Abstract
The P2U purinergic agonist ATP (0.3 mM) elicited an increase in [Ca2+]i due to Ca2+ release from intracellular stores in transfected Chinese hamster ovary cells that express the bovine cardiac Na+/Ca2+ exchanger (CK1.4 cells). The following observations indicate that ATP-evoked Ca2+ release was accompanied by a Ca2+- dependent regulatory activation of Na+/Ca2+ exchange activity: Addition of extracellular Ca2+ (0.7 mM) 0–1 min after ATP evoked a dramatic rise in [Ca2+]i in Na+-free media (Li+ substitution) compared to Na+-containing media; no differences between Na+- and Li+-based media were observed with vector-transfected cells. In the presence of physiological concentrations of extracellular Na+ and Ca2+, the ATP-evoked rise in [Ca2+]i declined more rapidly in CK1.4 cells compared to control cells, but then attained a long-lived plateau of elevated [Ca2+]i which eventually came to exceed the declining [Ca2+]i values in control cells. ATP elicited a transient acceleration of exchange-mediated Ba2+ influx, consistent with regulatory activation of the Na+/Ca2+ exchanger. The acceleration of Ba2+ influx was not observed in vector-transfected control cells, or in CK1.4 cells in the absence of intracellular Na+ or when the Ca2+ content of the intracellular stores had been reduced by prior treatment with ionomycin. The protein kinase C activator phorbol 12-myristate 13-acetate attenuated the exchange-mediated rise in [Ca2+]i under Na+-free conditions, but did not inhibit the ATP-evoked stimulation of Ba2+ influx. The effects of PMA are therefore not due to inhibition of exchange activity, but probably reflect the influence of protein kinase C on other Ca2+ homeostatic mechanisms. We conclude that exchange activity is accelerated during ATP-evoked Ca2+ release from intracellular stores through regulatory activation by increased [Ca2+]i. In the absence of extracellular Ca2+, the stimulation of exchange activity is short-lived and follows the time course of the [Ca2+]i transient; in the presence of extracellular Ca2+, we suggest that the exchanger remains activated for a longer period of time, thereby stabilizing and prolonging the plateau phase of store-dependent Ca2+ entry.
Keywords: fura-2, endoplasmic reticulum, Ba uptake, protein kinase C, Na/Ca exchange
introduction
In Chinese hamster ovary (CHO)1 cells, the P2U purinergic agonist ATP elicits the production of 1,4,5-inositol trisphosphate (InsP3) and the release of Ca2+ from InsP3-sensitive stores (Iredale and Hill, 1993; Pijuan et al., 1993). Ca2+ influx is accelerated after Ca2+ release from internal stores, and this leads to an extended period of gradually declining [Ca2+]i after the peak of the transient as intracellular stores refill and Ca2+ influx subsides. The Ca2+ influx pathway in many cells involves low conductance Ca2+ channels (store-operated channels) that are activated by organellar Ca2+ release (Hoth and Penner, 1993). The mechanism of activation is not well understood but may involve a low molecular weight diffusible second messenger (Parekh et al., 1993; Randriamampita and Tsien, 1993). The Ca2+ influx pathway has been termed capacitative Ca2+ entry (Putney, 1990) or store-dependent Ca2+ influx (Clementi et al., 1992).
Recent work in our laboratory has been directed toward understanding the regulation of the cardiac Na+/ Ca2+ exchange system and its interactions with other Ca2+ homeostatic mechanisms. The experimental approach we have used involves examining the effects of Na+ on Ca2+ mobilization in transfected CHO cells permanently expressing the bovine cardiac Na+/Ca2+ exchanger (CK1.4 cells; Pijuan et al., 1993). Since nontransfected (or vector-transfected) CHO cells do not normally express Na+/Ca2+ exchange activity, comparing the behavior of CK1.4 cells with vector-transfected cells provides a means of identifying functional roles of exchange activity in relation to other Ca2+ homeostatic processes (Chernaya et al., 1996).
In the accompanying manuscript (Condrescu et al., 1997), we described the advantages of using Ba2+ as a surrogate for Ca2+ in studies of exchanger regulation. We concluded that Ba2+ is transported by the exchanger but is not significantly accumulated by intracellular organelles and that the exchanger becomes deactivated as [Ca2+]i is lowered by the Ca2+ sequestering activities of intracellular organelles. We had previously presented indirect evidence, based on fura-2 measurements of cytosolic Ca2+ ([Ca2+]i), that exchange activity was accelerated during ATP-evoked Ca2+ release in CK1.4 cells (Chernaya et al., 1996); however, in that study the various contributions of exchange activity, Ca2+ channel activity and changes in organellar Ca2+ sequestration to the [Ca2+]i response could not be clearly defined. In the present report, we show that ATP elicits a transient increase in exchange-mediated Ba2+ influx and that this acceleration of exchange activity is dependent upon an increase in [Ca2+]i. Other results provide additional information on the ATP-evoked Ca2+ movements and suggest that activation of the exchanger stabilizes and prolongs the plateau phase of store-dependent Ca2+ entry.
methods
The cells, materials, solutions, and procedures used are essentially identical to those described in the accompanying report (Condrescu et al., 1997). Calibration of the fura-2 signal in representative cell preparations for Ca2+ at 340 and 380 nm excitation as described (Condrescu et al., 1997) yielded Sf/Sb = 4.8, Rmin = 0.9, Rmax = 12. Assuming K D = 224 nM for the Ca2+/fura-2 complex (Grynkiewicz et al., 1985), the following ratios correspond to the indicated values for [Ca2+]i: 1.5 (60 nM), 2.0 (120 nM), 3.0 (250 nM), 4.0 (420 nM), and 5.0 (630 nM). Calibrations for Ba2+ and for Ca2+ at the 350/390 excitation wavelengths used for the Ba2+ influx studies were reported in Condrescu et al. (1997).
