Abstract
Functional evaluation of chemically modified human erythrocytes has led to the proposal that amino acid residue E681 of the band 3 anion exchanger AE1 lies on the anion translocation pathway and is a proton carrier required for H+/SO4 2− cotransport. We have tested in Xenopus oocytes the functional consequences of mutations in the corresponding residue E699 of mouse AE1. Most mutations tested abolished AE1-mediated Cl− influx and efflux. Only the E699Q mutation increased stilbene disulfonate-sensitive efflux and influx of SO4 2−. E699Q-mediated Cl− influx was activated by elevation of intracellular SO4 2−, but E699Q-mediated Cl− efflux was undetectable. The DNDS (4,4′-dinitrostilbene-2,2′-disulfonic acid) sensitivity of E699Q-mediated SO4 2− efflux was indistinguishable from that of wt AE1-mediated Cl− efflux. The extracellular anion selectivity of E699Q-mediated SO4 2− efflux was similar to that of wt AE1-mediated Cl− efflux. The stoichiometry of E699Q-mediated exchange of extracellular Cl− with intracellular SO4 2− was 1:1. Whereas SO4 2− injection into oocytes expressing wt AE1 produced little change in membrane potential or resistance, injection of SO4 2−, but not of Cl− or gluconate, into oocytes expressing E699Q depolarized the membrane by 17 mV and decreased membrane resistance by 66%. Replacement of bath Cl− with isethionate caused a 28-mV hyperpolarization in SO4 2−-loaded oocytes expressing E699Q, but had no effect on oocytes expressing wt AE1. Extracellular Cl−-dependent depolarization of SO4 2−-preloaded oocytes was blocked by DNDS. AE1 E699Q-mediated inward current measured in the presence of extracellular Cl− was of magnitude sufficient to account for measured 35SO4 2− efflux. Thus, AE1 E699Q-mediated SO4 2−/ Cl− exchange operated largely, if not exclusively, as an electrogenic, asymmetric, 1:1 anion exchange. The data confirm the proposal that E699 resides on or contributes to the integrity of the anion translocation pathway of AE1. A single amino acid change in the sequence of AE1 converted electroneutral to electrogenic anion exchange without alteration of SO4 2−/Cl− exchange stoichiometry.
Keywords: band 3, chloride/bicarbonate exchange, Xenopus oocyte, BCECF, sulfate
introduction
The anion exchanger 1 (AE1)1 (band 3) erythroid anion exchanger is one of the most extensively studied membrane transport proteins (Passow, 1986; Tanner, 1993; Alper, 1994). Erythroid AE1-mediated exchange of Cl− for HCO3 − serves to increase the total CO2 carrying capacity of blood. Intracellular HCO3 − generated by red cell carbonic anhydrase from CO2 released from respiring tissues is exchanged for extracellular Cl− in the capillaries of the peripheral circulation. In the pulmonary capillaries the reverse sequence occurs, and extracellular HCO3 − enters the cell in exchange for intracellular Cl−. The newly entered HCO3 − is converted to CO2 by carbonic anhydrase, and the CO2 can diffuse from the blood cell across endothelium, interstitial space, and alveolar epithelium into the alveolar airspace for expiration.
Despite many years of study, the molecular mechanism by which AE1 mediates anion exchange remains unclear. Only recently has data emerged identifying amino acid residues that may participate directly in binding and translocating anions. Chemical modification studies of red cells have contributed to these identifications. The requirement for liganding anions focused initial attention on cationic amino acid residues. Indeed, chemical modification and pH titration of AE1 in red cells led to the hypothesis that one or more arginine residues play important roles in AE1 transport function (Wieth et al., 1982; Julien and Zaki, 1988). Reductive methylation of AE1 lysines inhibited transport (Jennings, 1982), whereas dansylation of red cells produced reciprocal changes in Cl− and SO4 2− transport (Lepke and Passow, 1982; Berghout et al., 1988). Mutation of the lysine residues that provide covalent attachment sites to the isothiocyanate moieties of cyanostilbene disulfonates reduced inhibition by these site-directed reagents, but did not change the K 1/2 for extracellular Cl− of AE1-mediated transport (Wood et al., 1992). A different lysine residue has also been identified as exposed to both intracellular and extracellular aqueous media by fluorescence quench of pyridoxal phosphate covalently bound to red cell AE1 (Bar-Noy and Cabantchik, 1990).
The mildly acidic pK of inhibition by protons of red cell Cl−/Cl− exchange led to the implication of histidine residues in AE1 transport function by study of the effect of diethylpyrocarbonate modification of red cells (Izuhara et al., 1989) and by site-directed mutagenesis (Muller-Berger et al., 1995b ). SO4 2− transport in red cells was found to be stimulated by acidification, with a pKa of 5.5, and was accompanied by proton uptake (Milanick and Gunn, 1982, 1984). The demonstration of H+-SO4 2− cotransport led to the proposal that the residue responsible for proton binding is a residue of acidic pKa. Both titration experiments and chemical modification studies have implicated carboxylate residues in AE1-mediated H+-SO4 2−/Cl− exchange.
