Abstract
The epithelial sodium channel is a multimeric protein formed by three homologous subunits: α, β, and γ; each subunit contains only two transmembrane domains. The level of expression of each of the subunits is markedly different in various Na+ absorbing epithelia raising the possibility that channels with different subunit composition can function in vivo. We have examined the functional properties of channels formed by the association of α with β and of α with γ in the Xenopus oocyte expression system using two-microelectrode voltage clamp and patch-clamp techniques. We found that αβ channels differ from αγ channels in the following functional properties: (a) αβ channels expressed larger Na+ than Li+ currents (INa+/ILi+ 1.2) whereas αγ channels expressed smaller Na+ than Li+ currents (INa+/ILi+ 0.55); (b) the Michaelis Menten constants (K m) of activation of current by increasing concentrations of external Na+ and Li+ of αβ channels were larger (K m > 180 mM) than those of αγ channels (K m of 35 and 50 mM, respectively); (c) single channel conductances of αβ channels (5.1 pS for Na+ and 4.2 pS for Li+) were smaller than those of αγ channels (6.5 pS for Na+ and 10.8 pS for Li+); (d) the half-inhibition constant (K i) of amiloride was 20-fold larger for αβ channels than for αγ channels whereas the K i of guanidinium was equal for both αβ and αγ. To identify the domains in the channel subunits involved in amiloride binding, we constructed several chimeras that contained the amino terminus of the γ subunit and the carboxy terminus of the β subunit. A stretch of 15 amino acids, immediately before the second transmembrane domain of the β subunit, was identified as the domain conferring lower amiloride affinity to the αβ channels. We provide evidence for the existence of two distinct binding sites for the amiloride molecule: one for the guanidium moiety and another for the pyrazine ring. At least two subunits α with β or γ contribute to these binding sites. Finally, we show that the most likely stoichiometry of αβ and αγ channels is 1α:1β and 1α:1γ, respectively.
Keywords: epithelial Na+ channel, amiloride, guanidinium, Xenopus oocyte
introduction
Epithelial sodium channels (ENaC)1 mediate sodium reabsorption in a variety of tissues (for review, see Garty and Benos, 1988; Rossier et al., 1994). Na+ channels are located in the apical membranes of epithelia such as the distal nephron and colon, the ducts of salivary and sweat glands, and the respiratory tract. Molecular cloning of ENaC has revealed that the channel is formed by three homologous subunits, α, β, and γ (Canessa et al., 1994), that share 34–37% identity at the amino acid level. Expression of the three subunits in a heterologous system such as Xenopus oocytes reconstitutes channels with properties similar to those present in native tissues: high selectivity of sodium over potassium (PNa+/PK+ > 20), half-inhibition constant (K i) of amiloride of 0.10 μM, and only a slight voltage dependence of the open probability (Hamilton and Eaton, 1985; Palmer and Frindt, 1986; Canessa et al., 1994). Canessa et al. (1994) have previously shown that co- expression of α, β, and γ subunits is necessary to induce maximal levels of channel activity. Single subunits produce amiloride-sensitive currents of small magnitude (α subunit expressed alone induces only 1% of the maximal whole-cell current) or no current at all (β or γ expressed alone or co-expression of the two subunits do not induce any current). However, co-expression of α and β subunits or of α and γ subunits results in about 15–20% of the maximal magnitude of amiloride-sensitive whole cell current.
The total number of subunits and the stoichiometry of the subunits in ENaC have not yet been determined. Additionally, it is not known whether the subunit composition can be varied to give channels with different functional properties. The physiological relevance of channels of different subunit composition is supported by the observation that not all Na+ absorbing epithelia express the three subunits of ENaC. In the lung there is a heterogeneity of expression with some cells having only α and γ subunits (Farman et al., 1997); in colonic epithelia, in the absence of stimulation by aldosterone, only the α subunit is expressed (Lingueglia et al., 1994; Asher et al., 1997). These findings raise the possibility that, at least in certain tissues, only one or two ENaC subunits may be sufficient to form functional sodium channels in vivo.
Because little is known about the properties of channels that consist of only two of the ENaC subunits, we describe the expression and functional properties of channels formed by the combination of α and β and of α and γ subunits. We found that αβ and αγ channels differ in sensitivity to the blocker amiloride, K m values for activation by external Na+ and Li+, and single channel conductances. Maximal currents were observed when the cRNAs injected of α and β, and α and γ were of 1:1 ratio; large variations from this ratio decreased the magnitude of the amiloride-sensitive currents, but the distinct functional properties of the αβ and αγ channels were maintained suggesting that the most likely stoichiometry of αβ and αγ channels is 1α:1β and 1α:1γ, respectively. In addition, we identified a short stretch of amino acids before the second transmembrane domain that determines amiloride affinity. We discuss a model that describes the presence of at least two distinct binding sites for the amiloride molecule, one site for the guanidinium moiety and another for the pyrazine ring.
methods
Expression of Sodium Channels in Oocytes
cDNAs were propagated in the transcription-competent vector pSD-5 in Escherichia coli DH5α. Capped cRNAs were transcribed in vitro from linearized cDNAs using SP6 RNA polymerase. Stage V-VI Xenopus oocytes were isolated by partial ovariectomy under tricaine anesthesia and then defolliculated by treatment with 1 mg/ml collagenase (Type 1A; Sigma Chemical Co., St. Louis, MO) in zero Ca2+ modified Barth's solution (MBS), containing in mmol/liter: 85 NaCl, 2.4 NaHCO3, 1 KCl, 0.8 MgSO4, 0.3 Ca(NO3), 0.4 CaCl2, and 5 HEPES (buffered to pH 7.2) for 1 h at room temperature. 2–24 h after defolliculation, oocytes were injected with 1–3 ng of each cRNA in a final volume of 50 nl. The amount of injected cRNA was changed to obtain five conditions with different ratios of α and β, or of α and γ cRNAs. Condition 10α:1β received 3 ng of α and 0.3 ng of β; condition 5α:1β received 3 ng of α and 0.6 ng of β; condition 1α:1β received 3 ng each of α and β; condition 1α:5β received 0.6 ng of α and 3 ng of β, and condition 1α:10β received 0.3 ng of α and 3 ng of β. Similar experimental protocols were used for the five corresponding αγ conditions. In experiments where the βα-dimer was injected, oocytes received 1 ng of βα-dimer cRNA alone or in addition to 1, 2, or 3 ng of α cRNA or 1, 2, or 3 ng of β cRNA. In experiments where the αγ-dimer was injected, oocytes received 1 ng of αγ-dimer cRNA alone or in addition to 1, 2, or 3 ng of α cRNA or 1, 2, or 3 ng of γ cRNA. Oocytes were kept in MBS with 1.8 mM Ca2+, supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) for 3–5 d at 19°C before experiments.
Electrophysiology
Oocyte whole-cell currents were measured using the two-microelectrode voltage clamp technique (Oocyte Clamp C-725B; Warner Instrument Corp., Hamden, CT). Epithelial sodium channel currents were calculated as the difference in whole-cell current before and after the addition of 100 μM amiloride to the bathing solution. The bath solution contained in mmol/liter: 1.8 CaCl2; 2 MgCl2; 4 KCl; 5 BaCl2; 5 HEPES, buffered to pH 7.2, and 0–150 mM Na+, Li+, or K+ gluconate. NMDG-gluconate (N -methyl- d-glucamine) was used to make the total concentration of cation-gluconate 150 mM. Pulse software (HEKA Elektronik, Lambrecht, Germany) was used to generate voltage pulses. To obtain I-V curves, the membrane potential was changed from −180 to 80 mV by 20-mV incremental steps of 200-ms duration. Data were collected and analyzed with a Power Macintosh computer.
The K ms for external Na+ and Li+ were calculated from the amiloride-sensitive component of whole-cell currents (I) induced by varying the concentration (C) of Na+ or Li+ in the bath solution from 0 to 150 mM. The data were fitted to the Michaelis-Menten equation:
 |
1 |
where I max is the maximal current and K m is the half-activation constant using a nonlinear fit program based on the simplex method.