results
Effect of Na+ o on ATP-induced Ca2+ Influx
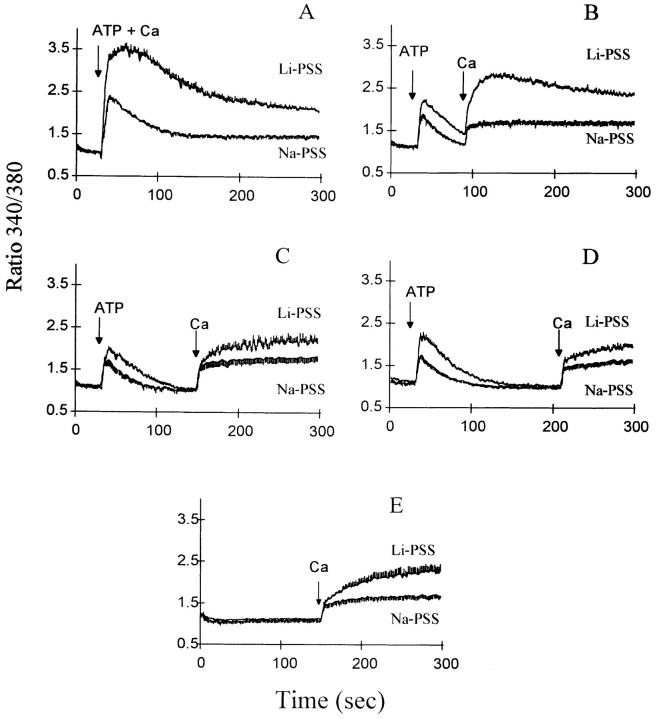
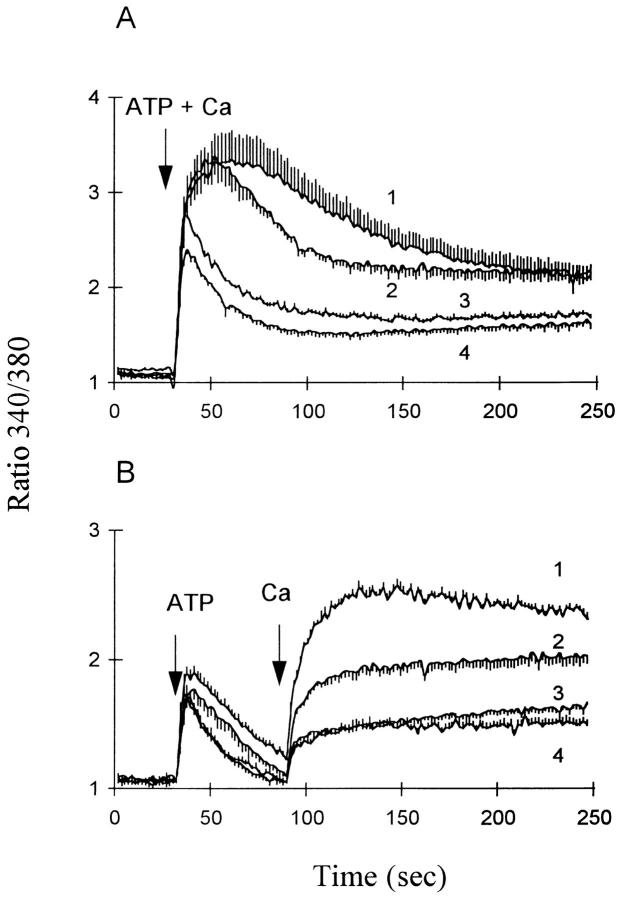
The expression of the Na+/Ca2+ exchanger in the transfected CHO cells profoundly affects their response to extracellular ATP. The data in Fig. 1 depict the changes in [Ca2+]i when ATP and Ca2+ are added to CK1.4 cells in either Na–physiological salts solution (PSS) or Li-PSS; traces are shown for the simultaneous addition of ATP and Ca2+ (Fig. 1 A), the addition of Ca2+ 1, 2 or 3 min after ATP (Fig. 1 B–D), or the addition of Ca2+ alone (E). As shown in Fig. 1, B, C, and D, the presence of Na+ accelerates the decline in the [Ca2+]i transient elicited by ATP under Ca2+-free conditions, as reported previously (Chernaya et al., 1996). Upon addition of Ca2+, the rise in [Ca2+]i was higher in Li-PSS than in Na-PSS, but the relative increase in Li-PSS was most pronounced when Ca2+ was added either simultaneously with ATP (cf. Chernaya et al., 1996) or within 1 min after ATP. When Ca2+ was added 2 or 3 min after ATP, the changes in [Ca2+]i in both media were similar to that observed upon the addition of Ca2+ alone.
Figure 1.
Effect of ATP on [Ca2+]i in CK1.4 cells. Cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and diluted 30-fold into cuvettes containing Na- or Li-PSS plus 0.3 mM EGTA. ATP (0.3 mM) was added to the cuvette at 30 s in each panel and 1 mM CaCl2 was added at various intervals after ATP as indicated in the individual panels; in A, ATP and CaCl2 were added simultaneously and in B, C, and D, CaCl2 was added 60, 120, and 180 s after ATP. In E, ATP was not added and CaCl2 was added at 150 s. Fura-2 fluorescence was monitored with alternate excitation at 340 and 380 nm. For all experiments, n = 3–4 except B, where n = 5–6.
In contrast to this behavior, control-transfected cells showed no increase in [Ca2+]i in Li-PSS compared to Na-PSS; indeed, the changes in [Ca2+]i tended to be somewhat reduced in Li-PSS (Fig. 2). Note that in Li-PSS, the increase in [Ca2+]i upon adding Ca2+ 1 min after ATP was much greater in CK1.4 cells than in control cells (compare B in Figs. 1 and 2); the average [Ca2+]i values in Li-PSS 30-60 s after the addition of Ca2+ were 221 ± 8 nM (n = 5) for CK1.4 cells and 71 ± 11 nM (n = 3) for control cells.
Figure 2.
Effect of ATP on [Ca2+]i in control cells. Conditions are identical to those described in Fig. 1 except that vector-transfected control CHO cells were used instead of CK1.4 cells. ATP and CaCl2 were added either simultaneously (A), or CaCl2 was added 60, 120, or 180 s after ATP in B, C, and D, respectively. Fura-2 fluorescence was monitored with alternate excitation at 340 and 380 nm. For all experiments, n = 1–2 (no error bars) except for B, where n = 3–4.
