Abstract
We have produced transgenic mice which overexpress cardiac Na+-Ca2+ exchange activity. Overexpression has been assessed by Western blot, Northern blot, and immunofluorescence. Functional overexpression was analyzed using membrane vesicles and isolated ventricular myocytes. In whole cell clamped myocytes dialyzed with 0.1–0.2 mM Fura-2, the magnitude of ICa and Ca2+ i -transient triggered by ICa or caffeine were not significantly different in transgenic vs. control myocytes. In transgenic myocytes, activation of ICa, however, was followed by a large slowly inactivating transient inward current representing INa-Ca. This current depended on Ca2+ release as it was abolished when sarcoplasmic reticulum (SR) Ca2+ was depleted using thapsigargin. Cai-transients triggered by rapid application of 5 mM caffeine, even though equivalent in control and transgenic myocytes, activated larger INa-Ca (∼5 pA/pF at −90 mV) in transgenic vs. control myocytes (1.5 pA/pF). The decay rate of caffeine-induced Ca2+ i -transient and INa-Ca was 2.5 times faster in transgenic than in control myocytes. 5 mM Ni2+ was equally effective in blocking INa-Ca in control or transgenic myocytes. In 9 out of 26 transgenic myocytes, but none of the controls, Ca2+ influx via the exchanger measured at +80 mV caused a slow rise in [Ca2+]i triggering rapid release of Ca2+ from the SR. SR Ca2+ release triggered by the exchanger at such potentials was accompanied by activation of transient current in the inward direction. In 2 mM Fura-2–dialyzed transgenic myocytes caffeine-triggered Cai-transients failed to activate INa-Ca, even though the kinetics of inactivation of ICa slowed significantly in caffeine-treated myocytes. In 0.1 mM Fura-2–dialyzed transgenic myocytes 100 μM Cd2+ effectively blocked ICa and suppressed Cai-transients at −10 or +50 mV. Our data suggests that in myocytes overexpressing the exchanger, the content of intracellular Ca2+ pools and the signaling of its release by the Ca2+ channel vis-à-vis the Na+-Ca2+ exchanger were not significantly altered despite an up to ninefold increase in the exchanger activity. We conclude that the exchanger remains functionally excluded from the Ca2+ microdomains surrounding the DHP/ryanodine receptor complex.
Keywords: ventricular myocytes, Ca2+ channel, whole cell patch clamp, immunofluorescence, isolated sarcolemmal vesicles
introduction
Regulation of Ca2+ fluxes in cardiac myocytes is a complex process involving multiple transporters, channels, and compartments. A key transport process is that mediated by the Na+-Ca2+ exchange protein. The Na+-Ca2+ exchanger catalyzes the countertransport of three Na+ ions for one Ca2+ ion across the sarcolemmal membrane and is the major Ca2+ efflux mechanism of myocardial cells. Ca2+ extrusion by the exchanger helps bring about muscle relaxation after contraction. Thus, while the Ca2+ channels of sarcolemma (DHP receptors) provide the primary route for entry of Ca2+, the Na+-Ca2+ exchanger serves as a primary route for Ca2+ extrusion. To maintain and regulate cytosolic Ca2+ concentrations during the contraction/relaxation cycle, the activity of the two pathways ultimately must be balanced. Although the role of the Na+-Ca2+ exchanger in the efflux of Ca2+ has been quantified and is universally accepted, the physiological role of the exchanger in the Ca2+ influx mode has been somewhat controversial and not generally agreed upon (Callewaert, 1992; Levesque et al., 1994; Lipp and Niggli, 1994; Sham et al., 1992). This controversy may be related, in part, to different levels of expression of the exchanger in different species (Sham et al., 1995b ). Ca2+ cross signaling experiments between the DHP and ryanodine receptors in myocytes dialyzed with high concentrations of Ca2+ buffers, however, suggest that the exchanger protein might be excluded from the “functional” microdomain of Ca2+ surrounding the DHP/ryanodine receptor complex (Sham et al., 1995a ; Adachi-Akahane et al., 1996), even though immunofluorescence techniques have suggested that the exchanger is located in the t-tubular system near diadic or triadic junctures (Frank et al., 1992; Kieval et al., 1992).
The availability of the cDNA clone for the Na+-Ca2+ exchanger (Nicoll et al., 1990) allows new approaches to physiological problems regarding the exchanger. For example, antisense oligonucleotides have been used to “knock out” exchange activity in both cardiac and arterial myocytes (Lipp et al., 1995; Sidzinski et al., 1995). Transgenic mouse technology offers another opportunity to manipulate Na+-Ca2+ exchange activity. Here we describe the production and characterization of transgenic mice overexpressing Na+-Ca2+ exchange activity specifically in cardiac muscle. Myocytes from the transgenic mice have increased INa-Ca and were used to further probe the ability of INa-Ca and the Ca2+ channel to trigger Ca2+ release. Even with the increased density of INa-Ca, the exchanger failed to trigger Ca2+ release from the sarcoplasmic reticulum (SR)1 in the physiological voltage range.
materials and methods
Production of Transgenic Mice
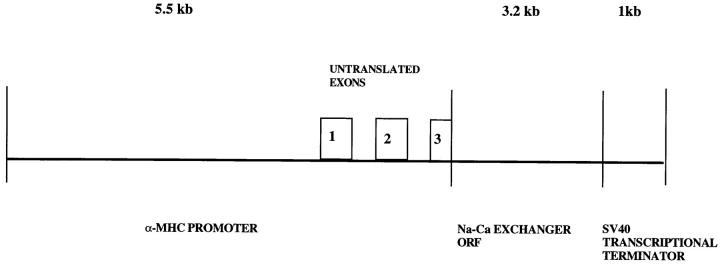
The transgene construct (Fig. 1) consisted of the open reading frame of the canine cardiac Na+-Ca2+ exchanger (Nicoll et al., 1990) under the control of the α-myosin heavy chain (α-MHC) promoter. The α-MHC promoter consisted of 4.5 kb of 5′ upstream sequence plus 1 kb of the α-MHC gene encompassing exons 1 through 3 of the 5′ untranslated region (Gulick et al., 1991; Subramaniam et al., 1991). The presence of upstream introns and exons may improve expression of a transgene (Palmiter et al., 1991). Downstream from the α-MHC promoter was the SV40 transcriptional terminator to provide a polyadenylation signal. The exchanger open reading frame was removed from pTB11 (Nicoll et al., 1990) by digestion with EcoRV and SnaBl and ligated into the SalI site between the α-MHC promoter and the SV40 transcriptional terminator. The SalI site had first been digested, blunted, and dephosphorylated. Proper orientation of the exchanger insert was confirmed by restriction mapping. The transgene was purified by GeneClean (Bio 101, La Jolla, CA) and microinjected into the nuclei of C57Bl/6xC3HF1 mice by the UCLA Transgenic Core Facility for transgenic mouse production.
Figure 1.
Transgene construct. The open reading frame (ORF ) of the Na+-Ca2+ exchanger was under the control of the α-MHC promoter. For details, see text.
Eight lines (A through H) of transgenic mice were generated. Southern blot analysis was used to confirm the presence of the transgene in the mouse genome. Genomic DNA was extracted from tail clippings, digested with EcoRI, and size fractionated on a 0.8% agarose gel. After transfer to nitrocellulose, the DNA was probed with the 0.4-kb EcoRI fragment from pTB11. This portion of the open reading frame of the Na+-Ca2+ exchanger is derived from a region of the NCX1 gene encompassing several exons (Kofuji et al., 1994). Thus, on the Southern blot, the 0.4-kb probe would hybridize with the 0.4-kb fragment derived from the EcoRI-digested transgene which contains no exchanger introns. Digestion of the endogenous mouse exchanger gene, however, with EcoRI did not produce a similar sized fragment. Prehybridization and hybridization were carried out as described previously (Li et al., 1994).
Northern Blot Analysis
Total RNA was isolated from mouse tissues by the method of Chomczynski and Sacchi (1987). The RNA was separated on a denaturing 1% agarose gel and transferred onto a Hybond-N nylon filter. The same 0.4-kb cDNA fragment used in the Southern blot analysis above was also used for Northern blot analysis. Hybridization conditions were also the same.
Western Blot Analysis
Proteins were first separated on a 7.5% gel by SDS-PAGE and transferred onto nitrocellulose for 30 min at 100 V. Washings and antibody incubations were carried out in the presence of 1% milk. The primary polyclonal antibody (π) was raised against the canine cardiac exchanger and has been described previously (Philipson et al., 1988). The secondary antibody was goat anti–rabbit IgG coupled to horseradish peroxidase. Diaminobenzidine was used as substrate for color development.