Dose-response curves for the blockers amiloride, benzamil, and guanidinium were obtained by measuring the change in current induced by adding increasing concentrations of blocker into the bath solution. The data were fitted to the equation:
 |
2 |
where A is the concentration of the blocker and K i is the inhibition constant. To assess the voltage dependence of amiloride block, the K i was measured at various membrane holding potentials ranging from −180 to 50 mV. As indicated, in certain experiments the K i of amiloride was measured in the presence of 150 or 30 mM Na+ in the bathing solution. Results are expressed as mean ± standard error (SEM), n = number of observations.
Patch Clamp Technique
Single channel recordings were obtained from membrane patches from Xenopus oocytes (Methfessel et al., 1986) injected with either (α, β, and γ), (α and γ), or (α and β) rat ENaC cRNAs. Recording pipettes were constructed from borosilicate glass capillaries (Dagan Corp., Minneapolis, MN) using a Narishige PP83 microelectrode puller (Narishige Scientific Instrument Laboratory, Tokyo, Japan) and were not fire polished. The pipettes had tip resistances of 2–5 MΩ and were partially filled with solutions containing, in mmol/liter: 150 NaCl or LiCl; 1 CaCl2; 1 MgCl2; 5 HEPES, buffered to a final pH of 7.4. Bath solutions contained, in mmol/liter: 150 NaCl or LiCl; 5 EDTA; 5 HEPES, buffered to a final pH of 7.4. Experiments were performed at room temperature (∼20–22°C).
Single channel currents were recorded from cell-attached patches with a patch clamp amplifier (Model EPC-7; List Electronics, Darmstadt, Germany, or Model PC-505; Warner Instrument Corp.). The signal was low pass filtered at 500 Hz with an 8-pole bessel filter and stored on video tape after pulse code modulation (Model PCM-501ES; Sony, Tokyo, Japan). For analysis, data were re-digitized (2 kHz), transferred to a PC, and analyzed using the pCLAMP6 (Axon Instruments Inc., Burlingame, CA) software system. Data were then filtered at 30–100 Hz for analysis and display. Single channel conductance was estimated with linear regression analysis from currents measured from −100 and −40 mV.
Construction of cDNAs
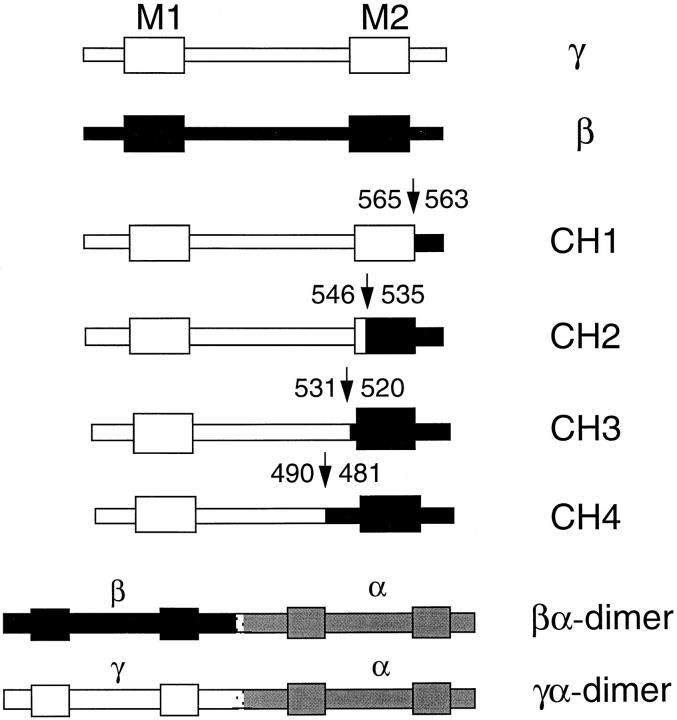
Chimeras of γ and β rat ENaC cDNAs were generated by a two-step PCR protocol. CH1 was constructed by amplification of wild-type γ template using a complementary sense primer 5′CATCTACAACGCTGCC3′ and an antisense γ-β hybrid primer 5′CCGCCTCCTGCGGGCGATGATAGAGAAG3′. The γ-β hybrid antisense primer has the 3′ half of the sequence complementary to γ and the 5′ half complementary to β sequences. The amplification protocol consisted of 20 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 45 s. 1 μl of the first PCR product was mixed with wild-type β template for the second PCR amplification, the sense primer of the first PCR reaction, and an antisense primer complementary to β 5′AATATTCTGGTACCAGC3′. The amplification protocol consisted of 25 cycles of 94°C for 45 s, 55°C for 50 s, and 72°C for 50 s. CH2 was constructed by amplification of γ template with a complementary sense primer 5′CATCTACAACGCTGCC3′ and an antisense γ-β hybrid 5′GATCTCCCCAAACTCAATGACACAGACGAC3′. 1 μl of the first PCR reaction was mixed with β template in a second amplification that contained the sense primer of the first reaction and a specific antisense β primer 5′AATATTCTGGTACCAGC3′. CH3 was constructed by amplification of γ template using a complementary sense primer 5′CATCTACAACGCTGCC3′ and an antisense γ-β hybrid primer 5′GGCCACCCAGGTTGGACAGGAGCATCTC3′. The second PCR amplification used the first PCR sense primer and an antisense primer complementary to β 5′AATATTCTGGTACCAGC3′. For the first PCR of CH4 a sense γ primer 5′CATCTACAACGCTGCC3′ with an antisense γ-β hybrid primer 5′GATATTTGAGCTCTGGTCCCAGGTGAGAACATT3′ were used to amplify γ template. The second amplification used β template, the same sense primer and an antisense β primer 5′CATCTACAACGCTGCC3′. The products of all the second PCR reactions were digested with two unique restriction enzymes BglII and KpnI, and subcloned into the vector pSD5.
Construction of α and β dimers was performed in the following way: The coding regions of the cDNAs of α and β subunits were linked to form a single polypeptide by the addition of a unique ClaI site after the last amino acid of the β subunit and before the first methionine of the α subunit using PCR. The α and β cDNAs were digested with ClaI and ligated into a single cDNA that encodes for the βα polypeptide. Construction of α and γ dimer was performed by the addition of a ClaI site at the end of the coding region of the γ cDNA and immediately before the first methionine of the α subunit using PCR. The α and γ cDNAs were digested with ClaI and ligated into a single cDNA that encodes for one γα polypeptide. The presence of the ClaI site introduced two amino acids (isoleucine and aspartic acid) between β and α subunits and between γ and α subunits.
All constructs were sequenced at the Yale facility using an ABI 373 automated DNA sequencer employing dye terminators following a standard protocol supplied by the manufacturer (Applied Biosystems, Inc., Foster City, CA).
results
Na+ and Li+ Selectivity of Channels with Different Subunit Composition
The native epithelial sodium channel has high selectivity for Na+ over other cations. The only other permeant ions are Li+ (with a PLi+/PNa+ 1.5) and H+ ions (Palmer, 1984). We examined the relative permeabilities for Na+ and Li+ of the amiloride-sensitive current in oocytes co-injected with equal amounts of α and β or of α and γ cRNAs. The magnitude of the observed amiloride-sensitive sodium current varied from 0.2 to 3 μA, a level of expression adequate for our whole-cell studies. The mean currents were 1.9 ± 0.7 μA for αβ and 2.7 ± 0.9 μA for αγ. Current-voltage relations were generated in the presence of 150 mM K+, Na+ or Li+ as the gluconate salt in the bathing solution. Membrane potentials were changed in 20 mV incremental steps from −180 to 80 mV. Fig. 1 A shows the relative currents of Na+, Li+, and K+ for αγ channels. Current measurements have been normalized to the amiloride-sensitive Na+ current obtained at −100 mV. At this potential and with this combination of subunits the inward Li+ current was 1.5-fold greater than the Na+ current. There was no significant inward current when the bath contained 150 mM K+. The small outward current at depolarizing membrane potentials is probably carried by Na+ because it is substantially reduced by exposing the cells to Na+ free solutions for 24 h (not shown). Fig. 1 B shows the results of the relative currents through αβ channels for the same three cations. The Na+ current at −100 mV was 0.8-fold larger than the Li+ mediated current, again with little or no measurable K+ current. Thus, the magnitude of the amiloride-sensitive current of αγ channels is Li+ > Na+ >> K+, a profile similar to that for wild-type αβγ channels (Canessa et al., 1994) and for homomeric α channels (Canessa et al., 1993). In contrast, the αβ channel currents were Na+>Li+>>K+.
