Abstract
We examined Ba2+ influx using isotopic and fura-2 techniques in transfected Chinese hamster ovary cells expressing the bovine cardiac Na+/Ca2+ exchanger (CK1.4 cells). Ba2+ competitively inhibited exchange-me diated 45Ca2+ uptake with a K i ∼ 3 mM. Ba2+ uptake was stimulated by pretreating the cells with ouabain and by removing extracellular Na+, as expected for Na+/Ba2+ exchange activity. The maximal velocity of Ba2+ accumulation was estimated to be 50% of that for Ca2+. When the monovalent cation ionophore gramicidin was used to equilibrate internal and external concentrations of Na+, Ba2+ influx was negligible in the absence of Na+ and increased to a maximum at 20–40 mM Na+. At higher Na+ concentrations, Ba2+ influx declined, presumably due to the competition between Na+ and Ba2+ for transport sites on the exchanger. Unlike Ca2+, Ba2+ did not appear to be taken up by intracellular organelles: Thus, 133Ba2+ uptake in ouabain-treated cells was not reduced by mitochondrial inhibitors such as Cl-CCP or oligomycin-rotenone. Moreover, intracellular Ca2+ stores that had been depleted of Ca2+ by pretreatment of the cells with ionomycin (a Ca2+ ionophore) remained empty during a subsequent period of Ba2+ influx. Ca2+ uptake or release by intracellular organelles secondarily regulated exchange activity through alterations in [Ca2+]i. Exchange-mediated Ba2+ influx was inhibited when cytosolic [Ca2+] was reduced to 20 nM or less and was accelerated at cytosolic Ca2+ concentrations of 25–50 nM. We conclude that (a) Ba2+ substitutes for Ca2+ as a transport substrate for the exchanger, (b) cytosolic Ba2+ does not appear to be sequestered by intracellular organelles, and (c) exchange-mediated Ba2+ influx is accelerated by low concentrations of cytosolic Ca2+.
Keywords: fura-2, endoplasmic reticulum, Ba uptake, Na/Ca exchange, CHO cells
introduction
The cardiac Na+/Ca2+ exchanger couples the transmembrane movement of 3 Na+ ions to that of a single Ca2+ ion in the opposite direction. It is the principal mechanism for mediating Ca2+ efflux in cardiac myocytes. It plays a critical role in regulating cardiac contractility by competing with the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA)1 for cytosolic Ca2+, thereby modulating the amount of releasable Ca2+ stored in the sarcoplasmic reticulum (reviewed in Reeves, 1995). Kinetic results are compatible with a consecutive exchange mechanism, in which Ca2+ and Na+ are translocated in separate steps (Khananshvili, 1990; Hilgemann et al. 1991).
Exchange activity is regulated by Ca2+- and/or ATP-dependent processes that affect the exchanger's distribution between an active state and either of two inactive states (Hilgemann et al., 1992a , b ). Entry of the exchanger into the first inactive state (I1 inactivation) is thought to occur when the exchanger is fully loaded with Na+ at the cytoplasmic membrane surface; this mode of inactivation is observed experimentally as a time-dependent decrease in “reverse” Na+/Ca2+ exchange (Na+ i-dependent Ca2+ influx) following a step increase in [Na+]i (Hilgemann, 1990). The second (I2) mode of inactivation is promoted by the absence of cytosolic Ca2+ and is detected experimentally as an activation of reverse exchange activity by submicromolar concentrations of cytosolic Ca2+ (secondary Ca2+ activation) (DiPolo, 1979; DiPolo and Beaugé, 1987). Cytosolic ATP counteracts both modes of inactivation, although the precise mechanism(s) involved have not been delineated. Despite the advances in our understanding of the regulatory behavior of the exchanger in subcellular or internally dialyzed cellular preparations, much less information is available on how exchange activity is regulated in intact cells, or how it interacts with other Ca2+ homeostatic mechanisms. Part of the difficulty in studying these issues stems from the technical limitations of Ca2+ influx measurements. Both 45Ca2+ fluxes and fura-2 measurements are greatly affected by the sequestration and release of Ca2+ from the endoplasmic reticulum and the mitochondria. A previous report (Chernaya et al., 1996) used Ba2+ as a substitute for Ca2+ to assess effects of thapsigargin (Tg) on Na+/Ca2+ exchange activity in transfected Chinese hamster ovary cells expressing the bovine cardiac Na+/Ca2+ exchanger. Here we describe detailed studies of Ba2+ transport by the Na+/Ca2+ exchanger in these cells. Consistent with results obtained with other cells, cytosolic Ba2+ is not significantly accumulated by either the endoplasmic reticulum or the mitochondria. Measurements of Na+ i-dependent Ba2+ influx therefore provide a more direct measure of exchange activity than the corresponding Ca2+ flux measurements. Moreover, regulatory activation of Nai-dependent Ba2+influx by [Ca2+]i can be readily observed under appropriate conditions. Our results suggest that Ca2+ i-dependent activation of exchange activity involves a complex interplay between [Ca2+]i and various intracellular Ca2+ compartments.
methods
Cells
CK1.4 cells were prepared by transfection of dhfr − CHO cells (CCL 61; American Type Culture Collection, Rockville, MD) with a mammalian expression vector (pcDNA I/Neo; Invitrogen Corp., San Diego, CA) containing a cDNA insert coding for the bovine cardiac Na+/Ca2+ exchanger (Aceto et al. 1992; Pijuan et al. 1993). Control cells were prepared by transfection of the CHO cells with pcDNA3 (Invitrogen Corp.), a closely related expression vector with no cDNA insert. The cells were grown in Iscove's modified Dulbecco's medium containing 500 μg/ml geneticin (G418; GIBCO, Gaithersburg, MD), either 10% FCS (JRH Biosciences, Lenexa, KS) or 10% supplemented calf serum (Cool Calf 2; Sigma Chemical Co., St. Louis, MO) and antibiotics as described (Pijuan et al., 1993). Unless otherwise specified, all biochemicals were obtained from Sigma Chemical Co.
Solutions
Na-PSS (physiological salts solution) contains 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, and 20 mM MOPS, pH adjusted to 7.4 (37°C) with Tris. NMDG-, Li-, and K-PSS have the same composition as Na-PSS, except that Na+ is replaced with NMDG+, Li+, and K+, respectively. The termination medium for the 45Ca2+ transport assay consists of 100 mM MgCl2, 10 mM LaCl3, and 5 mM MOPS, pH 7.4 (Tris).
45Ca2+ and 133Ba2+ Uptake
Cells were grown to confluence in 24-well plastic dishes and preincubated for 30 min at 37°C with 1 ml/well of nominally Ca2+-free Na-PSS with or without 0.4 mM ouabain as indicated. The preincubation medium was then aspirated and replaced with 200 μl of assay medium (Na- or NMDG-PSS, as indicated) containing 100 μM 45Ca2+ or 133Ba2+. Radioisotopes were obtained from DuPont NEN Research Products (Boston, MA). For cells preincubated with ouabain, the assay solutions also contained 0.4 mM ouabain. After the desired interval, the wells were washed four times with 1 ml of termination medium; the contents of the wells were then extracted with 1 ml of 0.1 N HNO3 and counted. Protein was determined in separate sample wells by the Lowry method (Lowry, 1951). Data are presented as the mean values ± SEM (error bars shown in figures) for the indicated number (n) of experiments.
