Abstract
The ability of scallop hyperpolarizing photoreceptors to respond without attenuation to repetitive flashes, together with their low light sensitivity, lack of resolvable quantum bumps and fast photoresponse kinetics, had prompted the suggestion that these cells may be constitutively in a state akin to light adaptation. We here demonstrate that their photocurrent displays all manifestations of sensory adaptation: (a) The response amplitude to a test flash is decreased in a graded way by background or conditioning lights. This attenuation of the response develops with a time constant of 200–800 ms, inversely related to background intensity. (b) Adapting stimuli shift the stimulus-response curve and reduce the size of the saturating photocurrent. (c) The fall kinetics of the photoresponse are accelerated by light adaptation, and the roll-off of the modulation transfer function is displaced to higher frequencies. This light-induced desensitization exhibits a rapid recovery, on the order of a few seconds. Based on the notion that Ca mediates light adaptation in other cells, we examined the consequences of manipulating this ion. Removal of external Ca reversibly increased the photocurrent amplitude, without affecting light sensitivity, photoresponse kinetics, or susceptibility to background adaptation; the effect, therefore, concerns ion permeation, rather than the regulation of the visual response. Intracellular dialysis with 10 mM BAPTA did not reduce the peak-to-plateau decay of the photocurrent elicited by prolonged light steps, not the background-induced compression of the response amplitude range and the acceleration of its kinetics. Conversely, high levels of buffered free [Ca]i (10 μM) only marginally shifted the sensitivity curve (Δσ = 0.3 log) and spared all manifestations of light adaptation. These results indicate that hyperpolarizing invertebrate photoreceptors adapt to light, but the underlying mechanisms must utilize pathways that are largely independent of changes in cytosolic Ca. The results are discussed in terms of aspects of commonalty to other ciliary sensory receptor cells.
Keywords: light adaptation, photoreceptors, calcium, light-dependent channels
introduction
The eyes of some invertebrate organisms display the remarkable feature of a double retina (Dakin, 1910), composed of a proximal layer of depolarizing photoreceptors, which possess a microvilli-covered light-sensitive lobe similar to that found in most invertebrates, and a distal layer of hyperpolarizing photoreceptors (Gorman and McReynolds, 1969; Mpitsos, 1973). In the latter, the light-sensing organelles are modified ciliary appendages (Miller, 1958) analogous to the outer segment of rods and cones (Tokuyasu and Yamada, 1959; Eakin, 1965). In a previous report we used patch-electrode recording to characterize the properties of the light-sensitive conductance in enzymatically isolated ciliary photoreceptors from two species, Lima scabra and Pecten irradians (Gomez and Nasi, 1994); subsequently, we demonstrated that these cells, like rods and cones, use the cyclic GMP cascade for transduction, whereas the inositol 1,4,5-trisphosphate (IP3)1/Ca signalling pathway appears not to play a role in the activation of the light-sensitive conductance (Gomez and Nasi 1995). In addition to underscoring an essential divergence in the visual excitation mechanisms across photoreceptor cell lines, these results raise questions about light adaptation, which in all other known photoreceptors is chiefly controlled by cytosolic calcium levels (reviewed by Fain and Matthews, 1990; Fein and Szuts, 1982): in addition to the absence of light-dependent IP3-mediated Ca release, no changes of Ca fluxes accompany the photocurrent in these cells (e.g., unlike rods and cones), because their light-sensitive conductance is highly selective for potassium ions (Cornwall and Gorman 1983; Gomez and Nasi, 1994). The possibility that the light response of ciliary photoreceptors may not be coupled to changes in intracellular Ca could simply point to a lack of normal adaptation processes. This prospect is not to be dismissed a priori, if one considers that the biological function of the distal retina in these organisms is to initiate a discharge of action potentials that triggers a defensive reflex when light is abruptly decreased (as when an approaching predator casts a shadow in the animal's visual field). This requires the ability to respond to continuous illumination, so that the inactivation of voltage-gated Ca channels (which occurs at the “dark” resting potential) is tonically removed by the hyperpolarizing receptor potential; in this way, the system is constantly primed to respond to light dimming, producing a rebound excitation when the membrane depolarizes. As such, this scheme may not necessarily benefit from a gain-control mechanism. An alternative possibility is that adaptation does occur, and the lack of calcium involvement may be indicative of a novel regulatory mechanism for the light response.
Little information is available on the modulation of the photoresponse in hyperpolarizing invertebrate visual cells. In one of their seminal descriptions of the physiology of the dual retina of Pecten, McReynolds and Gorman (1970) reported that when ciliary photoreceptors were stimulated with pairs of flashes, the response to the second light suffered no attenuation even with inter-stimulus intervals as short as 5 s; by contrast, rhabdomeric cells required minutes to regain responsiveness after the first flash. Although this phenomenon may indicate that ciliary cells lack the capability to desensitize, one cannot rule out the possibility that light-adaptation does occur, but recovers rapidly. In fact, Cornwall and Gorman (1979) briefly reported that the response to a test flash was reduced in amplitude when the stimulus was superimposed on a steady light. In the present report, we examined systematically the effect of background and conditioning lights on photoresponse sensitivity and kinetics; we have found that all the manifestations of light adaptation are present in ciliary photoreceptors, with similar characteristics to those of other visual cells. We then ascertained the effect of manipulations of calcium on light adaptation and determined that regulation of the photoresponse must use a pathway that is largely calcium-independent.
methods
Specimens of Pecten irradians were obtained from the Aquatic Resources Division of the Marine Biological Laboratory (Woods Hole, MA) and used immediately. The protocol for obtaining viable isolated hyperpolarizing photoreceptors by enzymatic dispersion of the retina has been described previously (Gomez and Nasi, 1994). Cells were plated in a recording chamber continuously superfused with artificial sea water (ASW) containing 480 mM NaCl, 10 mM KCl, 49 mM MgCl2, 10 mM CaCl2, 10 mM HEPES, and 5 mM glucose, pH 7.8 (NaOH). In 0-Ca ASW, calcium was replaced by magnesium. Patch electrodes were fabricated with thin-wall (1.5 mm o.d. 1.1 mm i.d.) borosilicate capillary tubing (type 7052; Garner Glass, Claremont, CA) pulled to a 2–3 μm tip diameter and fire-polished immediately before use. Their resistance, measured in ASW, was 2–4 MΩ. The “intracellular” filling solution contained 100 mM KCl, 200 mM K-aspartate or K-glutamate, 12 mM NaCl, 5 mM Na2ATP, 5 mM MgCl2, 10 mM HEPES, 1 mM EGTA, 300 mM sucrose, and 200 μM GTP, pH 7.3 (KOH). In some experiments, instead of EGTA a higher concentration (10 mM) of the rapid Ca chelator BAPTA (1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid) was used to provide stronger calcium buffering. In others, CaCl2 was added to the internal solution to yield a free concentration of 10 μM, as determined by the program Chelator (kindly provided by Dr. Theo Schoenmakers, University of Nijmegen, The Netherlands). The calculated concentration of free Mg was 0.99 mM for the internal solutions containing EGTA, and 0.94 mM for the solution containing BAPTA. In all recordings series resistance errors were electronically corrected (maximum residual error <2 mV). Currents were low-pass filtered with a Bessel 4-pole filter, using a cutoff frequency of 500–1,000 Hz and digitized on-line at 2–3 kHz sampling rate, 12-bit resolution by an analogue/digital computer interface board (2821; Data Translation, Marlboro, MA). All experiments were conducted at room temperature (22–24°C).
Light stimuli were provided by two independent photostimulators: one consisted of a halogen-quartz light source which delivered light to the preparation from above, through the microscope condenser (Nasi, 1991a , b ; Nasi and Gomez, 1992a ). The other one utilized a Xenon arc lamp (Photon Technologies Inc., South Brunswick, NJ) coupled by a fiber optics light-guide to the epifluorescence port of the inverted microscope and stimulated the cells from below, through the objective (Gomez and Nasi, 1994). The application of light stimuli was controlled by a microprocessor-based programmable stimulator (Stim 6; Ionoptix, Milton, MA) and electromechanical shutters and drivers (Vincent Associates, Rochester, NY). To provide sufficiently bright test flashes to saturate the photoresponse under light-adapted conditions, broad-band light was used in many of the experiments (515–670 nm); its effective intensity was calibrated in vivo in several cells (n = 9), by comparing it to monochromatic stimulation (500 nm peak, 10 nm half-width; Ditric Optics, Hudson, MA), as previously described (Gomez and Nasi, 1994). In the experiments described below, light intensity is expressed either in terms of flux of equivalent photons at 500 nm, or by specifying the relative attenuation with respect to a known standard (i.e., log10[I/Io], where Io is the maximum light intensity). Calibrated neutral-density filters (Melles Griot, Irvine, CA) provided controlled attenuation. For continuous modulation of the intensity of the light beam, an adjustable attenuator was used (Meadowlark Optics, Longmont, CO); this consisted of a liquid crystal variable retarder placed between two crossed polarizers, controlled by an arbitrary waveform generator (Hewlett Packard Mod. 33120A) which was programmed to modulate the light sinusoidally, with a defined depth and frequency. Monochromatic light (580 nm) was used for this purpose. During experimental manipulations the cells were illuminated through a near-infrared long-pass filter (λ > 780 nm; Andover Corp., Salem, NH) and viewed with a Newvicon TV camera (Mod. WV-1550; Panasonic, Secaucus, NJ). The infrared illuminator was turned off for several minutes before testing light responses.