Figure 1.
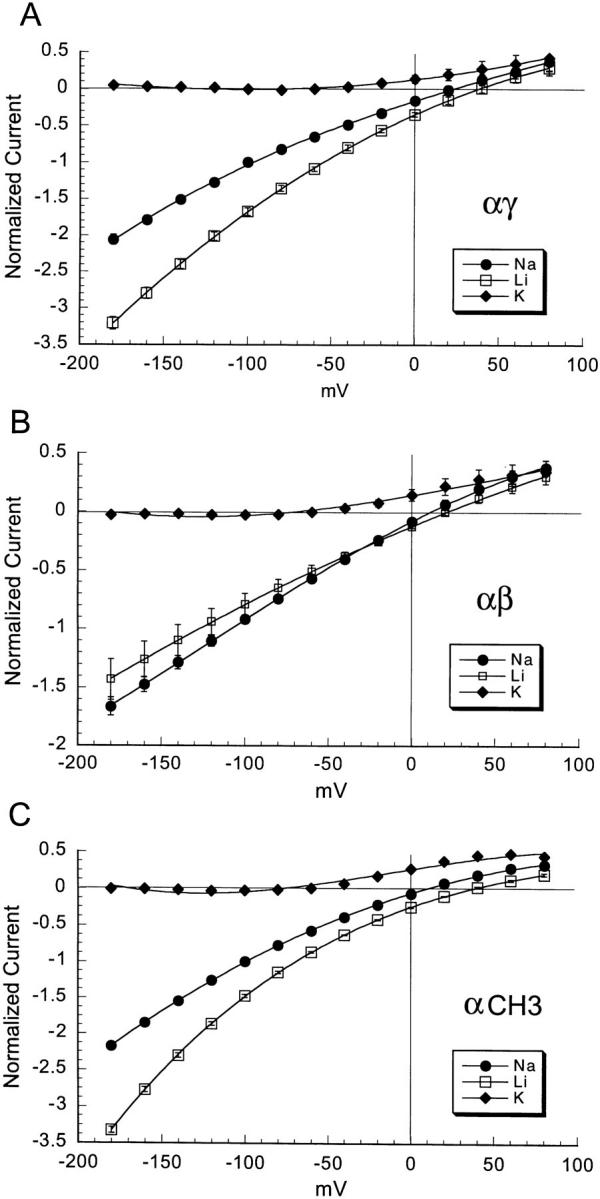
Current-voltage relationships of amiloride-sensitive currents of oocytes injected with equal amounts of cRNAs from α and γ subunits (A), α and β subunits (B), and CH3 with α subunit (C) measured in the presence of bathing solutions that contain 150 mM K+, Na+ or Li+ as the gluconate salt. Oocytes were voltage clamped and the membrane potential was changed in 20-mV steps from −180 to 80 mV before and after the addition of 50 μM amiloride to the bath. Curves represent the difference of whole-cell currents before and after the addition of amiloride. Values were normalized to the current measured in the presence of Na+ at −100 mV membrane potential. Each point is the mean of 8 different oocytes, error bars represent SEM.
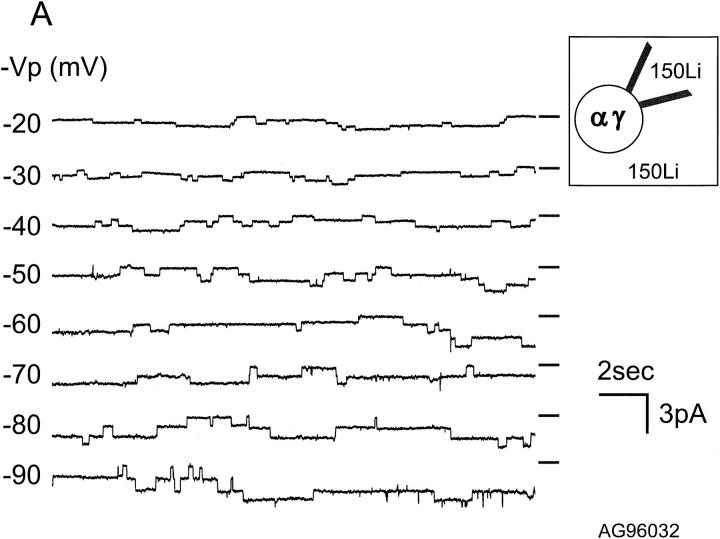
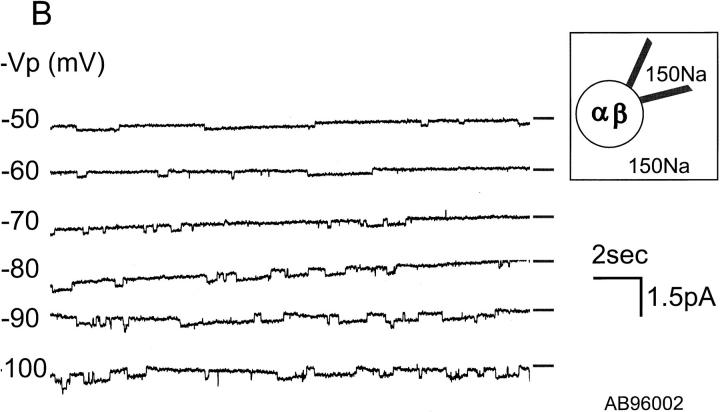
Characteristics of αβ and αγ Unitary Currents
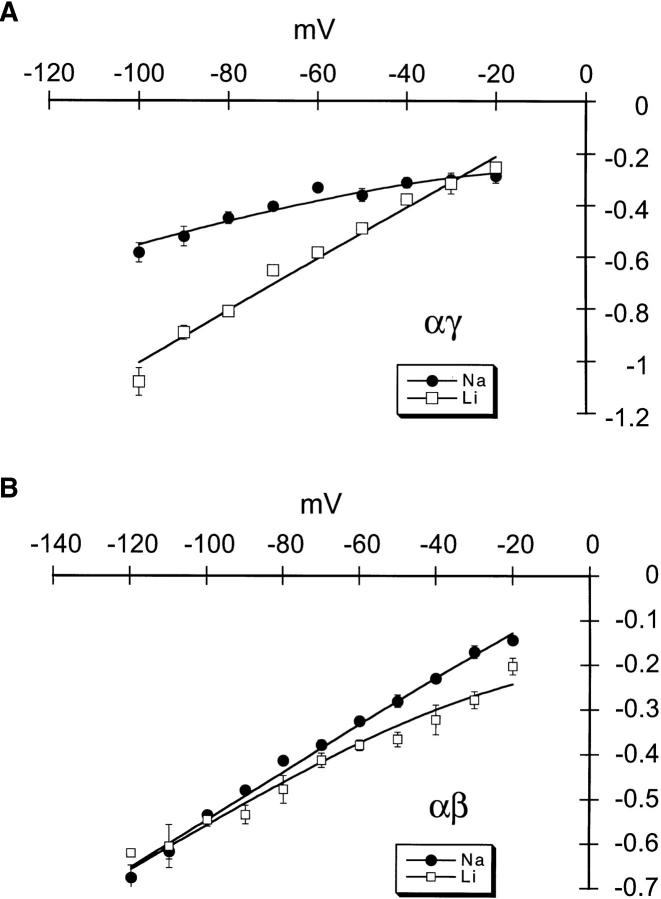
Single channel currents were examined in cell-attached patches from oocytes injected with αγ or αβ cRNAs. For patch clamp experiments, we selected oocytes that expressed >1 μA of whole-cell current. Fig. 2, A and B, show unitary currents for αβ and αγ channels, respectively, for a range of holding potentials (−Vp). These records with their long openings and closures are similar to those obtained from αβγ in oocytes (not shown). With 150 mM NaCl in the pipette, the single channel conductance of αγ channels was 6.5 pS (n = 9, Fig. 3 A) and, with 150 mM Li+, was 10.8 pS (n = 5, Fig. 3 A). Thus, the larger unitary conductance for Li+ than for Na+ of αγ channels is similar to αβγ channels (Canessa et al., 1994). In contrast, the single channel conductances of αβ channels were 5.1 pS (n = 5, Fig. 3 B) for Na+ and 4.2 pS (n = 6, Fig. 3 B) for Li+, therefore the Li+/Na+ current ratio of αβ is close to 1. The magnitude and Li+/Na+ ratios of unitary currents are in agreement with the data obtained with measurements of whole-cell currents shown in Fig. 1.