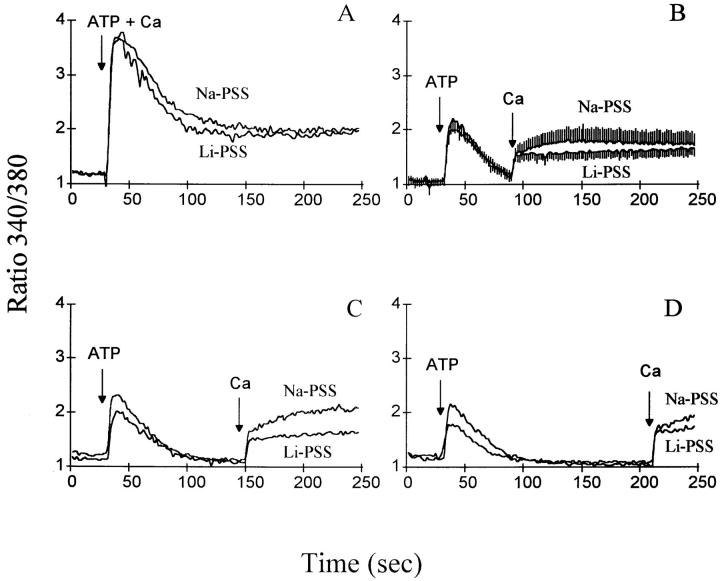
The responses of control and CK1.4 cells to ATP addition under physiological conditions, i.e., in Na-PSS containing 1 mM CaCl2, are directly compared in Fig. 3. The peak increase in [Ca2+]i upon ATP addition was higher for control cells than for CK1.4 cells. After the peak of the [Ca2+]i transient in the control cells, [Ca2+]i gradually declined toward the initial “resting” value (70–75 nM) over the time course of the experiment. With CK1.4 cells, the changes in [Ca2+]i elicited by ATP followed a markedly different time course: [Ca2+]i fell quickly after the peak of the transient and attained a stable plateau (100 ± 1 nM at 150–200 s; n = 6), which remained elevated compared to the initial [Ca2+]i (74 ± 2 nM). The more rapid decline in [Ca2+]i in the CK1.4 cells was clearly evident when the results were normalized for differences in the peak height of the transient (Fig. 3, inset). Although reduced in magnitude compared to control cells, the plateau of elevated [Ca2+]i in the CK1.4 cells was maintained for a longer period of time and indeed “crossed over” the declining [Ca2+]i trace for the control cells at ∼170 s in the figure.
Figure 3.
Comparison of control and CK1.4 cells: effect of ATP on [Ca2+]i in presence of extracellular Na+ and Ca2+. CK1.4 or control transfected cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and then diluted 30-fold into cuvettes containing Na-PSS + 1 mM CaCl2. Fura-2 fluorescence was monitored (340/380 excitation) and ATP (0.3 mM) was added as shown (n = 5–6).
ATP-induced Ba2+ Influx in CK1.4 Cells
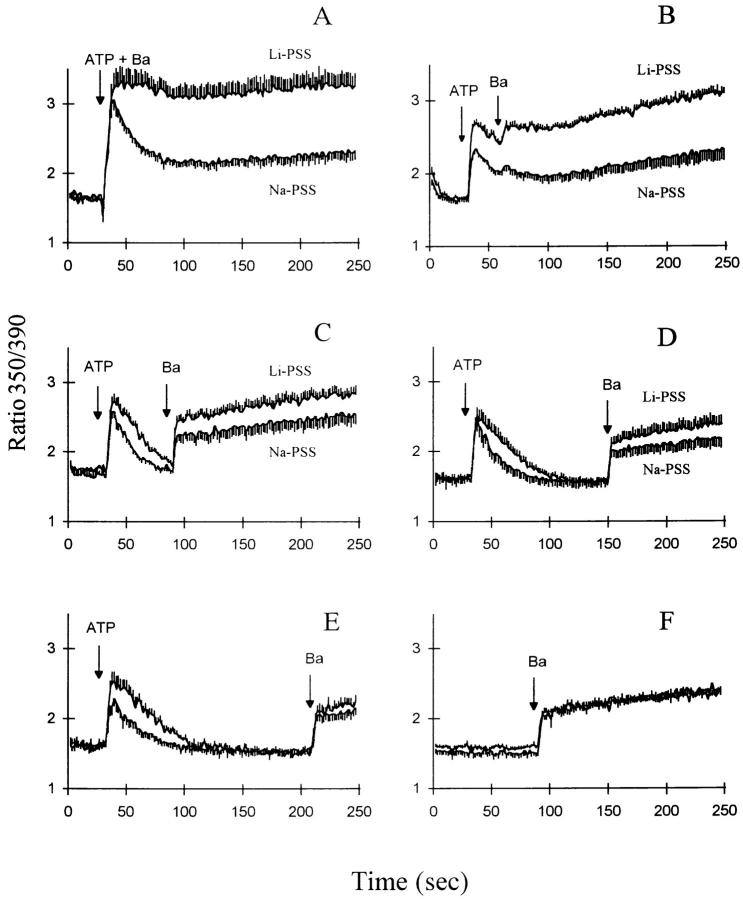
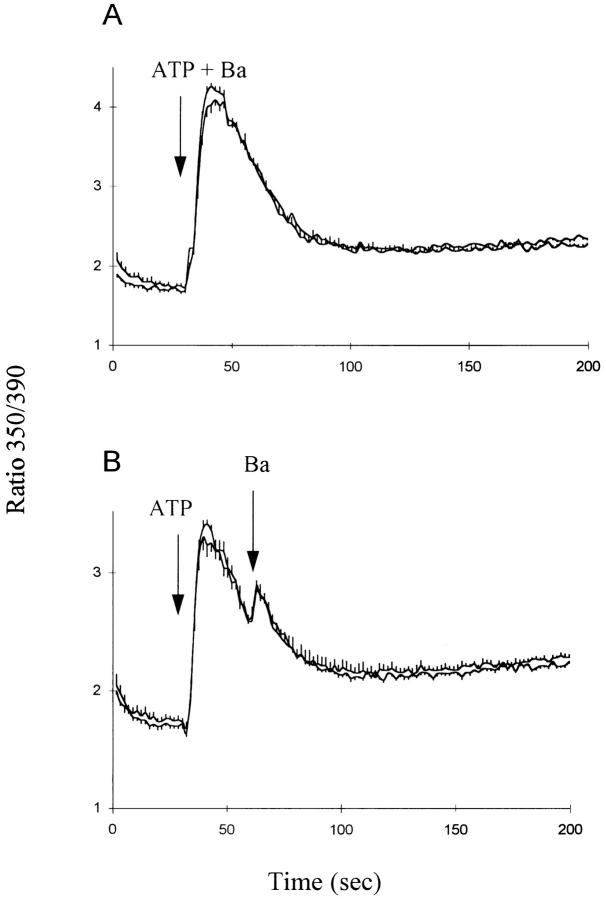
In the experiments depicted in Fig. 4, we examined the effects of extracellular ATP on Ba2+ influx. In general, ATP accelerated Ba2+ influx in Li-PSS compared to Na-PSS, and the time dependence of this acceleration was consistent with a transient activation of exchange activity which roughly coincided with the time course of the [Ca2+]i transient. The fura-2 traces in these experiments are highly complex because they reflect the combined influence of changes in [Ca2+]i and [Ba2+]i. For example, in the experiment where ATP and Ba2+ are added simultaneously (Fig. 4 A), the contribution of [Ca2+]i to the fura-2 signal would initially be expected to predominate because of the release of Ca2+ from InsP3-sensitive stores. The concentration of Ca2+ would then decline as Ca2+ is removed from the cytosol due to efflux or organellar sequestration. In Na-PSS, this is reflected in a decline in the fura-2 ratio, but in Li-PSS, the fura-2 ratio remains elevated because Ba2+ rapidly enters the cell via exchange activity. Two observations lend support to this interpretation: (a) The presence of extracellular or cytosolic Ba2+ does not interfere with the decline in [Ca2+]i after ATP addition (Condrescu et al., 1996). (b) When excess EGTA was added to the cells after Ba2+ influx under these conditions, the fura-2 signal did not decline (data not shown), as expected for cytosolic Ba2+ (Condrescu et al., 1997); in contrast, a rapid decline would be expected upon EGTA addition if the elevated fura-2 ratio were due to cytosolic Ca2+ (Chernaya et al., 1996).
Figure 4.
