5 EGTA-NMG, 10 HEPES, 4 MgCl2, 4 creatine phosphate, 4 ATP-Na, leupeptin, and creatine kinase, pH 7.3 (CsOH), osmolality = 305 ± 5 mosm/kg. Addition of 0.2 mM GTP to the pipet solution did not qualitatively influence the results. Substitutions are listed in the figure legends.
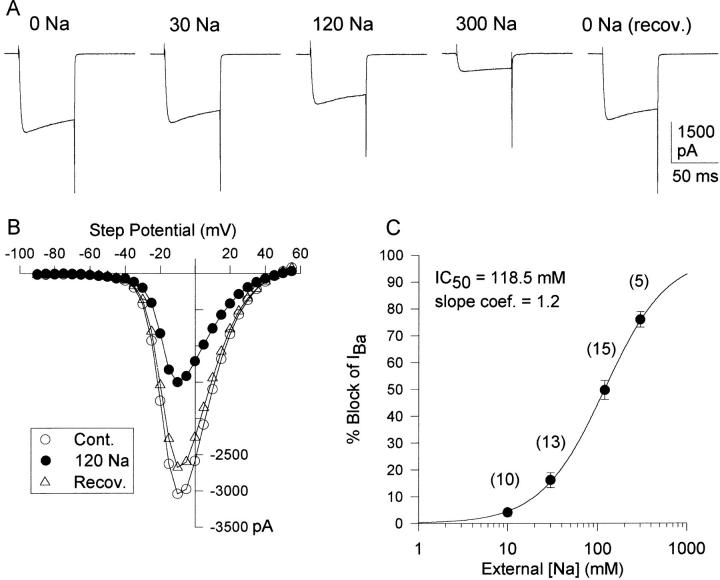
Figure 4.
Concentration dependence of Na+ block of Ba2+ currents. The external solution contained 2 mM Ba2+. (A) Representative currents evoked by 100-ms depolarization to 0 mV, in the presence of the external Na+ concentration shown above the current. External Na+ was added to the control bath solution, so there was an increase in osmolality with increasing [Na+]. Currents were recorded 10 s after addition of Na+. At Na+ concentrations of 30 and 120 mM, results were statistically identical when cells were exposed to added Na+ (n = 4 [30 mM], 7 [120 mM]) or substitution of Na+ for TEA (n = 9 [30 mM], 8 [120 mM]). (B) I-V curves recorded before, during and after addition of 120 mM Na+ in the presence of TEA. (C) Concentration dependence of block by Na+. Circles represent mean and SEM of pooled data for substitution and addition of Na+. The number of cells tested is shown in parentheses. The solid line represents the best fit of the data by Eq. 1.
Data Analysis
All curve fitting and statistics were done with SigmaPlot 2.0 for Windows (Jandel Scientific, Corte Madera, CA). Average values are expressed as mean ± standard error of the mean (SEM), with the number of samples given in parentheses. Statistical significance was tested by unpaired Student's t test. Data on percent block of Ca2+ channel currents by monovalent cations (Figs. 4 C and 8 C) were fit by the following equation:
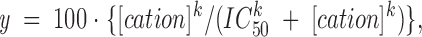 |
1 |
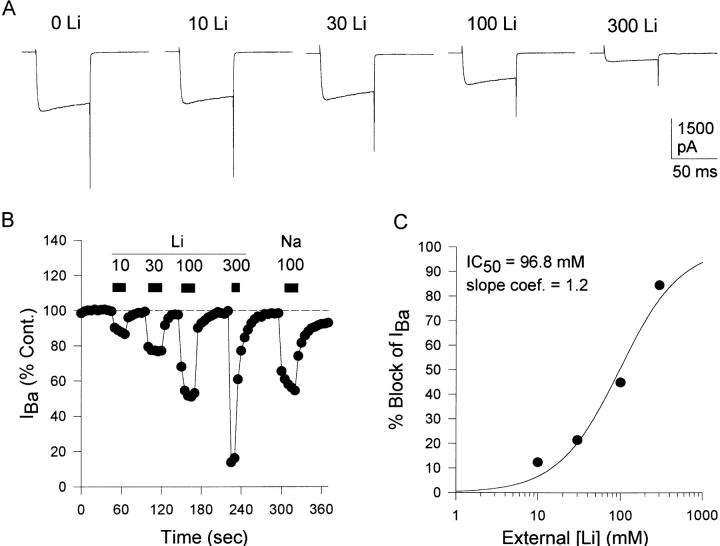
Figure 8.
Ba2+ current block by Li+. (A) Currents recorded in the presence of the Li+ concentrations shown. Li+ was added to the control external solution. Currents were evoked by 100-ms depolarizations from −80 to 0 mV. (B) Ba2+ currents recorded over time in the presence of 0, 10, 30, 100, and 300 mM [Li+]. After recovery from 300 mM Li+, cells were exposed to 100 mM Na+ for comparison. (C) Concentration dependence of Li+ block of Na+ currents. Circles represent the mean ± SEM for six cells in each condition (error bars are smaller than the symbols). The solid line represents the best fit of the data to Eq. 1. The calculated IC50 was 96.8 ± 17.2 mM and the slope coefficient was 1.16 ± 0.24.
where y is the percentage block, IC50 is the concentration for 50% block, and k is the slope coefficient, which represents the number of molecules acting in a cooperative manner. The data in Fig. 7 were fit to the complementary function.