Fura-2 Assays
Cells were grown to confluence in 75-cm2 plastic culture flasks and washed three times with Na-PSS. The cells were released from the flask with 5 ml of Na-PSS + 5 mM EDTA, centrifuged, and resuspended in 5 ml of Na-PSS + 1 mM CaCl2, centrifuged again, and resuspended in 4–5 ml of Na-PSS + 1 mM CaCl2 + 1% BSA; the BSA aids in solubilizing the added fura-2-AM (Thomas and Delaville, 1991). The cells were distributed in individual 0.3-ml aliquots to plastic tubes and allowed to incubate for 30 min at 37°C to recover from isolation. Individual tubes of cells were loaded at 10-min intervals for 30 min (37°C) with 3 μM fura-2-AM (Molecular Probes, Eugene, OR) and 0.25 mM sulfinpyrazone to retard transport of fura-2 out of the cell (DiVirgilio et al., 1988; Pijuan et al., 1993). The fura-2 and sulfinpyrazone were added as 1,000-fold concentrated stock solutions in dimethyl sulfoxide. Where indicated, ouabain (0.4 mM) was also added as a 1,000-fold concentrated solution in dimethyl sulfoxide. After the 30-min loading period, the cells were rapidly centrifuged in an Eppendorf Mini-centrifuge, washed, and preincubated in 0.1 ml of the desired medium for 1 min as specified in individual experiments. The cells were then added directly to a cuvette containing 3 ml of either Na-PSS or Li-PSS and fura-2 fluorescence was monitored at 510-nm emission with alternate excitation at 350/390 nm (Schilling et al., 1989), using a Photon Technology International (South Brunswick, NJ) RF-M 2001 fluorometer. All fluorescence values were corrected for autofluorescence using cells that had not been loaded with fura-2. Data are presented as the ratio of fluorescence values at the 350/390 excitation wavelengths and represent the mean values ± SEM (error bars shown in figures) for the indicated number (n) of experiments. Calibrations were conducted with digitonin-permeabilized cells according to the procedure of Grynkiewicz et al. (1985) and yielded values of Rmax = 7.5, Rmin = 1.4 and Sf/Sb = 2.75 under our experimental conditions. K D values for Ba2+ and fura-2 of 0.8 μM (Schilling et al., 1989), 1.4 μM (Kwan and Putney, 1990), and 2.4 μM (McCormack and Osbaldeston, 1990) have been reported; the average K D (1.5 μM) yields [Ba2+] values of 0.5, 1.5, 3.1, and 6.0 μM at 350/390 ratios of 2, 3, 4, and 5, respectively. Fura-2 also responds to Ca2+ at the excitation wavelengths used for the Ba2+ measurements. Assuming K D = 224 nM for the Ca2+/fura-2 complex (Grynkiewicz et al., 1985), the following 350/390 excitation ratios correspond to the indicated values for [Ca2+]i: 2.0 (67 nM), 2.5 (135 nM), 3.0 (219 nM), 4.0 (457 nM), and 5.0 (887 nM).
results
Isotope Flux Studies
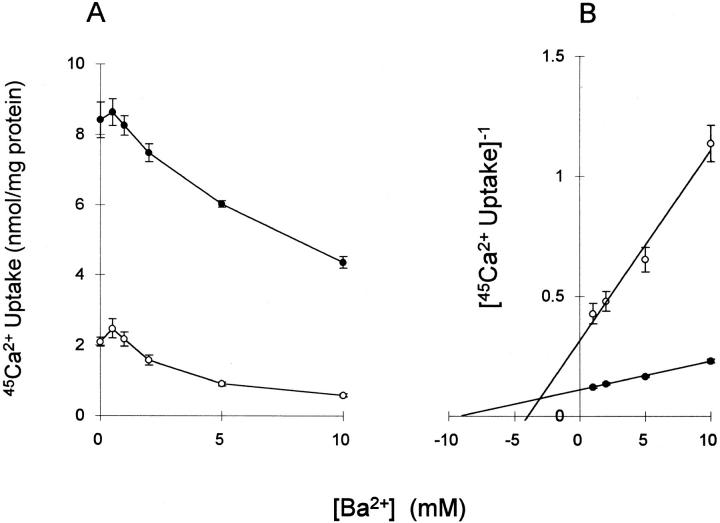
The data in Fig. 1 show the effects of various concentrations of Ba2+ on the rates of 45Ca2+ uptake by ouabain-treated CK1.4 cells in a Na+-free medium. Ba2+ inhibited 45Ca2+ uptake at both 0.1 and 1.0 mM [Ca2+], but was a more potent inhibitor at the lower Ca2+ concentration. Curiously, low concentrations of Ba2+ (0.1 mM) slightly stimulated 45Ca2+ uptake; the explanation for this is unclear, and this phenomenon was not investigated further. At Ba2+ concentrations >1 mM, the inhibition was competitive (K i = 3.1 mM) as indicated by the Dixon plot in the right panel of Fig. 1; including the full range of Ba2+ concentrations in the Dixon plot shifted the apparent K i to 4.5 mM (data not shown). The results imply that Ba2+ interacts with the Ca2+ transport site on the Na+/Ca2+ exchanger.
Figure 1.
Ba2+ inhibits exchange-mediated 45Ca2+ uptake by CK1.4 cells. (Left) Cells were pretreated with 0.4 mM ouabain and assayed for 45Ca2+ uptake (15 s) in NMDG-PSS containing the indicated concentrations of BaCl2. The concentrations of 45Ca2+ used are 1 mM (filled circles) and 0.1 mM (open circles). (Right) The data from the left panel for 1–10 min are presented as a Dixon plot. The point of intersection of the two lines yields an apparent K i for Ba2+ of 3 mM (n = 5).