ATP and Ba2+ influx in CK1.4 cells. CK1.4 cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and diluted 30-fold into cuvettes containing either Li- or Na-PSS plus 0.3 mM EGTA. Fura-2 fluorescence was monitored with alternate excitation at 350 and 390 nm. ATP (0.3 mM) was added at 30 s in each panel and BaCl2 (1 mM) was added at increasing intervals following ATP, as indicated; in A, ATP and BaCl2 were added simultaneously. (n = 4–6 [A, B]; 3–4 [D, E] and 6–8 [C]).
When Ba2+ was added 0.5, 1, 2, or 3 min after ATP, the difference between the fura-2 signals in Li-PSS and Na-PSS diminished (Fig. 4 B–E) as the delay between ATP and Ba2+ addition increased. We conclude that exchange activity decreased progressively after ATP addition, consistent with the results obtained with Ca2+ (Fig. 1) and that the acceleration of exchange activity corresponded closely with the period of elevated [Ca2+]i. In the absence of ATP, no difference in Ba2+ influx between Li-PSS and Na-PSS (Fig. 4 F) could be detected when Ba2+ was added at 90 s (compare with Fig. 4 C); this indicates that the exchanger is inactive at this time in the absence of ATP addition. When control-transfected cells were used instead of CK1.4 cells, we observed little or no stimulation of Ba2+ influx by ATP and could detect no differences between Li- and Na-PSS (Fig. 5). This result strongly supports our interpretation that Ba2+ influx in Li-PSS in the CK1.4 cells is due to exchange activity.
Figure 5.
ATP and Ba2+ influx in control cells. Vector transfected control cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and added to cuvettes containing Li- or Na-PSS plus 0.3 mM EGTA. ATP (0.3 mM) and BaCl2 (1 mM) were added to the cuvettes, either simultaneously (A), or with a 1 min interval between ATP and Ba2+ additions (B). The traces in Na-PSS and Li-PSS are indistinguishable (n = 4).
As an additional test of this conclusion, we examined the effects of removing cytosolic Na+, a treatment that should block Ba2+ influx via the exchange activity. To remove cytosolic Na+, the gramicidin-treatment protocol described in the accompanying manuscript (Condrescu et al., 1997) was used (Fig. 6). In this experiment, fura-2–loaded cells were added to a cuvette containing 20/120 Na/K-PSS and gramicidin (1 μM) was added to permeabilize the membrane to monovalent cations. Results for CK1.4 cells are shown in Fig. 6 A. In the absence of ATP addition, the rate of Ba2+ influx (trace b) was essentially identical to that shown in Fig. 5 in the accompanying manuscript (Condrescu et al., 1997) for gramicidin-treated cells under very similar conditions ([Na+] = 19 mM). However, when ATP was added 60 s before Ba2+, the rate of Ba2+ influx was dramatically accelerated (trace a) compared to that seen in the absence of ATP addition. Ba2+ influx was completely abolished when ATP was added to cells in the absence of Na+ (trace c), although the transient due to Ca2+ release from InsP3-sensitive stores was normal. When the same experiment was carried out with vector-transfected control cells (Fig. 6 B), Ba2+ influx was essentially the same under all experimental conditions, and was identical to that shown in the absence of Na+ (trace c, Fig. 6 A) for the CK1.4 cells.
Figure 6.