Figure 2.
Records of unitary currents from cell-attached patches of oocytes co-injected with α and γ (A) or with α and β (B) subunits. −Vp refers to the negative value of the pipette holding potential. The short solid line to the right of each trace represents the minimal current level observed (closed level). Downward deflections represent inward currents (channel opening). There are at least four channels present in the patch shown in A and two channels in the patch shown in B.
Figure 3.
Current-voltage (I-V) relationships for inward αγ (A) and αβ (B) unitary currents recorded from cell attached patches with either 150 mM NaCl in the pipette and the bath or 150 mM LiCl in the pipette and the bath. Data points are from a total of 5–9 separate experiments. The solid line represents the prediction from the constant field equation (Hille, 1992).
To correlate the incidence of channels with macroscopic currents under the different injection conditions, we assessed the number of channels per patch. Oocytes injected with αβγ had average macroscopic currents of 10 μA (ranged from 3 to 12 μA in 20 oocytes) and the number of channels per patch was at least 5. The average whole-cell current of αβ and αγ injected oocytes was 1.9 (ranged from 0.2 to 2.5 μA in 40 oocytes) and 2.7 μA (ranged from 0.15 to 3.5 μA in 30 oocytes), respectively. The αβ and αγ patches contained 1–4 channels which is less than the ≥5 per patch observed with αβγ. These data indicate that the density of active channels in the plasma membrane of αγ and αβ injected oocytes is less than that of αβγ injected oocytes. In summary, these results demonstrate that the lower magnitude of whole-cell currents when α and γ or α and β are co-expressed, compared with αβγ, is a result of fewer channels in the plasma membrane and not to marked reduction in single channel conductance or open probability.
Inhibition of αγ and αβ Channels by the Blocker Amiloride and Its Analogues
The epithelial sodium channel is reversibly blocked by the potassium sparing diuretic amiloride (3,5-diamino- N -(aminoiminomethyl) - 6 - chloropyrazinecarboxamide) (Garty and Benos, 1988). The amiloride molecule has a guanidinium group that is positively charged at physiological pH and a pyrazine ring. Aromatic substitutions on the guanidinium moiety (e.g., benzamil or phenamil) increase the potency of the block by approximately 10-fold (Kleyman and Cragoe, 1988). The half maximal concentration of amiloride block (K i) is in the order of 0.1 μM in the cortical collecting tubule, distal colon, and toad bladder. The amiloride and benzamil K is of the cloned ENaC measured in oocytes injected with αβγ cRNAs are also 0.1 and 0.01 μM, respectively (Canessa et al., 1994).
We obtained the K i of amiloride, benzamil, and guanidinium of αγ and αβ channels by measuring oocyte whole-cell currents after the addition to the bathing solution of increasing concentrations of these blockers from 0.001 to 100 μM (see Fig. 5). These measurements were performed with 150 mM Na+ in the bath solution and with the membrane potential held at −100 mV. For αγ channels the K i of amiloride was 0.13 ± 0.05 μM (see Fig. 5 A) and the K i of benzamil was 0.015 ± 0.004 μM (see Fig. 5 B). For αβ channels the K i of amiloride was 4 ± 0.4 μM (see Fig. 5 A), and the K i of benzamil was 0.4 ± 0.09 μM (see Fig. 5 B). Thus, αβ channels have lower affinity for amiloride and benzamil than αγ channels.
Figure 5.
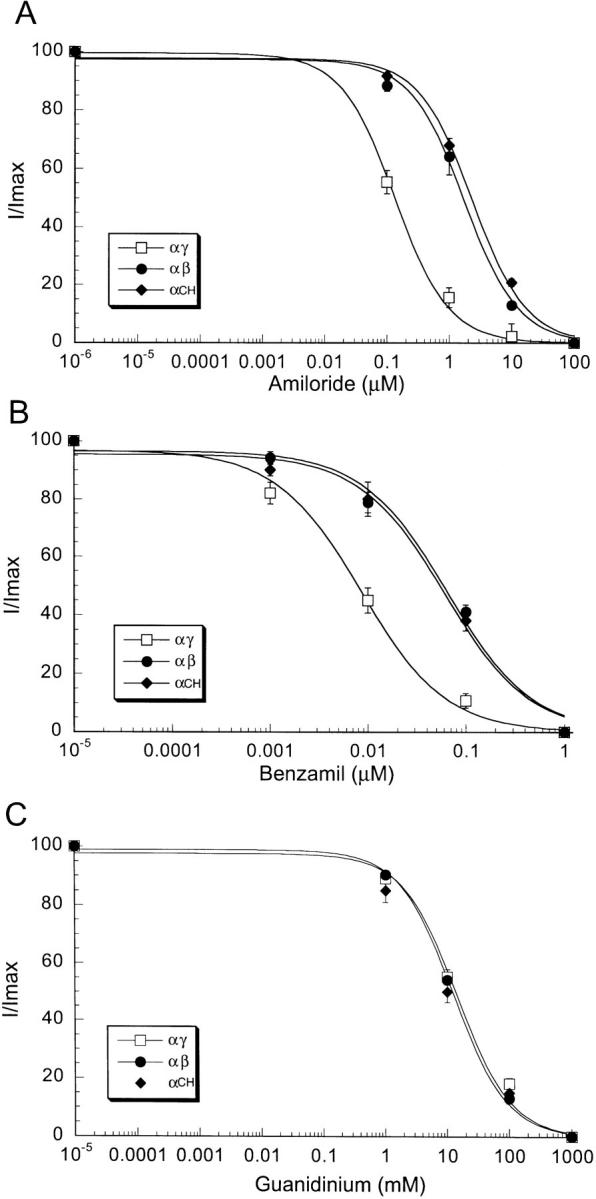
Dose-response curves of inhibition of Na+ currents by amiloride (A), benzamil (B), and guanidinium (C) of oocytes co-injected with α and γ cRNAs (□), α and β cRNAs (•), and CH3 and α cRNAs (♦). Similar response to CH3 was obtained with chimera CH4. Measurements were obtained with the two-electrode voltage clamp technique in the presence of 150 mM Na+ gluconate in the bath and at −100 mV membrane potential. Data points represent the mean of 10 different oocytes. Vertical bars represent the SEM.
Both amiloride and benzamil contain a guanidinium group, a monovalent positively charged molecule that acts as a pore blocker (Palmer, 1990). We measured the effectiveness of blockade by guanidinium for αγ and αβ channels. Fig. 5 C shows that, in contrast to amiloride and benzamil, guanidinium has an equal K i (10 ± 2.5 mM) for αγ and αβ channels.
To localize the structural domains that determine amiloride affinity we constructed several chimeric proteins of γ and β subunits. Fig. 4 shows linear representations of the γ-β chimeric constructs (CH). M1 and M2 represent the first and second transmembrane domains of the subunit proteins. Sequences corresponding to γ are shown in white and sequences corresponding to β are shown in black. The numbers at the left and right of the arrows indicate the amino acids of γ and β subunits at the junction between the two subunits, respectively. We also made the reciprocal β-γ chimeras that contained the amino terminus of β and the carboxy terminus of γ. All these constructs were not functional when expressed either alone or when they were co-injected with wild-type α subunit (data not shown).
Figure 4.