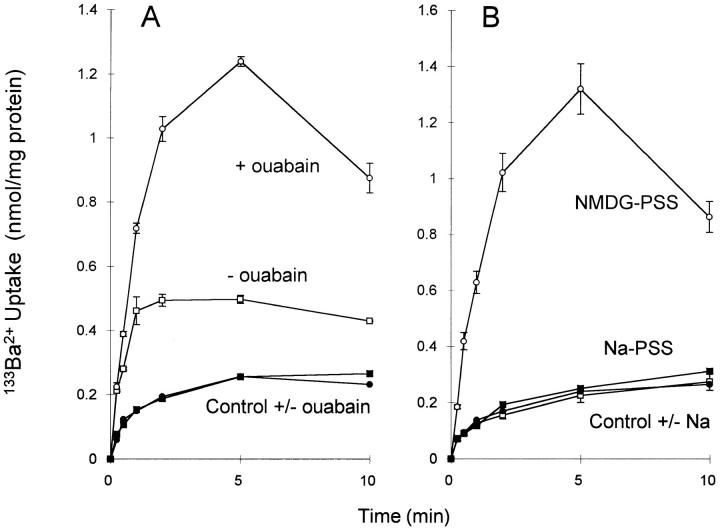
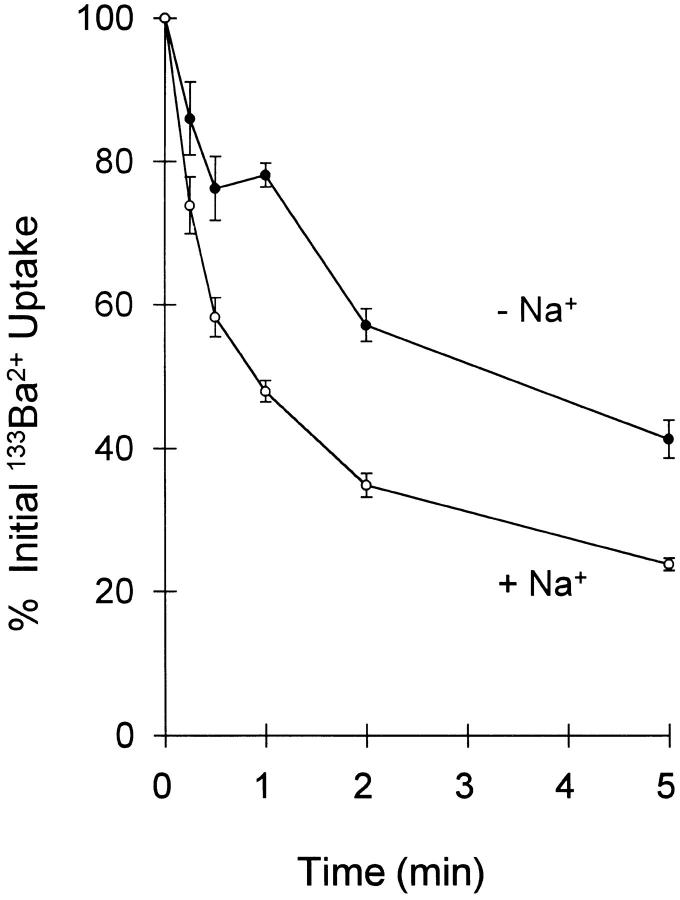
To determine whether Ba2+ is transported by the exchanger, we examined 133Ba2+ uptake. As shown in Fig. 2 A (open symbols), 133Ba2+ uptake in an Na-free medium was stimulated by prior treatment of the cells with ouabain to elevate intracellular Na+. In the presence of physiological concentrations of extracellular Na+, 133Ba2+ uptake by ouabain-treated cells was strongly inhibited (Fig. 2 B, open squares). For vector transfected control cells (Fig. 2, filled symbols), which do not exhibit Na+/ Ca2+ exchange activity (Pijuan et al., 1993; Chernaya et al., 1996), 133Ba2+ uptake was <20% of the maximal levels shown by CK1.4 cells and was unaffected by ouabain treatment or Na-free conditions. The results with 133Ba2+ are qualitatively similar to those obtained with 45Ca2+; the maximal levels of 45Ca2+ uptake, however, are generally three to five times higher than for 133Ba2+ (see, for example, Fig. 6). Based on the initial rates of exchange-mediated 133Ba2+ uptake in Fig. 2, and assuming a K m of 3 mM, we calculate a Vmax for Ba2+ uptake of 3.7 nmol/mg protein/15 s, or ∼50% of that reported previously for 45Ca2+ uptake (Condrescu et al., 1995). As shown in Fig. 3, extracellular Na+ stimulated 133Ba2+ efflux from preloaded CK1.4 cells, suggesting that the exchanger also transports Ba2+ out of the cells. The efflux data with 133Ba2+ are again qualitatively similar to those obtained using 45Ca2+.
Figure 2.
133Ba2+ uptake by CK1.4 cells and vector-transfected control cells. (A) Cells were preincubated with or without 0.4 mM ouabain, as indicated, and assayed for 133Ba2+ uptake in NMDG-PSS. (B) Cells were preincubated with ouabain and assayed for 133Ba2+ uptake in NMDG-PSS or Na-PSS, as indicated. Open symbols, CK1.4 cells (n = 3); closed symbols, control cells (n = 2).
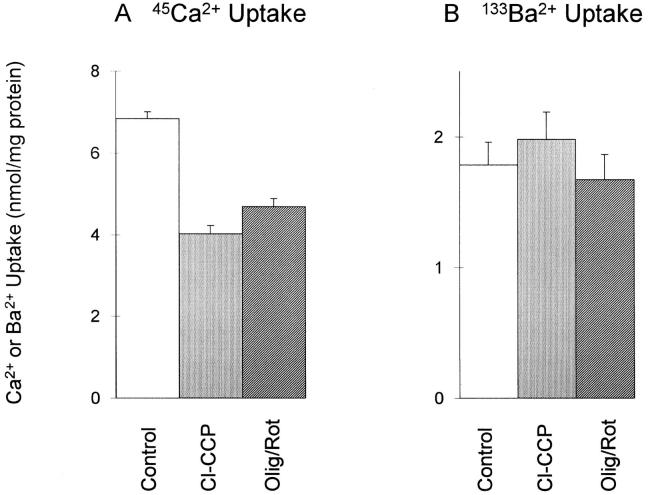
Figure 6.
Effect of mitochondrial antagonists on 45Ca2+ and 133Ba2+ uptake by CK1.4 cells. Cells were treated with ouabain and assayed for in NMDG-PSS for 45Ca2+ (0.1 mM) or 133Ba2+ (0.1 mM) uptake (5 min); where indicated 10 μM Cl-CCP or 2.5 μg/ml oligomycin + 2 μM rotenone were present in the assay medium (n = 6, 133Ba2+; n = 4, 45Ca2+).
Figure 3.
133Ba2+ efflux from CK1.4 cells. Ouabain-treated cells were allowed to accumulate 133Ba2+ for a period of 5 min and then placed in Na-PSS (open circles) or NMDG-PSS (closed circles) for the indicated intervals (n = 4).
Fura-2 Measurement of Ba2+ Influx
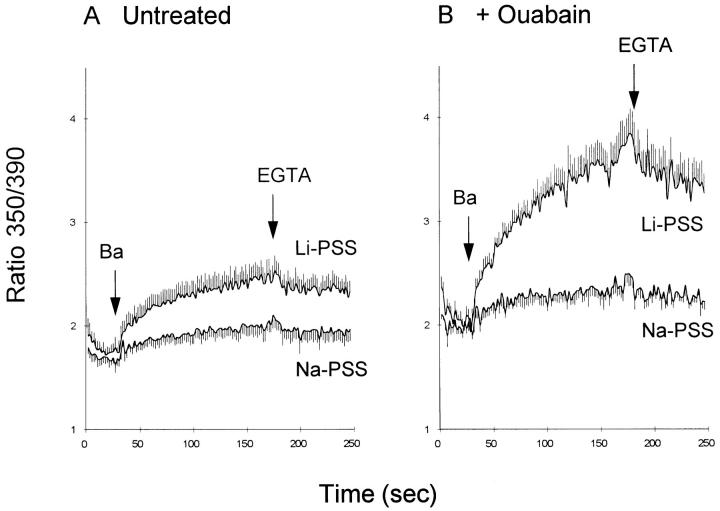
An alternate method of assaying Ba2+ movements is to use the Ca2+-indicating dye fura 2 (Schilling et al., 1989). As shown in Fig. 4 A, the addition of Ba2+ to fura 2–loaded CK1.4 cells produced an increase in the 350/ 390 excitation ratio which was enhanced by Na+ o-removal (Li+-substitution). Pretreating the cells with ouabain to elevate [Na+]i (Fig. 4 B) increased the 350/390 excitation ratio under Na+-free conditions, consistent with the expected increase in Na+ i-dependent Ba2+ influx via the exchanger. Similar results were obtained when N -methyl-d-glucamine was used as the Na+ substitute instead of Li+ (data not shown). When vector-transfected control cells were used instead of CK1.4 cells, the increase in the 350/390 ratio was not affected by Na+-removal or by ouabain-treatment and was approximately equivalent to that seen in the presence of Na+ for the CK1.4 cells (data not shown).