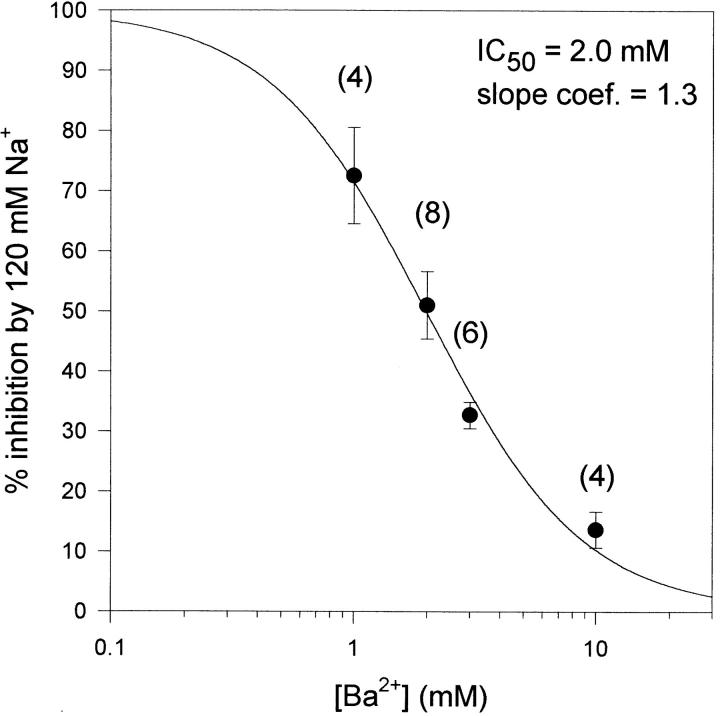
Figure 7.
Inhibition of Na+ block by Ba2+. Currents were recorded from cells bathed in one of four Ba2+ concentrations. I-V curves were generated as in Fig. 6, and the percent inhibition by 120 mM Na+ was measured at the peak of the I-V. The number of cells tested is shown in parentheses. The solid line represents the best fit of the data to the complement of Eq. 1. The calculated IC50 was 1.98 ± 0.14 mM with a slope coefficient of 1.34 ± 0.17.
results
Ca2+ Channel Type
It was previously demonstrated that Ca2+ currents through DRGs acutely isolated from 11–12-d-old chick embryos consist entirely of N-type Ca2+ channels (Cox and Dunlap, 1992). When cells were kept in culture for several days, however, other Ca2+ channel types appeared (Cox and Dunlap, 1992). Since we used cells isolated from slightly older embryos, we examined the sensitivity of currents in our preparation to ω-conotoxin GVIA, which selectively blocks N-type Ca2+ channels in chick DRGs at concentrations of 1–10 μM (Aosaki and Kasai, 1989; Cox and Dunlap, 1992). Conotoxin (10 μM) irreversibly blocked Ca2+ currents by 94.4 ± 2.7% (n = 18; inhibition = 100% in 14 of 18 cells) and Ba2+ currents by 93.1 ± 1.2% (n = 4). In four cells tested, 1 μM conotoxin also produced a 100% block of Ca2+ current. Also consistent with N-type Ca2+ channel properties, currents were completely inactivated by depolarization to −10 mV, with half-maximal inactivation at −64.4 ± 1.5 (n = 6). Identical pharmacological and kinetic results were obtained from cells ranging in size from 12 to 40 μm, which indicates that the channel population in cells of all sizes studied was identical in these respects. These results indicate that the channel population in our cells was composed almost exclusively of N-type Ca2+ channels.
Permeation Characteristics of the Chick N-type Ca2+ Channel
The theoretical framework for understanding Ca2+ channel permeation is derived almost exclusively from studies on L-type Ca2+ channels. To determine whether chick N-type Ca2+ channels used a fundamentally similar selectivity mechanism, we tested for the salient feature of Ca2+ channel permeation: conductance of Na+ in the absence of divalent cations and inhibition of Na+ conductance by μM [Ca2+].