results
Why the Susceptibility to Light Adaptation Is at Issue
To appreciate why the presence of light-adaptation mechanisms in ciliary photoreceptors has been called into question, it is helpful to compare some salient features of their photoresponses to those of rhabdomeric cells. In rhabdomeric photoreceptors of many species illumination with dim light evokes discrete waves of depolarization (or inward current, if measured under voltage clamp); statistical analysis reveals that these discrete waves, or “quantum bumps” originate from individual absorbed photons (Yeandle, 1958). When the photoreceptors are light adapted, the size of the bumps is dramatically reduced, an effect that accounts in part for the resulting desensitization (Wong, 1978). Fig. 1 A shows an example of light-induced current fluctuations recorded in an isolated rhabdomeric photoreceptor from Pecten. Membrane current was recorded at −50 mV in the dark, or during 10-s steps of dim light, the intensity of which was progressively increased. In ciliary photoreceptors, by contrast, quantum bumps cannot be resolved even at the lowest stimulating intensities (Fig. 1 B): in this case the brighter lights gave rise to a small sustained outward photocurrent with a smooth time course, lacking any resolvable discrete events. Similar results were obtained in three additional cells.
Figure 1.
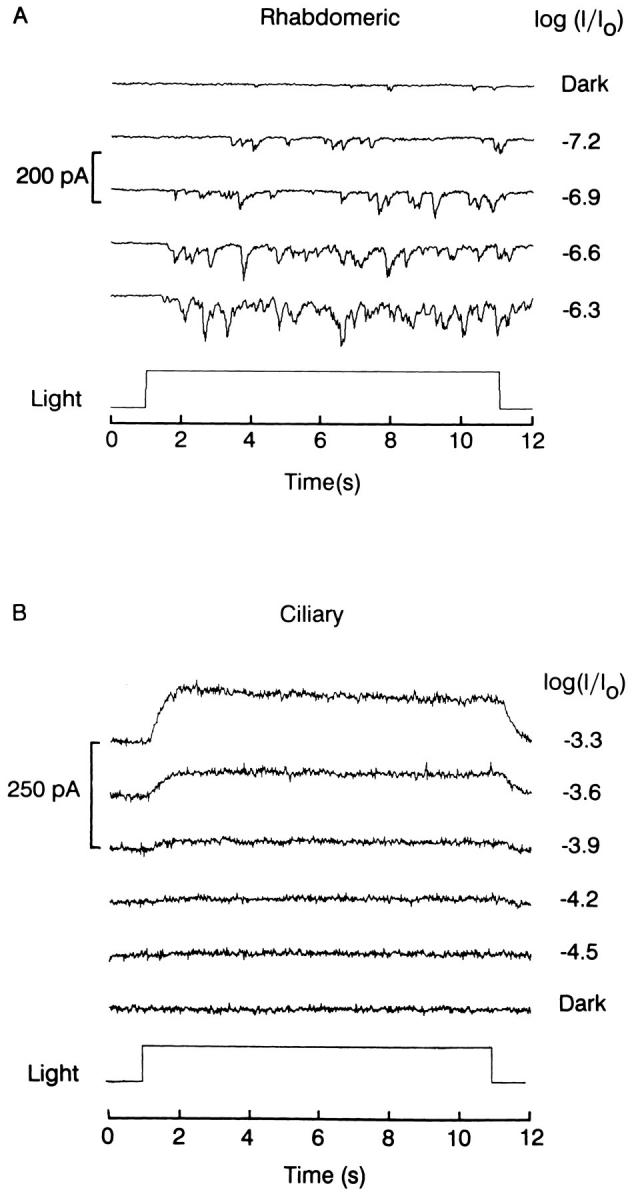
Lack of resolvable quantum bumps in ciliary cells. (A) Example of single-photon responses in a rhabdomeric photoreceptor voltage clamped at −50 mV. Light steps of the indicated relative intensities were presented for 10 s. (B) Smooth photocurrent evoked by threshold stimulation in a ciliary photoreceptor. Similar protocol as in A, except that the holding potential was −30 mV. Unattenuated light intensity: 3.8 × 1015 photons · cm−2 · s−1 (A), and 4.2 × 1015 photons · cm−2 · s−1 (B).
The minute size of single-photon currents is consistent with the observation that the light sensitivity of ciliary photoreceptors is substantially lower than that of their rhabdomeric counterparts (McReynolds and Gorman, 1970; Gomez and Nasi, 1994). Fig. 2 A directly compares families of responses to flashes of increasing intensity measured under voltage clamp in the two types of photoreceptors; in part (B) of the same figure, the normalized stimulus-response relations are plotted in semi-logarithmic coordinates, revealing a relative shift of ≈2.5 log units along the abscissa. Similar results were obtained in five additional cells of each class (see also Gomez and Nasi, 1994). On the assumption that the amount of rhodopsin is comparable (as suggested by measurements of the early receptor current; unpublished observations), one can calculate the relative transduction gain in rhabdomeric vs. ciliary cells taking into account the amplitude of their respective macroscopic light-induced conductances, as well as the unitary conductance (48 and 26 pS, respectively) and the mean open times of single-channels (Nasi and Gomez, 1992a ; Gomez and Nasi, 1994); the estimated number of channels activated per isomerized rhodopsin turns out to be at least 100-fold lower in ciliary cells. In addition, the photocurrent of ciliary cells also displays considerably more rapid kinetics than that of rhabdomeric cells from the same preparation (see also McReynolds and Gorman, 1970). Reduced quantal response size, low sensitivity, and fast kinetics are characteristic features of the behavior of a light-adapted photoreceptor. In fact, it has been suggested that ciliary cells may be constitutively in a state akin to light-adaptation (McReynolds, 1976). This called for demonstrating whether normal light-adaptation mechanisms are indeed operative in these cells.
Figure 2.
Comparison of photocurrent sensitivity and kinetics in rhabdomeric and ciliary photoreceptors. (A) Left: Light-evoked inward currents elicited by flashes of increasing intensity in a rhabdomeric photoreceptor voltage clamped at −50 mV. Right: Outward photocurrents in a ciliary photoreceptor; holding potential −30 mV. (B) Normalized peak amplitude of the photocurrent for the two families of recordings shown in A; sigmoidal functions of the form y(x) = S/{1 + exp[α(σ − x)]}, where S, α, σ > 0, were fitted to the two sets of data points by the method of least squares. The difference in half-saturating light for the two cells was 2.5 log. Unattenuated light intensity: 10.8 × 1015 photons · cm−2 · s−1, flash duration 100 ms.
Demonstration of Adaptation
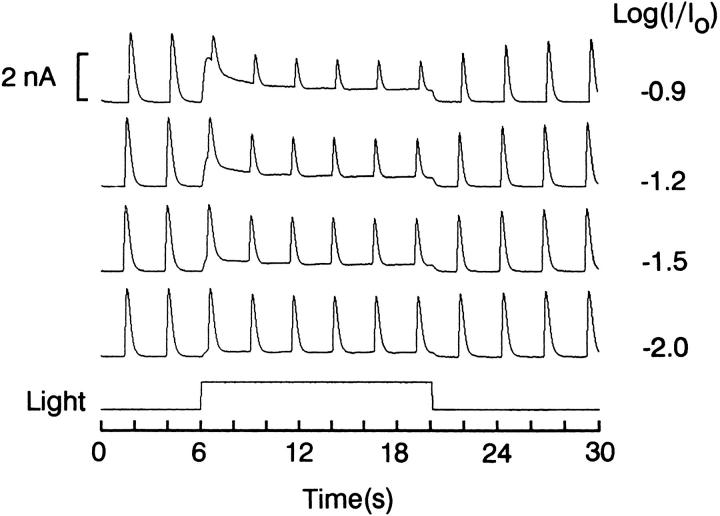
We first determined the effect of background light on the responses elicited by test flashes of fixed intensity. Fig. 3 shows recordings in a cell stimulated every 2.5 s; after the first two responses, a background light was turned on for 14 s. Four different background intensities were tested. In the presence of the sustained light step, the incremental responses to the test flashes grew smaller, the amount of reduction being directly related to background intensity. The test response amplitude rapidly recovered after termination of the adapting light. The decrease in light response cannot be accounted for by direct occlusive effects of the two lights (namely, the reduced number of light-dependent channels that remain available during background illumination), because in the presence of the adapting light the total current amplitude at the peak of the flash responses was lower than that attained when the flashes were presented alone (n = 3).
Figure 3.
Effect of background light on the response to brief test flashes. A ciliary photoreceptor was voltage clamped at −20 mV and stimulated by 20-ms flashes every 2.5 s (8.1 × 1015 equivalent photons · cm−2 · s−1). After 6 s of this regime, a steady light was turned on for 14 s; four different background intensities were examined (from 4.2 to 52 × 1013 photons · cm−2 · s−1). The sustained light caused a graded, reversible reduction in the amplitude of the responses to the test flashes. During the background light, the total membrane current at the peak of the responses to the test flashes was lower than the value attained in the absence of the adapting background.