Figure 4.
Ba2+ influx in CK1.4 cells detected with fura-2. Cells were loaded with fura-2 without (A) or with (B) 0.4 mM ouabain, washed, and preincubated for 1 min in Na-PSS + 1 mM CaCl2 and then diluted 30-fold into cuvettes containing either Li-PSS or Na-PSS, as indicated; 0.3 mM EGTA was included in both media. BaCl2 (1 mM) and EGTA (10 mM) were added as indicated by the arrows (n = 5).
Adding 10 mM EGTA after a period of Ba2+ accumulation in the Na-free medium resulted in little or no decline in the 350/390 ratio (Fig. 4) suggesting that cytosolic Ba2+ is transported poorly or not at all by the ATP-dependent Ca2+ pumps. In comparable experiments conducted with Ca2+ instead of Ba2+, EGTA addition results in a rapid decline in [Ca2+]i (see Condrescu et al., 1995; Chernaya et al., 1996). The results with Ba2+ in the fura-2 experiments differ from the results of the 133Ba2+ flux studies (Fig. 3), which indicated that 133Ba2+ was lost from the CK1.4 cells, even in the absence of extracellular Na+ (cf., discussion).
Dependence of Ba2+ Influx on [Na+]i
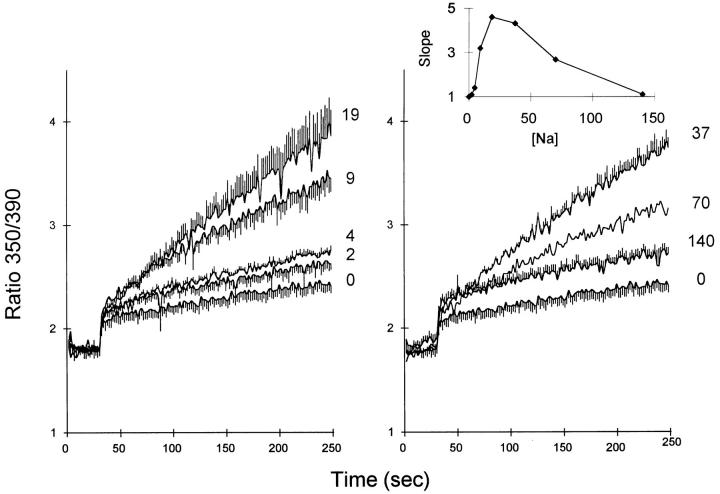
The effects of ouabain treatment indicate that Ba2+ entry is accelerated when [Na+]i is increased. The influence of [Na+]i is examined more directly in the experiments shown in Fig. 5. Fura-2–loaded CK1.4 cells were placed in cuvettes containing K-PSS with various concentrations of Na+ (mM concentrations given in Fig. 5 next to individual traces) and treated with 1 μM gramicidin to bring about rapid equilibration of monovalent cations across the plasma membrane. Thus, in the presence of gramicidin, Na+ concentrations should be equal on both sides of the cell membrane. In the absence of Na+, Ba2+ influx was negligible. (The initial, abrupt rise in the fura-2 ratio upon addition of Ba2+ is due to the presence of small amounts of extracellular fura-2.) Increasing concentrations of Na+ produced progressively higher rates of Ba2+ influx with maximal rates at 20–40 mM Na+. With higher Na+ concentrations, Ba2+ influx declined to a level that was only slightly higher, at 140 mM Na+, than that observed in the absence of Na+. The slopes of the fura-2 traces are presented as a function of [Na+] in the inset to the right panel of Fig. 5. The increasing rates of Ba2+ influx within the range of 0–20 mM [Na+] most likely reflect the stimulatory effects of cytosolic Na+ in activating exchange activity. The decline in Ba2+ influx rates at higher Na+ concentrations is probably due to competition between external Na+ and Ba2+ for transport sites on the exchange carrier. When control transfected cells were used in similar experiments, Ba2+ influx was low (comparable to that seen in the absence of Na+ for the CK1.4 cells), and variations in [Na+] had no effect. We conclude that the Na+/Ca2+ exchanger provides the major route of Ba2+ entry in the CK1.4 cells.
Figure 5.
Ba2+ influx in gramicidin-treated CK1.4 cells. Cells were loaded with fura-2, washed, and preincubated in K-PSS + 1 mM CaCl2, centrifuged, and resuspended in mixtures of K-PSS + Na-PSS that yielded the final Na+ concentrations (in mM) indicated next to each trace; each solution also contained 0.3 mM EGTA. Gramicidin (1 μM) was added immediately after the cells and 1 mM BaCl2 was added at 30 s. (n = 3–5; for 70 mM Na+, n = 2). Inset: Dependence of the rate of Ba2+ influx on [Na+]. The slopes of the traces at each [Na+], normalized to that for [Na+] = 0, are plotted on the ordinate scale.
Ba2+ Uptake and Organellar Sequestration
Previous reports have suggested that Ba2+ is not significantly sequestered by the endoplasmic reticulum (Kwan and Putney, 1990; Rasgado-Flores et al., 1986). Ba2+ uptake by mitochondria has been reported, but the results vary markedly with different cell types (cf., discussion). We conducted the following experiments to determine whether Ba2+ was sequestered by intracellular organelles in CK1.4 cells and to assess whether the presence of cytosolic Ba2+ interfered with other Ca2+ transport processes.
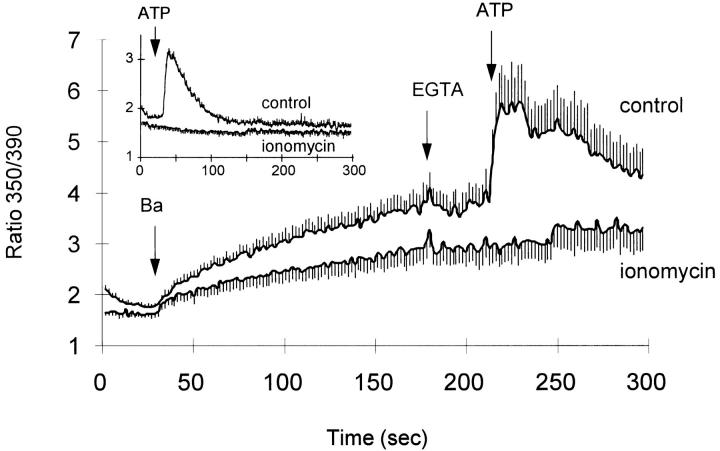
To examine mitochondrial accumulation of Ba2+, we measured the effects of mitochondrial inhibitors on 45Ca2+ and 133Ba2+ uptake. As shown in Fig. 6, the uncoupler Cl-CCP (2 μM) and the combination of oligomycin (2.5 μg/ml) + rotenone (2 μM) inhibited exchange-mediated 45Ca2+ uptake in ouabain-treated CK1.4 cells by 42 ± 2 and 32 ± 2%, respectively, but had no effect on 133Ba2+ uptake (−11 ± 3 and 6 ± 4% inhibition, respectively). The results suggest that any mitochondrial accumulation of Ba2+ was small compared to that observed with Ca2+, consistent with the reported selectivity of the mitochondrial uniporter toward divalent cations (Ca2+ >> Ba2+; Saris and Åkerman, 1980). To address the question of whether cytosolic Ba2+ could be sequestered by the endoplasmic reticulum, we depleted intracellular Ca2+ stores using the Ca2+ ionophore ionomycin and then asked whether the stores would refill with Ba2+ during a subsequent period of Ba2+ influx. We assessed the degree of filling of intra-cellular stores by measuring the response of fura-2– loaded cells to the addition of extracellular ATP. ATP binds to P2U receptors in Chinese hamster ovary cells and elicits the production of inositol (1,4,5)-trisphosphate (InsP3), leading to release of sequestered Ca2+ from InsP3-sensitive stores (Iredale and Hill, 1993; Pijuan et al., 1993). This is illustrated in the inset to Fig. 7, where ATP elicits a pronounced [Ca2+]i transient when added to CK1.4 cells (control trace). In contrast to this behavior, when an aliquot of cells was pretreated for 1 min with 10 μM ionomycin before adding the cells to the cuvette, no Ca2+ transient was observed (Fig. 7, inset). This indicates that the ionomycin had released essentially all the sequestered Ca2+ from the stores during the 1-min preincubation. It is important to note that in all traces shown in Fig. 7, 0.3% BSA was present in the cuvette to scavenge residual ionomycin (cf., Chernaya et al., 1996); this was done to ensure that the presence of the ionophore would not interfere with possible Ba2+ accumulation in the stores. In comparable experiments carried out with Ca2+, the InsP3-sensitive stores refilled rapidly in the presence of extracellular Ca2+ (Chernaya et al., 1996).