Linear representation of γ-β chimeras and dimer constructs. Numbers in the chimeras indicate the amino acid positions of the corresponding γ and β polypeptides from rat, and the arrows indicate the junction between γ and β subunits.
When we examined the γ-β chimeric constructs co-injected with α subunit, only channels containing chimeras CH1 and CH2 showed a high affinity for amiloride with K i of 0.12 μM. For channels containing CH3 and CH4 the K i of amiloride was 3.7 ± 0.41 μM (Fig. 5 A), similar to the value observed for αβ. The transition from high to low amiloride affinity occurred with chimeras CH2 and CH3. CH3 differs from CH2 only by 15 amino acids from the β subunit just proximal to the second transmembrane domain. Therefore, the results obtained with the expression of γ-β chimeras suggest that a short stretch of the amino acids before the second transmembrane domain of the β subunit are important in determining amiloride affinity.
In contrast to amiloride inhibition, measurements of guanidinium K i gave similar values for αγ, αβ, and α-chimeric (CH1, CH2, CH3, and CH4) channels indicating that the guanidinium binding site is similar in all these proteins. Since amiloride and benzamil differ from guanidinium in having an attached pyrazine ring, the data indicate the presence of two distinct binding sites in the channel protein; one site for the pyrazine ring and the other site for the guanidinium group.
Since the amiloride block of epithelial sodium channels is known to be voltage dependent (Palmer, 1985; Warncke and Lindemann, 1985), we examined whether the voltage dependence of the block by amiloride was similar in αγ and αβ channels. For this purpose, we measured the K i of amiloride at various holding potentials (K i(V)) ranging from −180 to 50 mV in the presence of only 30 mM Na+ in the bathing solution to enhance the outward currents at positive membrane potentials. Fig. 6 shows the K i for αγ and αβ channels normalized to the values obtained at −100 mV and plotted against membrane voltage. The amiloride block of αγ and αβ channels exhibited virtually identical linear relationships with respect to membrane voltage. We calculated the fraction of the electric field that the blocker traverses to reach the site (δ) according to the equation:
 |
Figure 6.
Effect of membrane potential on amiloride K i for αγ (□), and αβ (•) channels. Membrane potential was held between −180 and +50 mV. These measurements were obtained with 30 mM Na-gluconate in the bath solution. The K i were normalized to the values obtained at −100 mV. Data points represent the mean of 8 different oocytes. Vertical bars represent the SEM.
where K i (0) is the value of K i at 0 mV, Z is the charge valence of the blocker, which is set to 1, and F, R, and T have their usual meaning. At 22°C, the value of δ was 0.133 ± 0.012 for αγ and 0.153 ± 0.002 for αβ. These values are similar to those reported by Palmer (1985) in toad urinary bladder. From these results, it is reasonable to assume that amiloride senses the same magnitude of the transmembrane electrical field in both types of channels.
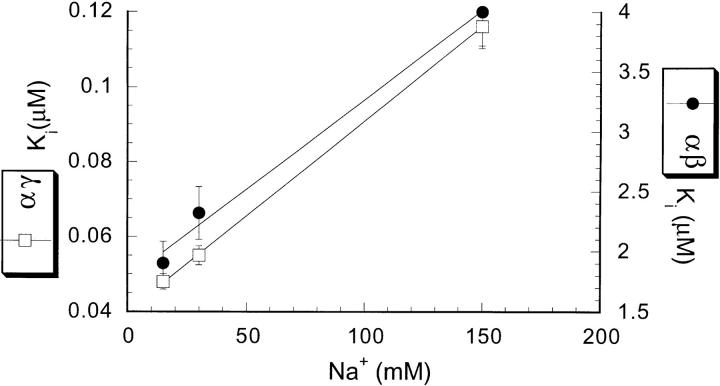
It has been shown for wild-type epithelial sodium channels, that external Na+ and amiloride interact competitively, so that the K i for amiloride increases in the presence of high external sodium concentration. Since αγ and αβ channels also differ significantly in their Na+ affinity, it was interesting to examine whether the two types of channels exhibit the same competitive effect with external Na+. We measured whole-cell currents in 15, 30, and 150 mM external Na+ at a membrane potential of −100 mV, the ionic strength was kept constant with NMDG gluconate. As shown in Fig. 7, increasing external Na+ concentration from 15 to 150 mM changed the K i of αγ channels from 0.045 ± 0.005 to 0.104 ± 0.025 μM and that of αβ channels from 2.11 ± 0.33 to 4.43 ± 0.66 μM. Thus, both αγ and αβ channels showed a two-fold increase in amiloride K i for a 10-fold increase in Na+ concentration.
Figure 7.
Effect of Na+ concentration on amiloride K i for αγ (□) and αβ (•) channels. Measurements of K i were made at a holding potential of −100 mV in 4 different oocytes.
In summary, αγ and αβ channels have a similar K i for guanidinium, a similar voltage dependence of amiloride inhibition, and a similar attenuation of amiloride block by external Na+. These observations are consistent with the notion that the guanidinium group on amiloride interacts with comparable site(s) in the outer mouth of the channel pore of both αβ and αγ channels.
Apparent Na+ and Li+ Affinities of αγ, αβ Channels and γ-β Chimeras
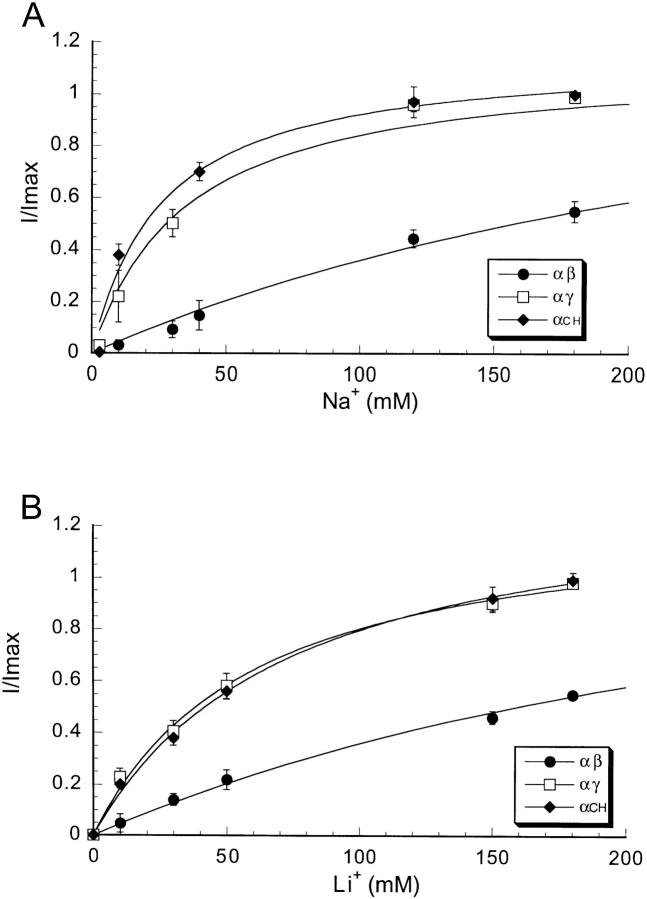
Macroscopic currents of wild-type channels can be described by Michaelis-Menten curves with half-maximal concentrations (K m) of 10–30 mM for Na+ and 30–50 mM for Li+, depending on the tissue and the method of measurements (Olans et al., 1984; Palmer and Frindt, 1988; Puoti et al., 1995). We investigated whether αγ and αβ channels differ in apparent affinities K m for external Na+ and Li+ ions from wild-type channels. We measured the amiloride-sensitive currents induced by increasing the concentrations of Na+ and Li+ gluconate from 0 to 180 mM in the bathing solution while the membrane potential was held at −100 mV. The results are shown in Fig. 8. We found that αγ channels had an apparent K m of 35 ± 6 mM for Na+ and of 60 ± 5 mM for Li+, values similar to those previously reported for αβγ channels when studied under similar conditions. In contrast, the apparent K m for Na+ and for Li+ of αβ channels were much larger, estimated values >180 mM. These values represent only an estimation of the actual K m since the largest cation concentration used in these measurements was 180 mM. Further increase in the bath concentration was not tolerated by the oocytes due to the high osmolality of the solution.