Jennings and colleagues have investigated further the involvement of glutamate residues in human AE1-mediated anion exchange. They reported that reduction of the E681 carboxylate to the corresponding alcohol by treatment with Woodward's reagent K (WRK) followed by reduction with borohydride (BH4) produced a complex pattern of changes in anion transport (Jennings and Anderson, 1987; Jennings and Smith, 1992). Cl−/ Cl− exchange was inhibited, whereas SO4 2− i/SO4 2− o and SO4 2− i/Cl− o exchange were stimulated 5- to 10-fold and 80-fold, respectively. Protons were no longer cotransported with SO4 2−, and the activation of SO4 2− transport by acidic pH was abolished. Moreover, in WRK-BH4 modified cells, trans Cl−-dependent efflux of 35SO4 2− was accelerated by cationophores, and ionophore-mediated 86Rb efflux was activated by extracellular Cl−. (Jennings and Al-Rhaiyel, 1988; Jennings, 1995). Residue E681 of AE1 was defined as the principal target for WRK-BH4 modification of intact human erythrocytes (Jennings and Smith, 1992). These workers proposed that E681 is exposed to both intracellular and extracellular aqueous spaces during the anion exchange cycle and is a proton binding site for H+/SO4 2− cotransport. The data further suggested that modification of the AE1 residue E681 to the corresponding alcohol conferred on modified AE1 the ability to mediate electrogenic exchange of intracellular SO4 2− for extracellular Cl− (Jennings, 1995).
We were prompted by these findings to examine the functional role of the corresponding glutamate residue in mouse AE1, E699, as expressed in the Xenopus oocyte from cRNA. The Xenopus oocyte was the first heterologous system used for functional expression of recombinant AE1 (Bartel et al., 1989; Garcia and Lodish, 1989) and has been developed by subsequent investigators for examination of structure-function relationships in the AE1 polypeptide (Kietz et al., 1991; Groves and Tanner, 1993; Chernova et al., 1995; Muller-Berger, 1995a, b). The Xenopus oocyte has been used to confirm the electroneutrality of wild-type (wt) AE1-mediated Cl−/anion exchange, but also to demonstrate the potential-dependence of transport rates (Grygorczyk et al., 1987).
We have found decreased Cl− transport in oocytes expressing mouse AE1 mutated at residue E699. Only one among the several amino acid substitutions tested, E699Q, displayed increased SO4 2− transport. Efflux of intracellular SO4 2− was easily detected and required the presence of extracellular Cl− or other appropriate anions. In contrast efflux of intracellular Cl− was undetectable regardless of the extracellular anion present. Whereas AE1 E699Q mediated 1:1 electroneutral SO4 2−/SO4 2− exchange, exchange of intracellular SO4 2− for extracellular Cl− by AE1 E699Q was accompanied by inward currents, consistent with electrogenic outflow of anions. E699Q-mediated anion fluxes and currents were inhibited by the stilbene disulfonate, DNDS (4,4′-dinitrostilbene-2,2′-disulfonic acid). The stoichiometry of SO4 2− efflux to Cl− influx to inward current was consistent with 1:1 electrogenic anion exchange.
Our results with recombinant mutant AE1 confirm and extend several earlier conclusions from the studies of Jennings and colleagues on red cells: (a) E699 serves as the proton binding site during H+SO4 2−/Cl− exchange by wt AE1; (b) charge neutralization at E699 leads to electrogenic exchange of SO4 2− for Cl− with a 1:1 stoichiometry; and (c) such electrogenic exchange is asymmetric. The results further show that electroneutrality and 1:1 stoichiometry are properties of the AE1 protein that can be uncoupled. Recent results from two additional groups address the functional role of E699. Muller-Berger et al. (1995a) showed that AE1 E699D expressed in Xenopus oocytes exhibits increased pK for Cl−/Cl− exchange. Sekler et al. (1995) found in microsomes prepared from transfected HEK293 cells that whereas AE1 E699D exhibited complete loss of SO4 2−/ SO4 2− exchange, AE1 E699Q exhibited increased SO4 2−/ SO4 2− exchange accompanied by loss of the pH-dependence characteristic of wt AE1.
methods
Materials
Female Xenopus laevis were purchased from NASCO (Madison, WI), maintained at room temperature in running distilled water, and fed with frog brittle (NASCO). DNDS was from Pfaltz & Bauer (Waterbury, CT). Bumetanide was obtained from Dr. P. Feit (Leo Pharmaceuticals Ballerup, Denmark). All salts were analytical grade and were purchased from Fluka Chemical Corp. (Ronkonkoma, NY) or Sigma Chemical Co. (St. Louis, MO) unless otherwise noted. Na36Cl was from Amersham Corp. (Arlington Heights, IL) or NEN-Dupont (Boston, MA); Na235SO4 was from ICN Biomedicals Inc. (Costa Mesa, CA).
Solutions
ND-96 contained (in mM): 96 NaCl, 2 KCl, 1.8 MgCl2, 1 CaCl2, and 5 HEPES hemisodium, pH 7.40. In some experiments 96 mM NaCl was replaced mole for mole with the sodium salt of either gluconate, isethionate, nitrate, bromide, or iodide. Alternatively, 96 mM NaCl was replaced with 64 mM Na2SO4 or with 70 mM sodium phosphate, pH 7.4. All Cl−-free solutions included the above mentioned concentrations of K+, Mg2+, and Ca2+ as the gluconate salts.
Construction of AE1 Mutants
The 563 nt Sma1/Sph1 fragment encoding murine erythroid AE1 nt 2117-2679 (Kopito and Lodish, 1985) was excised from the murine kidney AE1 plasmid pBL (Brosius et al., 1989) and subcloned into M13mp19. Mutations in AE1 E699 were constructed in this phage subclone by the dut−/ung− method (Kunkel et al., 1991) using the degenerate oligonucleotide 5′-CAT TTTCCTT[C,G,T,A][C,G,T,A]GTCT-3′ and the Muta-Gene T7 Kit (Bio-Rad Laboratories, Richmond, CA). Mutant phage were detected by sequencing across the mutation site. Double-stranded DNAs from phage in which E699 had been mutated to R, K, G, T, and Q were used to reconstruct full-length mutant AE1 cDNAs. Mutant plasmid cDNAs were again sequenced across the mutation site.