Indirect Immunofluorescent Labeling
Isolated mouse myocytes, either from control hearts or transgenic hearts, were fixed with 2% buffered formaldehyde for 15 min. The fixed cells were quenched in Na+ borohydrate, treated with Triton X-100, and exposed to blocking solution and the monoclonal antibody R3F1 (1/500 dilution) against the Na+-Ca2+ exchanger, as previously described (Frank et al., 1992; Chen et al., 1995). The cells were incubated with fluorescein-labeled goat anti–mouse secondary antibody for 45 min, rinsed, and mounted on glass slides with 90% glycerol plus a photobleaching inhibitor. The confocal fluorescence microscopy was carried out with a Nikon photomicroscope equipped with a molecular dynamic confocal imaging system.
Transport Measurements in Isolated Vesicles
A crude membrane fraction was prepared for measurement of Na+-Ca2+ exchange fluxes. Mouse hearts (∼100 mg) were homogenized in 1.4 ml of 560 mM NaCl, 10 mM Mops/Tris, pH 7.4, and spun in an Eppendorf centrifuge for 4 min at 11,000 rpm. The pellet was resuspended in 0.9 ml of 140 mM NaCl, 10 mM Mops/Tris, pH 7.4 and spun for 4 min at 11,000 rpm. The pellet was resuspended in 0.8 ml of the same solution and spun briefly (5 s) at 4,000 rpm to remove particulate material. The supernatant, containing Na+-loaded membrane vesicles was used directly for Ca2+ uptake measurements. The protein yield in the final fraction was identical for the control and transgenic mice.
To measure Na+ gradient–dependent 45Ca2+ uptake, 10 μl of the supernatant was rapidly diluted into a Ca2+ uptake medium containing 140 mM KCl, 10 μM 45Ca2+, 1 μM valinomycin, 10 mM Mops/Tris, pH 7.4. The reaction was quenched after 3 s and then filtered. A blank was subtracted in which the uptake medium contained NaCl instead of KCl. We have used this technique extensively in the past to quantitate vesicular Na+-Ca2+ exchange (Li et al., 1991).
Isolation of Adult Mouse Ventricular Myocytes
Adult mouse ventricular myocytes were isolated according to a previously described method (Mitra and Morad, 1985) with minor modification. After injection of heparin sodium (1,000 U/ kg, i.p.), mice were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.), hearts were quickly excised and perfused in a Langendorff apparatus (1.2–1.6 ml/min) first with nominally Ca2+-free Tyrode's solution composed of (in mM) NaCl, 137; KCl, 5.4; HEPES, 10; MgCl2, 1; glucose, 10; pH 7.3 at 37°C for 7 min, then with Ca2+-free Tyrode's solution containing collagenase (0.5–0.6 Us/ml) and protease (0.55 Us/ml) for 15 min, and finally with low Na+ Tyrode's solution. The ventricular part of the digested heart was then cut into several sections and gently agitated to dissociate cells. The freshly dissociated cells were stored at room temperature in low Na+ Tyrode's (in mM: 52.5 NaCl, 4.8 KCl, 1.19 KH2PO4, 1.2 Mg SO4, 11.1 glucose, 10 HEPES, 145 sucrose, pH 7.4) containing 0.2 mM Ca2+ and were used for up to 10 h after isolation. In all of the electrophysiological experiments, the transgenic mouse line H was used.
Current Recording
Ca2+ current was measured in the whole cell configuration of the patch-clamp technique (Hamill et al., 1981) using a DAGAN 8900 amplifier (Dagan Corp., Minneapolis, MN). The patch electrodes, made of borosilicate glass capillaries, were fire-polished to have a resistance of 1.2 to 2.0 MΩ when filled with the internal solution composed (in mM): CsCl, 110; tetraethylammonium chloride (TEA-Cl), 30; NaCl, 10; HEPES, 10; MgATP, 5; cAMP, 0.2; K5Fura-2, 0.1–2.0 and titrated to pH 7.4 with CsOH. Inward rectifier K+-current was suppressed by either addition of Ba2+ (0.1 mM) to or omission of K+ from the external solutions. Na+-current was mostly suppressed by addition of 10 μM tetrodotoxin to the external solution, and by including a high concentration (200 μM) of cAMP in the internal solution (Schubert et al., 1989). Myocytes were dialyzed with 200 μM cAMP not only to enhance ICa but also to fully activate Ca-ATPase activity through phosphorylation of phospholamban.
Generation of voltage-clamp protocols and acquisition of data were carried out using pCLAMP software (version 5.5-1; Axon Instruments, Inc., Foster City, CA). The leak currents were not digitally subtracted by the P/N method (N = 5–6) as to avoid suppression of maintained components of INa-Ca. Thus, we chose cells which had little or no leak currents. The series resistance was 1.5 to 3 times the pipette resistance and was electronically compensated through the amplifier. Sampling frequency was 0.5–2.0 kHz, and current signals were filtered at 10 kHz before digitization and storage.
Intracellular Calcium Measurements
Intracellular calcium activity was measured according to the method described earlier (Cleemann and Morad, 1991). Ventricular myocytes were dialyzed with 0.1–2.0 mM Fura-2 via the patch pipettes. Ultraviolet light originated from a 100 W mercury arc lamp, was split into two beams using a mirror vibrating at 1,200 Hz, and passed through the interference filters (410 and 335 nm, 20 nm bandwidth). The fluorescent light passed through a wide-band interference filter (510 nm, 70 nm bandwidth) and was detected with a photo-multiplier. The signal from the photo-multiplier was demultiplexed (Cleemann and Morad, 1991), yielding two signals corresponding to the two wavelengths of excitation. These signals were acquired simultaneously with the whole-cell currents using pCLAMP software. Cai -transients were quantified using FURA 2N program (Adachi-Akahane et al., 1996).
The data collected with dual wavelength excitation of Fura-2 were analyzed to determine the intracellular Ca2+ activity ([Ca2+]i) by the ratiometric method with a K d value of Fura-2 for Ca2+ as 220 nM (Grynkiewicz et al., 1985). The background fluorescences (F410,bg and F335,bg) were measured after making a giga-seal just before rupture of the membrane. Calibration measurements were performed with samples of 50 μM Fura-2 either saturated with 5 mM Ca2+ (F410,Ca and F335,Ca) or in free form with 10 mM EGTA (F410,EGTA and F335,EGTA).
Drugs were dissolved in the external Tyrode's solution, and applied rapidly using a concentration-clamp device (Cleemann and Morad, 1991).
All the experiments were performed at room temperature (22–25°C).
Collagenase (type A) was purchased from Boehringer-Mannheim Biochemicals (Indianapolis, IN), Protease (type XIV, pronase E) and MgATP from Sigma Chemical Co. (St. Louis, MO), thapsigargin and tetrodotoxin from Calbiochem Corp. (La Jolla, CA), and K 5Fura-2 salt from Molecular Probes, Inc. (Eugene, OR). Thapsigargin was dissolved in DMSO and stored as 10−3 M stock solution. The highest (0.1%) concentration of DMSO used had no effect by itself on ICa or Cai-transients.
results
Transgenic Mice, Their Molecular and Ultrastructured Characterization
Transgenic mouse lines were produced to overexpress the canine cardiac Na+-Ca2+ exchanger in mouse myocardium. The transgene used in these experiments (Fig. 1) contained the open reading frame of the canine sarcolemmal Na+-Ca2+ exchanger under the control of the α-MHC promoter. This promoter has been used previously in transgenic experiments for cardiac-specific expression (Milano et al., 1994; Soonpaa et al., 1994; Koch et al., 1995). Eight transgenic mouse lines, called lines A through H, were generated. The transgenic mice were all heterozygous so that in all cases nontransgenic littermates could be used for controls. The mice had no readily observable altered phenotype; body and heart weights were all normal. No differences between the transgenic mouse lines were detected unless specifically mentioned below. Table I compares some electrophysiological properties of the myocytes not directly related to the exchanger activity in control and transgenic mice. The size of the myocytes (cell capacitance), the resting Ca2+ concentrations (basal [Ca]i), the magnitude of triggered Cai-transients (ΔCai), and the Ca2+ current density (ICa) were not significantly altered in transgenic mice.
Table I.
Ca2+ Current (ICa) and Ca2+ i -transient (Δ[Ca2+]i Triggered by ICa in Nontransgenic (Control) and Transgenic Myocytes
| ICa(pA) | Capacitance (pF) | ICa Density (pA/pF) | Basal [Ca2+]i (nM) | Δ[Ca2+]i (nM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| control | 2,189 ± 207 | 192 ± 9 | 11.6 ± 1.1 | 126 ± 13 | 364 ± 22 | |||||
| (19) | (19) | (14) | (10) | (10) | ||||||
| transgenic | 2,129 ± 209 | 204 ± 7 | 10.5 ± 0.9 | 142 ± 21 | 337 ± 23 | |||||
| (31) | (31) | (16) | (15) | (15) |
ICa were activated by giving depolarizing pulses from −60 to −10 mV. Data are represented as the mean ± SEM (number of experiments).
Molecular Evidence for Overexpression of the Na+-Ca2+ Exchanger
Northern blot analysis.