Figure 8.
Amiloride-sensitive current of oocytes injected with equal amounts of cRNAs from α and γ subunits (□), α and β subunits (•), and α subunit and CH4 (♦) in the presence of increasing concentrations of Na+ (A) and Li+ (B) in the bath solution. Values were normalized to the maximal current. Data points were fitted with the Michaelis-Menten equation. Each curve represents the mean of 9 oocytes.
Interestingly, the γ-β chimeric constructs (CH1 to CH4) exhibit an apparent K m for Na+ and for Li+ (Fig. 4), and ILi+/INa+ at −100 mV (Fig. 1) similar to αγ channels. Channels formed by α and CH1 or CH2 showed amiloride K i and apparent K m for Na+ similar to αγ, whereas channels formed by α and CH3 or CH4 showed amiloride K i similar to αβ (Fig. 5) but an apparent K m for Na+ similar to αγ channels. The results indicate that in contrast to amiloride, the low apparent K m for external ions in αβ channels is determined by the first two-thirds of the β subunit.
Effect of Expression of Varied Ratios of ENaC Subunits
In additional experiments we investigated whether the presence of different ratios of α and β subunits would result in the formation of channels with different amiloride and cation affinities. For that purpose we co-injected α and β cRNAs into oocytes in the following ratios: 1α to 10β; 1α to 5β; 1α to 1β; 5α to 1β; and 10α to 1β. We then examined whether the amiloride K i or the apparent K m for Na+ of αβ channels changed in proportion to the ratios of α and β subunits. Similar experiments were performed with the corresponding ratios of α and γ subunit cRNAs.
There was no significant difference in either Na+ or Li+ affinities, or in amiloride K i for the various subunit ratios. This observation indicates that even with an excess of β or γ subunits, the properties of the expressed channels do not change, suggesting that the stoichiometry of both αβ and αγ channels is fixed.
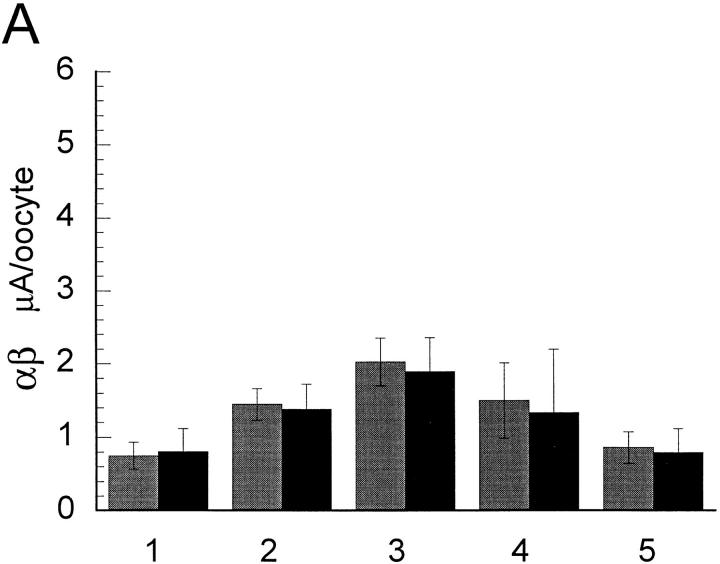
The magnitude of Na+ and Li+ amiloride-sensitive currents obtained with the various ratios of α/γ and α/β cRNAs are shown in Fig. 9. The largest currents were observed with the 1α/1γ and 1α/1β ratios and the magnitude of the currents decreased progressively when the ratios were made 10α/1β or 1α/10β (Fig. 9 A) or 10α/1γ or 1α/10 γ (Fig. 9 B).
Figure 9.
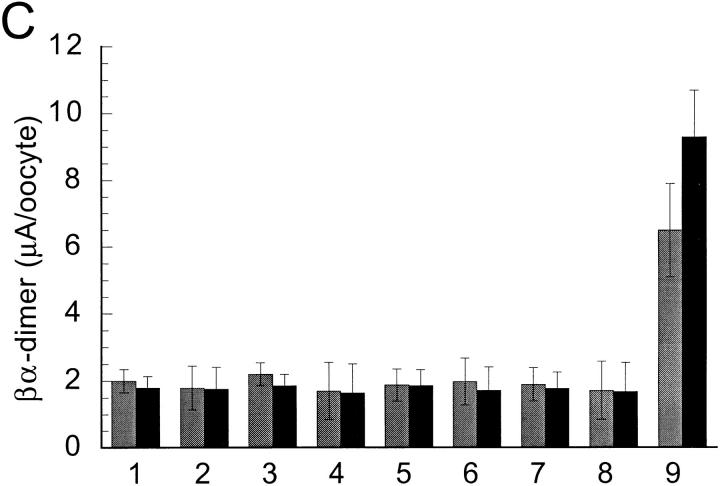
Effect of varying the concentration ratio of cRNAs in the magnitude of amiloride-sensitive whole cell Na+ (gray bars) and Li+ (black bars) currents: (A) α and β; (B) α and γ; (C) βα-dimer and α or β; (D) γα-dimer and α or γ. Oocytes were injected with equal amount of cRNA that was distributed in concentration ratios as follows (numbers in parentheses indicate the column on the appropriate graph). (A) 10α/1β (1), 5α/1β (2), 1α/1β (3), 1α/5β (4), and 1α/10β (5). (B) 10α/1γ (1), 5α/1γ (2), 1α/1γ (3), 1α/5γ (4), and 1α/ 10γ (5). (C) βα-dimer alone (1), 1α/1β (2), 1βα-dimer/1α (3), 1βα-dimer/2α (4), 1βα-dimer/3α (5), 1βα-dimer/1β (6), 1βα-dimer/2β (7), βα-dimer/3β (8), and 1βα-dimer/1γ (9). (D) 1γα-dimer alone (1), 1α/1γ (2), 1γα-dimer/1α (3), 1γα-dimer/2α (4), 1γα-dimer/3α (5), 1γα-dimer/1γ (6), 1γα-dimer/2γ (7), γα-dimer/3α (8), and 1γα-dimer/1β (9). Each bar represents the mean of 6–12 oocytes.
To examine further the ratio of subunits we constructed βα-dimers that contained all the amino acids of the β subunit linked to all the amino acids of the α subunit by the addition of a unique restriction site ClaI that introduced a short linker of two new amino acids: isoleucine and aspartic acid between α and β. We also constructed γα-dimers that contained all the γ subunit linked to all the α subunit by the same two amino acids. The βα and γα-dimers were functional when injected alone in oocytes in the absence of wild-type monomeric α subunits. The expressed amiloride-sensitive current was indistinguishable in magnitude and properties from the current induced by co-injection of α and β or of α and γ subunits, respectively. When βα dimers were co-injected with increasing amounts of α or β subunits, or when γα dimers were co-injected with increasing amounts of α or γ subunits the magnitude of the amiloride-sensitive current did not increase (Fig. 9, C and D). However, co-injection of αβ-dimers with γ subunit induced an eight-fold increase in current and changed the Li+/Na+ permeability to >1. Co-injection of αγ-dimers with β subunit also induced a large increment of current without changing the functional properties, indicating that the βα- and αγ-dimers appear to be competent for association with γ and β subunits, respectively to form functional hetero-multimeric channels.