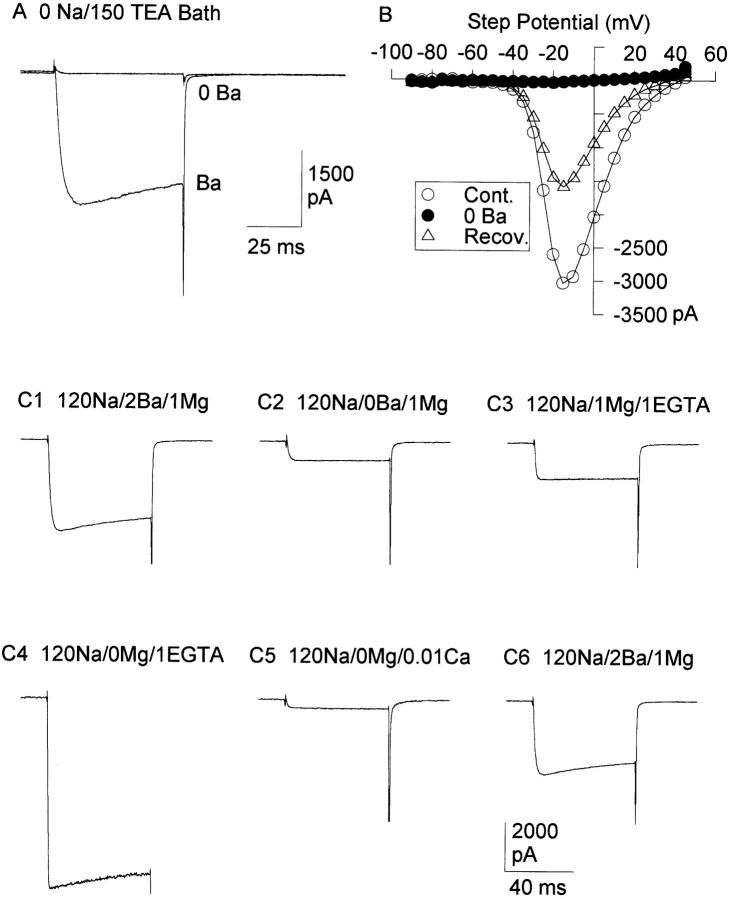
In the absence of Na+, removal of external Ba2+ completely abolished inward currents (Fig. 1 A and B; n = 4). When the Ba2+-free solution contained 120 mM Na+, however, a residual, voltage-activated current was always present (Fig. 1 C2). Our solutions made in the absence of experimentally added Ca2+ typically contain several μM Ca2+. Upon addition of 1 mM EGTA to chelate this residual Ca2+, the magnitude of the inward current increased (Fig. 1 C3). Removal of external Mg2+ in this Ca2+-free, Ba2+-free solution resulted in an additional large increase in Na+ current magnitude (Fig. 1 C4). Finally, addition of just 10 μM Ca2+ to the Ba2+-free, Mg2+-free bath solution inhibited the inward current by 95.0 ± 0.1% (n = 3; Fig. 1 C5). Increasing the Ca2+ concentration to 100 μM resulted in an additional 2–3% inhibition of inward current magnitude (n = 3; not shown). Upon return to the control solution, the current magnitude returned nearly to control values (Fig. 1 C6). These results demonstrate that in the absence of Ca2+ (or Ba2+) Na+ conducts well through the chick N-type Ca2+ channel, and that both Mg2+ and low concentrations of Ca2+ inhibit Na+ conductance through the channel. Thus, these data suggest that the chick N-type Ca2+ channel selects for Ca2+ over Na+ by a qualitatively similar mechanism as the L-type Ca2+ channel.
Figure 1.
Permeation of Na+ through N-type Ca2+ channels. (A) Two superimposed currents recorded in a Na+-free bath, in 2 mM Ba2+, and after the external solution was switched to one containing 0 Ba2+. The 0 Ba2+ solution contained 1 mM Mg2+ and was nominally free of Ca2+ (we assume a [Ca2+] between 5–10 μM). (B) I-V curves recorded with 2 mM Ba2+ (Cont., Recov.) and 0 Ba2+. (C) Currents were recorded from a different cell in the conditions described above the trace. All external solutions contained (in mM): 30 TEA, 10 glucose, 10 HEPES, and 1 μM TTX. (C1) Control (external solution as described in methods). (C2) After removal of Ba2+. (C3) After addition of 1 mM EGTA (0 Ba2+). (C4) After removal of 1 mM Mg2+ (0 Ba2+, 0 Mg2+, 1 EGTA). (C5) After removal of EGTA and addition of 10 μM Ca2+ (0 Ba2+, 0 Mg2+). (C6) Recovery (control solution). In A and C, all currents were evoked by 100-ms depolarizations from −80 to 0 mV.
Ca2+ Channel Block by External Na+
Fig. 2 A shows representative currents recorded in the presence of external TEA (Cont.) and Na+. Replacement of external TEA by Na+ (150 mM) reversibly reduced Ca2+ current magnitude with no shift in activation voltage or reversal potential (Fig. 2 B). With Ca2+ as the charge carrier, we were rarely able to record pure Ca2+ channel currents. Despite using a variety of solutions designed to eliminate other currents, depolarization usually resulted in activation of two large currents other than Ca2+ currents. One of these was a delayed rectifier K+ channel that conducts Na+ in the absence of K+ (Callahan and Korn, 1994). The tail current marked by the dotted arrow in Fig. 2 A illustrates an Na+ current through the K+ channel. Many DRG neurons also contain a Ca2+-dependent Cl− channel (see Callahan and Korn, 1994). To minimize the contamination of Ca2+ currents by these other currents, the remaining experiments were conducted with Ba2+ as the permeating cation. Ba2+ does not activate the Ca2+-dependent Cl− channel (Korn and Weight, 1987), and Ba2+ blocks Na+ currents through the Na+-conducting K+ channel, with an IC50 of ∼1 mM (Callahan and Korn, 1994). To block the remaining currents through the K+ channel, we added 30 mM Cs+ to the pipet solution (to block Na+ currents) and 30 mM TEA to both the internal and external solutions (to block Cs+ current). With this combination of ions, there was little or no shift in reversal potential after application of Na+, no detectable Na+ or Cl− tail currents at Ba2+ concentrations of 2 mM or more (e.g., see Figs. 3 B and 4 A), and no detectable outward currents upon removal of Ba2+ and/or Na+. This indicated that the currents observed were essentially pure Ca2+ channel currents.
Figure 2.