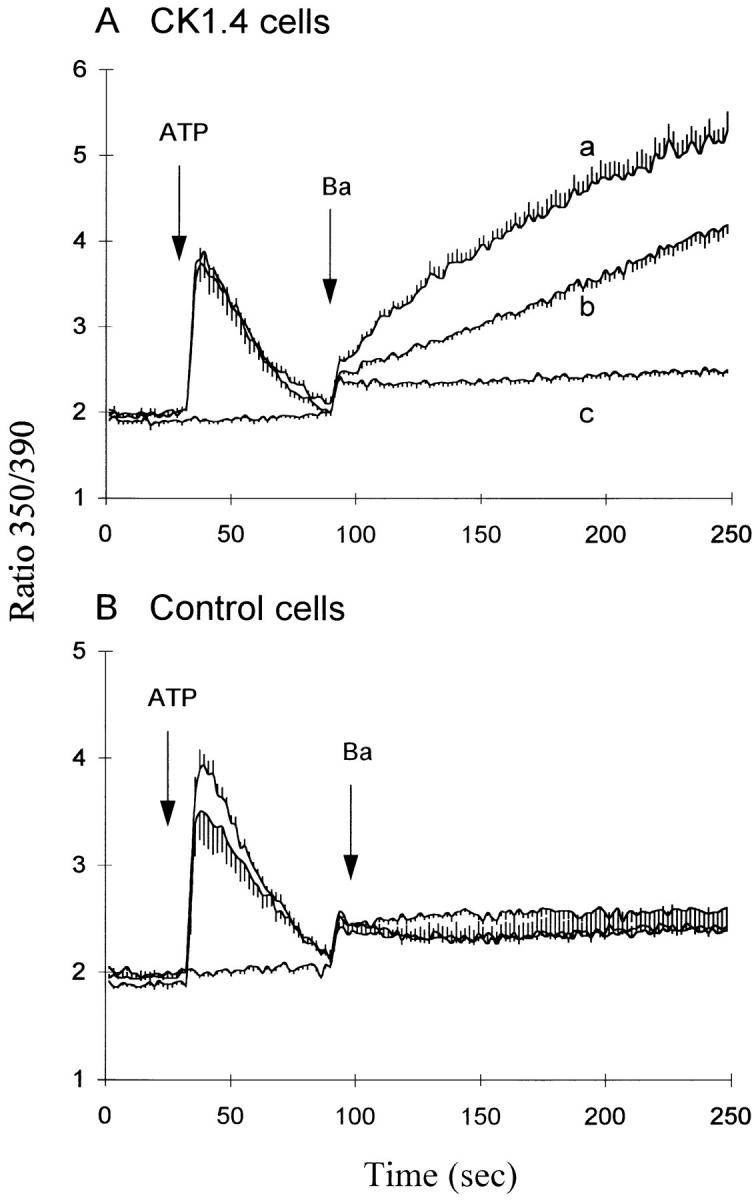
ATP and Ba2+ influx in gramicidin-treated cells. CK1.4 (A) or vector-transfected control (B) cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and diluted 30-fold into cuvettes containing 20/120 Na/K-PSS + 0.3 mM EGTA (traces a and c). For trace c, the cells were preincubated in K-PSS + 1 mM CaCl2 and then transferred to cuvettes containing K-PSS + 0.3 mM EGTA. Gramicidin (1μM) was added immediately after adding the cells to the cuvette. ATP (0.3 mM final concentration) was added at 30 s for traces a and c and was omitted for trace b ; 1 mM BaCl2 was added for all traces at 90 s (n = 5).
In the experiments with gramicidin-treated cells, considerable exchange-mediated Ba2+ influx was observed even in the absence of ATP addition (trace b; Fig. 6). This appears to reflect stimulation of the exchanger by the increase in [Ca2+]i brought about by gramicidin through its uncoupling action on mitochondria: A similar increase in [Ca2+]i was observed with the uncoupler carbonyl cyanide m-chlorophenylhydrazone (2 μM) or with the combination of oligomycin (2.5 μg/ml) plus rotenone (2 μM), and these agents also accelerated exchange activity (data not shown).
[Ca2+]i and Exchange Activity
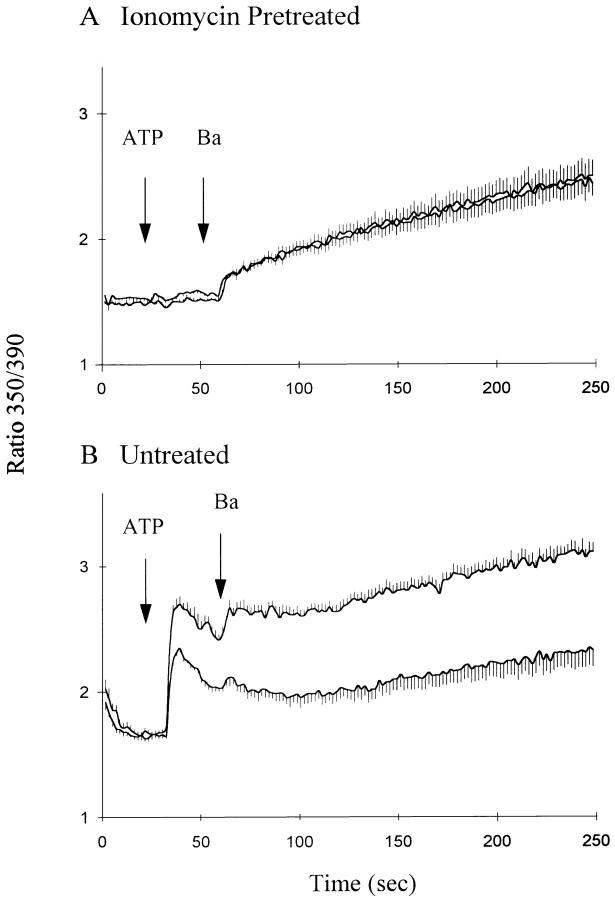
The effect of ATP on exchange-mediated Ba2+ influx is dependent upon the release of Ca2+ from internal stores. Thus, as shown in Fig. 7, when intracellular Ca2+ stores were depleted by pretreatment with ionomycin and the ionomycin was subsequently removed with BSA (Fig. 7 A), ATP elicited no increase in [Ca2+]i and the addition of Ba2+ 30 s later revealed no differences in Ba2+ influx between Li- and Na-PSS. The data in the Fig. 7 B are for cells in which the ionomycin treatment was omitted and show the expected increase in Ba2+ influx in Li- vs. Na-PSS. Thus, a rise in cytosolic Ca2+ appears to be necessary for the activation of exchange activity evoked by extracellular ATP.
Figure 7.