Figure 7.
Test for Ba2+ sequestration by InsP3-sensitive stores. (control) Cells were preloaded with fura-2 in the presence of 0.4 mM ouabain, washed, and incubated for 1 min in Na-PSS + 1 mM CaCl2 and then diluted 30-fold into cuvettes containing Li-PSS + 0.3 mM EGTA + 0.3% BSA. BaCl2 (1 mM), EGTA (10 mM) and ATP (0.3 mM) were added as indicated. (ionomycin) Cells were loaded with fura-2 in the presence of ouabain, washed, and incubated for 1 min in Na-PSS + 0.3 mM EGTA containing 10 μM ionomycin; the cells were then centrifuged and resuspended in Li-PSS + 0.3 mM EGTA + 0.3% BSA. BaCl2, EGTA, and ATP were added as in the control trace. (Inset) Experimental conditions are as described for the control and ionomycin-treated cells above, except that ATP (0.3 mM) was added as indicated (n = 6).
In the main panel of Fig. 7, ouabain-treated CK1.4 cells with intact Ca2+ stores were allowed to accumulate Ba2+ for 2.5 min in an Na+-free medium; then 10 mM EGTA was added to block any further Ba2+ influx, and ATP was subsequently added (after a 30-s delay) to measure the amount of Ca2+ present in the stores. As shown, ATP evoked a robust [Ca2+]i transient under these conditions (control trace). These results indicate that the InsP3-sensitive stores had retained their Ca2+ load during the period of Ba2+ accumulation and responded normally to ATP addition. Note that after the peak of the [Ca2+]i transient, the fura-2 signal declined toward the level seen before ATP addition; continued incubation resulted in stabilization of the fura-2 signal at the level observed before ATP addition, consistent with the absence of Ba2+ removal from the cytosol (data not shown). Similar results were seen when 2 μM ionomycin was used to elicit Ca2+ release instead of ATP (data not shown). Thus, the presence of cytosolic Ba2+ did not block either the ATP-evoked Ca2+ release pathway or Ca2+ removal from the cytosol. The latter process may occur through either the SERCA or the plasma membrane Ca2+ pumps, or both. In the case where Ca2+ release was elicited by ionomycin, only the plasma membrane Ca2+ pump would be expected to contribute to Ca2+ removal from the cytosol since intracellular organelles would be unable to accumulate Ca2+ in the presence of the ionophore. These results therefore indicate that Ca2+ extrusion by the plasma membrane Ca2+ ATPase is not blocked by the presence of cytosolic Ba2+.
When ionomycin-pretreated cells (with depleted Ca2+ stores) were subjected to the protocol described above, ATP did not elicit an increase in the 350/390 ratio (Fig. 7, main panel). The absence of an increase in [Ba2+]i indicates that the InsP3-sensitive stores remained empty during the period of Ba2+ uptake. Note that Ba2+ is rapidly conducted by InsP3-gated Ca2+ channels (Bezprozvanny and Ehrlich, 1994) and would therefore have been released from the stores if present. The results indicate that Ba2+ is not sequestered by InsP3-sensitive stores in these cells, probably due to its inability to serve as a transport substrate for the SERCA Ca2+ pump.
An unexpected feature of these results was that the ionomycin-treated cells showed a sharply reduced rate of Ba2+ influx compared to control cells that had not been treated with ionomycin. As described in more detail below, this appears to be a secondary consequence of the reduced [Ca2+]i in the cells with depleted Ca2+ stores: following removal of the ionomycin with BSA, the stores would be expected to re-sequester residual cytosolic Ca2+ and lower [Ca2+]i. The average value of [Ca2+]i during the 15 s before the addition of Ba2+ in this experiment was 23 ± 7 nM for the ionomycin/BSA-treated cells vs. 41 ± 5 nM for the control cells (P < 0.001, paired t test; n = 6).
Store Depletion and Ba2+ Influx
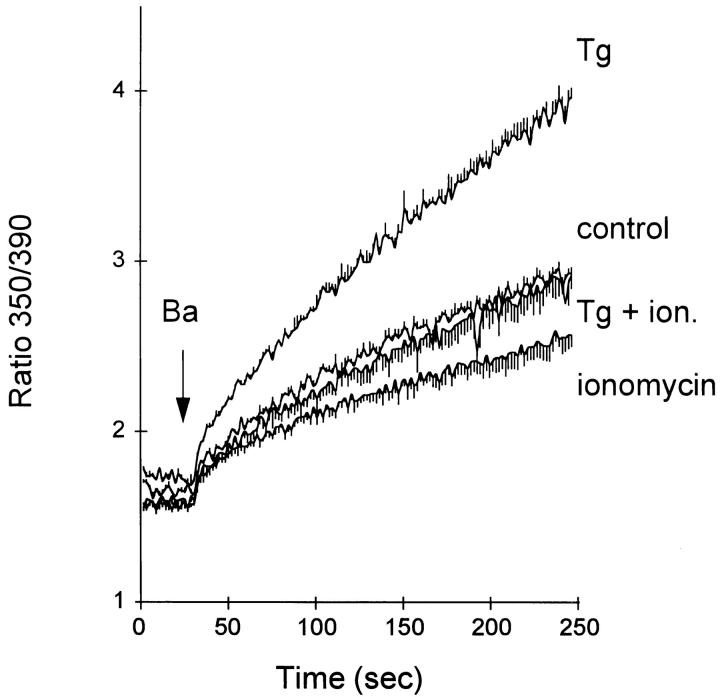
To examine the issues raised above in greater detail, we studied the effects of ionomycin on exchange-mediated Ba2+ influx in cells treated with thapsigargin (Tg), a selective blocker of SERCA Ca2+ pumps (Lytton et al., 1991). CK1.4 cells were pretreated with ionomycin, Tg, or both agents and then washed with 1% BSA to scavenge residual ionomycin. The cells were then placed in Li-PSS + 0.3 mM EGTA and fura-2 fluorescence was monitored after the addition of 1 mM BaCl2. In each case, the cells had also been loaded with cytosolic Na+ by including 0.4 mM ouabain in the fura-2 loading medium (see legend to Fig. 8 for further details).
Figure 8.