Inhibition of Ca2+ currents by extracellular Na+. (A) Three superimposed Ca2+ currents recorded during control (0 external Na+), after equimolar replacement of external TEA by 150 mM Na+, and during recovery (0 Na+). The stimulus was a 100-ms depolarization from −80 to −10 mV. The dashed arrow points to a tail current carried by Na+ through a delayed rectifier K+ channel. (B) Current-voltage curves in the three conditions mentioned. Pipet solution (in mM): 150 NMG, 10 EGTA, 20 HEPES, 4 MgCl2, 4 creatine phosphate, 4 ATP-Na, creatine kinase and leupeptin, pH 7.3 with NMG, osmolality 310. External solution (in mM): 150 TEA-Cl or NaCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 0.001 TTX, pH 7.3 with TEAOH.
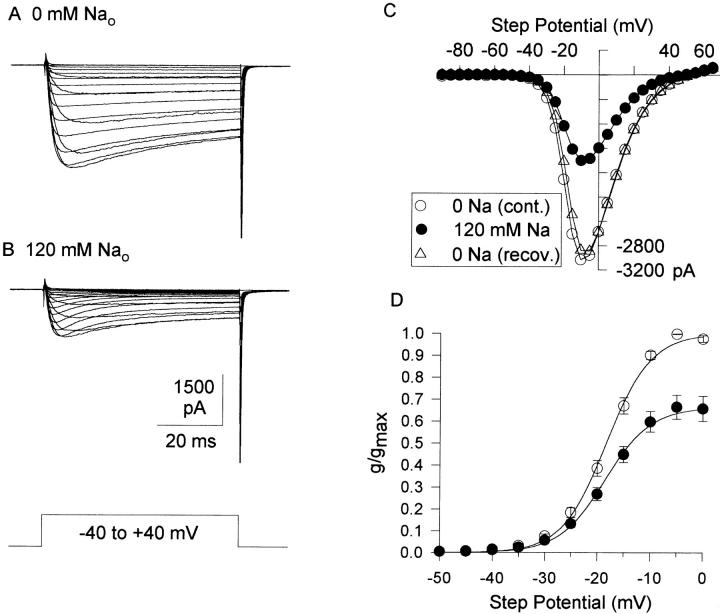
Figure 3.
Inhibition of Ba2+ currents by extracellular Na+. The external solution contained 3 mM Ba2+. (A) Control family of currents, evoked by depolarizations between −40 and +40 mV. (B) Currents following substitution of 120 mM Na+ for TEA. (C) I-V curves before, during and following removal of 120 mM Na+. (D) Activation curves, normalized to the peak control current at −5 mV. Symbols represent mean ± SEM for five cells in each condition. Solid lines represent the best fit to the data, using a modified Boltzmann equation, {1 + exp[(V 1/2 − V)/K]}−1. Values for V 1/2 (in mV) were −18.3 ± 0.16 (Cont.) and −18.6 ± 0.17 (120 mM Na+). Values for K (in mV) were 4.18 (Cont.) and 4.33 (120 mM Na+).
Ba2+ Current Block by Na+
Replacement of external TEA with Na+ (120 mM) reversibly inhibited Ba2+ currents (Fig. 3) with no change in reversal potential (Fig. 3 C), activation voltage (Fig. 3 D), or voltage-dependence of inactivation (not shown). The concentration dependence of Ba2+ current block by Na+ was examined in two ways. In one set of experiments, Na+ concentration was increased to 10, 30, or 120 mM by equimolar replacement of external TEA (as in Fig. 3). In the second set of experiments, Na+ concentration was increased to 30, 120, or 300 mM in the presence of 150 mM TEA (Fig. 4 A and B). This latter approach resulted in an increase in osmolality with increasing [Na+] but eliminated the possibility that removal of TEA (rather than addition of Na+) caused the change in Ba2+ current magnitude. There was no shift in the current-voltage relationship with increasing osmolality with either 120 mM Na+ (Fig. 4 B) or 300 mM Na+ (not shown). The percent inhibition by 30 and 120 mM Na+ in each of these types of experiments was statistically identical, and the data were pooled for the analysis in Fig. 4 C. With 2 mM Ba2+ as the charge carrier, Na+ block of the channel was well fit by Eq. 1, with an IC50 of 119 mM and a slope coefficient near 1.
Block of Ca2+ channel currents by Ca2+, Ba2+, Cd2+, and Mg2+ is voltage dependent (Fukushima and Hagiwara, 1985; Lansman et al., 1986; Rosenberg and Chen, 1991; Kuo and Hess, 1993a), which places the location of the high affinity Ca2+ binding site inside of the membrane field. Although the site of Li+ interaction in the Ca2+ channel may be external to the membrane field (Kuo and Hess, 1993a ), evidence strongly suggests that the interaction between divalents and monovalents in the Ca2+ channel occurs inside the pore (Fukushima and Hagiwara, 1985; Lansman et al., 1986; Yamashita et al., 1990; Kuo and Hess, 1993a , b ). To examine the voltage dependence of Na+ block of Ba2+ currents, we examined block of tail currents at different repolarization potentials (Fig. 5). Panels A and B illustrate currents from one cell evoked in TEA bath (Fig. 5 A) and Na+ bath (Fig. 5 B). The tail I-V curve was reasonably linear between −70 and 0 mV (Fig. 5 C), and block by Na+ was almost identical at all potentials. Fig. 5 D plots the percent block by 120 mM Na+ as a function of voltage, averaged over six cells. Between −70 and 0 mV, block was essentially voltage independent. Block between −30 and +30 mV, measured during the depolarizing voltage step, was similarly voltage independent (not shown).