Acceleration of Ba2+ influx by ATP requires Ca2+ release. CK1.4 cells were preincubated for 1 min in Na-PSS + 0.3 mM EGTA + 10 μM ionomycin (A), or Na-PSS + 1 mM CaCl2 (B). The cells in A were centrifuged and then incubated for an additional 1 min in Na-PSS + 0.3 mM EGTA + 1% BSA to scavenge residual ionomycin; after a final centrifugation, the cells were resuspended in either Na- or Li-PSS + 0.3 mM EGTA and ATP (0.3 mM) and BaCl2 (1 mM) were added as indicated. The data in B were obtained using the same batch of cells as in A. In this case, however, the ionomycin pretreatment and BSA wash were omitted and the cells were added directly to the cuvette containing Na- or Li-PSS + 0.3 mM EGTA and additions were made as in A. The data in B are reproduced in B of Fig. 4 (n = 4–6).
Protein Kinase C and Exchange-mediated Ca2+ Influx
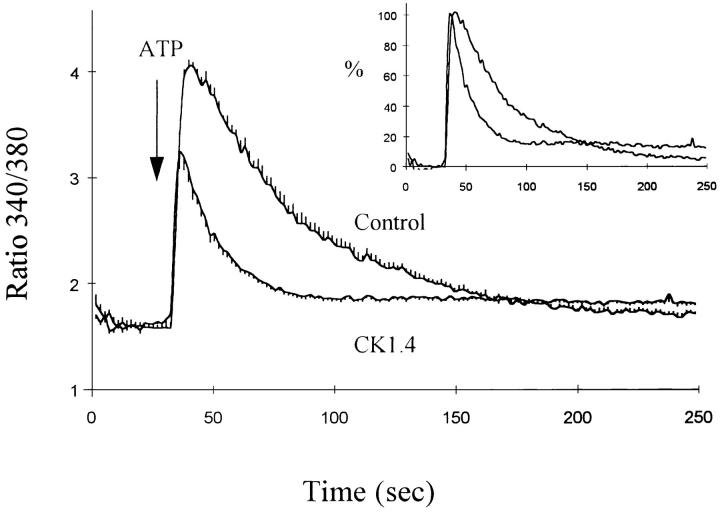
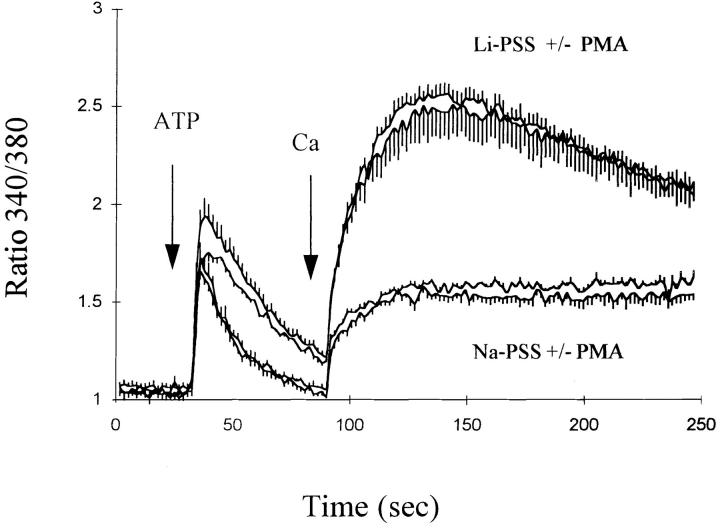
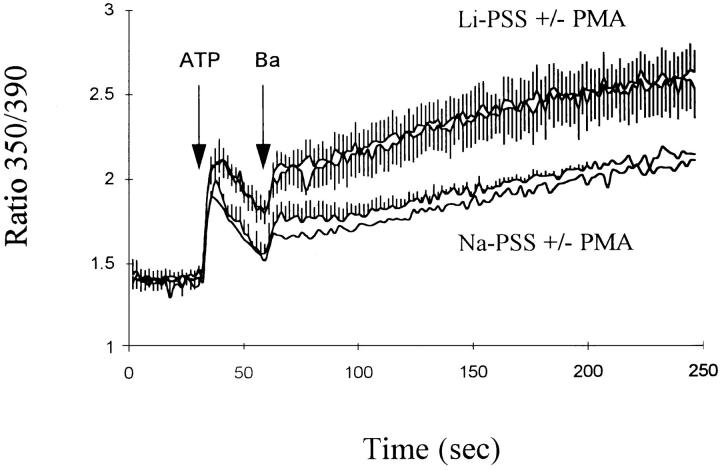
The protein kinase C activator phorbol 12-myristate 13-acetate (PMA) attenuated the effects of ATP on exchange-mediated Ca2+ entry in the CK1.4 cells. The data in Fig. 8 show that Ca2+ influx in Li-PSS was reduced in CK1.4 cells treated with 100 nM PMA (trace 2 in each panel); the effect was observed when ATP and Ca2+ were added simultaneously (Fig. 8 A) but appeared to be more pronounced in experiments where Ca2+ is added 1 min after ATP (Fig. 8 B). To determine whether the effect of PMA is mediated by protein kinase C, cells were treated overnight with 100 nM PMA, a procedure which down-regulates most isoforms of protein kinase C in many different cell types (Jaken et al., 1981). As shown in Fig. 9, overnight treatment with PMA completely blocked the acute effects of PMA on Ca2+ influx in response to ATP, consistent with the conclusion that PMA was acting through protein kinase C in the earlier experiments (Fig. 8). Down-regulation of protein kinase C did not appear to enhance exchange-mediated Ca2+ influx in comparison to untreated cells (compare traces in Fig. 9 with those in the lower panel of Fig. 8).
Figure 8.
PMA attenuates ATP-evoked Ca2+ influx in CK1.4 cells. CK1.4 cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and diluted 30-fold to cuvettes containing Li- or Na-PSS plus 0.3 mM EGTA. ATP (0.3 mM) and CaCl2 (1 mM) were added simultaneously (A) or with a 1 min interval between ATP and CaCl2 additions (B). For traces 2 and 4, 100 nM PMA was included in the cuvette solutions. Li-PSS: traces 1 and 2; Na-PSS: traces 3 and 4. In B, the order of the traces before the addition of Ca2+ are (from top) 1, 2, 3, and 4; the latter two are indistinguishable (n = 3–5).