We first analyzed RNA from control and transgenic mouse hearts for levels of exchanger transcript derived from the transgene in the different transgenic mouse lines. The native mouse exchanger has a transcript size of 7 kb; most of the size of the transcript is due to extensive 5′- and 3′-untranslated regions with the open reading frame of the exchanger being only 3 kb. Most of the untranslated regions have been removed in the construction of the transgene and the expected size of the transcript in this case is 3.2 kb. Thus, it is straightforward to distinguish the two transcripts by Northern blot analysis.
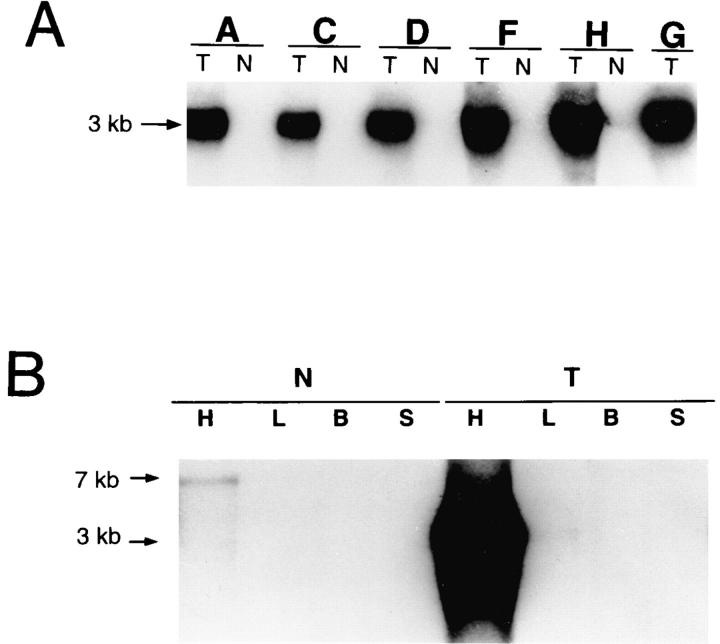
Fig. 2 A shows a Northern blot with RNAs isolated from the hearts of transgenic mice and their nontransgenic littermates from six different lines probed with an exchanger cDNA probe. A strong signal from RNA isolated from the transgenic hearts is seen at 3 kb after an exposure of only 1 h. With such short exposure times, no signal is observed with the RNA from the control mice. A signal from the native exchanger becomes visible at 7 kb in all lanes after longer exposures (not shown, but see Fig. 2 B). Similar results were obtained for transgenic mouse lines B and E. Clearly, substantial amounts of RNA are being transcribed from the transgene.
Figure 2.
Na+-Ca2+ exchanger transcript expression in transgenic mice (A) RNA was isolated from the hearts of transgenic (T ) mice or nontransgenic (N ) littermates from transgenic mouse lines A, C, D, F, H, and G, as indicated. After hybridization, the Northern blot was exposed to film for 1 h. (B ) Northern blot analysis was performed using RNA isolated from the heart (H ), lung (L), brain (B), and skeletal muscle (S) of transgenic mice (T ) or nontransgenic littermates (N ) from line H. After hybridization with an NCX1 cDNA probe, the Northern blot was exposed to film for 6 h. 10 μg of total RNA was run in each lane.
The α-MHC promoter is supposed to permit gene expression only in cardiac tissue, therefore we next assessed the tissue specificity of exchanger transgene expression. Fig. 2 B shows the results of a Northern blot analysis using RNA isolated from the heart, lung, brain, or skeletal muscle of transgenic mice or nontransgenic littermates from line H. In this case, a longer exposure time was used to permit visualization of both the native and transgenic Na+-Ca2+ exchangers. In the tissues from the nontransgenic mice, a weak signal at 7 kb from the native exchanger is seen only in the RNA from the cardiac tissue. (Upon longer exposure, 7-kb bands also become visible in the RNA from brain and lung.) In the RNAs from the transgenic mice, a strong signal is seen only with the cardiac RNA at 3 kb (transgenic exchanger) and a weak signal is seen at 7 kb (native exchanger). Not visible in the photograph, but discernible by eye, is a low level of expression of the 3-kb transgenic exchanger in the lung of transgenic mice. This was also seen in lung RNA from other transgenic mouse lines. Thus, the α-MHC promoter was not completely silent in lung tissue. In one transgenic mouse line (line E), expression of transgene transcripts were much higher in lung than in the other transgenic lines though still many fold lower than the expression level in heart. Line E was also the only line in which transgene transcripts could also be weakly detected in brain RNA. Subramaniam et al. (1991) have previously noted a low level of α-MHC gene expression in lung tissue, specifically in the thick wall of the pulmonary veins of the lung. Nevertheless, of those tissues tested, high levels of transgenic Na+-Ca2+ exchanger transcript were found only in the myocardium.
Western blot analysis.
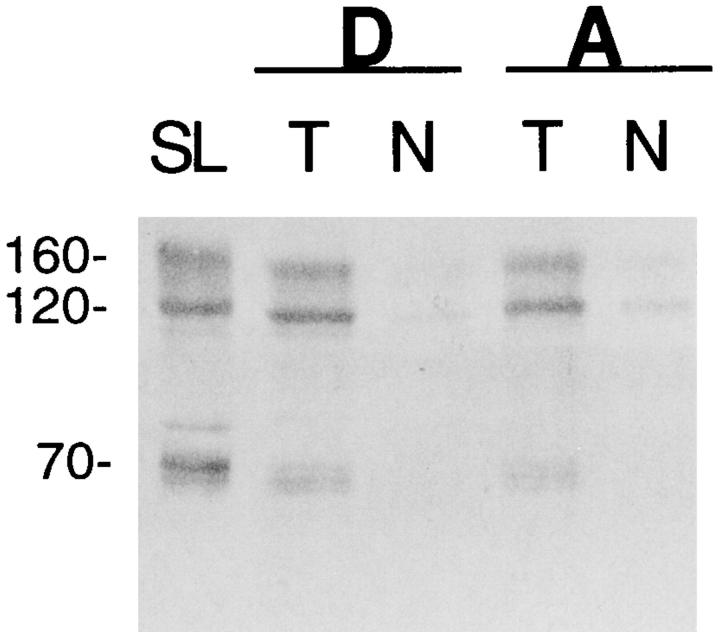
Immunoblots were performed to assess the level of Na+-Ca2+ exchanger protein in the hearts of the transgenic mice. The proteins of myocardial homogenate were separated by SDS-PAGE and probed with a polyclonal antibody to the canine sarcolemmal Na+-Ca2+ exchanger. Strong immunoreactivity is seen in the transgenic hearts but not in the hearts from control littermates (Fig. 3). The protein bands which immunoreact have a similar pattern to that seen with isolated canine sarcolemmal membranes, a positive control (Philipson et al., 1988). The transgenic exchanger protein bands, however, appear to be of slightly smaller molecular weight than those of the isolated sarcolemma, perhaps due to a small difference in amount of glycosylation. We have previously demonstrated that glycosylation does not affect exchanger function (Hryshko et al., 1993).
Figure 3.
Immunoreactivity of Na+-Ca2+ exchanger protein in transgenic mouse hearts. Shown is Western blot reacted with a polyclonal antibody to the canine cardiac Na+-Ca2+ exchanger. Loaded in each lane was canine cardiac sarcolemmal membranes (10 μg) as a positive control or 50 μg of cardiac homogenate protein from transgenic (T ) mice or nontransgenic (N ) littermates from mouse lines D and A. Antibody dilution was 1/1,000.
The native mouse exchanger of the control mouse hearts produces only a weak immunoreactivity under these conditions, though at the same apparent molecular weight as the transgenic exchanger protein. The strength of the immunoreactivity, however, cannot be used to estimate quantitatively the amount of exchanger overexpression. The antibody was produced using the canine heart exchanger as antigen and thus may react more weakly with the native mouse heart exchanger than with the canine exchanger encoded by the transgene. Qualitatively, however, the Western blot clearly demonstrates that a substantial amount of Na+-Ca2+ exchanger protein is being translated from transgene transcripts.