Ba2+ influx in CK1.4 cells pretreated with Tg, ionomycin or both. Aliquots (0.3 ml) of CK1.4 cells were loaded with fura-2 in the presence of 0.4 mM ouabain, washed, and preincubated for 1 min in 0.1 ml of Na-PSS + 0.3 mM EGTA containing either 200 nM Tg, 10 μM ionomycin, both Tg and ionomycin, or neither agent (control). The cells were then centrifuged, resuspended in 0.3 ml of Na-PSS + 0.3 mM EGTA + 1% BSA and incubated for an additional 1 min. After an additional centrifugation, the cells were resuspended in Li-PSS + 0.3 mM EGTA in the cuvette; BaCl2 (1 mM) was added as indicated (n = 3).
As shown in Fig. 8, Ba2+ influx was greatest in the Tg-treated cells and least in the cells pretreated with ionomycin; in the latter case, Ba2+ influx was essentially identical to that seen in Na-PSS (data not shown), implying that the exchanger was inactive under these conditions. Remarkably, Ba2+ influx in cells that had been treated with both ionomycin and Tg was greatly reduced compared to cells treated with Tg alone. Control cells that had been subjected to the same preincubation protocol, but in the absence of either ionomycin or Tg, also showed reduced Ba2+ influx; this is due to the mechanical manipulations involved in the protocols, which lead to release of ATP, depletion of the Ca2+ stores, and a decline in [Ca2+]i (unpublished observations; cf. below). The rate of Ba2+ influx shows a reasonably good correlation with the values of [Ca2+]i observed during the 15 s interval before the addition of Ba2+. The [Ca2+]i values corresponding to Tg, control, Tg + ionomycin or ionomycin treatments were 34 ± 3, 26 ± 1, 20 ± 3, and 17 ± 1 nM respectively (n = 3 in each case).
Our interpretation of these results (cf. discussion) is that exchange activity is regulated secondarily through the influence of intracellular organelles on the cytosolic Ca2+ concentration. Release of Ca2+ from internal stores elevates [Ca2+]i and accelerates exchange activity (cf. Chernaya et al., 1996), whereas the exchanger is deactivated when these organelles resequester residual cytosolic Ca2+ and lower [Ca2+]i. The effects of organellar Ca2+ release and sequestration are further documented in the accompanying manuscript (Vázquez et al., 1997), which describes time-dependent changes in exchange activity associated with ATP-induced Ca2+ release from InsP3-sensitive Ca2+ stores. Two surprising aspects of results in Figs. 7 and 8 deserve special emphasis: (a) exchange activity becomes activated at quite low values of [Ca2+]i (25–50 nM) and (b) exchange activity can be deactivated even when the SERCA pumps are blocked by Tg, implying that Tg-resistant Ca2+ pumps also participate in regulation of cytosolic Ca2+.
discussion
The results presented here show that Ba2+ provides an advantageous alternative to Ca2+ for measurements of exchange activity in intact cells. Ba2+ competitively inhibited 45Ca2+ uptake by Na+/Ca2+ exchange and therefore presumably binds to the Ca2+ transport sites on the exchanger (Fig. 1). 133Ba2+ uptake was stimulated by ouabain-treatment and was completely suppressed by 140 mM extracellular Na+ (Fig. 2); similar behavior was observed using fura-2–loaded cells to assess Ba2+ influx (Fig. 4). The effects of Na+ in the gramicidin-treated cells (Fig. 5) indicated that Ba2+ influx was blocked in the absence of cytosolic Na+ and was accelerated by increasing concentrations of cytosolic Na+. We conclude that the exchanger provides the major pathway for Ba2+ entry in these cells. The data with gramicidin-treated cells also suggests that exchange activity is quite sensitive to Na+ i; a small, but significant increase in Ba2+ entry was observed at concentrations as low as 2.3 mM Na+ (Fig. 5).
Previously published studies addressed the question of whether Ba2+ is transported by the Na+/Ca2+ exchanger. Trosper and Philipson (1983) reported that 45Ca2+ efflux from preloaded cardiac sarcolemmal vesicles was accelerated in the presence of Ba2+ and suggested that this was due to Ca2+/Ba2+ exchange. Tibbits and Philipson (1985) measured exchange-mediated 133Ba2+ uptake by cardiac sarcolemmal vesicles; maximal rates of uptake were 20-fold lower than for Sr2+ or Ca2+. Measurements of outward exchange currents in cardiac myocytes indicated that Ba2+ was transported only poorly, if at all, by the exchanger (Kimura et al., 1987; Shimoni and Giles, 1987). However, more recent experiments with excised membrane patches from cardiac myocytes and/or frog oocytes expressing the cardiac exchanger indicate that significant outward exchange currents can be observed with Ba2+ as the transported substrate (personal communications from Dr. Donald Hilgemann, University of Texas Southwestern Medical Center, Dallas, TX and Dr. Larry Hryshko, St. Boniface General Hospital Research Center, Winnipeg, Canada). In contrast to reports that Ba2+ is transported only poorly by the exchanger, our estimated maximal influx rates with 133Ba2+ are ∼50% of those observed with 45Ca2+. It is not clear why there is such a disparity among the different reports on the relative rates of Ca2+ and Ba2+ as exchange substrates. We have recently observed that exchange-mediated Ba2+ influx in CHO cells is much more temperature sensitive than Ca2+ influx (unpublished observations); while this in itself does not explain the differing results among the various experimental reports, it does suggest that Ba2+ translocation by the exchanger might involve mechanistic constraints that do not apply to Ca2+. Thus, the varying results obtained among different investigators might reflect subtle differences in experimental conditions, species differences, or variations in membrane composition that exert disproportionate effects on exchange-mediated Ba2+ movements.
Approximately 1–2 nmol/mg protein of 133Ba2+ were accumulated by ouabain-treated cells under Na+-free conditions (Figs. 2 and 6). With a cellular water content of 6 μl/mg protein, this is equivalent to 200–300 μM total intracellular Ba2+. In fura-2 experiments, the 350/390 ratios during Ba2+ influx approached 4.0, which is equivalent to a cytosolic concentration of approximately 3 μM. Thus, much of the intracellular Ba2+ is buffered, although it is unclear which cellular constituents are involved in this process. With 45Ca2+, the cells accumulated 7 nmol/mg protein under the conditions of Fig. 6; with different conditions (1 mM 45Ca2+, 40 mM Na+ + 100 mM K+ in the assay media), 45Ca2+ accumulations of up to 40 nmol/mg protein have been observed (data not shown). [Ca2+]i rarely exceeded 1 μM during exchange-mediated Ca2+ influx, even under conditions favoring extensive Ca2+ accumulation. Thus, the ratio of total cellular cation to the free cytosolic concentration was much higher for Ca2+ than for Ba2+, a result consistent with a greater degree of organellar sequestration of Ca2+ (cf. below).
An unexpected disparity was observed between the efflux data obtained with 133Ba2+ and fura-2. In the 133Ba2+ studies, preaccumulated Ba2+ was lost from the cells with a half-time of 3–4 min under Na+-free conditions (Fig. 3). With fura-2–loaded cells, however, little or no decline in cytosolic [Ba2+] could be detected when 10 mM EGTA was added (under Na+-free conditions) following a period of Ba2+ influx (Fig. 4). This ratio of EGTA to Ba2+, under the conditions of our experiments, yields a free [Ba2+] of 1.6 μM (MAXC program; Bers et al., 1994) and completely blocks Ba2+ influx. Moreover, despite the data in Fig. 3 indicating that extracellular Na+ stimulates 133Ba2+ efflux, we were unable to demonstrate an Na+ o-dependent decline in c ytosolic [Ba2+] in fura-2–loaded cells using several different protocols to preload cells with Ba2+ (data not shown). This behavior remains unexplained at the present time.