Competition between Ba2+ and Na+
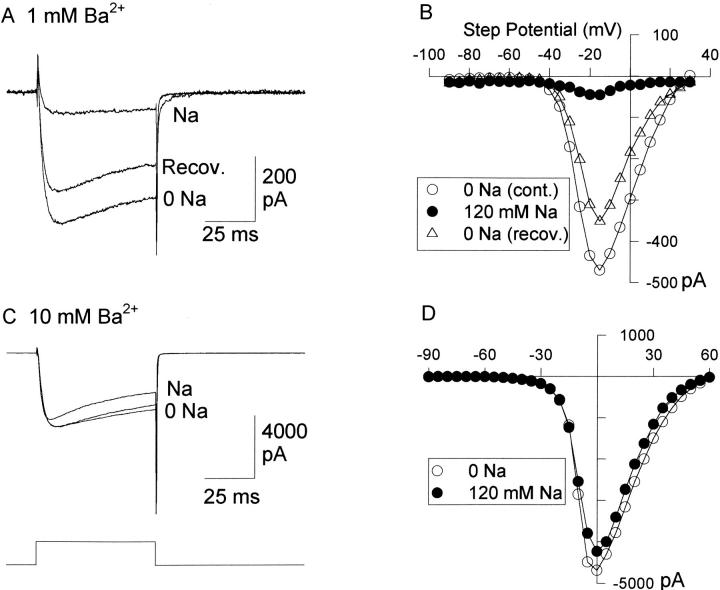
If Na+ blocked Ba2+ currents by binding to a Na+-selective regulatory binding site, Na+ block would be predicted to persist regardless of Ba2+ concentration. In contrast, if Na+ and Ba2+ bound to a common site, Na+-induced inhibition should be dependent on Ba2+ concentration. We tested these alternative hypotheses by examining block by 120 mM Na+ in the presence of different Ba2+ concentrations. In the presence of 1 mM Ba2+, 120 mM Na+ inhibited Ba2+ currents by 72.5 ± 8.0% (n = 4; Fig. 6 A and B). In the presence of 10 mM Ba2+, 120 mM Na+ had little effect on Ba2+ currents, with an average inhibition of 13.6 ± 2.2% (n = 4; Fig. 6 C and D). Channel block by 120 mM Na+ was half maximal at a Ba2+ concentration of 2 mM (Fig. 7). These data, in combination with those of Fig. 4 C, suggest that Na+ blocks Ba2+ currents via a competitive interaction with Ba2+ for a binding site.
Figure 6.
Na+ block of Ba2+ currents in low and high [Ba2+]. (A) Currents recorded in 1 mM Ba2+, with 0 Na2+ (0 Na and Recov.), and with 120 mM Na+. (B) I-V curves from cell in A. (C) Currents recorded in 10 mM Ba2+, before, during (Na) and after substitution of 120 mM Na+ for TEA. (D) I-V curves for the cell in C. The stimulus in A and C was a 100-ms depolarization from −80 to −15 mV (A) or 0 mV (C).
Ba2+ Current Block by Li+
L-type Ca2+ channels are somewhat selective for Li+ over Na+, both in the presence and absence of external Ba2+ (Hess et al., 1986). In addition, the single channel conductance is lower for Li+ than Na+ (Hess et al., 1986). These observations are consistent with a model in which Li+ has a somewhat higher affinity for the channel than Na+. If N-type Ca2+ channel permeation is indeed similar to that of L-type channels, Li+ would be expected to inhibit Ba2+ currents at similar or slightly lower concentrations than Na+. Fig. 8 illustrates that this was the case. Application of increasing concentrations of Li+ in the presence of a constant concentration of TEA produced a concentration-dependent, reversible inhibition of the Ba2+ current (Fig. 8, A and B). The IC50 for Ba2+ current block by Li+, derived from the best fit of the data to Eq. 1, was 97 mM, with a slope coefficient near 1 (Fig. 8 C).
discussion
The primary result described in this paper is that at physiological concentrations of Ca2+ and Na+, external Na+ blocks Ca2+ (and Ba2+) currents through N-type Ca2+ channels. This observation is of interest from a physiological perspective, since it suggests that Ca2+ channels are normally conducting at <100% efficiency. Our results also indicate that the permeation selectivity mechanism in chick N-type Ca2+ channels is similar to that of L-type Ca2+ channels. Consequently, these experiments provide additional insight into the Ca2+ channel permeation mechanism.
Comparison of Permeation Mechanism in N-type and L-type Ca2+ Channels
Ca2+ channel permeation has been studied almost exclusively in L-type Ca2+ channels. These channels select for Ca2+ over Na+ via differential affinity of intrapore binding sites for these ions. At high [Ca2+], Ca2+ channels are almost perfectly selective for Ca2+ over monovalent cations. Reduction of external Ca2+ to submicromolar levels permits Na+ and other monovalent cations to conduct. Indeed, Na+ conducts almost 10 times better than Ca2+ (Hess et al., 1986). Addition of μM external [Ca2+] (K d = 0.7 μM) prevents monovalent cations from carrying inward current (Kostyuk et al., 1983; Almers et al., 1984; Fukushima and Hagiwara, 1985; Hess et al., 1986; Matsuda, 1986), and external Mg2+ blocks Na+ currents with an IC50 of about 60 μM (Matsuda, 1986). A similar mechanism appears to operate in a T-like Ca2+ channel in a B lymphocyte cell line (Fukushima and Hagiwara, 1985).