Immunofluorescence
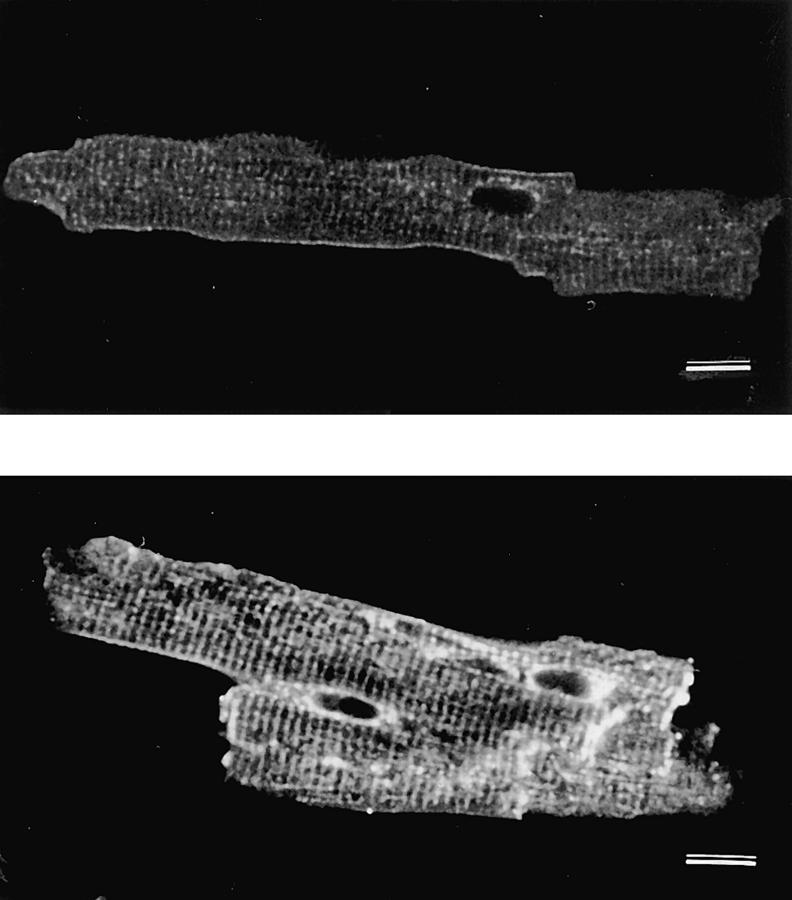
Overexpression of the Na+-Ca2+ exchanger is also clearly demonstrated by immunofluorescence (Fig. 4). The confocal micrographs of the control (Fig. 4 A) and transgenic (Fig. 4 B) myocytes were taken under identical conditions. In the transgenic myocytes, there is intense labeling of both the surface and t-tubular sarcolemma as well as the area surrounding the nucleus which is presumably the Golgi apparatus involved in protein trafficking. As described above, the antibody reactions cannot be used for quantitative comparison of exchanger expression.
Figure 4.
Confocal microscope images of isolated mouse myocytes immunolabeled with monoclonal antibodies (R3F1) against the Na+-Ca2+ exchange protein. Both cells were incubated under the same conditions. The gain of the confocal microscope was set to the lowest level to allow the control cell (top) to be just visible. Under these conditions the transgenic myocytes (bottom) have intense labeling of the surface sarcolemma and t-tubules indicating considerable expression of Na+-Ca2+ exchanger protein in the membrane. Also intensely labeled is the area surrounding the nuclei which presumably represents the Golgi. Magnification, 660×.
We had previously found a preferential localization of the Na+-Ca2+ exchanger in that part of the sarcolemma which forms the t-tubules in guinea pig and rat myocytes (Frank et al., 1992). This preferential localization is much less obvious in mouse myocytes in which strong staining of the peripheral sarcolemma is also observed. This species difference may be related to the large amount of peripheral sarcoplasmic reticulum found in mouse myocytes ( J.S. Frank, unpublished observations). Thus, even the Na+-Ca2+ exchangers found in surface sarcolemma in mouse myocytes may be in close proximity to underlying sarcoplasmic reticulum.
Evidence for functional overexpression of the Na+-Ca2+ exchanger.
Two types of experimental protocols using freshly isolated ventricular myocytes and one set of experiments using a crude membrane preparation were employed to examine the exchanger activity in control and transgenic mice.
In the first set of experiments we prepared a crude myocardial membrane fraction and assayed for Na+ gradient-dependent 45Ca2+ uptake. In a second set of experiments, Fura-2-dialyzed myocytes were clamped at holding potentials of −90 mV and were subjected to rapid (<50 ms) application of 5 mM caffeine to induce Ca2+ release from the SR to activate a transient Ni2+-sensitive inward INa-Ca (Ca2+-extrusion mode of the exchanger; Callewaert, Cleemann and Morad, 1989). In a third set, long (1–2 s) depolarizing pulses to positive potentials were used to measure the Ca2+ influx mode of the exchanger. The maintained outward Ni2+-sensitive current and the accompanying rise in [Ca2+]i were quantified to represent Ca2+ transported by the exchanger (Kimura et al., 1986; Näbauer and Morad, 1992).
Na+-Ca2+ exchange activity in cardiac membrane fraction.
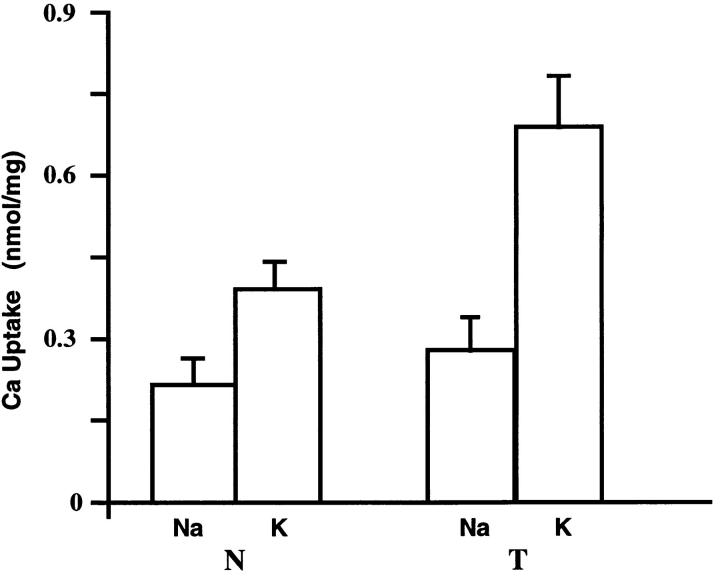
We prepared crude membrane fractions and assayed Na+ gradient-dependent 45Ca2+ uptake. Results obtained using hearts from transgenic mouse line H are shown in Fig. 5. Na+-Ca2+ exchange activity is 148% higher in vesicles from transgenic hearts than in vesicles from the hearts of control littermates. The level of overexpression in vesicles from transgenic hearts from other mouse lines averaged about 100%, though due to scatter, it was not clear if there were significant differences. The level of overexpression as assessed electrophysiologically from line H mice (see Table II) appears to be somewhat larger than that measured in vitro by isotope flux.
Figure 5.
Na+-Ca2+ exchange activity in isolated membranes. 45Ca2+ uptake into Na+-loaded membrane vesicles was determined in the presence (K+) or absence (Na+) of an outwardly directed Na+ gradient. The external uptake media in these cases contained KCl or NaCl, respectively. The difference in 45Ca2+ uptake from the K+ or Na+ media is taken as Na+-Ca2+exchange activity. Vesicles were prepared from the hearts of transgenic ( T ) or nontransgenic littermates (N). Media contained 10 μM Ca2+ and the uptake period was 3 s. n = 3 and error bars represent the S.D.
Table II.
Caffeine-induced Na-Ca Exchange Current and Cai-transient at Holding Potential of −90 mV in Ventricular Myocytes from Nontransgenic (Control) and Transgenic Mice
| INa-Ca Density (pA/pF) | τINa-Ca-decay (ms) | Δ[Ca2+]i (nM) | τCai-decay (ms) | [Ca2+]i/dt (μM/s) | Capacitance (pF) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| control | 1.61 ± 0.16(14) | 304 ± 23 (9) | 410 ± 26(14) | 1,186 ± 282(12) | 4.8 ± 0.4(14) | 206 ± 11(14) | ||||||
| transgenic | 4.96 ± 0.63*(16) | 126 ± 13*(10) | 437 ± 31(16) | 486 ± 54*(16) | 5.1 ± 0.4(16) | 213 ± 9.0(16) | ||||||
| control | 1.32 ± 0.22 (3) | 388 ± 19 (3) | 764 ± 131 (3) | 5.1 ± 0.6 (3) | 1.88 ± 19 (3) | |||||||
| control + Ni2+ | 0.08 ± 0.08 (3) | 458 ± 13‡ (3) | 3,511 ± 1659 (3) | 4.2 ± 0.3 (3) | ||||||||
| transgenic | 5.52 ± 2.07 (4) | 422 ± 48 (4) | 457 ± 113 (4) | 6.2 ± 1.1 (4) | 206 ± 8 (4) | |||||||
| transgenic + Ni2+ | 0.13 ± 0.07 (4) | 698 ± 125§ (4) | 6,278 ± 1375§ (4) | 8.3 ± 1.2§ ‖ (4) |
Data are represented as the mean ± SEM (number of experiments).
Significantly different from control at P < 0.05.
Significantly different from control at P < 0.05.
Significantly different from transgenic at P < 0.05.
Significantly different from control + Ni2+ at P < 0.05.
Activation of exchanger by caffeine and Ca2+ channel-induced Ca2+ release.