Expression of cRNA in Xenopus Oocytes
Transcription template was made by linearizing plasmids with HindIII. cRNA transcription with T7 RNA polymerase was performed with the Megascript Kit (Ambion Inc., Austin, TX). Manually defolliculated oocytes prepared as previously described (Humphreys et al., 1994) were microinjected with 20 ng cRNA and incubated in ND-96 at 19°C for 1–14 d.
Immunoprecipitation of Total and Surface AE Proteins from Xenopus Oocytes
Groups of 10–12 oocytes previously injected with water or with 20 ng cRNA were incubated in ND-96 containing 1–1.5 mCi/ml of 35S-methionine (20–30 μM) for 48–72 h. Metabolically labelled oocytes were washed in modified ND-96, pH 8.0, then incubated for 1 h at 4°C in the same medium in the presence or absence of 5 mg/ml chymotrypsin. Oocytes were then washed three times in 10 ml ND-96, pH 7.4 containing 2 mM PMSF and 1 mg/ml BSA Fraction V, and once more in the same medium containing 100 μg/ml chymostatin and 1 mg/ml BSA. Groups of washed oocytes were manually homogenized at 4°C with a fitted Teflon® pestle (Kontes, Vineland, NJ) in microfuge tubes with 100 μl oocyte immunoprecipitation (IP) buffer containing (in mM) 50 Tris-HCl, 1 EDTA, 1 PMSF, and 0.04 each of leupeptin, pepstatin, and antipain, pH 7.6. The extract was incubated with shaking for 30 min at 4°C, then centrifuged 10 min in a microfuge. The resultant supernatants were brought to 500 mM NaCl and precleared with 5% normal rabbit serum. Precleared supernatants were incubated 1 h with rabbit polyclonal antiserum raised against mouse AE1 aa 214-228 (Alper et al., 1989), followed by precipitation with protein A-agarose. The protein A-agarose pellets were washed six times in 1 ml IP buffer containing 500 ml NaCl, six more times in 1 ml IP buffer without NaCl, then analyzed by SDS-PAGE fluorography.
Isotopic Flux Studies
Measurement of 35SO4 2− influx was carried out as follows: Oocytes were injected 10 min before initiation of influx measurements with 50 nl of a solution containing (in mM) 130 Na2SO4, 50 HEPES, pH 7.4. The flux assay was initiated by transfer of groups of 6–10 water-injected or 8–12 cRNA-injected oocytes into microtiter wells containing 149 μl of Na isethionate influx medium containing 2 mM Na2SO4 and 1 μl (5 μCi) Na2 35SO4. Influx assays were carried out at room temperature for 15 min, then terminated by rapid transfer of groups of oocytes through three 25-ml room temperature washes in isotonic Na gluconate medium. In experiments designed to measure the stoichiometry of SO4 2−/ SO4 2− exchange, influx medium contained 64 mM Na2SO4 instead of 96 mM Na isethionate.
Assay of 35SO4 2− efflux was carried out as follows: Oocytes were injected with 50 nl of a solution containing Na2 35SO4 (0.25–0.5 μCi, 3–6 μM) in 50 mM HEPES, pH 7.4, with or without 130 mM Na2SO4, and maintained for 10 min post-injection in SO4-free, Cl-free medium containing 96 mM Na isethionate. Efflux was initiated by transfer of individual oocytes into 1 ml of efflux medium. At regular intervals, 950 μl of this medium was removed for scintillation counting and replaced with fresh medium. All efflux experiments ended with a final period of efflux into medium containing 100 μM DIDS or DNDS before solubilization of the washed oocyte in 100 μl 1% SDS. The sum of efflux fractions and residual cpm in the oocyte was >98% of originally injected cpm. Data were plotted as ln (% cpm remaining) vs. time. Efflux rate constants were measured from linear least squares regressions calculated from the last three time points for each experimental condition, and corrected by subtraction of the efflux rate constant of water-injected oocytes studied in the same experiment. Effluxes were calculated as products of the measured rate constants and intracellular ion concentration (determined as described below). Oocyte water space was assumed to be 450 nl (500 nl acutely following 50 nl microinjection of isotope). As described in results, endogenous oocyte SO4 2− concentration was assumed to be 1.1 mM (1.0 mM following microinjection of 50 nl fluid).
Measurement of 36Cl− influx was carried out as described by Humphreys et al. (1994) in the presence of 10 μM bumetanide. Influx periods were 15–30 min, during which uptake was linear. For some experiments, individual oocytes kept in isethionate medium were injected with 50 nl of 50 mM HEPES, pH 7.40, with or without 130 mM Na2SO4, then maintained 10 min in isethionate medium before initiation of the influx experiment in ND-96 or other media as indicated.
Measurement of 36Cl− efflux was carried out as described previously (Humphreys et al., 1994), modified only in that oocytes were placed into isethionate medium for 10 min after isotope injection. Efflux was initiated by transfer of individual oocytes into medium containing ND-96 or other media as indicated.
Peak anion transport activities were exhibited by wt AE1 at 3–4 d post-injection of cRNA, as noted previously (Brosius et al., 1989; Humphreys et al., 1995). However, anion transport activities of oocytes expressing AE1 E699Q required up to 7 d to reach peak values. Wt AE1-expressing oocytes began to die as soon as 4 d post-injection and were unusable after 10 d. In contrast, AE1 E699Q-expressing oocytes maintained in ND-96 remained healthy and functional for as long as 15 d or more post-injection with cRNA.