These results suggest that αβ and αγ channels are formed by a 1:1 ratio of α and β and of α and γ subunits, respectively. However, the number of each subunit contributing to functional channels cannot be determined from our data.
discussion
Physiological Relevance for Diversity of Epithelial Sodium Channels
Hetero-multimeric channels such as ligand-gated receptors (Nakanishi et al., 1990), cyclic nucleotide-gated channels (Bradley et al., 1991; Chen et al., 1993), and certain voltage-gated potassium channels (Isacoff et al., 1990; Krapivinsky et al., 1995) associate their subunits in various combinations to generate different channels, each with unique functional properties. In the present study, we have examined whether the three subunits of ENaC, α, β, and γ, can also associate in various combinations to form channels with distinct functional characteristics. The existence of a diversity of sodium channels is suggested by reports describing amiloride-sensitive sodium channels with properties that do not correspond to those of ENaC (Cantiello et al., 1989; MacGregor et al., 1994; Yue et al., 1994). For instance, it has been shown that in fetal lung epithelial cells there are two populations of amiloride-sensitive sodium channels, one with high affinity (K i = 0.019 μM) and the other with low affinity (K i = 1.5 μM) for benzamil (Matalon et al., 1993). Sodium-selective channels with ILi+/INa+ current ratio <1 and relative low amiloride affinity (K i = 0.87 mM) have been described in membranes of rat macrophages (Negulyaev and Vedernikova, 1994). In the apical membrane of rabbit proximal tubules, amiloride-sensitive highly sodium-selective channels have been observed that have a larger single channel conductance than those present in the distal tubule (Gögelein and Greger, 1986).
These sodium-selective channels may be formed by new subunit proteins from the ENaC family or by combinations of the already known ENaC subunits. Waldmann et al. (1995a) have cloned a δ subunit that can associate with β and γ but not with α to form functional channels. The ILi+/INa+ ratio of δ is <1, and the K i for amiloride is 2.6 μM. These properties are similar to those of αβ channels. The expression of the δ subunit overlaps with the expression of α, β, and γ in several tissues including the kidney, although it is not yet known which segments of the nephron express the δ subunit or whether there is colocalization with the other ENaC subunits to form functional channels in vivo.
Recently, Farman and colleagues (1997) have reported heterogeneity at the level of expression of the subunits of ENaC in respiratory epithelia. A large prevalence of α and γ expression was found in a subpopulation of alveolar cells, in tracheal epithelium as well as in nasal and tracheal gland acini, with little or no β subunit expression. In other tissues, such as the epithelium of the distal colon, the level of expression of individual subunits is highly regulated by aldosterone. Under normal conditions these cells only express the α subunit; following stimulation by aldosterone, however, large amounts of β and γ cRNAs are expressed (Asher et al., 1997). Differential expression of ENaC subunits could represent a mechanism of regulating Na+ reabsorption in tissues. For instance, cells that express only α and γ or α, β, and γ subunits will form channels with high sodium affinity and high amiloride sensitivity, however the maximal capacity for Na+ absorption in α and γ cells will be much smaller than in cells that express simultaneously the three subunits (Canessa et al., 1994). The high affinity for external Na+ of αγ and αβγ channels is important for efficient Na+ absorption in tissues such as the cortical-collecting tubule of the kidney where the luminal Na+ concentration reaches very low levels in states of Na+ depletion. On the other hand, cells that only express α and β subunits will form channels with low affinity for Na+, which will limit Na+ absorption unless the concentration of external Na+ is large. Therefore, αβ channels will only be active when exposed to high Na+ concentrations such as in the lumen of proximal tubules, in the pulmonary airways, or in surrounding macrophages (although the functional significance of ENaC in blood cells is still unknown).
In summary, our results indicate that the ENaC subunits can indeed generate a diversity of channels with different functional and pharmacological characteristics. Epithelial Na+ channels like other multimeric channel proteins have the potential for complex regulation by differential expression of their subunits during development and under the influence of various hormones and stimuli.
Ki of Amiloride
The most specific inhibitor of Na+ channels in high- resistance epithelia is the potassium-sparing diuretic drug amiloride. Several observations indicate that this drug acts by obstructing the conducting pore: (a) it has been demonstrated that the positive charge on the guanidinium moiety is essential for blocking; (b) the K i of amiloride is sensitive to the luminal Na+ activity indicating an amiloride-ion competition effect; and (c) the channel-amiloride association/dissociation rate constants are voltage dependent. These findings are consistent with the notion that amiloride blocks the channel from the outer part of the conducting pore. However, the molecular site(s) on the channel protein to which the blocker binds has not yet been defined.
We have shown that the amiloride affinity of αβ channels is significantly lower than that exhibited by αβγ and αγ channels and have presented evidence that a short stretch of 15 amino acids before the second transmembrane domain of the β subunit determines the amiloride K i of αβ channels. In spite of a 25-fold difference in amiloride and benzamil K i of αγ and αβ, both types of channels exhibited the same affinity for guanidinium with a K i of 15 mM. The results indicate that the amiloride molecule interacts with the channel protein in at least two distinct sites. At one site, guanidinium binds loosely to the outer pore of the channel. At another distinct site, the pyrazine group binds, increasing the affinity of the blocker by several hundred-fold. Three key observations support the notion that guanidinium binds to a similar site in αβ and αγ channels: (a) similar guanidinium K i; (b) a similar electrical distance (δ) sensed by the charged moiety of amiloride; and (c) similar competitive effect of external Na+ on amiloride binding. In contrast, the 25-fold difference in amiloride affinity of αγ and αβ channels supports the idea that the pyrazine binding site is different in these two channels.
Our results show that β subunits also contribute to determining the amiloride affinity of channels. It is known that α subunits are important for amiloride binding, channels formed only by α subunits bind amiloride with high affinity, and mutations in the human α subunit at positions S598 and S581 decrease the K i for amiloride (Waldmann et al., 1995b ). The region we identified in the β subunit that determines the K i of amiloride of αβ channels is highly conserved in all subunits, suggesting that amiloride may also bind to β and γ, although with different affinity. According to this interpretation amiloride binds to more than one subunit and therefore the arrangement of the subunits around the channel affects the final affinity for amiloride. This notion is consistent with the finding that αβγ channels exhibit high amiloride affinity in spite of having β subunits in their composition. Alternatively, amiloride only makes contact with the α subunit but the binding site is modified by the adjacent subunits.
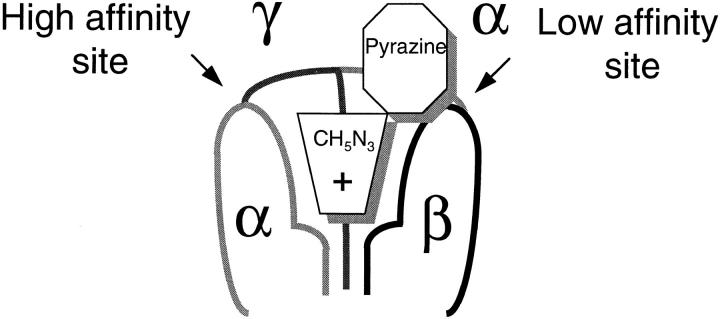
Fig. 10 depicts a model of the outer channel pore where the guanidinium group blocks the entrance of the pore and the pyrazine group makes contact with a second site. The pyrazine binding site is shown between two subunits αβ or αγ to emphasize the notion that more than one subunit contributes to the site or alternatively, the site formed by the α subunit is affected by the neighboring subunits.
Figure 10.
Proposed model for amiloride binding site in the epithelial sodium channel. The three subunits (α, β, and γ) arranged around the channel pore are shown. The positively charged guanidinium group blocks loosely the entrance of the channel while the pyrazine ring binds to a second site in the protein. Two types of pyrazine-binding sites are shown, the one with high affinity is formed by α and γ subunits, whereas the one with low affinity is formed by α and β subunits.
Apparent Km for Na+ and Li+ of αβ and αγ Channels
The amiloride-sensitive whole-cell currents shown in Fig. 8 are described by the Michaelis-Menten equation. We demonstrate that the K m values for Na+ and Li+ for αβ channels are at least 10-fold larger than those for αγ channels. Since these values were derived from whole-cell currents, they represent an ensemble of processes that include single channel conductance, open probability and/or number of conductive channels any of which could change as a function of increasing external cation concentrations. Our present data cannot distinguish between these possibilities. A detailed study of single channel kinetics at different Na+ concentrations is required to establish the different effect of external Na+ in αβ and αγ channels.