N-type channels from bullfrog sympathetic neurons and chick sensory neurons also conduct Na+ in the absence of Ca2+ (Kuo and Bean, 1993; Cox and Dunlap, 1994), which suggests that the permeation mechanism in these channels is similar to that in L-type channels. Our experiments extend these observations. Removal of divalent cations resulted in large Na+ currents through the Ca2+ channel that were inhibited 95% by 10 μM Ca2+ (consistent with an IC50 near 0.7 μM). Addition of 1 mM external Mg2+ inhibited Na+ currents through N-type channels by 80%, consistent with an IC50 near 250 μM. Although not examined in great detail, these results suggest that the selectivity mechanism in N-type Ca2+ channels is quite similar to that of L-type Ca2+ channels.
Simultaneous Binding of Ca2+ and Na+ in the Pore
There is a wealth of evidence to suggest that Ca2+ channels are multi-ion pores (Almers et al., 1984; Hess and Tsien, 1984; Fukushima and Hagiwara, 1985; Friel and Tsien, 1989; Yue and Marban, 1990; Kuo and Hess, 1993a , b ). Although the total number and location of binding sites in the pore is still somewhat controversial, molecular and biophysical studies suggest that a single molecular location near the outer mouth of the pore binds Ca2+ with high affinity, and constitutes the selectivity filter (Heinemann et al., 1992; Tang et al., 1993; Yang et al., 1993; Ellinor et al., 1995; Parent and Gopalakrishnan, 1995). This single locus is postulated to form a structure that is capable of binding of two Ca2+ ions (Yang et al., 1993; Ellinor et al., 1995). In low [Ca2+], the on-rate of a single Ca2+ to this site from the exterior of the pore is similar when the channel is conducting monovalent cation currents in either inward or outward directions, and the on-rate of Ca2+ to the pore is nearly diffusion-limited (Kuo and Hess, 1993b ). This suggests that the Ca2+ blocking site is easily accessible from the external solution. Ca2+ channel block by divalent cations is voltage dependent (Fukushima and Hagiwara, 1985; Lansman et al., 1986; Rosenberg and Chen, 1991; Kuo and Hess, 1993a ), which places the high affinity binding site inside the membrane field.
Although many studies argue against the single-site allosteric model of Kostyuk et al. (1983), other models of Ca2+ permeation have been proposed that include only a single high affinity Ca2+ binding site (Armstrong and Neyton, 1991; Yang et al., 1993; Dang and McCleskey, 1996). The Armstrong and Neyton model is conceptually quite similar to the Kuo and Hess model; in the former, two ions can bind to one site, in the latter, two cation binding sites are separated by little or no energy barrier. The Yang et al. (1993) model similarly postulates that a single location can bind either one or two ions. In all three models, the first Ca2+ in does not leave the high affinity site until it is “knocked off” by an incoming cation. The Dang and McCleskey model suggests that a single high affinity site flanked by low affinity sites could account for much of the Ca2+ channel permeation data. Although the energies that propel the ions through the pore are derived from different sources, each of these models postulates that two ions bind in very close proximity at a single location. Molecular studies have identified the EEEE locus as the likely location of this binding (Heinemann et al., 1992; Tang et al., 1993; Yang et al., 1993; Ellinor et al., 1995; Parent and Gopalakrishnan, 1995).
At very low [Ca2+], high external Li+ can impede the outward exit of Ca2+ from the channel (Kuo and Hess, 1993b ), which suggests that Li+ can bind in the pore externally to Ca2+. Whether this occurs at the EEEE locus while it is singly bound by Ca2+, or binds to an independent site external to the EEEE locus, is unknown. At [Ca2+] near its K d, addition of high external Li+ reduced the on-rate of Ca2+ for the high affinity site, with an apparent K d for Li+ of ∼100 mM. This reduction of on-rate presumably results from binding of Li+ to a pore unoccupied by Ca2+. Although the conditions of these experiments do not reflect a true equilibrium situation, this suggests that the K d of Li+ for the binding site in the absence of Ca2+ is on the order of 100 mM.
Our data extend these observations to the interaction of Ca2+ and Na+ in Ca2+ channels at physiological [Ca2+] and [Na+]. Although our data do not directly address the issue of whether the interaction occurs inside the pore, our data are consistent with the framework laid by many others that monovalent and divalent cations do indeed interact inside the pore. Consequently, we will restrict our discussion to this assumption.
At 2 mM Ca2+, one Ca2+ is always bound to the high affinity binding site. The observation that Na+ inhibits Ca2+ currents indicates that occupancy of the channel by Ca2+ does not prevent Na+ from entering the pore. The observation that Na+ block of Ca2+ channel currents is reduced by divalent cations in a concentration-dependent manner suggests that Na+ is binding to a Ca2+ binding site. We observed little or no voltage dependence of block by Na+. Taken together, these observations suggest that Na+ is binding externally to a Ca2+ ion bound to a high affinity Ca2+ binding site. Largely on indirect evidence, Kuo and Hess (1993a) also concluded that the site of Li+ occupancy in a Li+-conducting Ca2+ channel was voltage insensitive, and therefore outside the membrane field.