Determination of Oocyte (Ovary) Intracellular SO4 2− Concentration
Samples for sulfate determination were prepared as follows. Resected fragments of Xenopus ovary were centrifuged in a microcentrifuge at 18,000 rpm for 1 h. 130 μl of the supernatant fraction were diluted with water to 500 μl and filtered through a Centricon 3 filter (Amicon Corp., Danvers, MA) in a Sorvall RT 6000B at 3,000 rpm for 4 h. This crude cytosol fraction was subjected to sulfate analysis by a barium precipitation method (Greenberg et al., 1980). Costa et al. (1989) have shown that intracellular Na and Cl activities and contents determined in ovary extracts and in pooled, isolated oocytes did not differ.
Measurements of Membrane Potential as a Function of Intracellular Anion
Defolliculated oocytes were incubated for 2–4 d at 19°C after injection of cRNA or water. Oocytes were placed in a superfusion chamber, impaled with a 3 M KCl-filled microelectrode, and allowed to recover for several minutes until a stable membrane potential was observed. Pulses of 10 nA were injected at 3-s intervals to monitor input resistance. Monitoring of membrane potential and current injection were performed with a CA 100 voltage clamp amplifier (Biologic, Echirolles, France). A second microelectrode was then introduced into the oocyte, through which the designated K+ salts (40–60 nl, titrated to pH 7.4) were injected into the oocytes with 10 s pressure pulses from a pneumatic picopump (WPI, Hertfordshire, UK). Membrane potential and input resistance were monitored for at least 10 min after pressure injection. Data were printed on a thermal array recorder (Graphtec, Japan) or processed through an analog-digital converter at 5 kHz, then transferred to computer for further analysis with custom-written software.
Measurements of Membrane Potential and Current as a Function of Extracellular Anion
After injection of cRNA or water, oocytes were incubated in ND96 for 4–14 d at 19°C. Oocytes were then injected with 50 nl of solution containing (in mM) 130 Na2SO4, 50 HEPES, pH 7.40, and placed in medium in which 96 mM Na isethionate replaced NaCl. Between 1 and 6 h afterward, single oocytes were placed in a 5-ml bath chamber (Model RC-11, Warner Instrument Corp., Hamden, CT) on the stage of a dissecting microscope and impaled with microelectrodes under direct view. Current and potential-sensing electrodes were pulled from borosilicate glass (1.2-mm outer diameter, 0.94-mm inner diameter; Sutter Instrument Co., Novato, CA) on a Flaming/Brown Model P-97 micropipette puller (Sutter Instrument Co.). The electrodes were filled with 3 M KCl and had resistances of 2–3 MΩ.
After impalement, oocyte membrane potential was monitored until it had stabilized (typically 3–7 min). Oocytes were voltage clamped using a Geneclamp 500 amplifier (Axon Instruments, Foster City, CA) interfaced to an 80486 50 MHz IBM-compatible computer (Dell 450/ME, Austin, TX) via a DigiData 1200 AD/D board (Axon Instruments). Electrical connections between the electrodes and the amplifier were made using Ag/AgCl pellets or wires and 3 M KCl/3% agarose bridges. Current was measured as that flowing to ground via the bath reference electrode. Errors induced by voltage drops across the bath ground were eliminated by use of a second reference electrode and a virtual-ground circuit. Current signals were filtered at 200 Hz before digitization. Oocytes were maintained in open-circuit conditions except during measurement of whole oocyte currents, for which membrane potential was clamped at a holding potential of −50 mV, and test potential steps at 20-mV intervals between −100 or −80 mV to +80 mV were imposed for 800-ms time periods. Whole oocyte currents recorded at each test potential were acquired to hard disk for later analysis with pClamp software (Axon Instruments). Oocytes clamped to test potentials of +80 mV or higher displayed activation of endogenous outward currents.
Estimate of Proton Flux Accompanying Sulfate Efflux
Oocytes previously injected with 50 nl of 130 mM Na2SO4 were placed on coverslips in 1 μl droplets of modified ND-96 containing 5 μM BCECF free acid and 0.5 mM MOPS, pH 7.4, as buffer (Jaisser et al., 1993). The coverslips were mounted in a customized chamber on an inverted microscope stage, and the oocytes in their droplets were alternately irradiated at 440 and 495 nm. BCECF fluorescence excitation ratios were acquired at 530 nm from the extracellular fluid and recorded to optical disk with an Image 1 digital ratio imaging system (Universal Imaging, West Chester, PA) as previously described (Humphreys et al., 1994, 1995). Calibration of the BCECF free acid fluorescence ratio was performed as described (Thomas et al., 1979). JH + from oocyte into the surrounding droplet was calculated by multiplying the measured dpHi/dt of the droplet by the calculated buffer capacity of the extracellular medium. AE1 E699Q-mediated proton efflux was estimated by subtracting outward JH + of water-injected oocytes from that of E699Q-expressing oocytes.
results
Isotopic Chloride Transport by AE1 Mutants
Site-directed mutagenesis was used to modify the wt glutamate residue at position 699 in recombinant murine AE1. Two mutations reversed the wt negative charge with arginine and lysine substitutions. One mutation neutralized the charge with glycine. Two more mutations attenuated the negative charge with the negative dipoles of threonine and glutamine. The threonine hydroxyl resembled the hydroxyl group of the hydroxynorvaline product of WRK-BH4 modification, whereas the gluta-mine side chain more closely imitated the steric bulk of the hydroxynorvaline. cRNAs encoding the full-length mutant AE1 polypeptides were expressed in Xenopus oocytes. The ability of the mutant AE1 polypeptides to transport Cl− was compared with that of wt AE1 48 h after cRNA injection. Fig. 1 A shows a 64-fold activation of unidirectional Cl− influx by wt AE1, from 0.1 to 6.4 nmol Cl− per oocyte · 15 min. In contrast, none of the mutants mediated Cl− uptake under these conditions.