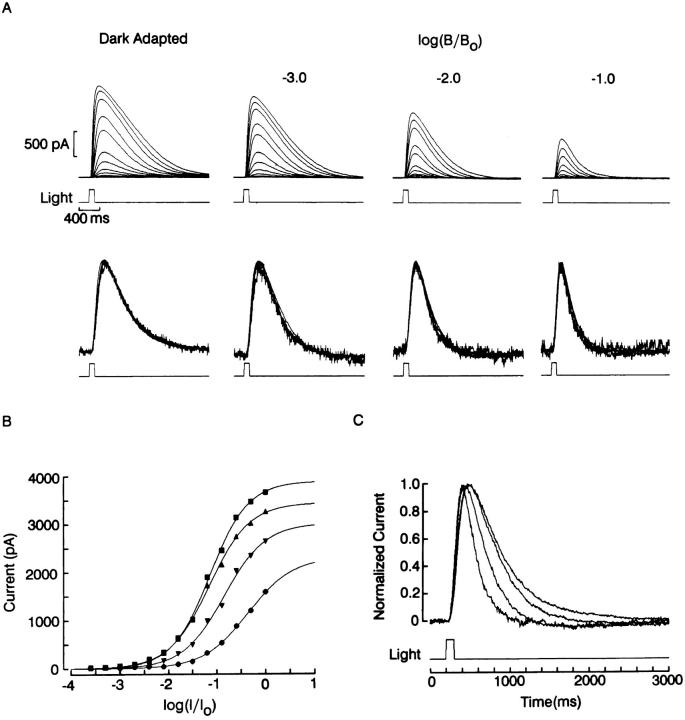
To examine systematically the adapting effects of sustained illumination, the full sensitivity curve of the flash response was determined with a standard background-adaptation protocol. The top row of Fig. 4 A shows recordings obtained in a ciliary photoreceptor voltage-clamped at −20 mV and stimulated with 100-ms flashes of light, the intensity of which was increased at 0.3 log increments. After this dark-adapted series, a steady background light was turned on, and a similar intensity series was administered. The procedure was repeated, increasing the intensity of the background light by one log unit each time. At the end a second control series was run to verify that the dark-adapted responsiveness recovered (peak amplitudes were within 10% of control) so that the photocurrent reduction in the presence of the background light could not be attributed to response run-down. In Fig. 4 B, the peak amplitude of the photocurrent is plotted as a function of log attenuation of the test light for each of the four background conditions. Sigmoidal functions were least-squares fitted to the data to estimate the saturating photocurrent amplitude (S) and the half-maximal stimulus intensity (σ). The resulting curves exhibit two essential features of light adaptation: (a) as the background light intensity is increased, the asymptotic value of the response amplitude is reduced and (b) the stimulus-response curves are progressively shifted in the positive direction along the abscissa. We next examined whether this desensitization was accompanied by changes in photocurrent kinetics. A remarkable feature observed when brief flashes are used to stimulate ciliary photoreceptors is the similarity in the time course of the responses as the light intensity is varied over a substantial range, spanning over 1.5 log units; this can be appreciated in the second row of Fig. 4 A, in which the photocurrent records in each family have been normalized and superimposed. There is a barely noticeable acceleration of the rising phase of the response (which becomes discernible at higher temporal resolution), but the overall shape of the response changes very little. Such near-constancy of kinetics justifies comparing across the four background conditions, because it makes comparisons independent of which particular trace is selected from each family. Fig. 4 C shows the superimposed, normalized half-saturating photocurrents of each group. As the intensity of the background illumination is increased, the response kinetics become faster; the effect is particularly pronounced in the decay phase, whereas the acceleration of the rising phase is comparatively more modest. Complete intensity series in the presence of different background lights were measured in four other cells, with similar results.
Figure 4.
Photoresponse sensitivity and kinetics changes induced by background illumination. (A) Intensity series with brief flashes (100 ms) measured in the dark adapted state (left) and in the presence of a progressively more intense background light. Holding potential was −20 mV; test flash intensity stepped at 0.3 log increments (unattenuated test light intensity 8.1 × 1015 photons · cm−2 · s−1; unattenuated background intensity 4.2 × 1015 photons · cm−2 · s−1). Flashes were spaced 1 min apart and were followed by a 3-s red light (λ > 630 nm, 14.8 mW · cm−2) to ensure constancy of chromatic adaptation throughout the procedure. Second row : normalized superimposed responses to flashes varying over 1.5 log units, for each of the background conditions. In each set of recordings the photocurrent follows a similar time course, irrespective of flash intensity. (B) Peak amplitude of the photocurrent plotted as a function log attenuation of the test light, for each of the four background conditions. Sigmoidal functions were least-squares fitted to the data points via a Simplex minimization algorithm. Increasing background illumination induced a reduction of the asymptotic response amplitude and a shift of the stimulus- response curves along the abscissa. (C) Superimposed, normalized photocurrents from each group in response to a half-saturating light. Adaptation induced an acceleration of the response kinetics, graded with the intensity of the background light.
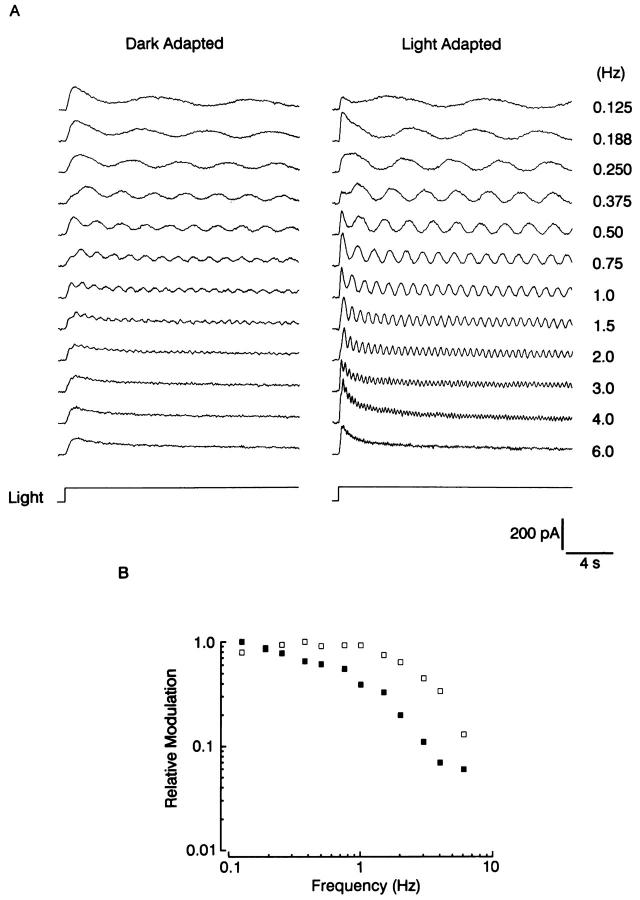
The faster kinetics of the flash response in the presence of a background light reflects the trade-off of speed for sensitivity as the ambient illumination level is varied, a well-known phenomenon in other classes of photoreceptors (Fain and Cornwall, 1994). A convenient quantification of this process entails measuring the temporal modulation transfer function (MTF) at different states of adaptation. Fig. 5 A shows the responses to 20-s steps of 580 nm light, whose intensity was modulated sinusoidally with a depth of 15% and a frequency that varied between 0.125 Hz and 6 Hz. In the recordings on the left, the light was dim, and produced a maximum response <5% of the saturating photocurrent. As the frequency was increased, the modulation of the response was quickly lost, and above 2 Hz only the mean photocurrent is seen (last 4 sweeps). Subsequently, the cell was exposed to a steady background that caused ≈0.5 log desensitization; the test light was made brighter by 1.2 log units in order to produce an incremental response of comparable size as the dark adapted response (right). In the light-adapted state the photoreceptor was capable of tracking more rapid temporal fluctuations in light intensity, and the response modulation faded only for the highest frequency (e.g., 6 Hz). The transfer function is shown in Fig. 5 B: the response modulation is plotted as a function of frequency in double-logarithmic coordinates. The 50% drop-off occurred at ≈0.75 Hz for the dark-adapted measurement, whereas with light adaptation it was attained at ≈2.5 Hz. Similar observations were obtained in a total of eight cells; the average shift in the 50% roll off frequency with light adaptation was 248 ± 64% SD.
Figure 5.
Changes in the temporal modulation transfer function induced by light adaptation. (A) The test light, λ = 580 nm, was presented for 20 s, and its intensity was sinusoidally modulated with a 15% depth, at the frequencies indicated in the right column. The recordings on the left were obtained with a dim light (average intensity 16.5 × 1012 photons · cm−2 · s−1), whereas on the right the cell was exposed to a steady adapting background (3.8 × 1013 photons · cm−2 · s−1), and the superimposed modulated light step intensity was increased by 1.2 log, to produce an incremental response of amplitude comparable to the control response. Light adaptation substantially increased the ability of the photocurrent to follow higher frequencies. (B) Plot of the modulation transfer function for the two conditions. In the dark-adapted state (filled squares) the response modulation dropped to 50% of the low-frequency asymptote around 0.75 Hz. With light adaptation, the roll-off was shifted to ≈2.5 Hz.