Figure 9.
Down-regulation of protein kinase C abrogates acute affect of PMA. CK1.4 cells were incubated overnight with 100 nM PMA to down-regulate protein kinase C. The cells were then loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and diluted 30-fold into cuvettes containing Li- or Na-PSS + 0.3 mM EGTA with or without 100 nM PMA, as indicated. ATP (0.3 mM) and CaCl2 were added where indicated (n = 3–4).
The data in Fig. 10 show the effects of PMA on measured exchange-mediated Ba2+ influx after ATP addition. Ba2+ influx in Li-PSS was the same whether or not PMA was present, suggesting that the effects of PMA on Ca2+ influx (Fig. 8) were not brought about by an alteration in exchange activity itself.
Figure 10.
Effect of PMA on Ba2+ influx in CK1.4 cells. CK1.4 cells were loaded with fura-2, preincubated for 1 min in Na-PSS + 1 mM CaCl2 and diluted 30-fold into cuvettes containing Li- or Na-PSS plus 0.3 mM EGTA, with or without 100 nM PMA as indicated. Fura-2 fluorescence was monitored (350/390 nm excitation) and ATP (0.3 mM) and BaCl2 (1 mM) were added where indicated. (n = 2–4; duplicate experiments are shown in the trace without error bars).
discussion
ATP interacts with P2U receptors in CHO cells, promoting Ca2+ release from internal stores and an increased influx of extracellular Ca2+ (Iredale and Hill, 1993; Pijuan et al., 1993). The expression of the cardiac Na+/Ca2+ exchanger in the CK1.4 cells altered the cellular response to added ATP in comparison to control cells. In Ca2+-free media, the [Ca2+]i transient evoked by ATP declined more rapidly in the presence of extracellular Na+ than in its absence (Fig. 1; see also Chernaya et al., 1996), whereas Na+ had no effect on the [Ca2+]i transient in control cells (Fig. 2). The effects of Na+ in the CK1.4 cells undoubtedly reflect accelerated Ca2+ efflux due to exchange activity. Previously reported experiments (Chernaya et al., 1996) indicate that Na+ accelerates Ca2+ efflux two- to threefold in the CK1.4 cells.
In the absence of Na+ o, influx of Ca2+ or Ba2+ occurs by “reverse mode” exchange, i.e., Na+ i-dependent Ca2+ or Ba2+ influx. ATP accelerated Ca2+ influx in an Na+-free medium, but the relative contributions of store- operated Ca channels and reverse Na+/Ca2+ exchange to this process could not be estimated. Since Ba2+ does not pass through store-operated channels in these cells (Chernaya et al., 1996), the acceleration of Ba2+ influx after ATP-induced Ca2+ release (Fig. 4) clearly demonstrated that exchange activity had been activated; no acceleration of Ba2+ influx was observed under identical conditions for vector transfected control cells (Fig. 5). In cells treated with gramicidin to equalize Na+ concentrations across the plasma membrane, Ba2+ influx for the CK1.4 cells was negligible in the absence of Na+ and was virtually absent in control-transfected cells, regardless of the Na+ concentration (Fig. 6). These results provide compelling evidence that enhanced Ba2+ influx evoked by ATP reflects a regulatory activation of the Na+/Ca2+ exchanger.
The effects of ATP on exchange activity are most simply explained by secondary activation of the exchanger through increased [Ca2+]i. Although additional mechanisms cannot be ruled out, this interpretation is supported by the following observations: (a) The period of accelerated exchange activity appeared to follow the time course of the [Ca2+]i transient. Thus, the difference in Ba2+ influx between Li- and Na-PSS diminished as the interval between the ATP and Ba2+ additions increased (Fig. 4 A–C), although a small difference was still evident after [Ca2+]i had returned to its resting level (Fig. 4 D and E). (b) In cells pretreated with ionomycin to deplete intracellular Ca2+ stores, the exchanger was essentially inactive after removal of the ionophore with BSA (cf. Condrescu et al., 1997). Addition of ATP to ionomycin-treated cells did not elicit a [Ca2+]i transient, as expected because of the depleted Ca2+ stores, and did not accelerate Ba2+ influx (Fig. 7). (c) Experiments to be presented elsewhere indicate that chelation of [Ca2+]i with cytosolic EGTA (introduced by hydrolysis of the EGTA-acetoxymethyl ester) markedly reduced both the Ca2+ transient and the acceleration of Ba2+ influx by ATP. (In these experiments, EGTA has such a low affinity for Ba2+ that it does not affect the fura-2 signal for Ba2+.) (d) In preliminary results with cells expressing exchanger mutants that are not regulated by [Ca2+]i, the rate of Ba2+ influx was independent of [Ca2+]i and was not affected by ATP. Overall, the results are consistent with the conclusions of the accompanying manuscript (Condrescu et al., 1997) and suggest that the exchanger is deactivated as intracellular organelles resequester cytosolic Ca2+. As in the accompanying manuscript, small increases in [Ca2+]i (before Ba2+ addition) are associated with quite large increases in exchange activity (compare traces a and b in Fig. 6, for example).
When both extracellular Na+ and Ca2+ were present, i.e., under physiological conditions, the [Ca2+]i transient elicited by ATP declined more rapidly in CK1.4 cells than in control cells (Fig. 3 inset) but then achieved a stable plateau of elevated [Ca2+]i which outlasted the corresponding phase of elevated [Ca2+]i in the control cells (Fig. 3). Although the precise mechanism underlying this phenomenon remains to be determined, the following speculative scenario provides a reasonable interpretation of the results: (a) In comparison to the control cells, Ca2+ efflux via the exchanger in the CK1.4 cells accelerates the decline in [Ca2+]i after the peak of the transient; this leads to a greater depletion of the InsP3-sensitive stores in the CK1.4 cells and also retards refilling of the stores (Chernaya et al., 1996). (b) As a result of the greater store depletion, the period during which store-operated Ca2+ channels remain active is prolonged in the CK1.4 cells. (c) Ca2+ influx through store-operated channels elevates [Ca2+]i beneath the plasma membrane and maintains the exchanger in an activated state. Thus, we suggest that the exchanger and store-dependent Ca2+ entry mechanisms mutually reinforce each other's activity so as to prolong the sustained phase of Ca2+ entry during continuous receptor stimulation. Although highly speculative, this interpretation suggests a potential mechanism by which the Na+/Ca2+ exchanger could modulate and sustain Ca2+ signalling processes.