Figure 1.
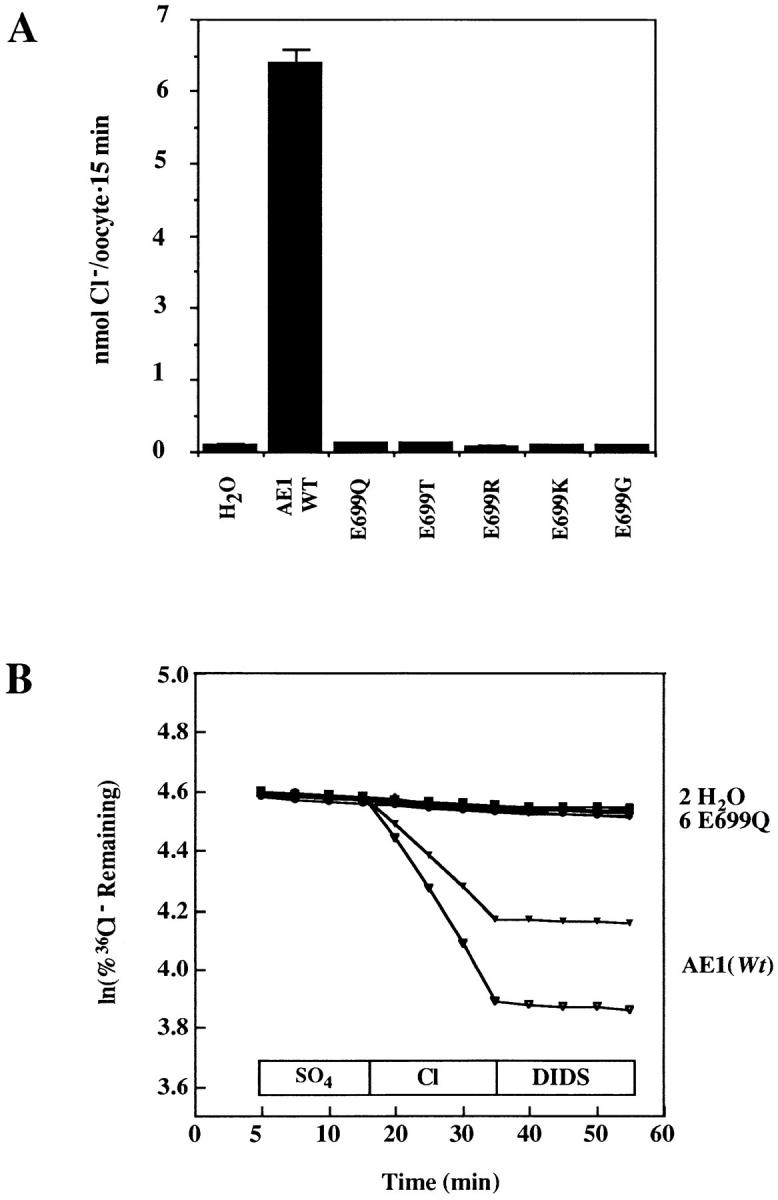
Cl− transport by mutant AE1 polypeptides. (A) 48 h after injection of cRNA or water, groups of 8–12 oocytes were incubated in 36Cl− for 15 min to determine AE1-mediated Cl− influx. None of the mutants displayed Cl−-uptake activity. Representative of three similar experiments for all mutants and three additional experiments comparing wt AE1 with AE1 E699Q. (B) 48 h after injection of cRNA or water, oocytes were acutely injected with Na36Cl and subjected to isotopic efflux assay as in methods. Extracellular condition noted above x axis. DIDS was added in the continued presence of Cl−. E699Q displayed no Cl− efflux activity. One of two similar experiments. Each trace represents a single oocyte.
36Cl− efflux mediated by these AE polypeptides is shown in Fig. 1 B. Cl− efflux was evident in wt AE1-expressing oocytes but not in oocytes expressing the AE1 mutants. Exchange of intracellular Cl− for extracellular sulfate was not observed with either wt AE1 or any of the mutants (Fig. 1 B). This is consistent with a relative rate of red cell Cl−/sulfate exchange >103 slower than Cl−/ Cl− exchange, and with a detection threshold of the oocyte 36Cl− efflux assay of >1% of the rate of Cl−/Cl− exchange (Humphreys et al., 1994).
Isotopic Sulfate Transport by AE1 Mutants
AE1 E699Q-mediated 35SO4 2− efflux was not reproducibly detectable 48 h after cRNA injection. However, by 5 d after cRNA injection, E699Q-expressing oocytes exhibited sulfate/sulfate exchange (Fig. 2 A). In contrast, neither oocytes expressing wt AE1 nor those expressing E699 mutants in which K, G, R, or T was substituted showed detectable sulfate/sulfate exchange. AE1 E699Q-mediated sulfate/sulfate exchange was DIDS-sensitive. Replacement of extracellular 64 mM Na2SO4 with 96 mM NaCl accelerated AE1 E699Q-mediated 35SO4 2− efflux 1.7-fold (Fig. 2 C) but did not produce detectable wt AE1-mediated 35SO4 2− efflux, unlike the acceleration of wt AE1-mediated 36Cl− efflux from oocytes (Fig. 1 B) or from red cells (Hanke-Baier et al., 1988; Passow et al., 1992) produced by the same extracellular substitution.