Time Course of the Development of Adaptation and Its Recovery
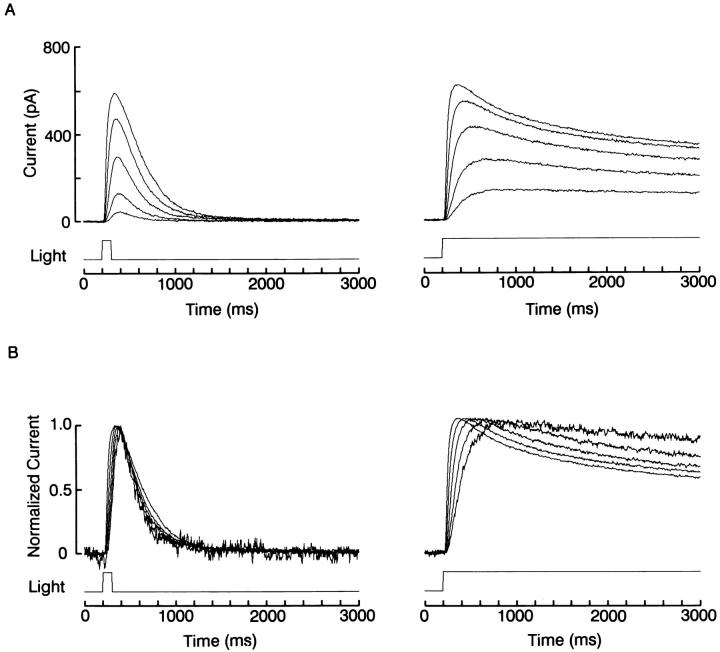
When background illumination is turned on, the process of adjusting the transduction gain must require a defined amount of time. In Fig. 3, above, the amplitude of the first flash response after the onset of the background light was already reduced, and no further changes occurred in subsequent responses. This suggests that light adaptation developed more rapidly than the inter-stimulus interval; the time resolution afforded by this protocol could not be increased substantially, because reduction in the temporal lag between flashes would result in response overlap. On the other hand, the observation that the time course of the photocurrent elicited by brief flashes changes little when the stimulus intensity is varied over a wide range (e.g., Fig. 4 A) indicates that adaptation must require at least a few hundred milliseconds to become manifest. As a consequence, one would predict that with more prolonged light steps the kinetics of the photocurrent should change as a function of light intensity. Such a comparison is presented in Fig. 6. In part A (left) a photoreceptor was stimulated with 100-ms flashes of increasing intensity, spanning 2.4 log; on the right, the protocol was repeated in the same cell, but duration of the stimulus was increased to 2,800 ms. To better compare the time course of the photocurrent at different light intensities, the responses in each family of traces were normalized with respect to their peak amplitude, and superimposed in part B of the figure. Whereas the currents evoked by flashes are almost superimposable (except for a slight acceleration in the rising phase with brighter lights), those evoked by the sustained steps show the progressive development of a relaxation to a reduced plateau level (the differences in the rising phase reflect chiefly the integration time of the cell). Similar results were replicated in three other cells.
Figure 6.
Comparison of flash vs. step response kinetics. (A) Effects of increasing light intensity over a range spanning 2.4 log (from 8.3 × 1012 to 2.1 × 1015 photons · cm−2 · s−1) in a cell stimulated with 100-ms flashes (left) or with sustained light steps (right). (B) Superimposed, normalized traces from the two families of recordings, showing that with brief stimuli the time course of the responses changed little, whereas with prolonged illumination the photocurrent developed a distinct decay phase as the intensity of the stimulus was increased. Holding potential was −20 mV.
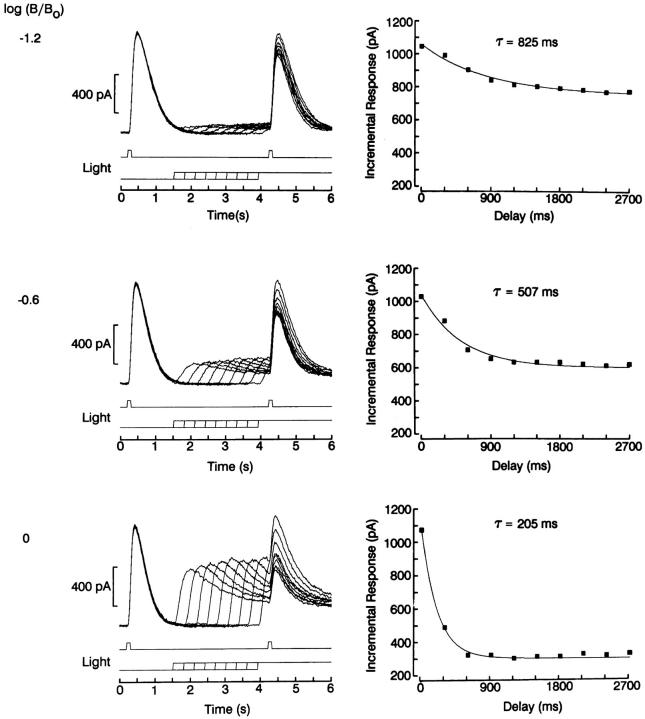
To quantify the time course of the development of light adaptation, we used a more complex protocol, which provided a substantially finer time resolution. A pair of identical test flashes, 100-ms in duration, were presented separated by 4 s, a sufficient temporal lag to allow any desensitization by the first light to dissipate (see also Fig. 7 below). On successive trials a steady background light was turned on during the period between the two test flashes, preceding the second one by an interval that varied between 300 and 2,700 ms. The second flash response, therefore, was always superimposed on the adapting background. As shown in Fig. 7, the amplitude of this incremental response decreased monotonically as the interval between background onset and test flash delivery was increased; this behavior reflects the gradual development of desensitization induced by the adapting light. The constancy of the responses to the first flash indicates that no response run-down occurred during the experiment. The entire sequence was repeated in the same cell, increasing the intensity of the background light at 0.6 log increments. On the right side of the same figure, the peak amplitude of the incremental response is plotted as a function of temporal lag, for the various conditions. Each set of data points was least-squares fitted by a single exponential function. As one would expect, the asymptotic level of response reduction, which measures the extent of desensitization, is directly related to the background light intensity. In addition, a striking change occurred in the exponential relaxation, which reflects the time course of the onset of light adaptation: as the intensity of the background light was increased, the time constant of the process decreased, in the range ≈800–200 ms (n = 3).
Figure 7.
Time course of the onset of light adaptation. Left: Two identical test flashes, 100 ms in duration were delivered 4 s apart. In the time between the two stimuli, a steady background light was turned on, preceding the second test flash by an interval that varied, on successive trials, between 300 and 2,700 ms. As this interval was made longer, the amplitude of the incremental response to the second test flash decreased progressively. The sequence was repeated three times in the same cell, reducing the attenuation of the background light at 0.6 log increments, as indicated on the left of each panel. Right: Peak amplitude of the incremental response plotted as a function of temporal lag between onset of the adapting background and delivery of the second test flash. Data points were least-squares fitted by a single exponential function by means of a Simplex algorithm. The time constant of the desensitization process and the asymptotic amplitude of the response decreased as the intensity of the background increased. Holding potential: −40 mV; test flash intensity: 16.4 × 1014 photons · cm−2 · s−1; unattenuated background intensity: 7.3 × 1014 photons · cm−2 · s−1.
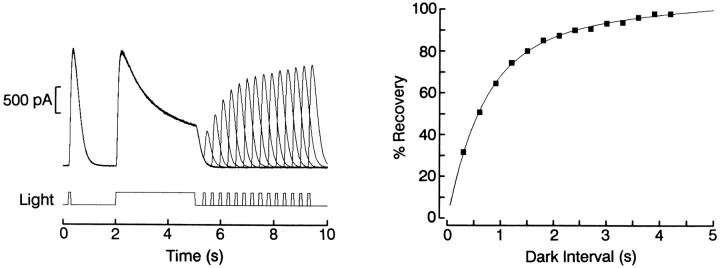
The photoresponse amplitude changes, the shift in sensitivity, and the acceleration of kinetics induced by background lights, demonstrated in Figs. 1–5, clearly indicate that all salient manifestations of light adaptation are present in ciliary photoreceptors. This conclusion implies that the previously mentioned observation by McReynolds and Gorman (1970), concerning the unabated responsiveness to closely spaced light stimuli, must be due to a very rapid recovery of sensitivity, rather than lack of desensitization. We corroborated this notion by examining the time course of dark adaptation, using the triple-stimulus protocol illustrated in Fig. 8: a photoreceptor was stimulated with a 100-ms test flash, which served as a reference, then exposed to a 3-s adapting light of the same intensity; recovery of sensitivity was measured by presenting another 100-ms test flash at different intervals, in the range of 300–4,200 ms, after the termination of the adapting light. A period of darkness, 1 min in duration, was interposed between trials to allow any residual desensitization to dissipate completely. In the left part of the figure one can appreciate that the responses to both the pre-test and the adapting lights are superimposable, indicating that no drift in responsiveness occurred during the experiment. During the 3-s light step a pronounced decay of the photocurrent occurred, chiefly reflecting the adaptation process; when the test flash was delivered shortly afterwards, the response was greatly depressed, but as the dark interval was progressively lengthened the response increased monotonically. The peak amplitude of the test photocurrent is plotted in the right side of the figure as a function of time elapsed since termination of the adapting light, and fitted to a two-time constant exponential function. Recovery was ≈99% complete in about 4 s (n = 3). Considering that the light intensity employed was nearly saturating, these results fully account for the apparent failure to desensitize the photoresponse with pairs of stimuli separated by a few seconds. Similar procedures were used with dimmer lights; in this case the recovery process followed a single-exponential function, lacking the rapid phase (data not shown).
