Abstract
Regulation of hexitol catabolism was investigated in Streptococcus mutans, a cariogenic human dental plaque bacterium. Induction of hexitol catabolic enzymes and phosphoenolpyruvate:hexitol phosphotransferase and hexitol phosphate dehydrogenase activities was regulated by an inducer exclusion mechanism initiated by D-glucose and 2-deoxy-D-glucose. Kinetic analysis of the inhibitory effect of 2-deoxy-D-glucose on initial hexitol uptake illustrated that this was a noncompetitive type of inhibition. In mutant strains of S. mutans lacking phosphoenolpyruvate:glucose phosphotransferase activity, 2-deoxy-D-glucose was unable to inhibit hexitol uptake. These observations provide evidence for possible molecular mechanisms for the exclusion process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. T., Patterson C. E. Ethanol production and alcohol dehydrogenase activity in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):127–131. doi: 10.1016/0003-9969(73)90027-7. [DOI] [PubMed] [Google Scholar]
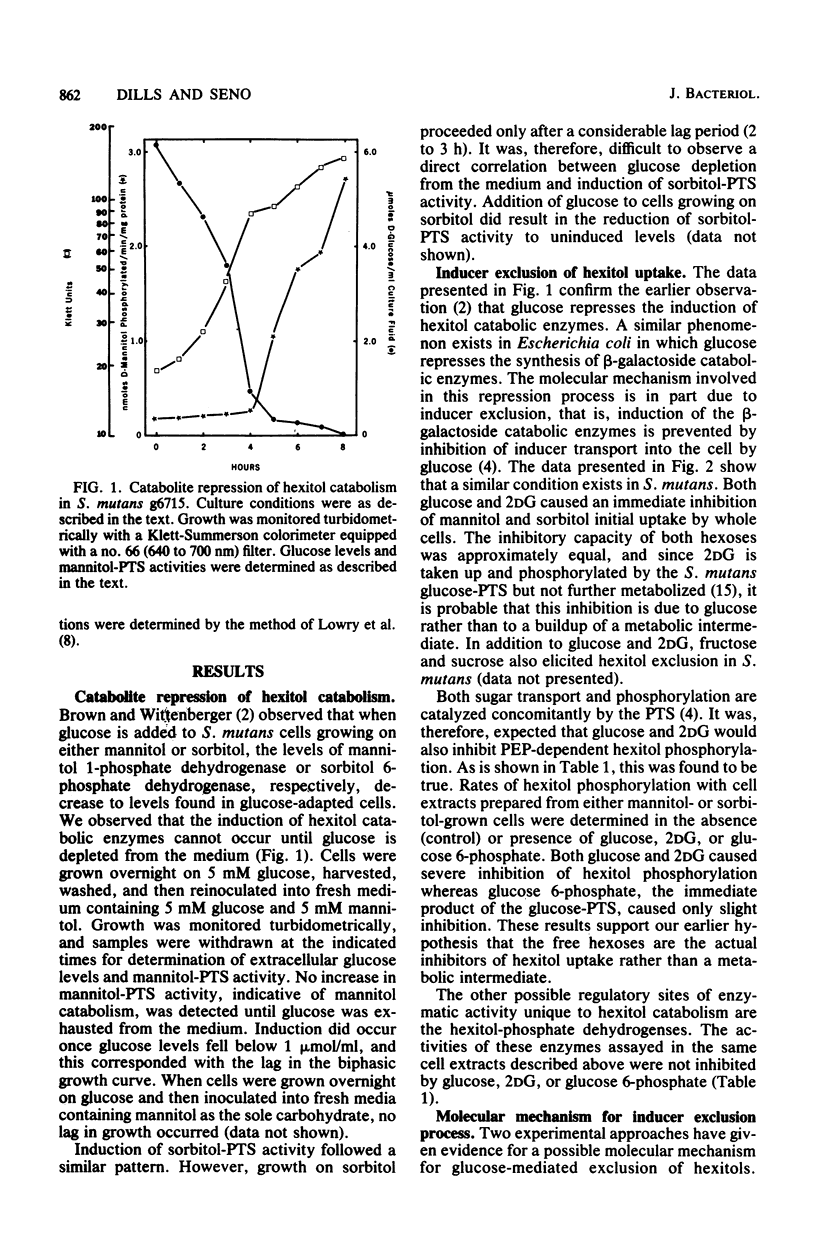
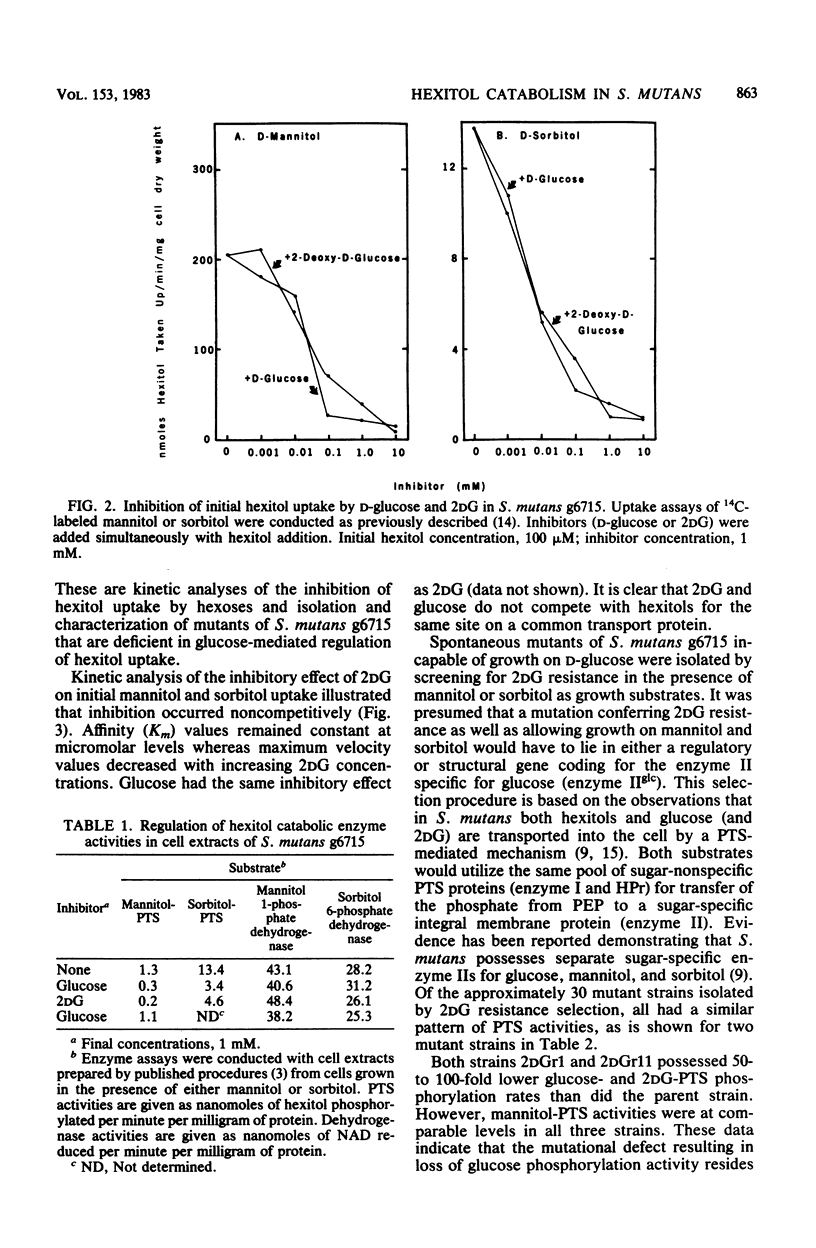
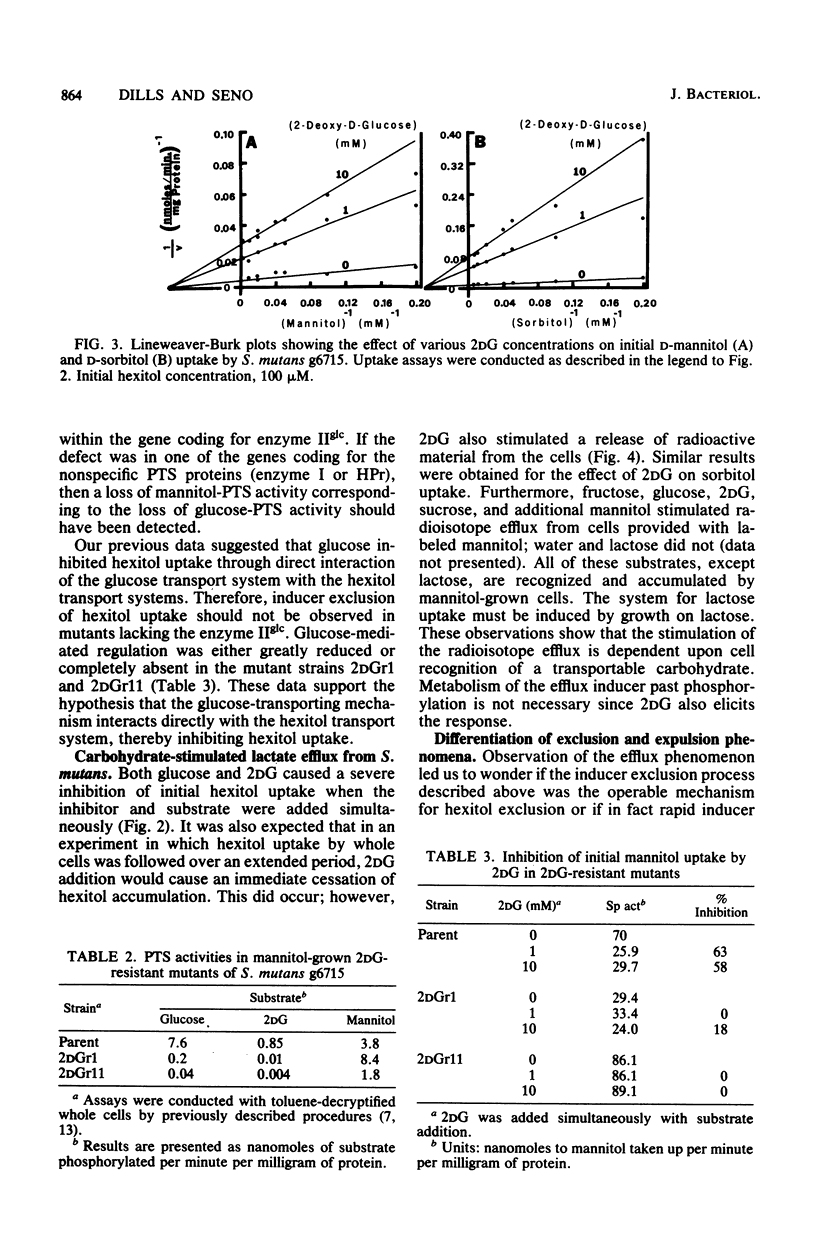
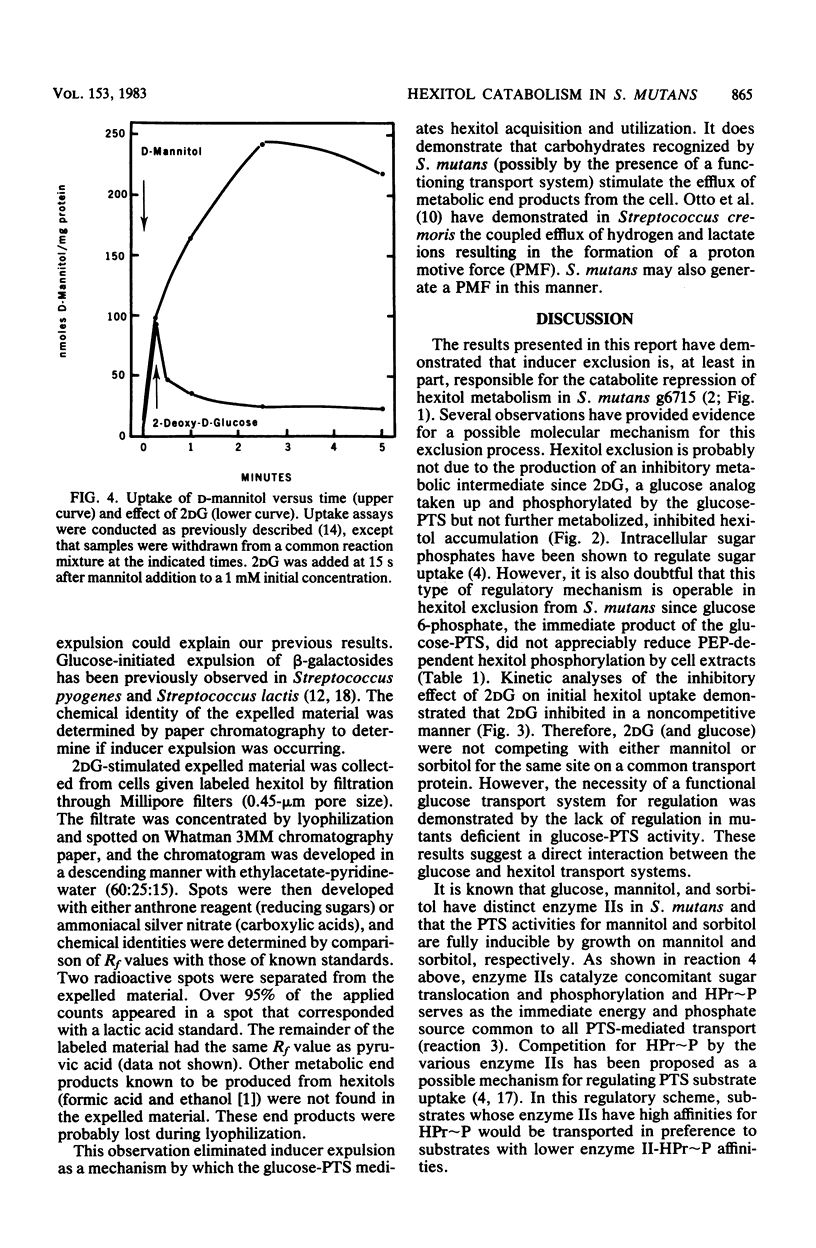
- Brown A. T., Wittenberger C. L. Mannitol and sorbitol catabolism in Streptococcus mutans. Arch Oral Biol. 1973 Jan;18(1):117–126. doi: 10.1016/0003-9969(73)90026-5. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem Biophys Res Commun. 1979 Jul 12;89(1):307–314. doi: 10.1016/0006-291x(79)90979-3. [DOI] [PubMed] [Google Scholar]
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maryanski J. H., Wittenberger C. L. Mannitol transport in Streptococcus mutans. J Bacteriol. 1975 Dec;124(3):1475–1481. doi: 10.1128/jb.124.3.1475-1481.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Sonnenberg A. S., Veldkamp H., Konings W. N. Generation of an electrochemical proton gradient in Streptococcus cremoris by lactate efflux. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5502–5506. doi: 10.1073/pnas.77.9.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider E., Wagner E. F., Schweiger M. Control of phosphoenolpyruvate-dependent phosphotransferase-mediated sugar transport in Escherichia coli by energization of the cell membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5529–5533. doi: 10.1073/pnas.76.11.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Schachtele D. F., Leung W. L. Effect of sugar analogues on growth, sugar utilization, and acid production by Streptococcus mutans. J Dent Res. 1975 May-Jun;54(3):433–440. doi: 10.1177/00220345750540030301. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Postma P. W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114(1):51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]
- Schäfer A., Schrecker O., Hengstenberg W. The staphylococcal phosphoenolpyruvate-dependent phosphotransferase system. Purification and characterisation of the galactoside-specific membrane-component enzyme II. Eur J Biochem. 1981 Jan;113(2):289–294. doi: 10.1111/j.1432-1033.1981.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