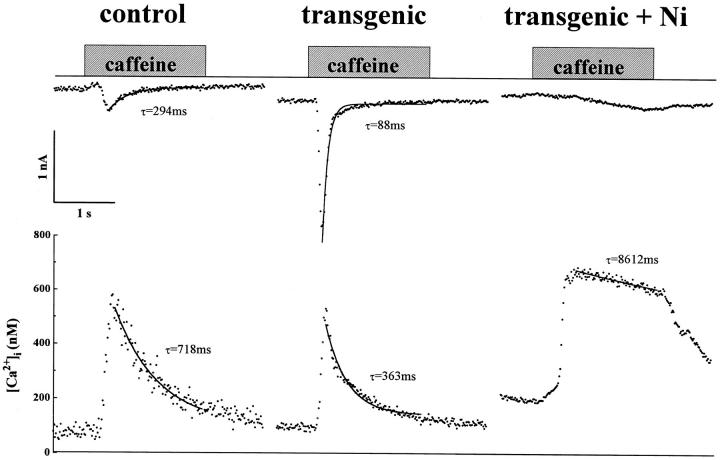
Fig. 6 compares the Ca2+ transients triggered by rapid (<50 ms) application of caffeine in two isolated whole-cell clamped ventricular myocytes dialyzed with 0.2 mM Fura-2. In control mice, 5 mM caffeine triggered a Cai-transient and a slowly activating exchanger current of about 250 pA decaying with a time constant of 300 ms. In transgenic myocytes, although the caffeine-triggered Cai-transient was similar in magnitude, the accompanying exchanger current was three times larger than in control mice. In addition, the kinetics of decay of both the exchanger current and Cai-transients in transgenic myocytes were two to threefold faster than those of control myocytes. In control as well as transgenic myocytes, 5 mM Ni2+ effectively blocked the caffeine-induced INa-Ca but allowed the Cai-transient to develop. Fig. 6 (transgenic + Ni2+) shows that the strong suppression of INa-Ca by Ni2+ in transgenic mice is accompanied by a 20-fold reduction in the rate of decay of Ca2+ transients, confirming the prominent role of the exchanger in the Ca2+ efflux process. Comparison of the rates of decay of Cai-transients and INa-Ca suggests a two to threefold increase in the kinetics of Ca2+ extrusion in transgenic myocytes (Table II). In 14 control and 16 transgenic myocytes dialyzed with 100–200 μM Fura-2, the Ni2+-sensitive current (INa-Ca) averaged about 1.6 and 5.0 pA/pF, respectively (see Table II). Though the magnitude of Ca2+ release was not significantly different in control and transgenic myocytes, the exchanger activity was enhanced in transgenic myocytes by all three criteria tested: the magnitude of INa-Ca, the kinetics of INa-Ca, and the rate of decay of Cai-transients.
Figure 6.
Caffeine-induced Cai-transients and INa-Ca in control and transgenic myocytes. Na+-Ca2+ exchange current was activated by Ca2+-release from the SR triggered by rapid application of 5 mM caffeine at a holding potential of −90 mV. INa-Ca (top) and Ca2+ i -transient (bottom) recorded from a control myocyte. The timing of caffeine application is indicated by the shaded bar. Similar recordings from a transgenic myocyte in the absence and presence of 5 mM NiCl2 as indicated. Cell capacitance of control and transgenic myocytes were 257.9 pF and 239.7 pF, respectively. Fura-2 concentration was 0.2 mM. Data was obtained at room temperature (∼25°C).
Table II quantifies some of the parameters of INa-Ca and the accompanying Cai-transients in transgenic and control myocytes. The size of the caffeine-triggered Ca2+ pool was found to be equivalent in control and transgenic myocytes. Comparison of the caffeine-releasable pool in normal and transgenic mice in the presence of 5 mM Ni2+ revealed a somewhat larger caffeine-sensitive pool in transgenic. In addition, the rate of release of Ca2+ (an indirect indication of flux of Ca2+ through the ryanodine receptor), though equivalent in control and transgenic mice, was enhanced in the presence of Ni2+ only in transgenic myocytes (Table II). These findings suggest that the higher exchanger activity reduces the cytosolic Ca2+ concentrations very rapidly in transgenic mice. It is premature to suggest possible compensatory Ca2+ adaptive pathways, as we do not have direct data on the activity or regulation of SR and SL Ca2+ pumps, the ryanodine receptors, and, in particular, Ca2+ storing proteins such as calsequestrin in myocytes overexpressing the exchanger. Some indication as to the activity of SR Ca2+ pump in transgenic myocytes was obtained by comparing the rate of relaxation of Cai-transient, triggered by brief (50 ms) pulses of caffeine, in Na+-free solutions. Yao and Barry (personal communication) found no significant differences in the rate of relaxation of Ca2+-transients between control and transgenic myocytes. Although such studies are complicated by the rate of dissociation of Ca2+ from the dye, they nevertheless do not support compensatory enhancement of Ca2+ ATPase activity in transgenic myocytes. Compensatory responses in transgenic mice showing no phenotypic changes, as was the case here, are likely to be subtle and multifaceted. Upregulation of calsequestrin, for example, could serve as a possible mechanism by which the SR could maintain its Ca2+ load in the face of overexpression of exchanger protein. Such an idea is supported by a recent study that reports upregulation of Ca2+ release pools in transgenic mice overexpressing cardiac calsequestrin (Suzuki et al., 1997).
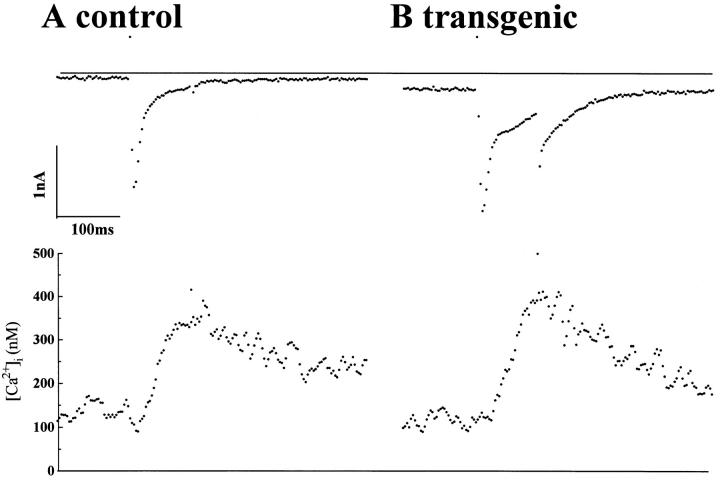
Fig. 7 illustrates ICa-gated Ca2+ release in two myocytes obtained from transgenic and non-transgenic littermates. In both cell types the magnitude of ICa and Cai-transients were similar (see also Table I). In transgenic cells, however, ICa measured between −20 and +20 mV was consistently followed by a slowly activating “transient inward current” during the rise of [Ca2+]i. Further, a slowly decaying tail current was observed on repolarization of the membrane. Both the “transient inward current” and the slowly decaying tail currents were abolished by depletion of SR Ca2+ stores by incubation of myocytes in thapsigargin or caffeine (data not shown). A similar (intracellular Ca2+ store-dependent) transient inward current and large slowly inactivating inward tail currents, abolished by replacement of extracellular Na+ with Li+, were also reported in myopathic hamster myocytes overexpressing the exchanger (Hatem et al., 1994).
Figure 7.
ICa-induced Ca2+ release activated “transient inward current” in transgenic but not in control myocytes. Whole cell ICa and Cai-transient were simultaneously recorded from nontransgenic (control, A) and transgenic (B) myocytes. Myocytes were dialyzed with 0.1 mM Fura-2 through patch pipette. ICa were activated by giving depolarizing pulses to −10 mV from a holding potential of −60 mV every 10 s. Cell capacitance in A was 151 pF and in B was 235 pF.
Measurement of Exchanger Current in the Ca2+ Influx Mode
Normal and transgenic myocytes dialyzed with 10 mM Na+ were depolarized to +60 or +80 mV to minimize the influx of Ca2+ through the Ca2+ channel and enhance Ca2+ influx via the exchanger.