Figure 8.
Time course of the recovery from light adaptation. Left: A photoreceptor was stimulated with a triple-stimulus protocol, consisting of a 100-ms test flash which served as a reference, a 3-s adapting light, and a second test flash. The interval between the termination of the adapting light and the second test flash was varied between 300 and 4,200 ms, at 200-ms increments. A 1-min period of dark adaptation was interposed between trials. The responses to both the pre-test flash and the adapting light are superimposable, indicating that no response rundown occurred. Right: Peak amplitude of the test response plotted as a function of time elapsed since termination of the adapting light. The data points were fitted by a two-time constant exponential (τ1 = 590 ms; τ2 = 4 s). Light intensity 2.1 × 1015 photons · cm−2 · s−1 for all stimuli. Holding potential was −40 mV.
Effects of Calcium Manipulations on Light Adaptation and Recovery
A long-standing tenet in photoreceptor physiology is that the modulation of sensitivity is controlled by changes in intracellular Ca levels. A simple demonstration of this proposition entails superfusing cells with a solution lacking Ca. In rhabdomeric cells from several species this treatment transiently enhances the response amplitude and slows down its kinetics, an effect that has been attributed to the sensitization of the transduction cascade (Limulus : Millecchia and Mauro, 1969; Balanus : Brown et al., 1970; Apis : Raggenbass, 1983). In ciliary photoreceptors 0-Ca ASW increases the amplitude of the light response but has no significant effect on the intensity-response relation (not shown), nor on the kinetics of the photocurrent (Gomez and Nasi, 1995). Because extracellular Ca and Mg were previously shown to block the light-dependent conductance in these cells (Gomez and Nasi 1994; Nasi and Gomez, 1996), the larger responses measured in 0-Ca indicate relief from blockage, rather than a change in the state of adaptation. To further confirm this contention, we examined the effect of Ca removal on the desensitization induced by background lights of different intensities.
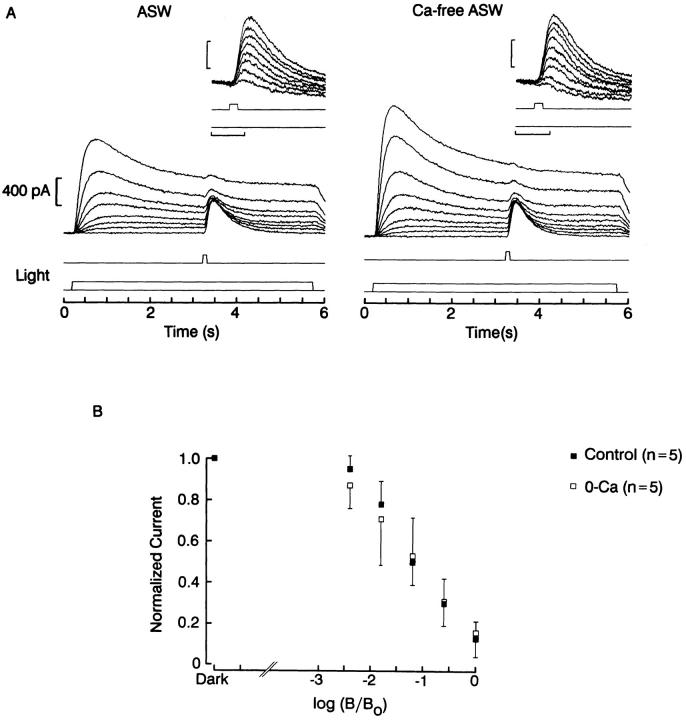
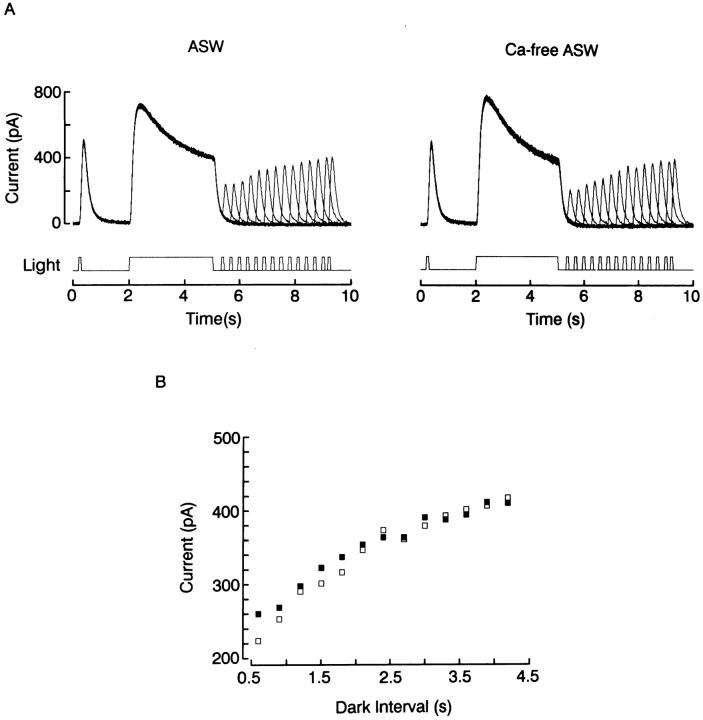
Fig. 9 shows the results of an experiment in which an adapting light step of variable intensity was presented for 5.5 s, and a fixed test flash was superimposed on it during the plateau phase of the response. The incremental response to the flash decreased monotonically as the background intensity was increased. This dependency was not significantly different in 10 mM vs. 0 Ca (n = 5). Exposure to Ca-free solution also failed to affect the recovery from light adaptation. In Fig. 10, a triple-stimulus protocol similar to that of Fig. 8 was used, but with dimmer lights designed to optimize the detection of changes in sensitivity. As the interval elapsed since the termination of the adapting light was increased, the response to the test flash recovered its amplitude; this recovery, however, followed a similar time course in the presence and in the absence of extracellular Ca (n = 2).
Figure 9.
Effects of removal of extracellular Ca on adaptation. (A) The response to 100-ms test flashes delivered in the dark or superimposed upon a 5-s background light was examined in standard ASW (left) and after superfusing the cell with Ca-free solution (right). The amplitude of the photocurrents increased in 0-Ca, but in both conditions the incremental flash response decreased as the background light intensity increased (insets). (B) Peak amplitude of the incremental responses normalized with respect to the dark-adapted value, plotted as a function of the attenuation of the background light. The relative reduction of sensitivity induced by the adapting light did not differ significantly in the two conditions. Test flash intensity: 16 × 1015 photons · cm−2 · s−1; unattenuated background intensity: 4.2 × 1015 photons · cm−2 · s−1.
Figure 10.
Effects of removal of extracellular Ca on recovery from adaptation. (A) A photoreceptor was tested with a triple-stimulus protocol, in which the delay between the termination of an adapting light presented for 3 s and a 100-ms test flash was varied systematically. Superfusion with 0-Ca did not alter the time course of the recovery from adaptation, as shown in part (B), in which the peak amplitude of the test response is plotted as a function of the time between adapting and test lights in ASW (filled squares) and in 0-Ca ASW (empty squares). Light intensity: 4.7 × 1014 photons · cm−2 · s−1; holding potential: −20 mV.
The insensitivity of ciliary photoreceptors to removal of extracellular Ca stands in striking contrast with the effects observed in rhabdomeric photoreceptors. A possible explanation could be that such manipulation simply fails to change [Ca]i significantly, because in these cells (a) light-dependent channels are highly selective for potassium (Gomez and Nasi, 1994),(b) internal Ca release is unlikely to occur, as these cells are unresponsive to IP3 and unaffected by IP3 antagonists (Gomez and Nasi, 1995), and (c) voltage clamping forestalls any contribution by voltage-sensitive Ca channels (Cornwall and Gorman, 1979). An alternative strategy, therefore, is to directly alter intracellular Ca by dialysis via the patch pipette. In a previous report, we demonstrated that the exchange between the pipette solution and the cytosol is rapid and efficient: equilibration occurs with a time constant on the order of 30 s, and the dialysate unambiguously reaches the site of phototransduction (Gomez and Nasi, 1995). We first examined the effects of strongly buffering intracellular calcium on light adaptation.