At 2 mM Ba2+, we observed an IC50 of Na+ and Li+ for the pore that was similar to that observed by Kuo and Hess (1993b) for reduction of Ca2+ on-rate to the high affinity binding site with [Ca2+] near 1 μM. These measurements were made differently, in that the apparent K ds in our studies were derived from near equilibrium measurements whereas those of Kuo and Hess were derived from experiments in which [Li+] was in steady state. Nonetheless, the similarity between these two measurements is intriguing. The measurement of apparent K d made by Kuo and Hess reflected the binding of Li+ to a pore occupied by Li+ but unoccupied by Ca2+. Kuo and Hess argued, however, that the binding affinity of Li+ to the second site may be similar whether the first site is bound by Ca2+ or Li+. Our measurement of apparent K d reflected binding of Li+ (or Na+) in a pore always occupied by at least one Ca2+. The similar apparent K ds suggest that the affinity of Na+ (or Li+) for the pore is not dramatically influenced by the occupancy of the first site by Ca2+. Consequently, these data suggest that the binding site for monovalent cations is unchanged by occupancy of the first Ca2+. Whereas these results do not preclude the hypothesis that binding of the second cation (either Ca2+ or Na+) has a lower affinity than the first due to electrostatic repulsion considerations, they do suggest that the binding affinity does not change due to an allosteric effect on the cation binding site produced by binding of the first Ca2+.
Comparison with Other Published Results
Almers et al. (1984) observed a slight reduction of Ca2+ channel currents in frog skeletal muscle upon partial replacement (32 mM) of TEA with Na+. This reduction is similar in magnitude to that which we observed. In contrast, replacement of external Na+ with Tris (Matsuda and Noma, 1984) or choline (Yamashita et al., 1990) did not influence Ca2+ currents in guinea pig ventricular cells or neoplastic lymphocytes. While these differences may, of course, be due to different Ca2+ channel preparations, they may also be related to the choice of ion substitute. As observed by Kuo and Hess (1993b) in L-type Ca2+ channels, NMG produced some block of the Ca2+ current in our experiments (data not shown). Thus, as with NMG, Tris and choline may also inhibit Ca2+ channel conductance, and thus mask inhibitory effects of Na+. Our results are also consistent with the possibility that cations did not block the Ca2+ channel but that TEA potentiated currents through the channel. We tested this in two ways. First, Na+ reduced the Ca2+ current when substituted for NMG (not shown), which indicates that the effect did not depend on the initial presence of TEA. More importantly, we observed an identical concentration-dependence of block whether Na+ isosmotically replaced TEA or was applied in addition to already present TEA.
Finally, our results may be considered in light of those obtained by Yamashita et al. (1990), which demonstrated that, at positive potentials, internal Na+ could pass outward current through Ca2+ channels, even in the presence of 2.5 mM external Ca2+. The differential ability of Na+ to conduct in the outward vs. the inward direction in the presence of normal external [Ca2+] may be partially due to the different competitive situations at the internal and external face of the channel. Thus, the very low internal [Ca2+], combined with positive voltages, may create a competitive advantage for Na+ over Ca2+ not possible at the external face of the channel.
Physiological Significance
Our data suggest that in physiological solutions, open Ca2+ channels are conducting submaximally when compared with the conductance expected for 2 mM Ca2+. Since it is not clear that external [Na+] would ever vary dramatically, the significance of these findings must be speculative. There are, however, situations where the monovalent cation-sensitivity of the channel could become meaningful. First, K+ blocks Ca2+ currents similarly to Na+ (data not shown). It is possible that under conditions of extreme increase in extracellular [K+], perhaps coupled with a small decrease in local [Ca2+] (for example, during high frequency neuronal activity or ischemia; cf. Hansen and Zeuthen, 1981), extracellular K+ could inhibit Ca2+ influx. Second, both Cs+ and Na+ will pass outward current through Ca2+ channels (Fenwick et al., 1982; Yamashita et al., 1990). It is intriguing to consider that intracellular K+, or local changes in intracellular [Na+] during high frequency activity, could influence Ca2+ channel permeation properties in a physiologically meaningful way. Finally, the sensitivity of the Ca2+ channel to Na+ suggests that compounds that bind to Na+ binding sites may also inhibit Ca2+ channels. For example, amiloride analogs, which inhibit many Na+-dependent processes, inhibit N-type, T-type and L-type Ca2+ channels (Tang et al., 1988; Garcia et al., 1990; Polo-Parada et al., 1996). Indeed, amiloride analogs are considered of potential use in the treatment of cardiac ischemia, especially during reperfusion (cf. Scholz et al., 1992). These drugs have both cardioprotective and antiarrythmic properties, which may be due, in part, to inhibition of Ca2+ influx through either pre- or postsynaptic Ca2+ channels. An understanding of the Na+ binding site in Ca2+ channels in physiological [Ca2+] and [Na+] may lead to a novel approach to the modulation of Ca2+ channel function.
Acknowledgments
Supported in part by the NSF and the University of Connecticut Research Foundation.