Activation of protein kinase C with PMA attenuated the rise in [Ca2+]i associated with Ca2+ influx via the exchanger after ATP administration (Fig. 8). This effect was not exerted directly on exchange activity, however, since PMA failed to affect exchange-mediated Ba2+ influx after ATP addition (Fig. 10); other experiments showed that PMA also had no affect on Ba2+ influx by ouabain-loaded cells under Na+-free conditions (data not shown). These results are consistent with previous results from our laboratory (Condrescu et al., 1995), but contrast sharply with a recent report by Iwamoto et al.(1996) who observed that PMA stimulated phosphorylation of the exchanger, and modestly accelerated exchange-mediated 45Ca2+ fluxes, in cardiac myocytes and transfected CCL39 cells. At present, the difference between the two sets of results is unexplained. We conclude that the effects of PMA in the CK1.4 cells reflect a secondary influence of protein kinase C activity through Ca2+ homeostatic mechanisms other than Na+/Ca2+ exchange. Recent results with other cell types provide evidence for at least two such mechanisms: protein kinase C activity inhibits influx of Ca2+ through store- operated Ca2+ channels (Parekh and Penner, 1995) and accelerates the activity of the plasma membrane Ca2+ ATPase (see Balasubramanyam and Gardner [1995] and references cited therein).
Our results suggest that Na+/Ca2+ exchange activity in these cells is regulated in a sensitive and dynamic fashion through Ca2+ uptake and release by intracellular stores. The reduction in exchange activity by intracellular stores as they sequester cytosolic Ca2+ and the corresponding activation of the exchanger as the stores fill to capacity would act to maintain both [Ca2+]i and the Ca2+ content of InsP3-sensitive stores at relatively constant levels (Condrescu et al, 1997). Factors which modulate the driving force for Na+/Ca2+ exchange and/or the affinity of the exchanger's regulatory sites for Ca2+ would therefore be expected to exert corresponding influences on the Ca2+ content of the stores. For cells which exhibit both Na+/Ca2+ exchange and InsP3-sensitive Ca2+ stores (e.g., vascular smooth muscle cells, chromaffin cells, neutrophils, pancreatic β cells) these feedback relations are likely to be important components of the Ca2+ signalling process.
Acknowledgments
We are grateful to Drs. Madalina Condrescu, Galina Chernaya and Masayuki Kimura for helpful discussions during the course of these studies.
This work was supported by National Institutes of Health grant HL 49932.
Footnotes
Abbreviations used in this paper: CHO, Chinese hamster ovary; InsP3, inositol (1,4,5)trisphosphate; PSS, physiological salts solution.
references
- Balasubramanyam M, Gardner JP. Protein kinase C modulates cytosolic free calcium by stimulating calcium pump activity in Jurkat T cells. Cell Calcium. 1995;18:526–541. doi: 10.1016/0143-4160(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Chernaya G, Vázquez M, Reeves JP. Sodium-calcium exchange and store-dependent calcium influx in transfected Chinese hamster ovary cells expressing the bovine cardiac sodium-calcium exchanger. Acceleration of exchange activity in thapsigargin-treated cells. J Biol Chem. 1996;271:5378–5385. doi: 10.1074/jbc.271.10.5378. [DOI] [PubMed] [Google Scholar]
- Clementi E, Scheer H, Zacchetii D, Fasolato C, Pozzan T, Meldolesi J. Receptor-activated Ca2+influx. J Biol Chem. 1992;267:2164–2172. [PubMed] [Google Scholar]
- Condrescu M, Gardner JP, Chernaya G, Aceto JF, Kroupis C, Reeves JP. ATP-dependent regulation of sodium-calcium exchange in Chinese hamster ovary cells transfected with the bovine cardiac sodium-calcium exchanger. J Biol Chem. 1995;270:9137–9146. doi: 10.1074/jbc.270.16.9137. [DOI] [PubMed] [Google Scholar]
- Condrescu M, Chernaya G, Kalaria V, Reeves JP. Barium influx mediated by the cardiac sodium-calcium exchanger in transfected Chinese hamster ovary cells. J Gen Physiol. 1997;109:41–51. doi: 10.1085/jgp.109.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Peonie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release activated calcium current in rat mast cells. J Physiol (Lond) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale PA, Hill SJ. Increases in intracellular calcium via activation of an endogenous P2-purinoceptor in cultured CHO-K1 cells. Br J Pharmacol. 1993;110:1305–1310. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HE, Shigekawa M. Phosphorylation-dependent regulation of cardiac Na/Ca exchanger via protein kinase C. J Biol Chem. 1996;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- Jaken S, Tashjian AH, Jr, Blumberg PM. Characterization of phorbol ester receptors an their down-modulation in GH4C1 rat pituitary cells. Cancer Res. 1981;41:2175–2181. [PubMed] [Google Scholar]
- Parekh AB, Penner R. Depletion activated calcium current is inhibited by protein kinase in RBL-2H3 cells. Proc Natl Acad Sci USA. 1995;92:7907–7911. doi: 10.1073/pnas.92.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Terlau H, Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+current by means of a phosphatase and a diffusible messenger. Nature (Lond) 1993;364:814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Pijuan V, Shuang Y, Smith L, Kroupis C, Condrescu M, Aceto JF, Reeves JP, Smith JB. Stable expression of the cardiac sodium-calcium exchanger in CHO cells. Am J Physiol. 1993;264:C1066–C1074. doi: 10.1152/ajpcell.1993.264.4.C1066. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Receptor-regulated calcium entry. Pharmacol Ther. 1990;48:427–434. doi: 10.1016/0163-7258(90)90059-b. [DOI] [PubMed] [Google Scholar]
- Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that simulates Ca2+influx. Nature (Lond) 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]