Figure 2.
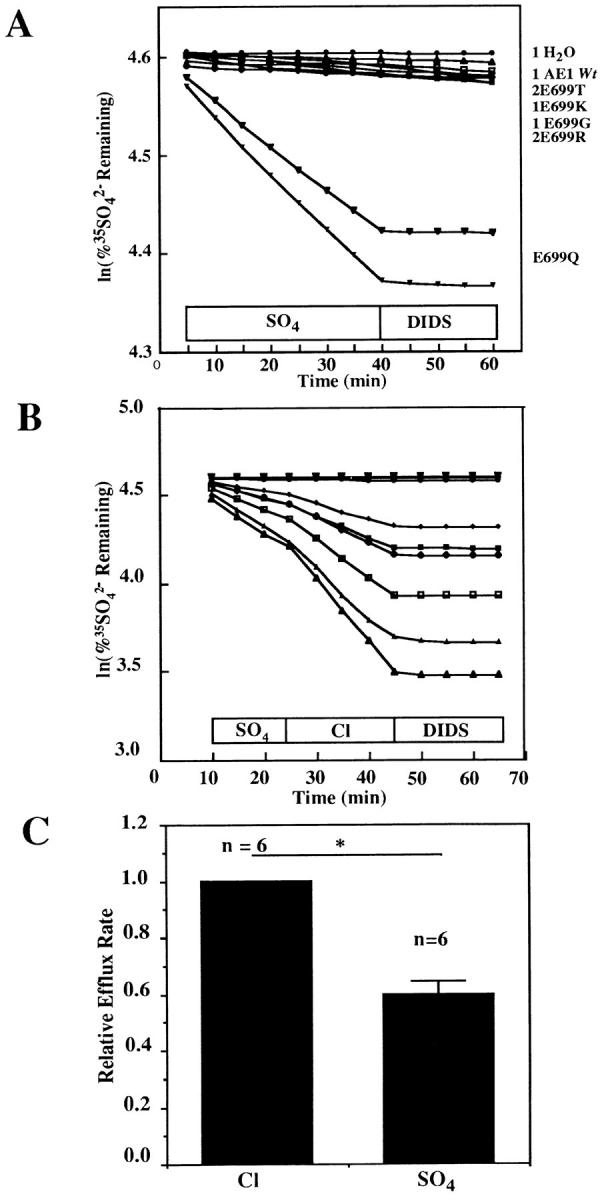
SO4 2− efflux by mutant AE1 polypeptides. (A) Extracellular SO4 2− supported 35SO4 2− efflux from AE1 E699Q-expressing oocytes (lower two traces) but not from oocytes expressing wt AE1 or AE1 mutants with T, K, G, or R in place of E699 (upper cluster of 8 traces). Extracellular condition shown above x axis. DIDS was added in the continued presence of SO4 2−. Representative of 3 similar experiments performed 4–5 d after cRNA injection. Each trace represents a single oocyte. (B) SO4 2−/Cl− exchange mediated by AE1 E699Q was 70% faster than SO4 2−/SO4 2− exchange. DIDS inhibited efflux in the continued presence of Cl−. The two uppermost traces show efflux from water-injected oocytes. The six traces below show efflux from AE1 E699Q-expressing oocytes. (C) Bar graph of the data in B, (n) for each condition above bars. *P < 0.02.
35SO4 2− influx into wt AE1-expressing oocytes was undetectable in seven experiments with extracellular [SO4 2−] ranging from 2 to 64 mM, consistent with the low SO4 2−/Cl− exchange rate of unmodified intact red cells. 35SO4 2− influx into AE1 E699Q-expressing oocytes was detectable at low level in about half of experiments performed 48 h after cRNA injection.
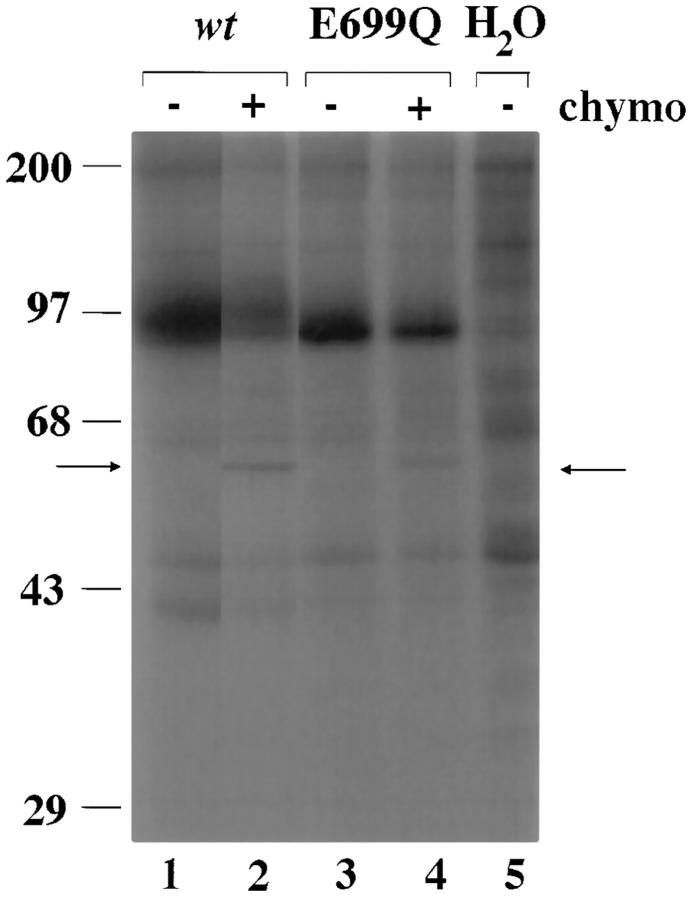
Biosynthesis and Surface Expression of AE1 E699Q
Since the only AE1 E699 mutant which transported sulfate in the Xenopus oocyte 48 h after cRNA injection was E699Q, further studies focused on this mutant. Total accumulation of AE1 E699Q polypeptide was at least half that of the wt AE1 polypeptide (Fig. 3, lanes 1 and 3). In Fig. 3, 19% of wt AE1 was cleaved by extracellular chymotrypsin (lane 2), as was 15% of AE1 E699Q (lane 4). These figures represent biochemical estimates of the steady-state proportion of total oocyte AE polypeptide at the oocyte surface. In four similar experiments using oocytes from two frogs, 32 ± 7% of wt AE1 was exposed to chymotrypsin digestion at the oocyte surface. The comparable figure for AE1 E699Q was 29 ± 9%. Therefore, surface accumulation of wt and of E699Q AE1 polypeptides in Xenopus oocytes was of comparable efficiency.