Abbreviation used in this paper
- DRG
dorsal root ganglion
references
- Almers W, McCleskey EW, Palade PT. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol (Lond) 1984;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Neyton J. Ion permeation through calcium channels. A one-site model. Ann NY Acad Sci. 1991;635:18–25. doi: 10.1111/j.1749-6632.1991.tb36477.x. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kasai H. Characterization of two kinds of high-voltage-activated Ca channel currents in chick sensory neurons: different sensitivity to dihydropyridines and ω-conotoxin GVIA. Pflüg Arch. 1989;414:50–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Korn SJ. Permeation of Na+ through a delayed rectifier K+channel in chick dorsal root ganglion neurons. J Gen Physiol. 1994;104:747–771. doi: 10.1085/jgp.104.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH, Dunlap K. Pharmacological discrimination of N-type from L-type calcium current and its selective modulation by transmitters. J Neurosci. 1992;12:906–914. doi: 10.1523/JNEUROSCI.12-03-00906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH, Dunlap K. Inactivation of N-type calcium current in chick sensory neurons: calcium and voltage dependence. J Gen Physiol. 1994;104:311–336. doi: 10.1085/jgp.104.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang T, McCleskey EW. A Ca2+channel model without ionic interaction. Biophys J. 1996;70:A184. [Google Scholar]
- Ellinor PT, Yang J, Sather WA, Zhang J-F, Tsien RW. Ca2+ channel selectivity at a single locus for high-affinity Ca2+interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol (Lond) 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. Voltage-gated calcium channels: direct observation of the anomalous mole fraction effect at the single-channel level. Proc Natl Acad Sci USA. 1989;86:5207–5211. doi: 10.1073/pnas.86.13.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol (Lond) 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ML, King VF, Shevell JL, Slaughter RS, Suarez-Kurtz G, Winquist RJ, Kaczorowski G. Amiloride analogs inhibit L-type calcium channels and display calcium entry blocker activity. J Biol Chem. 1990;265:3763–3771. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüg Archiv. 1981;381:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hansen AJ, Zeuthen T. Extracellular ion concentration during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand. 1981;113:437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Heinemann SH, Terlau H, Stuhmer W, Imoto K, Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature (Lond) 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hess P, Tsien RW. Mechanism of ion permeation through calcium channels. Nature (Lond) 1984;309:453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic channels in Excitable Membranes. 2nd ed. Sinauer Associates, Inc. Sunderland, MA. pp. 607.
- Korn SJ, Weight FF. Patch-clamp study of the calcium-dependent chloride current in AtT-20 pituitary cells. J Neurophysiol. 1987;58:1431–1451. doi: 10.1152/jn.1987.58.6.1431. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Mironov SL, Shuba YM. Two ion-selecting filters in the calcium channel of the somatic membrane of mollusc neurons. J Membr Biol. 1983;76:83–93. [Google Scholar]
- Kuo C-C, Bean BP. G-protein modulation of ion permeation through N-type calcium channels. Nature (Lond) 1993;365:258–262. doi: 10.1038/365258a0. [DOI] [PubMed] [Google Scholar]
- Kuo C-C, Hess P. Ion permeation through the L-type Ca2+channel in rat phaeochromocytoma cells: two sets of ion binding sites in the pore. J Physiol (Lond) 1993a;466:629–655. [PMC free article] [PubMed] [Google Scholar]
- Kuo C-C, Hess P. Characterization of the high-affinity Ca2+ binding sites in the L-type Ca2+channel pore in rat phaeochromocytoma cells. J Physiol (Lond) 1993b;466:657–682. [PMC free article] [PubMed] [Google Scholar]
- Lansman JB, Hess P, Tsien RW. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986;88:321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Sodium conductance in calcium channels of guinea-pig ventricular cells induced by removal of external calcium ions. Pflüg Arch. 1986;407:465–475. doi: 10.1007/BF00657502. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol (Lond) 1984;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent L, Gopalakrishnan M. Glutamate substitution in repeat IV alters divalent and monovalent cation permeation in the heart Ca2+channel. Biophys J. 1995;69:1801–1813. doi: 10.1016/S0006-3495(95)80050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L, Callahan MJ, Korn SJ. Does sodium-hydrogen exchange influence calcium channel currents in dorsal root ganglion neurons? . Biophys J. 1996;70:A314. [Google Scholar]
- Rosenberg RL, Chen X-H. Characterization and localization of two ion-binding sites within the pore of cardiac L-type calcium channels. J Gen Physiol. 1991;97:1207–1225. doi: 10.1085/jgp.97.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz W, Albus U, Linz W, Martonrana P, Lang HJ, Scholkens BA. Effects of Na+ / H+exchange inhibitors in cardiac ischemia. J Mol Cell Cardiol. 1992;24:731–740. doi: 10.1016/0022-2828(92)93387-y. [DOI] [PubMed] [Google Scholar]
- Tang CH, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science (Wash DC) 1988;240:213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Tang S, Mikala G, Bahinski A, Yatani A, Varadi F, Schwartz A. Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel. J Biol Chem. 1993;268:13026–13029. [PubMed] [Google Scholar]
- Tsien RW, Hess P, McCleskey EW, Rosenberg RL. Calcium channels: mechanisms of selectivity, permeation and block. Annu Rev Biophys Chem. 1987;16:265–290. doi: 10.1146/annurev.bb.16.060187.001405. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Ciani S, Hagiwara S. Effects of internal Na+on the Ca channel outward current in mouse neoplastic B lymphocytes. J Gen Physiol. 1990;96:559–579. doi: 10.1085/jgp.96.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ellinor PT, Sather WA, Zhang J-F, Tsien RW. Molecular determinants of Ca2+ channel selectivity and ion permeation in L-type Ca2+channels. Nature (Lond) 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- Yue DT, Marban E. Permeation in the dihydropyridine-sensitive calcium channel. J Gen Physiol. 1990;95:911–939. doi: 10.1085/jgp.95.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]