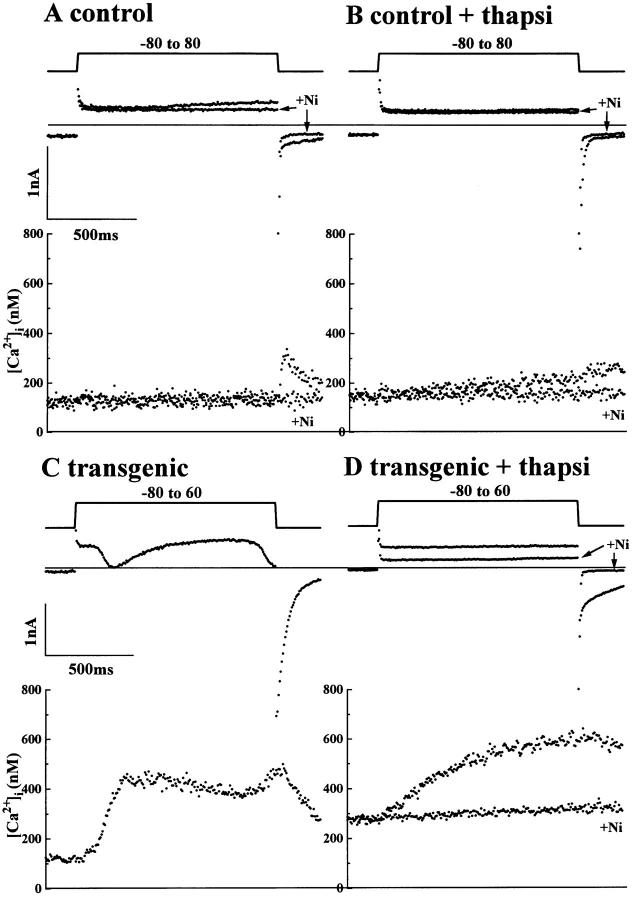
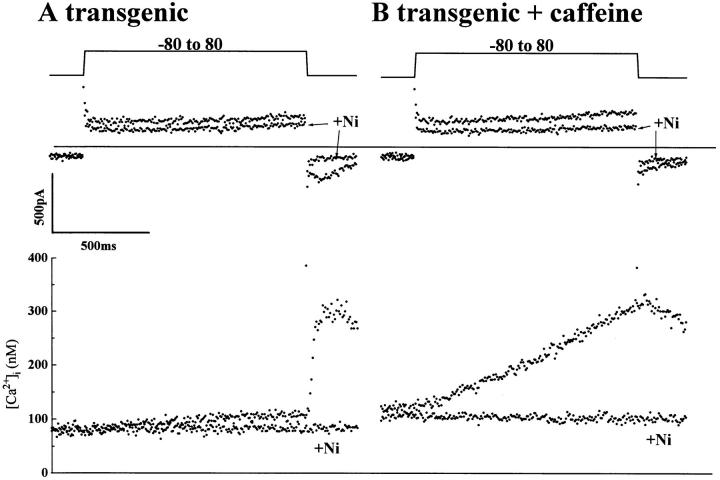
In myocytes from control mice (Fig. 8 A) a small Ni2+-sensitive INa-Ca consistently accompanied a small rise in [Ca2+]i in thapsigargin-treated myocytes (Fig. 8 B). Even though the rise in myoplasmic [Ca2+] induced by the exchanger was small in control myocytes (Fig. 8 A), repolarizing to −80 mV activated ICa “tails” triggering significant release of Ca2+. In thapsigargin-treated myocytes, the rise in [Ca2+]i in response to depolarization was somewhat larger than in control myocytes (Fig. 8 B), but Ca2+ release triggered by Ca2+ channel tail current was absent (compare Fig. 8 A with B). These results suggest that the small rise of [Ca2+]i induced by the influx of Ca2+ via the exchanger in control mice is blunted by the SR activity. In transgenic myocytes, however, depolarizing pulses to less positive voltages (+60 mV, Fig. 8, C and D) produced much larger rises in [Ca2+]i, often triggering Ca2+ release from the SR (Fig. 8 C) which activated a transient current in the inward direction at +60 mV, representing Ca2+ extrusion by the exchanger (Fig. 8 C). 6–8 min exposure of transgenic myocytes to 1.0 μM thapsigargin completely suppressed Ca2+ release and the inwardly directed transient current deflections (Fig. 8 D). Instead, intracellular Ca2+ slowly but continuously increased to values of 500–600 nM during the depolarizing pulses. 5 mM Ni2+ blocked INa-Ca, completely suppressed the rise in intracellular Ca2+, and abolished the slowly decaying exchanger-generated tail currents following the repolarization (Fig. 8 D).
Figure 8.
Cai-transients and INa-Ca currents in control and transgenic myocytes in the presence and absence of thapsigargin. The activity of Na+-Ca2+ exchanger activity in the Ca2+ influx mode was measured by applying depolarizing pulses to positive potentials near ECa. (A) Superimposed current traces (top) and the simultaneously measured [Ca2+]i (bottom) during the application of a depolarizing pulse to +80 mV in control myocyte in the presence and absence of 5 mM NiCl2. (B) Similar recordings obtained from the myocyte shown in A after treatment of myocyte with 1.0 μM thapsigargin. (C) Cai-transients and ICa measured at +60 mV in a transgenic myocyte overexpressing Na-Ca exchanger. (D) The superimposed recordings from the same transgenic myocyte as shown in C after treatment with 1.0 μM thapsigargin in the presence and absence of 5 mM NiCl2. Cell capacitance was 151 pF (A and B) and 228 pF (C and D). Myocytes were dialyzed with 0.2 mM Fura-2 concentration.
Thus, in sharp contrast to myocytes from control mice, the transgenic myocytes produce large and rapid rises in myoplasmic Ca2+ in response to activation of the Na+-Ca2+ exchanger. In 9 of 26 transgenic myocytes, the influx of Ca2+ via the Na+-Ca2+ exchanger caused rapid rise in myoplasmic Ca2+ (SR Ca2+ release) at +60 to +80 mV, the rate of which was strongly suppressed by caffeine. In 11 myocytes significant rise of [Ca2+]i was observed by depolarization without triggering Ca2+ release. In the remaining 6 myocytes, the large depolarizations failed to cause significant rise of [Ca2+]i even though large caffeine-induced INa-Ca was recorded. In contrast to transgenic myocytes, in only 5 of 15 control myocytes, significant rise of [Ca2+]i was observed upon application of large and long depolarizations. In the other 10 myocytes no significant change of [Ca2+]i could be observed at all, and in none of the 15 cells could we trigger Ca2+ release from the SR even at +80 mV.
Fig. 9 shows that Ca2+ influx via the exchanger is significantly blunted by a functional SR even in transgenic mice. In this myocyte, depolarization from −80 to 80 mV caused only slight increase in [Ca2+]i, although repolarization from 80 to −80 mV triggered Ca2+ release via Ca2+-influx through the deactivating L-type Ca2+ channels. Ni2+ at 5 mM concentration blocked both Ca2+ influx transported via the exchanger and the Ca2+ channels. The same myocyte, treated with 5 mM caffeine however, showed significant rise in [Ca2+]i during the pulse to +80 mV (Fig. 9 B). This large rise of [Ca2+]i was completely suppressed by 5 mM Ni2+.
Figure 9.
Enhancement of Ca2+ influx by Na+-Ca2+ exchanger after impairing the Ca2+ storage capacity of the SR by caffeine. (A) Exchanger-dependent currents and rise in [Ca2+]i in transgenic myocyte were elicited by depolarization from −80 to 80 mV. In this myocyte, large depolarization did not cause significant rise in [Ca2+]i, although repolarization from 80 to −80 mV triggered ICa-dependent Ca2+ release, which, in turn, activated INa-Ca inward current. (B) Membrane currents and changes in [Ca2+]i recorded for the same transgenic myocyte (shown in A) in the presence of 5 mM caffeine. The intracellular rise in Ca2+ with depolarization was significantly higher in the presence of caffeine and repolarization no longer triggered Ca2+-release because SR Ca2+ pools were depleted in presence of caffeine. Cell capacitance in A and B was 214 pF. Fura-2 concentration in the patch pipette was 0.2 mM.
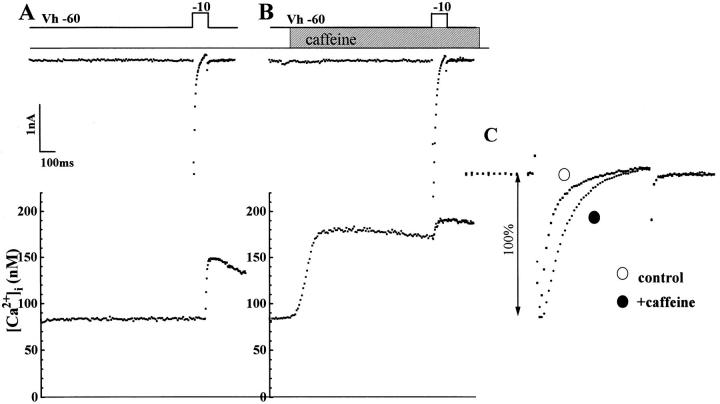
Relative Contributions of the Na+-Ca2+ Exchanger and Ca2+ Channel in Signaling of Ca2+ Release in Transgenic Mice
Fig. 10 examines the ability of the exchanger and Ca2+ channel to detect local rises in [Ca2+]i in transgenic myocytes dialyzed with 2 mM Fura 2 (high concentrations of Ca2+ buffer were used to prevent significant rise in global cytoplasmic Ca2+ concentrations; Adachi-Akahane et al., 1996; Sham et al., 1995a ). The experiment was designed to induce Ca2+ release first by caffeine and then by ICa. Thus it was possible to measure almost simultaneously the magnitude of INa-Ca activated by caffeine, or ICa activated by depolarization, and their respective Cai-transients. Although 2 mM Fura-2 completely suppressed the activation of caffeine-induced INa-Ca, it did not suppress cross signaling between DHP and ryanodine receptors (Fig. 10 B). Fig. 10 B also shows that ICa, activated following the depletion of Ca2+ pools by caffeine, was somewhat larger, triggered a small Cai-transient, and inactivated significantly more slowly (Fig. 10 C ). These results were similar to those observed in control mice myocytes and suggest that even though the density of the exchanger current is strongly enhanced in transgenic mice, the exchanger remains more susceptible to cytoplasmic Ca2+-buffering than the Ca2+ channel. Thus, in the presence of 2 mM Fura-2, the Ca2+ channel continues to signal Ca2+ release (Fig. 10 A), and is in turn regulated by Ca2+ released via the ryanodine receptors (Fig. 10 C ). We conclude that increasing the density of INa-Ca by at least threefold does not give the exchanger the type of access to the ryanodine receptor as that of the Ca2+ channel.