A major advantage of Ba2+ over Ca2+ in measuring exchange activity is that Ba2+ was not sequestered by the endoplasmic reticulum in these cells (Fig. 7). This conclusion confirms results reported previously using other cell types (Rasgado-Flores et al., 1987; Kwan and Putney, 1990). Moreover, Ba2+ did not appear to be significantly accumulated by mitochondria, as judged by the absence of an effect of mitochondrial inhibitors on 133Ba2+ uptake (Fig. 6). Previous studies of Ba2+ uptake by mitochondria yielded conflicting results. Uptake of divalent cations by isolated mitochondria showed a selectivity sequence Ca2+ > Sr2+ >> Mn2+ > Ba2+ (Saris and Åkerman, 1980; Vanio et al., 1970). In rat liver mitochondria, Ba2+ uptake was further inhibited by K+ and Mg2+ (Vanio et al., 1970), suggesting that the cytosolic concentrations of these ions would greatly reduce the capacity of mitochondria to accumulate Ba2+. Studies with permeabilized synaptosomes also showed little if any Ba2+ accumulation by mitochondria (Rasgado-Flores et al., 1987). On the other hand, Ba2+ accumulation was readily detectable in fura-2–loaded rat heart mitochondria (McCormack and Osbaldeston, 1990). Mitochondria in pancreatic B cells (Howell and Tyhurst, 1976) and rabbit vascular smooth muscle (Somlyo et al., 1974) showed dense Ba2+ deposits when the cells were exposed to high external concentrations of Ba2+. While our results do not rule out mitochondrial Ba2+ accumulation in the CHO cells, they suggest that this process is likely to be negligible compared to Ca2+ sequestration. Thus, measurements of Ba2+ uptake by either isotopic flux measurements or fura-2 should be a more direct indicator of exchange activity than the corresponding measures of Ca2+ uptake.
A second advantage of using Ba2+ for studies of exchanger regulation is that activation of exchange activity by [Ca2+]i can be readily observed (cf. below). This is experimentally difficult to demonstrate with Ca2+ influx measurements because Ca2+ entering the cells would itself accelerate exchange activity through positive feedback involving secondary Ca2+ activation. This implies that cytosolic Ba2+ is considerably less effective than Ca2+ for activating exchange activity at the Ca2+ regulatory sites. Recent measurements of exchange currents in excised patches from frog oocytes expressing the exchanger (NCX1) have verified this conclusion (personal communication, Dr. L. Hryshko).
The effects of Tg and ionomycin shown in Figs. 7 and 8 indicate that exchange activity depends secondarily on the Ca2+ sequestering activities of intracellular organelles through their influence on [Ca2+]i. Two aspects of these results deserve special mention. First, the exchanger becomes activated at surprisingly low cyto-solic Ca2+ concentrations. The experiments with fura-2 (Figs. 4 B, 5, 7, and 8) indicate that high levels of exchange-mediated Ba2+ influx are observed when cyto-solic Ca2+ levels (during the period just before the addition of Ba2+) were between 35 and 70 nM; on the other hand, exchange activity was greatly reduced when [Ca2+]i declined below 20 nM (Figs. 7 and 8). Note that these experiments were carried out in the absence of extracellular Ca2+, and so the lower values of [Ca2+]i were substantially below “resting” values under physiological conditions (50–75 nM; Pijuan et al., 1993; Vázquez et al., 1997). Because of the relatively simple calibration procedure used, the values cited for [Ca2+]i may be slightly inaccurate, but any errors are unlikely to be large enough to affect the general conclusions drawn below.
The [Ca2+]i values that activate exchange activity in our experiments were clearly much lower than values obtained with excised sarcolemmal patches, where concentrations of 300–600 nM are required for half-maximal activation of outward exchange currents (Hilgemann et al., 1992b ). On the other hand, our results agree closely with those of Miura and Kimura (1989) and of Noda et al.(1988), who observed half-maximal activation of outward exchange currents in guinea pig myocytes at [Ca2+]i = 22 and 47 nM, respectively. The reasons for the differences between the behavior of excised patches and intact cells are a matter for speculation: They might involve the loss of regulatory cellular components in the patches, or local interactions with intracellular Ca2+ storage organelles that elevate [Ca2+]i in the vicinity of the exchanger above the value in the bulk cytosol. In any event, our results imply that in cells under resting physiological conditions, where [Ca2+]i is typically 50–75 nM, the exchanger is at least partially activated.
The second aspect of our results that merits detailed consideration is the complex interaction between exchange activity and intracellular organelles. The data in Fig. 7 and 8 indicate that when intracellular Ca2+ stores were depleted with ionomycin, subsequent removal of the ionophore allowed resequestration of cytosolic Ca2+ by the stores, resulting in a reduction of [Ca2+]i below levels needed to activate the exchanger. Deactivation of exchange activity by organellar Ca2+ sequestration was not observed in preliminary experiments with cells expressing an exchanger deletion mutant that is not regulated by [Ca2+]i. The unexpected finding that exchange activity was reduced in ionomycin-treated cells where the SERCA Ca2+ pump had been blocked with Tg (Fig. 8) suggests that Tg-resistant Ca2+ pumps contributed to reducing [Ca2+]i; at present, the identity of these pumps is unknown. It should be noted that these experiments were conducted in the absence of extracellular Ca2+; if Ca2+ had been present externally, Ca2+ entry through store-dependent influx pathways (Putney, 1990) would have increased [Ca2+]i and activated exchange activity. These considerations raise the possibility that the exchanger participates in a capacitative feedback mechanism for regulating the filling state of intracellular stores. Thus, when intracellular stores are filled to capacity with Ca2+, their ability to sequester additional Ca2+ is reduced, leading to a rise in [Ca2+]i and activation of exchange activity. Activation of the exchanger under physiological conditions would stimulate Ca2+ efflux, thereby reducing net Ca2+ entry into the cell and limiting any further increase in store size. Conversely, when the stores contain a reduced Ca2+ load, sequestration of Ca2+ from the cytosol would reduce [Ca2+]i and attenuate exchange activity, thereby allowing additional filling of the stores. Capacitative feedback between intracellular stores and exchange activity, in conjunction with capacitative Ca2+ entry mechanisms (Putney, 1990), could be an important mechanism for controlling the Ca2+ content of InsP3-sensitive stores in neutrophils, pancreatic β cells and vascular smooth muscle cells.
Acknowledgments
We thank Drs. Abraham Aviv, Masayuki Kimura and Jeffrey P. Gardner for helpful discussions and advice during the course of this work. We are especially grateful to Dr. Larry Hryshko, Division of Cardiovascular Sciences, St. Boniface General Hospital Research Center, Winnipeg, Canada and Dr. Donald Hilgemann, Department of Physiology, University of Texas Southwestern Medical Center, Dallas, TX for sharing with us their unpublished data on Ba2+ and Na+/Ca2+ exchange activity.
Supported by National Institutes of Health grant HL 49932.
Footnotes
Portions of this work were presented previously in abstract form (Chernaya, G., V.G. Patel, M. Condrescu, and J.P. Reeves. 1996. Barium influx via sodium-calcium exchange is stimulated by intracellular store depletion in transfected CHO cells. Biophys. J. 70:A206).