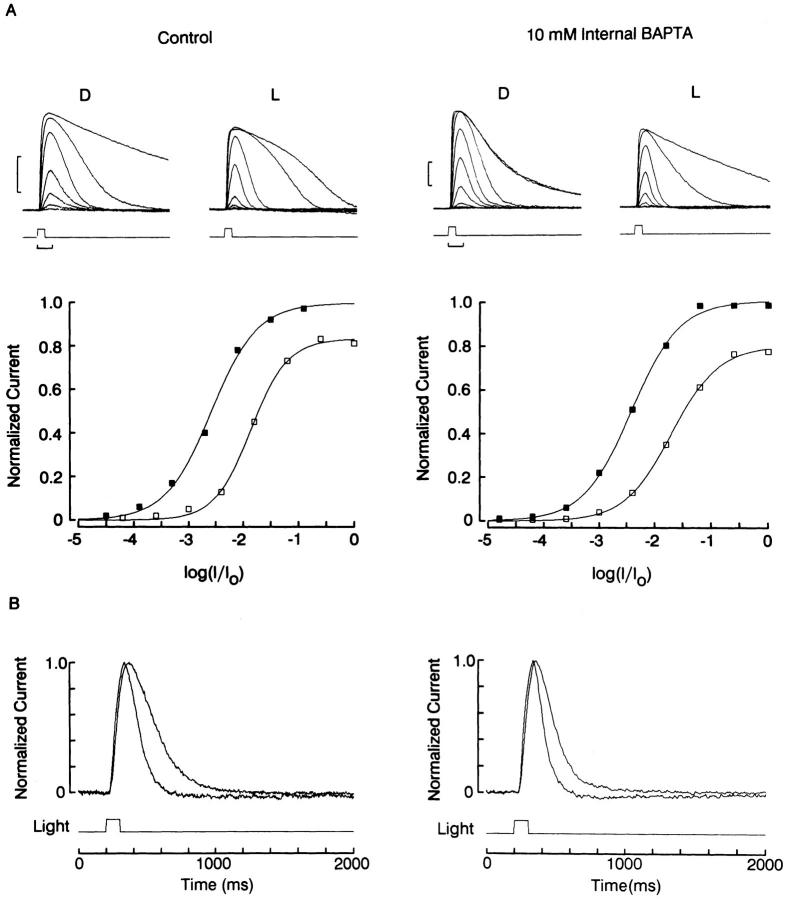
The left side of Fig. 11 A shows a standard background adaptation procedure in a control cell voltage clamped at −30 mV with an electrode filled with the standard intracellular solution containing 1 mM EGTA; light intensity series were measured in the dark-adapted state and in the presence of steady illumination. The adapting light reduced the saturating photoresponse amplitude to 84% and shifted the sensitivity curve (Δσ = 0.73 log); in addition, the response kinetics were significantly accelerated (Fig. 11 B, left). The same effects occurred in a quantitatively comparable manner in the presence of 10 mM intracellular BAPTA, as shown by the panels on the right: the background- induced response reduction was 80%, and Δσ was 0.74 log. Similar results were obtained in 10 cells (5 in each condition). We also compared the effects of different intensities of background illumination in the presence or absence of intracellular BAPTA. In Fig. 12 a cell dialyzed with 10 mM BAPTA was exposed to a 5-s light step of increasing intensity, upon which a fixed 100-ms test flash was superimposed. The incremental response decreased in size as the background was made brighter; this is shown in greater detail in the panel on the right. In Fig. 12 B the average normalized amplitude of the test response, pooled for several control cells and several BAPTA-treated cells, is plotted as a function of the intensity of the adapting light. Again, there is no indication of any systematic change in susceptibility to adaptation in the presence of the calcium chelator.
Figure 11.
Effect of intracellular dialysis with BAPTA. (A) Photoreceptor cells were stimulated with flashes of increasing intensity in the dark-adapted state (D) or in the presence of a steady background illumination (L). The two families of recordings on the left were obtained with the standard internal solution containing 1 mM EGTA; the ones on the right were obtained with 10 mM BAPTA in the patch pipette. In both conditions the background light compressed the amplitude range of the response (calibration bars: left, 200 ms/200 pA; right, 200 ms/100 pA). The corresponding plots of the peak response amplitude as a function of the log of test light attenuation show that in both conditions the background light caused a similar reduction of the saturating flash response (80–85%), and a nearly identical shift of the light intensity eliciting a half-maximal photocurrent (Δσ = 0.73 log). (B) Normalized half saturating responses measured in the dark-adapted and the light-adapted state. Both the control and the BAPTA-treated photoreceptor exhibit a pronounced acceleration of the photoresponse kinetics in the presence of the background light. Test flashes were 100 ms in duration; unattenuated intensity: 11 × 1016 photons · cm−2 · s−1. Background light intensity: 5.3 × 1014 photons · cm−2 · s−1. Holding potential: −20 mV; cells superfused with standard ASW.
Figure 12.
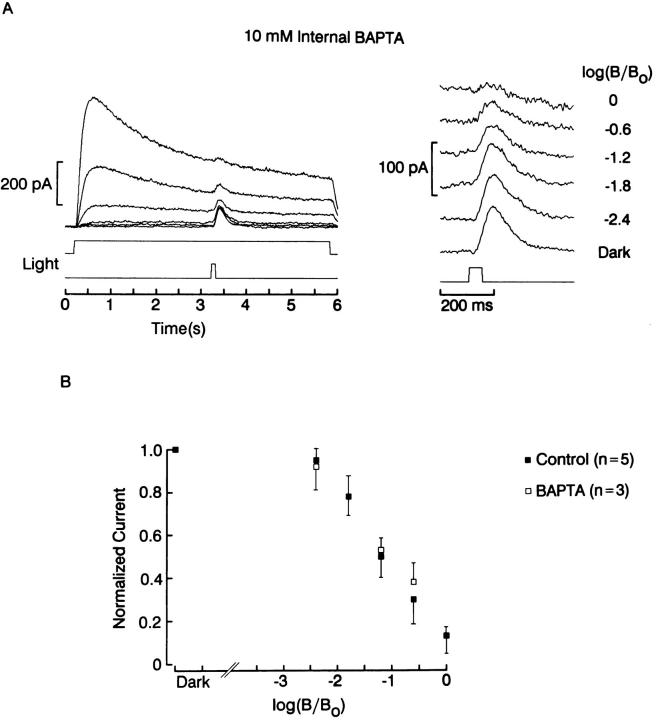
Susceptibility to adaptation by background light, in the presence or absence of intracellular BAPTA. (A) A photoreceptor voltage-clamped at −30 mV with a patch pipette containing 10 mM BAPTA was exposed to a 5-s light at 0.6 log increments of intensity, with a superimposed test flash 100 ms in duration (16.4 × 1013 photons · cm−2 · s−1). The incremental response (right) decreased in amplitude as the background was made brighter. (B) Normalized photocurrent, averaged for three cells treated with BAPTA and five control cells, plotted as a function of the intensity of the adapting light. There were no significant differences in susceptibility to adaptation in the presence of BAPTA. Unattenuated background intensity: 7.3 × 1014 photons · cm−2 · s−1.
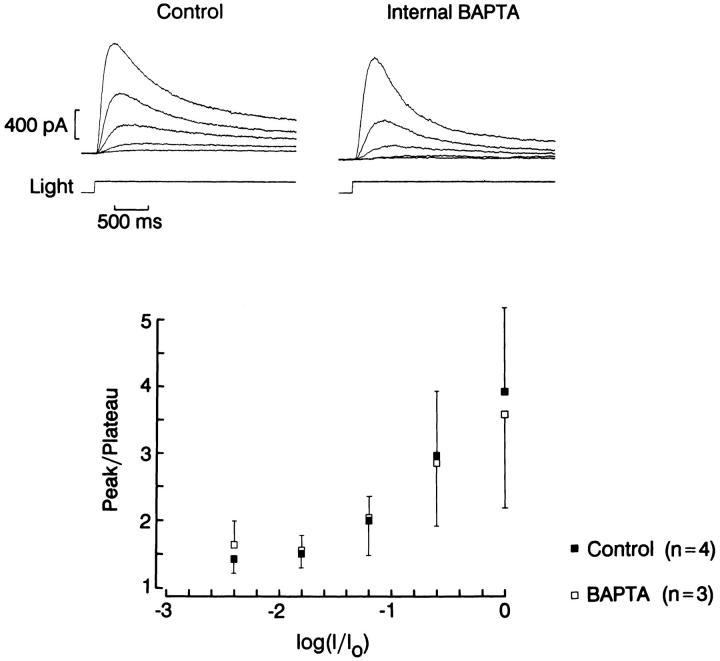
A final criterion is to compare the peak-to-plateau decay of the photocurrent elicited by a sustained light in cells treated with 10 mM BAPTA vs. 1 mM EGTA. Because the relaxation reflects in part the development of adaptation, if Ca changes play an important role in the process, the decay phase should be attenuated by the high concentration of the Ca chelator. Fig. 13 illustrates such a comparison. Light stimuli of increasing intensity were presented for a period of 3 s, separated by a 1-min interval of dark adaptation. The ratio of peak amplitude vs. plateau is plotted in the bottom graph of the figure, and shows no significant differences at any of the light intensities used. The converging results obtained with different approaches strongly indicate that chelating internal Ca has little effect on light response in ciliary cells; by contrast, identical treatments administered to rhabdomeric cells from the same retina profoundly influence the amplitude sensitivity and kinetics of the photocurrent (data not shown).