Figure 3.
Biosynthesis and cell surface expression of AE1 E699Q and wt AE1. 72 h after cRNA injection, metabolically labelled oocytes expressing wt AE1 (lanes 1 and 2), AE1 E699Q (lanes 3 and 4), or no cRNA (lane 5, water-injected) were incubated for 3 h in the absence (lanes 1, 3, and 5) or presence (lanes 2 and 4) of 5 mg/ml chymotrypsin as described in methods. Triton X100 extracts of the oocytes were subjected to immunoprecipitation with anti-AE1 antibody, SDS-PAGE, and autoradiography. Arrows indicate holoprotein and the 60-kD NH2-terminal chymotryptic fragment of AE1 (p60). Representative of four similar experiments.
Stilbene Sensitivity of AE1 E699Q
Wt AE1–mediated 36Cl− efflux into chloride medium was reversibly inhibited in a dose-dependent manner by the stilbene disulfonate inhibitor, DNDS, with a concentration for half-maximal inhibition of 13 + 8 μM (Fig. 4 B). This value is similar to the value of 3–5 μM reported by Passow et al. (1992). Half-maximal inhibition by DNDS of AE1 E699Q-mediated 35SO4 2− efflux into chloride medium was 8 ± 2 μM (Fig. 4, A and B), a value not significantly different from that of wt AE1 (P > 0.1). Thus, the apparent affinity of AE1 for an impermeant reversible antagonist applied outside the oocyte was not changed by the single E699Q mutation.
Figure 4.
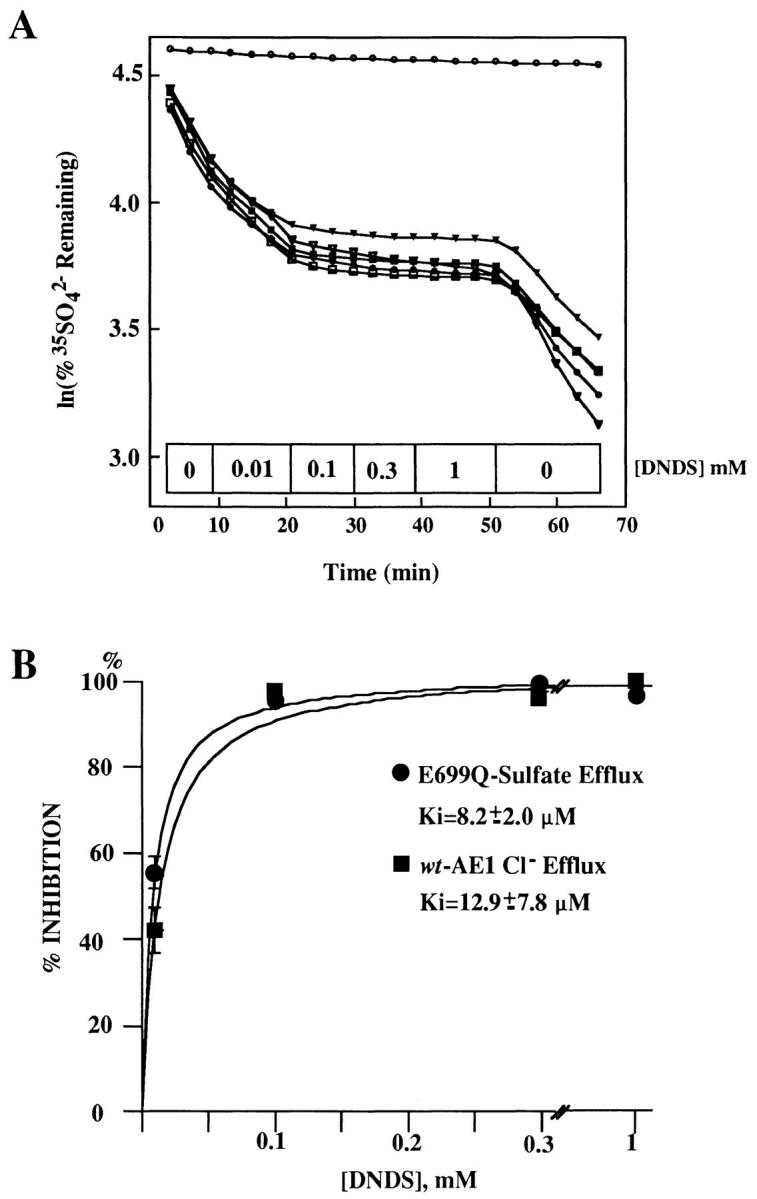
DNDS inhibits anion exchange by wt AE1 and by E699Q AE1 with similar potency. (A) Effect of increasing concentrations of DNDS on AE1 E699Q-mediated 35SO4 2− efflux into Cl− medium. Blockade was reversible. Each trace represents an individual oocyte. Upper trace is water-injected oocyte. (B) DNDS inhibition dose-response curves of wt AE1-mediated 36Cl− efflux and of AE1 E699Q-mediated 35SO4 2− efflux. Each of 5 oocytes was exposed for 9 or 12 min to incrementally increasing DNDS concentrations.