Since channels formed by α and chimeras CH1 to CH4 exhibit apparent K m for Na+ similar to αγ channels we can conclude that at least some of the structural determinants of the K m are encoded by the first two-thirds of the β subunit. Similar to the situation with amiloride, where more than one subunit contributes to the amiloride K i, more than one subunit is also involved in the determination of the apparent K m for Na+: αβγ and αγ channels have a K m for Na+ of 30 mM whereas αβ have ten fold larger K m values.
Stoichiometry of αγ and αβ Channels
All three subunits, α, β, and γ, associate to form ENaC although the number of subunits and their stoichiometry are presently unknown. We have examined the composition of channels formed only by two subunits, α and β, and α and γ, and whether the functional properties of these channels can be modified by injecting various ratios of the cRNAs. Varying the ratio of injected cRNA's between 0.1 and 10 did not change the properties of αβ or αγ channels. The largest amplitude of whole-cell amiloride-sensitive currents were obtained with ratios 1α:1β and 1α:1γ. These results suggest a most likely stoichiometry of 1α:1β and of 1α:1γ for αβ and for αγ channels, respectively. In addition, expression of βα-dimers and γα-dimers induced currents with properties indistinguishable from those induced by injection of single subunits, and the whole-cell currents were of similar magnitude than those induced by co- injection of single subunits with a 1:1 ratio. Since the addition of single subunits to the dimers did not increase the current, it seems likely that αβ and αγ channels have an even number of subunits, either 4, 6, or more; our data can not discriminate among these possibilities.
In conclusion, the results of these studies indicate that the stoichiometry and the order of the subunits around the pore are structural features that define the functional properties of the channel; when they are altered the properties of the channels such as amiloride affinity, apparent K m for Na+, INa+/ILi+ ratio are also changed.
Acknowledgments
This work was supported by the Edward Mallinckrodt, Jr. Foundation (C.M. Canessa) and the National Kidney Foundation (C.M. McNicholas).
Footnotes
Address correspondence to: Dr. C.M. Canessa, Department of Cellular and Molecular Physiology, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520-8026. Fax: 203-785-4951; E-mail: Cecilia_Canessa@yale.edu
Abbreviations used in this paper: CH, chimeric constructs; ENaC, epithelial Na+ channel; NMDG, N-methyl-d-glucamine.
references
- Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+channel subunits. Am J Physiol. 1997;271:C605–C611. doi: 10.1152/ajpcell.1996.271.2.C605. [DOI] [PubMed] [Google Scholar]
- Bradley J, Davidson N, Lester HA, Zinn K. Heterotrimeric olfactory cyclic nucleotide-gated channels: a subunit that confers increased sensitivity to cAMP. Proc Natl Acad Sci USA. 1991;91:8890–8894. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature (Lond) 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild LM, Buell G, Thorens B, Gautschl I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na+channel is made up of three homologous subunits. Nature (Lond) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Cantiello HF, Patenaude CR, Ausiello DA. G protein subunit, αi-3, activates a pertussis toxin-sensitive Na+channel from the epithelial cell line, A6. J Biol Chem. 1989;264:20867–20870. [PubMed] [Google Scholar]
- Chen TY, Peng YW, Dahallan RS, Ahamed B, Reed RR, Yau KW. A new subunit of the cyclic nucleotide gated cation channel in retinal rods. Nature (Lond) 1993;362:764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Farman N, Talbot CR, Boucher RC, Canessa CM, Rossier BC, Bonvalet JP. Non-coordinated expression of α, β and γ subunit mRNAs of the epithelial sodium channel along the rat respiratory tract. Am J Physiol. 1997;272:C131–C141. doi: 10.1152/ajpcell.1997.272.1.C131. [DOI] [PubMed] [Google Scholar]
- Garty H, Benos DJ. Characteristics and regulatory mechanisms of the amiloride sensitive Na+channel. Physiol Rev. 1988;68:309–373. doi: 10.1152/physrev.1988.68.2.309. [DOI] [PubMed] [Google Scholar]
- Gögelein H, Greger R. Na+selective channels in the apical membrane of rabbit late proximal tubules (pars recta) Pflüg Arch. 1986;406:198–203. doi: 10.1007/BF00586683. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Eaton DC. Single-channel recordings from amiloride-sensitive epithelial sodium channel. Am J Physiol. 1985;249:C200–C207. doi: 10.1152/ajpcell.1985.249.3.C200. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA.
- Isacoff E, Jan YN, Jan LY. Evidence for the formation of heteromultimeric potassium channels in Xenopusoocytes. Nature (Lond) 1990;345:530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature (Lond) 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Renard S, Waldmann R, Voilley N, Champigny G, Plass H, Lazdunski M, Barbry P. Different homologous subunits of the amiloride-sensitive Na+channel are differentially regulated by aldosterone. J Biol Chem. 1994;269:13736–13739. [PubMed] [Google Scholar]
- MacGregor GG, Olver RE, Kemp PJ. Amiloride-sensitive Na+channels in fetal type II pneumocytes are regulated by G proteins. Am J Physiol. 1994;267:L1–L8. doi: 10.1152/ajplung.1994.267.1.L1. [DOI] [PubMed] [Google Scholar]
- Matalon S, Bauer ML, Benos DJ, Kleyman TR, Lin CL, Cragoe E, Jr, O'Brodovich H. Fetal lung epithelial cells contain two populations of amiloride sensitive Na+channels. Am J Physiol. 1993;264:L357–L364. doi: 10.1152/ajplung.1993.264.4.L357. [DOI] [PubMed] [Google Scholar]
- Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp measurements on Xenopuslaevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflüg Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Schneider NA, Axel R. A family of glutamate receptor genes - evidence for the formation of heteromultimeric receptors with distinct channel properties. Neuron. 1990;5:569–581. doi: 10.1016/0896-6273(90)90212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulyaev YA, Vedernikova EA. Sodium-selective channels in membranes of rat macrophages. J Membr Biol. 1994;138:37–45. doi: 10.1007/BF00211067. [DOI] [PubMed] [Google Scholar]
- Olans L, Sariban-Sohraby S, Benos DJ. Saturation behaviour of single, amiloride sensitive Na+channels in planar lipid bilayers. Biophys J. 1984;46:831–835. doi: 10.1016/S0006-3495(84)84082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG. Voltage-dependent block by amiloride and other monovalent cations of apical Na channels in the toad urinary bladder. J Membr Biol. 1984;80:153–165. doi: 10.1007/BF01868771. [DOI] [PubMed] [Google Scholar]
- Palmer LG. Interactions of amiloride and other blocking cations with the apical Na channel in the toad urinary bladder. J Membr Biol. 1985;87:191–199. doi: 10.1007/BF01871218. [DOI] [PubMed] [Google Scholar]
- Palmer LG. Epithelial Na channels: the nature of the conducting pore. Renal Physiol Biochem. 1990;13:51–58. doi: 10.1159/000173347. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci USA. 1986;83:2767–2770. doi: 10.1073/pnas.83.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Conductance and gating of epithelial Na+channels from rat cortical collecting tubule. J Gen Physiol. 1988;92:121–138. doi: 10.1085/jgp.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A, Canessa CM, Horisberger J-D, Schild LM, Rossier BC. The highly selective low-conductance epithelial Na channel of Xenopuslevis A6 cells. Am J Physiol. 1995;38:C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Canessa CM, Schild L, Horisberger J-D. Epithelial sodium channels. Curr Opin Nephrol Hypertens. 1994;3:487–496. doi: 10.1097/00041552-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+channel. J Biol Chem. 1995a;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Lazdunski M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+channel function. J Biol Chem. 1995b;270:11735–11737. doi: 10.1074/jbc.270.20.11735. [DOI] [PubMed] [Google Scholar]
- Warncke J, Lindemann B. Voltage dependence of the blocking rate constants of amiloride at apical Na channels. Pflüg Arch. 1985;405:89–94. doi: 10.1007/BF00581786. [DOI] [PubMed] [Google Scholar]
- Yue G, Shoemaker RL, Matalon S. Regulation of low-amiloride-affinity sodium channels in alveolar type II cells. Am J Physiol. 1994;267:L94–L100. doi: 10.1152/ajplung.1994.267.1.L94. [DOI] [PubMed] [Google Scholar]