Figure 10.
Dialysis of transgenic myocytes with high concentrations of Ca2+ buffer does not block cross signaling between Ca2+ channel and ryanodine receptor but blocks activation of exchanger with caffeine-induced Ca2+ release. Ca2+ release by caffeine (5 mM) did not activate inward Na+-Ca2+ exchange current at −90 mV (middle, in a cell dialyzed with 2 mM Fura-2) but altered the rate of inactivation of ICa in panel A (normalized Ca2+ current tracings before and after 600-ms exposure to caffeine are compared in panel C ). Depletion of SR Ca2+ pools by caffeine slowed the inactivation kinetics of ICa even though the rise in [Ca2+]i failed to activate INa-Ca. Cell capacitance was 178.1 pF.
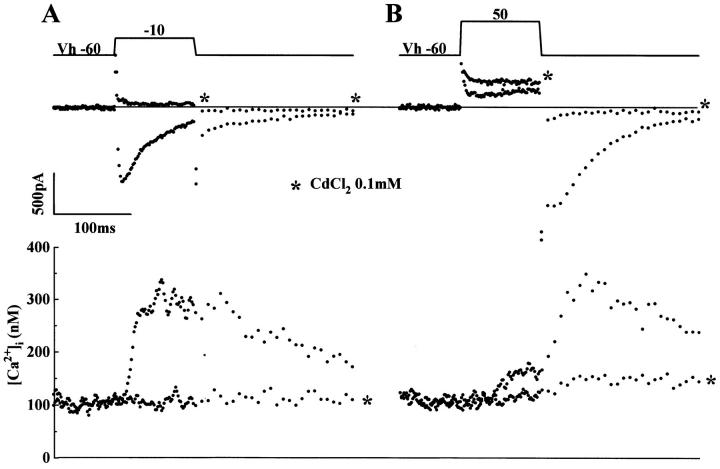
Fig. 11 illustrates an experimental protocol to determine the fractional contribution of the Ca2+ channel and the exchanger in signaling Ca2+ release from the SR in transgenic myocytes dialyzed with low concentrations of Ca2+ buffer (0.1 mM Fura 2). Transgenic myocytes were voltage clamped at 10-s intervals, from −60 to −10 mV and from −60 to +50 mV in control solutions and following 10–20-s application of 0.1 mM Cd2+. Fig. 11 A shows that in control solution activation of Ca2+ currents triggers Ca2+ release in transgenic myocytes and that rapid application of Cd2+ blocks ICa and the rise in [Ca2+]i (traces marked with *). In panel B, the cell is clamped to +50 mV activating an outward current accompanied by a small rise in [Ca2+]i. Upon repolarization, a slowly decaying inward INa-Ca is measured following the triggering of Ca2+ release by the Ca2+ channel tail current. The traces marked (by *) were obtained in the presence of 0.1 mM Cd2+ and show that Cd2+ blocked the Ca2+ channel tail current, the rapid rise in [Ca2+]i, and the accompanying INa-Ca. This is similar to findings in normal mice myocytes (not shown) and reported previously in rat heart (Sham et al., 1992). Thus it appears that the Ca2+ channel, even in myocytes overexpressing the exchanger, remains the primary pathway for gating the ryanodine receptor.
Figure 11.
Cd2+ blocks Ca2+ release by blocking ICa at −10 and +50 mV in transgenic myocytes. Membrane currents and Cai-transients activated by depolarizing pulses in transgenic myocytes dialyzed with 0.2 mM Fura-2 were recorded at 10-s intervals before and following addition of 0.1 mM Cd2+(*). ICa, tail current, and Ca2+ i transient activated by test pulses from −60 to −10 mV (A) or to 50 mV (B) were abolished within a few 100 ms by 100 μM Cd2+. Cd2+ increased the net membrane in the outward direction (*) at +50 mV and blocked the Ca2+ transient. The current traces were not leak-subtracted, so as not to suppress the approximately linear components of INa-Ca. Cell capacitance was 228 pF. Fura-2 concentration in dialyzing patch pipette was 0.2 mM.
discussion
The main finding of this report is that we have succeeded in producing transgenic mice that overexpress the canine sarcolemmal Na+-Ca2+ exchanger (NCX1) specifically in heart tissue. The report provides both biochemical and functional evidence for overexpression in ventricular myocytes. The Na-Ca exchange current density in transgenic mice myocytes was three times higher, on the average, compared to that measured in control myocytes (Table II). Overexpression of the exchanger did not significantly alter the Ca2+ content of Ca2+-release pools or enhance the contribution of Na+-Ca2+ exchanger to the Ca2+ release process. Overexpression of the exchanger did, however, accelerate the rate of removal of Ca2+ from the cytosol, when SR uptake was impaired by caffeine. Even though overexpression of the exchanger failed to trigger Ca2+ release at physiological membrane potentials, the exchanger did appear to blunt the rate of Ca2+ release gated by ICa in transgenic myocytes.
Consequences of Overexpression of the Exchanger
Since the exchanger is a major pathway for Ca2+ extrusion from the cytosol, it may be expected that its overexpression would reduce the Ca2+ content of the SR. Table II clearly shows that there was no significant decrease in Ca2+ content of SR as assessed from the magnitude of caffeine-induced Ca2+ release (Δ[Cai], column 3). Such a finding is consistent with the absence of significant changes in cardiovascular phenotypic parameters (e.g., heart rate and blood pressure) in transgenic mice overexpressing exchange activity (H. Rockman, personal communication). Table I, also, shows that there were no significant differences in the density of Ca2+ channel current in control vs. the transgenic myocytes. This finding supports the observation of a recent report (Silverman et al., 1995) that the duration of the action potential in these transgenic myocytes does not change significantly at 50% duration, a period where ICa may be the predominant inward current. Prolongation of the action potential measured at 90% of its duration reported in the same study may have been caused by the larger inward exchanger current (see Fig. 7).
Overexpression of the Exchanger and Ca2+ Microdomains of DHP/Ryanodine Receptors
Recent data using high concentrations of Ca2+ buffers suggests that Ca2+ signaling in cardiac muscle occurs via microdomains of Ca2+ (Sham et al., 1995a ; Adachi-Akahane et al., 1996). Fig. 10 shows that the release of Ca2+ by rapid application of caffeine in transgenic myocytes failed to activate the inward exchanger current in the presence of 2 mM Fura-2 (e.g. Fig. 6), even though the kinetics of inactivation of ICa were markedly altered after the release of Ca2+ from the SR. One possible interpretation is that the release of Ca2+ from the ryanodine receptor is effectively buffered by 2 mM Fura-2, placing the exchanger at microdomains outside of those surrounding DHP and the ryanodine receptor. The differential sensitivity of the exchanger for Ca2+ transport and the Ca2+ channel to Ca2+-induced inactivation, however, may also contribute to the data of Fig. 10. The sensitivity of Ca2+ sites on the two proteins, however, suggests about 5 μM affinity for the Ca2+ transport site of the exchanger (Matsuoka and Hilgemann, 1992) vs. much higher Ca2+ for Ca2+ channel inactivation (10–15 μM Haack and Rosenberg, 1994; and 50–100 μM Morad et al., 1988). Thus the failure to activate INa-Ca while strongly modulating the kinetics of inactivation of Ca2+ channel is more consistent with the idea that the exchanger remains excluded from the Ca2+ microdomains surrounding the DHP/ryanodine receptor complex, even in the transgenic mice.
Physiological Role of the Exchanger in Transgenic Myocytes
One reason for developing these transgenic mice was to enhance the exchanger activity in the Ca2+-influx mode. The activity of the exchanger was enhanced in most myocytes to levels where the density of current carried by the exchanger in the Ca2+ efflux mode was about 5 pA/pF, compared to 1.6 pA/pF in control myocytes (see Table II). Assuming that a similar increase in the activity of the exchanger takes place at positive potentials (Ca2+ influx mode of the exchanger), densities of current equivalent to those of Ca2+ current may be generated by the exchanger.