Figure 13.
Effect of intracellular BAPTA on the peak-to-plateau decay of the photocurrent. A sustained light was used to stimulate photoreceptors dialyzed with the control (left) or the BAPTA-containing intracellular solution (right). BAPTA did not attenuate the relaxation of the photocurrent as the light intensity increased. The ratio peak/plateau photocurrent amplitude at each light intensity is plotted on the bottom. No statistically significant differences were detected. Unattenuated light intensity: 7.3 × 1014 photons · cm−2 · s−1; holding potential: −30 mV.
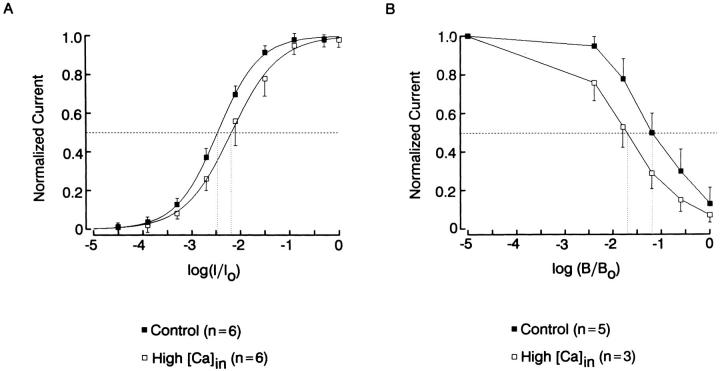
A complementary approach is to induce an elevation of intracellular Ca; we used 10 μM free Ca in the electrode solution, a concentration than in rhabdomeric cells causes a loss of sensitivity of at least 3 orders of magnitude, and a marked acceleration of the response kinetics. Fig. 14 A compares the intensity-response function averaged for six control cells (1 mM EGTA, no added Ca) and six cells internally perfused with 10 μM Ca2+. In the high-Ca condition the sensitivity curve shifted marginally, by ≈0.3 log. This shift, however, is quite modest by comparison to that induced by background illumination and is equivalent to the desensitization produced by an adapting light ≈20-fold dimmer than the half-saturating intensity. Fig. 14 B shows a comparison of the desensitization of the response to a fixed test flash in control conditions vs. elevated intracellular Ca, as a function of background light intensity. In the presence of high [Ca]i the cells retained the full dynamic range of modulation by the adapting light; and the background light intensity required to reduce the size of the incremental photoresponse by 50% was only slightly lower (by 0.49 log).
Figure 14.
(A) Intensity-response function averaged for six control cells (1 mM EGTA in the intracellular solution, no added Ca), and six cells internally perfused with 10 μM free Ca. In the high-Ca condition the curve shifted marginally to the right (Δσ = 0.3 log). (B) Reduction in the amplitude of the incremental response to a fixed test flash (100 ms, 16.4 × 1013 photons · cm−2 · s−1) as a function of the intensity of background illumination. In the presence of elevated internal Ca the background intensity causing a 50% reduction in the test response was shifted to the left by 0.49 log. Unattenuated intensity of the background light 7.3 × 1014 photons · cm−2 · s−1.
discussion
The results presented above indicate that changes of ambient illumination modulate the photocurrent sensitivity and kinetics in Pecten ciliary photoreceptors, in a way that resembles in all respects the light adaptation process found in other photoreceptor types, both vertebrate and invertebrate. However, cytosolic calcium appears to have little influence on this modulatory process, in sharp contrast with its pivotal role in several crucial aspects of light adaptation in other visual cells. In amphibian rods a decrease in intracellular Ca, such as it occurs during illumination (Yau and Nakatani, 1985; McNaughton et al., 1986; Ratto et al., 1988), modulates, via Ca-binding proteins, the activity of guanylate cyclase (Koch and Stryer, 1988), thus partially restoring the dark current and light responsiveness (reviewed by Fain and Matthews, 1990). In addition, Ca also regulates the lifetimes of activated rhodopsin (and, consequently, stimulation of PDE; Kawamura, 1993), the catalytic activity of rhodopsin (Lagnado and Baylor, 1994), and the affinity of the channels for cGMP (Hsu and Molday, 1993). These coordinated functions decrease sensitivity and speed-up the dark-current recovery during background illumination. In rhabdomeric photoreceptors of Limulus and Apis, where light causes an increase in internal Ca (Brown and Blinks, 1974; Walz et al., 1994), the photoresponse is transiently enhanced by lowering [Ca]o (Millecchia and Mauro, 1969; Raggenbass, 1983) or by intracellular application of Ca chelators (Lisman and Brown, 1975; Bader et al., 1976), whereas direct Ca injection causes desensitization (Lisman and Brown, 1972; Bader et al. 1976; Fein and Charlton, 1977). The mechanisms by which Ca mediates some key aspects of light adaptation in rhabdomeric photoreceptors are presently poorly understood, but the possible involvement of Ca-triggered protein phosphorylation mediated by a protein kinase C (PKC) was recently suggested by observations on a Drosophila mutant, inaC, which lacks an eye-specific PKC (Smith et al., 1991) and displays reduced desensitization by background illumination (Hardie et al., 1993).
We should emphasize that the present observations in ciliary visual cells point to the existence of a powerful alternative pathway for light adaptation but do not necessarily rule out all involvement of Ca under physiological conditions: for example, manipulations of cytosolic calcium could be rendered ineffective by the loss of some required soluble co-factor, as a result of cell dialysis. In principle, it should be possible to prevent such wash-out effects by using the perforated-patch variant of whole-cell clamp (Horn and Marty, 1988). Although this approach has been successfully applied to other isolated molluscan photoreceptors (Nasi and Gomez, 1992b ), control of internal Ca becomes problematic, because the best characterized pore-forming agents used for this purpose, such as Nystatin and Amphotericin B, only allow small monovalent ions to exchange between pipette and cytosol.
The nature of the Ca-independent pathway that modulates the photoresponse in Pecten ciliary photoreceptors is presently unknown. Possible leads are provided by experiments in which the light-sensitive conductance was directly stimulated by intracellular dialysis with cGMP: this treatment decreased the response to subsequent test flashes, as one would expect if light and cGMP tap the same effector (Gomez and Nasi, 1995); however, the observed decrease in responsiveness appeared to exceed the reduction in available channels, and the residual photocurrent displayed faster kinetics, suggesting that the nucleotides may have also desensitized the cell. Possible mechanisms that may mediate such an effect include protein phosphorylation by a cyclic-nucleotide–dependent kinase (e.g., PKG). In vertebrate rods two small phosphopeptides are phopsphorylated in a light-dependent manner (Polans et al., 1979); this phosphorylation has been shown to be regulated by cyclic nucleotides (Hermolin et al., 1982) probably by a cAMP-dependent protein kinase (Hamm, 1990). Cyclic-nucleotide–dependent protein phosphor-ylation was indeed proposed to contribute to light adaptation (Hermolin et al., 1982). A precedent for a negative feedback loop in which the ligand for the channels that underlie the receptor potential also down-regulates the transduction process via protein phosphorylation has recently been demonstrated: in olfactory cells that possess two separate transduction pathways, cAMP (one of the internal transmitters that gates odorant-dependent channels) also activates a PKA which, in turn, inhibits the stimulus-induced increase in intracellular cAMP. Conversely, a PKC is involved in the termination of the IP3-mediated signal (Boekhoff and Breer, 1992).
The present data raise the question of whether a regulatory scheme that operates independently of cytosol-ic Ca changes is unique to molluscan hyperpolarizing photoreceptors or if it may be shared by other visual cells. In spite of the powerful case for Ca mediation of adaptation in vertebrate rods, during the course of the last few years evidence has been emerging that some manifestations of light adaptation occur independently of Ca changes: for instance, an adapting light is capable of inducing an acceleration of the rising phase of the photoresponse, even under conditions in which cytosolic Ca is manipulated over a wide range of concentrations (Nicol and Bownds, 1989). Conversely, the acceleration of flash response recovery induced by background illumination does not obtain if an equivalent change of [Ca]i is imposed in the dark (Gray-Keller and Detwiler, 1996). Furthermore, the state of phosphorylation of light dependent channels, which determines their responsiveness to cyclic nucleotides, is controlled by endogenous phosphatases in a manner that is insensitive to Ca over a concentration range spanning 4 orders of magnitude (Gordon et al., 1992). Thus, the presence of alternative modulatory mechanisms of visual transduction may be of wide generality. Isolation of modulatory mechanisms that operate in a Ca-independent way is difficult in most photoreceptor cells because Ca changes are usually a natural concomitant of the photoresponse; in the case of invertebrates, they may in fact be inextricably linked to the visual excitation process (Bolsover and Brown, 1985; Werner et al., 1992; Shin, Richard and Lisman, 1993). In this respect, ciliary invertebrate photoreceptors provide a uniquely advantageous situation, because the two processes can be easily uncoupled.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 EY07559.