Abbreviations used in this paper: CHO, Chinese hamster ovary; InsP3, inositol (1,4,5)trisphosphate; PSS, physiological salts solution; SERCA, sarco(endo)plasmic reticulum Ca2+ ATPase; Tg, thapsigargin.
references
- Aceto JF, Condrescu A, Kroupis C, Nelson H, Nelson N, Nicoll D, Philipson KD, Reeves JP. Cloning and expression of the bovine cardiac sodium-calcium exchanger. Arch Biochem Biophys. 1992;298:553–560. doi: 10.1016/0003-9861(92)90449-7. [DOI] [PubMed] [Google Scholar]
- Bers, D., C. Patton, and R. Nuccitelli. 1994. A practical guide to the preparation of Ca buffers. In A Practical Guide to the Study of Ca2+ in Living Cells: Methods Cell Biology, Vol. 40. Academic Press, New York. 3–29. [DOI] [PubMed]
- Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernaya G, Vázquez M, Reeves JP. Sodium-calcium exchange and store-dependent calcium influx in transfected Chinese hamster ovary cells expressing the bovine cardiac sodium-calcium exchanger. Acceleration of exchange activity in thapsigargin-treated cells. J Biol Chem. 1996;271:5378–5385. doi: 10.1074/jbc.271.10.5378. [DOI] [PubMed] [Google Scholar]
- Condrescu M, Gardner JP, Chernaya G, Aceto JF, Kroupis C, Reeves JP. ATP-dependent regulation of sodium-calcium exchange in Chinese hamster ovary cells transfected with the bovine cardiac sodium-calcium exchanger. J Biol Chem. 1995;270:9137–9146. doi: 10.1074/jbc.270.16.9137. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Calcium influx in internally dialyzed squid giant axons. J Gen Physiol. 1979;73:91–113. doi: 10.1085/jgp.73.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R, Beaugé L. Characterization of the reverse Na/Ca exchange in squid axons and its modulation by Cai and ATP. Cai-dependent Nai/Cao and Nai/Naoexchange modes. J Gen Physiol. 1987;90:505–525. doi: 10.1085/jgp.90.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVirgilio F, Fasolato C, Steinberg TH. Inhibitors of membrane transport system for organic anions block fura-2 excretion from PC12 and N2A cells. Biochem J. 1988;256:959–963. doi: 10.1042/bj2560959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hilgemann DW, Collins A, Cash DP, Nagel GA. Cardiac Na+/Ca2+exchange system in giant membrane patches. Ann NY Acad Sci. 1991;639:126–139. doi: 10.1111/j.1749-6632.1991.tb17296.x. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW. Regulation and deregulation of cardiac Na+/Ca2+exchange in giant excised sarcolemmal membrane patches. Nature (Lond) 1990;344:242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Matsuoka S, Nagel GA, Collins A. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol. 1992a;100:905–932. doi: 10.1085/jgp.100.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Collins A, Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol. 1992b;100:933–961. doi: 10.1085/jgp.100.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SL, Tyhurst M. Barium accumulation in rat pancreatic B cells. J Cell Sci. 1976;22:455–465. doi: 10.1242/jcs.22.2.455. [DOI] [PubMed] [Google Scholar]
- Iredale PA, Hill SJ. Increases in intracellular calcium via activation of an endogenous P2-purinoceptor in cultured CHO-K1 cells. Br J Pharmacol. 1993;110:1305–1310. doi: 10.1111/j.1476-5381.1993.tb13960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khananshvili D. Distinction between the two basic mechanisms of cation transport in the cardiac Na+/Ca2+exchange system. Biochemistry. 1990;29:2437–2442. doi: 10.1021/bi00462a001. [DOI] [PubMed] [Google Scholar]
- Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol (Lond) 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C-Y, Putney JW., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr+ and Ba2+of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990;265:678–684. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- McCormack JG, Osbaldeston NJ. The use of Ca2+-sensitive intramitochondrial dehydrogenases and entrapped fura-2 to study Sr2+ and Ba2+transport across the inner membrane of mammalian mitochondria. Eur J Biochem. 1990;192:239–244. doi: 10.1111/j.1432-1033.1990.tb19221.x. [DOI] [PubMed] [Google Scholar]
- Miura Y, Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989;93:1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda, M., R.N. Shepherd, and D.C. Gadsby. 1988. Activation by [Ca2+]i, and block by 3′,4′-dichlorobenzamil, of outward Na/Ca exchange current in guinea-pig ventricular myocytes. Biophys. J. 53:342a. (Abstr.).
- Pijuan V, Zhuang Y, Smith L, Kroupis C, Condrescu M, Aceto JF, Reeves JP, Smith JB. Stable expression of the cardiac sodium-calcium exchanger in CHO cells. Am J Physiol. 1993;264:C1066–C1074. doi: 10.1152/ajpcell.1993.264.4.C1066. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Receptor-regulated calcium entry. Pharmacol Ther. 1990;48:427–434. doi: 10.1016/0163-7258(90)90059-b. [DOI] [PubMed] [Google Scholar]
- Rasgado-Flores H, Sanchez-Armass S, Blaustein MP, Nachsen DA. Strontium, barium and manganese metabolism in isolated presynaptic nerve terminals. Am J Physiol. 1986;252:C604–C610. doi: 10.1152/ajpcell.1987.252.6.C604. [DOI] [PubMed] [Google Scholar]
- Reeves, J.P. 1995. Cardiac sodium-calcium exchange system. In Physiology and Pathophysiology of the Heart. N. Sperelakis, editor. Kluwer Academic Publishers, Norwell, MA. 309–318.
- Saris NE, Åkerman KEO. Uptake and release of bivalent cations in mitochondria. Curr Topics Bioenerg. 1980;10:103–160. [Google Scholar]
- Schilling WP, Rajan L, Stroble-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity and kinetics. J Biol Chem. 1989;264:12838–12848. [PubMed] [Google Scholar]
- Shimoni Y, Giles W. Separation of Na-Ca exchange and transient inward currents in heart cells. Am J Physiol. 1987;253:H1330–H1333. doi: 10.1152/ajpheart.1987.253.5.H1330. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV, Devine CE, Peters PD, Hall TA. Electron microscopy and electron probe analysis of mitochondrial cation accumulation of smooth muscle. J Cell Biol. 1974;61:723–742. doi: 10.1083/jcb.61.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, A.P., and F. Delaville. 1991. The use of fluorescent indicators for measurements of cytosolic-free calcium concentration in cell populations and single cells. In Cellular Calcium. A Practical Approach. J.G. McCormack and P.H. Cobbold, editors. Oxford University Press, Oxford. 1–54.
- Tibbits GF, Philipson KD. Na+-dependent alkaline earth metal uptake in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1985;817:327–332. doi: 10.1016/0005-2736(85)90035-5. [DOI] [PubMed] [Google Scholar]
- Trosper TL, Philipson KD. Effects of divalent and trivalent cations on Na+/Ca2+exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1983;731:63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Vanio H, Mela L, Chance B. Energy dependent bivalent cation translocation in rat liver mitochondria. Eur J Biochem. 1970;12:387–391. doi: 10.1111/j.1432-1033.1970.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Vázquez M, Fang Y, Reeves JP. Acceleration of sodium-calcium exchange activity during ATP-induced calcium release in transfected Chinese hamster ovary cells. J Gen Physiol. 1997;109:53–60. doi: 10.1085/jgp.109.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]