Ca2+ influx via the exchanger when activated by large and long depolarizing pulses did trigger Ca2+ release in 9 out of 26 transgenic myocytes. However, ∼100–300 ms were required for the exchanger to activate the Ca2+-induced Ca2+ release mechanism (Fig. 8). In part, because of relatively low capacity of the exchanger versus that of the SR Ca2+ pump (which may prevent significant accumulation of cytosolic Ca2+), the exchanger fails to trigger Ca2+ release on beat-to-beat basis, especially at high mouse heart rates (∼6 Hz). In the physiological range of membrane potentials, −10 to +20 mV, we consistently failed to produce sufficient influx of Ca2+ via the exchanger to trigger Ca2+ release in transgenic myocytes dialyzed with 0.1 mM Fura 2 (Fig. 11), even though Ca2+ release triggered by Ca2+ current induced a large inward exchanger current (Fig. 7). Even though the exchanger may not have direct access to Ca2+ microdomains of the DHP/ryanodine receptor complex even when overexpressed, the finding that Ca2+ release in transgenic myocytes was significantly enhanced when the exchanger was blocked by Ni2+ (Table II), places the exchanger within distances close enough to the ryanodine receptor to blunt the Ca2+-induced Ca2+-release process. Thus, the overexpressed exchanger appears to produce functional consequences in the Ca2+ efflux, but not in the Ca2+ influx mode.
Acknowledgments
We thank Dr. D. Nicoll for assistance with molecular biology and Dr. H. Cheroutre and K. Williams (UCLA) for producing the transgenic mice. We thank Dr. Lars Cleemann for many useful discussions with measurements of Fura-2.
This work was supported by National Institutes of Health grant HL48509 and Laubisch Fund to K. Philipson and HL16152 to M. Morad.
Footnotes
A preliminary report of this work has been previously published in abstract form (Adachi-Akahane, S., K.D. Philipson, and M. Morad. 1996. Biophys. J. 70:A270).
Satomi Adachi-Akahane's present address is Department of Toxicology and Pharmacology, Faculty of Pharmaceutical Sciences, University of Tokyo, Tokyo, Japan.
Abbreviations used in this paper: α-MHC, α-myosin heavy chain; SR, sarcoplasmic reticulum.
references
- Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G. Excitation-contraction coupling in mammalian cardiac cells. Cardiovasc Res. 1992;26:923–932. doi: 10.1093/cvr/26.10.923. [DOI] [PubMed] [Google Scholar]
- Callewaert G, Cleemann L, Morad M. Caffeine-induced Ca2+-release activates Ca2+ extrusion via Na+-Ca2+exchanger in cardiac myocytes. Am J Physiol. 1989;257:C147–152. doi: 10.1152/ajpcell.1989.257.1.C147. [DOI] [PubMed] [Google Scholar]
- Chen F, Mottino G, Klitzner TS, Philipson KD, Frank JS. Distribution of the Na+/Ca2+exchange protein in developing rabbit myocytes. Am J Physiol. 1995;268:C1126–C1132. doi: 10.1152/ajpcell.1995.268.5.C1126. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleemann L, Morad M. Role of Ca2+ channel in cardiac excitation-contraction coupling in the rat: evidence from Ca2+transients and contraction. J Physiol. 1991;432:283–312. doi: 10.1113/jphysiol.1991.sp018385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleemann, L., and M. Morad. 1992. Fura-2 measurements of intracellular calcium in single rat ventricular myocytes. In Quantitative Spectroscopy in Tissue. K. Frank and M. Kessler. PMI Verlaggruppe, Frankfurt am Main. 141–154.
- Frank JS, Mottino G, Reid D, Molday RS, Philipson KD. Distribution of the Na+Ca2+exchange protein in mammalian cardiac myocytes: an immunofluorescence and immunocollodial gold-labeling study. J Cell Biol. 1992;117:337–345. doi: 10.1083/jcb.117.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Gulick J, Subramaniam A, Neumann J, Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- Haack JA, Rosenberg RL. Calcium-dependent inactivation of L-type calcium channels in planar lipid bilayers. Biophys J. 1994;66:1051–1060. doi: 10.1016/S0006-3495(94)80886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammil OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflüg Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hatem SN, Sham JS, Morad M. Enhanced Na+-Ca2+exchange activity in cardiomyopathic Syrian hamster. Circ Res. 1994;74:253–261. doi: 10.1161/01.res.74.2.253. [DOI] [PubMed] [Google Scholar]
- Hryshko LV, Nicoll DA, Weiss JN, Philipson KD. Biosynthesis and initial processing of the cardiac sarcolemmal Na+-Ca2+exchanger. Biochim Biophys Acta. 1993;1151:35–42. doi: 10.1016/0005-2736(93)90068-b. [DOI] [PubMed] [Google Scholar]
- Kimura J, Noma A, Irisawa H. Na-Ca exchange current in mammalian heart cells. Nature (Lond) 1986;319:596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- Kieval RS, Bloch RJ, Lindenmayer GE, Ambesi A, Lederer WJ. Immunofluorescence localization of the Na-Ca exchanger in heart cells. Am J Physiol. 1992;263:C545–C550. doi: 10.1152/ajpcell.1992.263.2.C545. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Rockman HA, Samama P, Hamilton R, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the β-adrenergic receptor kinase or a βARK inhibitor. Science (Wash DC) 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Lederer WJ, Schulze DH. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J Biol Chem. 1994;269:5145–5149. [PubMed] [Google Scholar]
- Levesque PC, Leblanc N, Hume JR. Release of calcium from guinea pig cardiac sarcoplasmic reticulum induced by sodium-calcium exchange. Cardiovasc Res. 1994;28:370–378. doi: 10.1093/cvr/28.3.370. [DOI] [PubMed] [Google Scholar]
- Li Z, Matsuoka S, Hryshko LV, Nicoll DA, Bersohn MM, Burke EP, Lifton RP, Philipson KD. Cloning of the NCX2 isoform of the plasma membrane Na+-Ca2+exchanger. J Biol Chem. 1994;269:17434–17439. [PubMed] [Google Scholar]
- Li Z, Nicoll DA, Collins A, Hilgemann DW, Filoteo AG, Penniston JT, Weiss JN, Tomich JM, Philipson KD. Identification of a peptide inhibitor of the cardiac sarcolemmal Na+-Ca2+exchanger. J Biol Chem. 1991;266:1014–1020. [PubMed] [Google Scholar]
- Lipp P, Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol (Lond) 1994;474:439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Schwaller B, Niggli E. Specific inhibition of Na-Ca exchange function by antisense oligodeoxynucleotides. FEBS Lett. 1995;364:198–202. doi: 10.1016/0014-5793(95)00391-l. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Hilgemann D. Steady-state and dynamic properties of cardiac sodium calcium exchanger. J Gen Physiol. 1992;100:963–1001. doi: 10.1085/jgp.100.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science (Wash DC) 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Morad M, Davies NW, Kaplan JH, Lux HD. Inactivation and block of Ca2+ channel by photo-released Ca2+in dorsal root ganglion neurons. Science (Wash DC) 1988;241:842–844. doi: 10.1126/science.2457253. [DOI] [PubMed] [Google Scholar]
- Näbauer M, Morad M. Modulation of contraction by intracellular Na+ via Na+-Ca2+exchange in single shark (Squalus Acanthias) ventricular myocytes. J Physiol (Lond) 1992;457:627–637. doi: 10.1113/jphysiol.1992.sp019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD. Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+exchanger. Science (Wash DC) 1990;250:562–565. doi: 10.1126/science.1700476. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Sandgren EP, Avarbock MR, Allen DD, Brinster RL. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci USA. 1991;88:478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson KD, Longoni S, Ward R. Purification of the cardiac Na+-Ca2+exchange protein. Biochim Biophys Acta. 1988;945:298–306. doi: 10.1016/0005-2736(88)90492-0. [DOI] [PubMed] [Google Scholar]
- Schubert B, VanDongen AMJ, Kirsch GE, Brown AM. β-Adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science (Wash DC) 1989;245:516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Sham JSK, Cleemann L, Morad M. Gating of the cardiac Ca2+ release channel: The role of Na+ current and Na+-Ca2+exchange. Science (Wash DC) 1992;255:850–853. doi: 10.1126/science.1311127. [DOI] [PubMed] [Google Scholar]
- Sham JSK, Cleemann L, Morad M. Functional coupling of Ca2+channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci USA. 1995a;92:121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham JSK, Hatem SN, Morad M. Species differences in the activity of the Na+Ca2+exchanger in mammalian cardiac myocytes. J Physiol (Lond) 1995b;488:623–631. doi: 10.1113/jphysiol.1995.sp020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidzinski MK, Juhaszova M, Blaustein MP. Antisense inhibition of Na+Ca2+exchange in primary cultured arterial myocytes. Am J Physiol. 1995;752:C1340–C1345. doi: 10.1152/ajpcell.1995.269.5.C1340. [DOI] [PubMed] [Google Scholar]
- Silverman, H.S., M.C.P. Haigney, E.J. Griffiths, S.K. Wei, C.J. Ocampo, K.D. Philipson, and M.D. Stern. 1995. Excitation-contraction coupling in ventriculocytes from transgenic mice with high level expression of canine sarcolemmal sodium-calcium exchanger. Circulation. 92:(Suppl.)I–236.
- Soonpaa MH, Koh YG, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science (Wash DC) 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- Subramaniam A, Jones KW, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the α-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- Suzuki YJ, Kobayashi M, Morad M, Jones L. Excitation-contraction coupling in transgenic myocytes overexpressing cardiac calsequestrin. Biophys J. 1997;72:A234. [Google Scholar]