Footnotes
Abbreviations used in this paper: ASW, artificial sea water; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N ′,N ′-tetraacetic acid; IP3, inositol 1,4,5-trisphosphate.
Portions of these results have been previously published in abstract form (Nasi, E., and M. Gomez. 1995. Biophys. J. 68:A18).
references
- Bader CR, Baumann F, Bertrand D. Role of intracellular calcium and sodium in light adaptation on the retina of the honey bee drone (Apis mellifera, L.) . J Gen Physiol. 1976;67:475–491. doi: 10.1085/jgp.67.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I, Breer H. Termination of second messenger signaling in olfaction. Proc Natl Acad Sci USA. 1992;89:471–474. doi: 10.1073/pnas.89.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsover SR, Brown JE. Calcium ion, an intracellular messenger of light adaptation, also participates in excitation of Limulusphotoreceptors. J Physiol (Camb) 1985;364:381–393. doi: 10.1113/jphysiol.1985.sp015751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Blinks JR. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. J Gen Physiol. 1974;64:643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HM, Hagiwara S, Koike H, Meech RM. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J Physiol (Camb) 1970;208:385–413. doi: 10.1113/jphysiol.1970.sp009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Gorman ALF. Contribution of calcium and potassium permeability changes to the off response of scallop hyperpolarizing photoreceptors. J Physiol (Camb) 1979;291:207–232. doi: 10.1113/jphysiol.1979.sp012808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall MC, Gorman ALF. The cation selectivity and voltage dependence of the light-activated potassium conductance in scallop distal photoreceptor. J Physiol (Camb) 1983;340:287–305. doi: 10.1113/jphysiol.1983.sp014763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin WJ. The eye of Pecten. . Q J Microsc Sci. 1910;55:49–112. [Google Scholar]
- Eakin RM. Evolution of photoreceptors. Cold Spring Harbor Symp Quant Biol. 1965;30:363–370. doi: 10.1101/sqb.1965.030.01.036. [DOI] [PubMed] [Google Scholar]
- Fain, G.L., and M.C. Cornwall. 1994. Light and dark adaptation in vertebrate photoreceptors. In Contrast Sensitivity. R. Shapley and D. Lam, editors. M.I.T. Press, Cambridge, MA. 3–32.
- Fain GL, Matthews HR. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990;13:378–384. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Fein A, Charlton S. A quantitative comparison of the effects of intracellular calcium injection on the photoresponse of Limulusventral photoreceptors. J Gen Physiol. 1977;70:591–600. doi: 10.1085/jgp.70.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein, A., and E. Szuts. 1982. Photoreceptors: their role in vision. Cambridge University Press, Cambridge, UK. pp 212.
- Gomez M, Nasi E. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptors: a patch-clamp study. J Gen Physiol. 1994;103:939–956. doi: 10.1085/jgp.103.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M, Nasi E. Activation of light-dependent potassium channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca cascade. Neuron. 1995;15:607–618. doi: 10.1016/0896-6273(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- Gorman ALF, McReynolds JS. Hyperpolarizing and depolarizing receptor potentials in the scallop eye. Science (Wash DC) 1969;165:309–310. doi: 10.1126/science.165.3890.309. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB. Ca2+dependence of dark- and light-adapted flash responses in rod photoreceptors. Neuron. 1996;17:323–331. doi: 10.1016/s0896-6273(00)80163-4. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Peretz A, Suss-Toby E, Rom-Glas A, Bishop SA, Selinger Z, Minke B. Protein kinase C is required for light adaptation in Drosophilaphotoreceptors. Nature (Lond) 1993;363:634–637. doi: 10.1038/363634a0. [DOI] [PubMed] [Google Scholar]
- Hamm H. Regulation by light of cyclic nucleotide-dependent protein kinases and their substrates in frog rod outer segments. J Gen Physiol. 1990;95:545–567. doi: 10.1085/jgp.95.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermolin J, Karell MA, Hamm HE, Bownds MD. Calcium and cyclic GMP regulation of light-sensitive protein phosphorylation in frog photoreceptor membranes. J Gen Physiol. 1982;79:633–655. doi: 10.1085/jgp.79.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature (Lond) 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature (Lond) 1993;362:855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch W-H, Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature (Lond) 1988;334:64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Baylor DA. Calcium controls light-triggered formation of catalytically active rhodopsin. Science (Wash DC) 1994;367:273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Brown JE. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulusventral photoreceptors. J Gen Physiol. 1972;59:701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Brown JE. Effects of intracellular injection of calcium buffers on light adaptation in Limulusventral photoreceptors. J Gen Physiol. 1975;66:489–506. doi: 10.1085/jgp.66.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton PA, Cervetto L, Nunn BJ. Measurement of the intracellular free calcium concentration in salamander rods. Nature (Lond) 1986;322:261–263. doi: 10.1038/322261a0. [DOI] [PubMed] [Google Scholar]
- McReynolds, J.S. 1976. Hyperpolarizing photoreceptors in invertebrates. In Neural principles in Vision. F. Zettler and R. Weiler, editors. Springer-Verlag, Berlin. 394–409.
- McReynolds, J.S., and A.L.F. Gorman. 1970. Photoreceptor potentials of opposite polarities in the eye of the scallop, Pecten irradians. J. Gen. Physiol. 56:376–391. [DOI] [PMC free article] [PubMed]
- Millecchia R, Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969;54:310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W.H. 1958. Derivatives of cilia in the distal sense cells of the retina of Pecten. J. Biophys. Biochem. Cytol. 4:227–228. [DOI] [PMC free article] [PubMed]
- Mpitsos, G.J. 1973. Physiology of vision in the mollusk Lima scabra. J. Neurophysiol. 36:371–383. [DOI] [PubMed]
- Nasi E. Electrophysiological properties of isolated photoreceptors from the eye of Lima scabra. . J Gen Physiol. 1991a;97:17–34. doi: 10.1085/jgp.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi E. Two light-dependent conductances in the membrane of Limaphotoreceptor cells. J Gen Physiol. 1991b;97:55–72. doi: 10.1085/jgp.97.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi, E., and M. Gomez. 1992a Light-activated ion channels in solitary photoreceptors from the eye of the scallop Pecten irradians. J. Gen. Physiol. 99:747–769. [DOI] [PMC free article] [PubMed]
- Nasi E, Gomez M. Electrophysiological recordings from solitary photoreceptors isolated from the retina of the squid Loligo pealei. . Vis Neurosci. 1992b;8:349–358. doi: 10.1017/s0952523800005083. [DOI] [PubMed] [Google Scholar]
- Nasi, E., and M. Gomez. 1996. Kinetics of divalent cation blockage of light-dependent K channels: requirement for an open pore. Biophys. J. A137. (Abstr.). [DOI] [PMC free article] [PubMed]
- Nicol GD, Bownds MD. Calcium regulates some, but not all aspects of light adaptation in rod photoreceptors. J Gen Physiol. 1989;94:233–259. doi: 10.1085/jgp.94.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polans AS, Hermolin J, Bownds MD. Light-induced dephosphorylation of two proteins in frog rod outer segments: influence of cyclic nucleotides and calcium. J Gen Physiol. 1979;74:595–613. doi: 10.1085/jgp.74.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggenbass M. Effects of extracellular calcium and of light adaptation on the response to dim light in honey bee drone photoreceptors. J Physiol (Camb) 1983;344:525–548. doi: 10.1113/jphysiol.1983.sp014955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto GM, Payne R, Owen WG, Tsien RY. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with Fura-2. J Neurosci. 1988;8:3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Richard EA, Lisman JE. Ca2+ is an obligatory intermediate in the excitation cascade of Limulusphotoreceptors. Neuron. 1993;11:845–855. doi: 10.1016/0896-6273(93)90114-7. [DOI] [PubMed] [Google Scholar]
- Smith DP, Ranganathan R, Hardy RW, Marx J, Tsuchida T, Zuker CS. Photoreceptor deactivation and retinal degeneration mediated by a photoreceptor-specific protein kinase C. Science (Wash DC) 1991;254:1478–1484. doi: 10.1126/science.1962207. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K, Yamada E. The fine structure of the retina studied with the electron microscope. IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol. 1959;6:225–230. doi: 10.1083/jcb.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz B, Zimmermann B, Seidl S. Intracellular Ca2+ concentration and latency of light-induced Ca2+changes in photoreceptors of the honeybee drone. J Comp Physiol A Sens Neural Behav Physiol. 1994;174:421–431. [Google Scholar]
- Werner U, Suss-Toby E, Rom A, Minke B. Calcium is necessary for light excitation in barnacle photoreceptors. J Comp Physiol. 1992;170:427–434. doi: 10.1007/BF00191459. [DOI] [PubMed] [Google Scholar]
- Wong F. Nature of light-induced conductance changes in ventral photoreceptors of Limulus. . Nature (Lond) 1978;276:76–79. doi: 10.1038/276076a0. [DOI] [PubMed] [Google Scholar]
- Yau KW, Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature (Lond) 1985;313:579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yeandle S. Evidence of quantized slow potentials in the eye of Limulus. . Am J Opthalmol. 1958;46:82–87. [Google Scholar]