Extracellular Anion Selectivity of AE1 E699Q
Fig. 5 shows the effect on SO4 2− efflux of complete replacement of extracellular Cl− with various anions. SO4 2− efflux rates relative to those observed in extracellular Cl− were 0.97 ± 0.12 (nitrate), 0.67 ± 0.04 (bromide), 0.20 ± 0.03 (gluconate), 0.20 ± 0.04 (isethionate), 0.16 ± 0.02 (iodide), and 0.04 ± 0.01 (phosphate). The values for nitrate, bromide, and iodide were close to those reported previously for wt AE1-mediated Cl− efflux from Xenopus oocytes and intact mouse red cells (Passow et al., 1992). The relative rates of AE1 E699Q-mediated SO4 2− exchange with gluconate, isethionate, and phosphate were higher than expected for AE1-mediated Cl− exchange with the same anions in human red cells, but these rates had not been reported for wt AE1 expressed in Xenopus oocytes. Therefore, relative rate constants were also measured for wt AE1-mediated efflux of 36Cl− into extracellular gluconate and isethionate, and were found to be 0.06 ± 0.02 (n = 13 oocytes, 3 frogs) and 0.11 ± 0.03 (n = 17 oocytes, 4 frogs), respectively. Though slightly lower than the corresponding values for AE1 E699Q, these values were consistent with the rates of Cl−/gluconate exchange exhibited by trout AE1 (Fievet et al., 1995) and by mouse AE2 (Humphreys et al., 1994).
Figure 5.
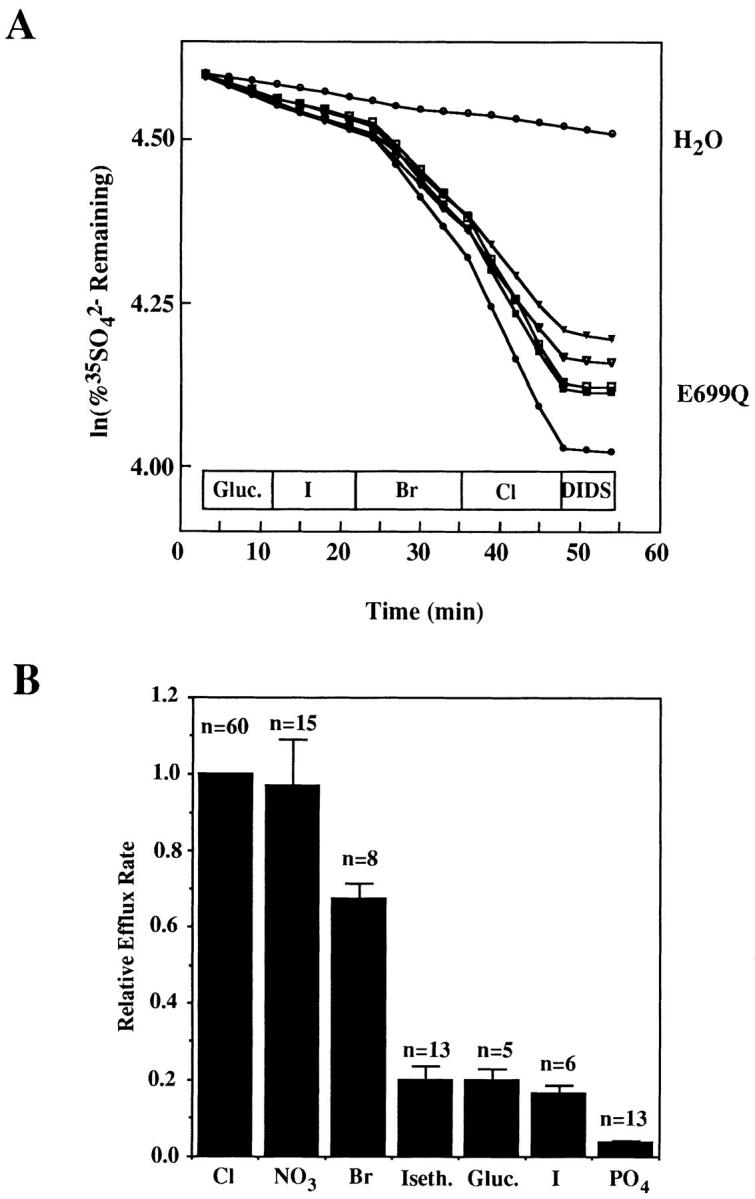
(A) Extracellular halide dependence of AE1 E699Q-mediated SO4 2− efflux. Each line represents a single oocyte. (B) Extracellular anion dependence of AE1 E699Q-mediated 35SO4 2− efflux. Summary of three similar experiments, with number oocytes evaluated in each condition noted above columns. Relative efflux into iodide medium lacking gluconate salts of Mg2+, Ca2+, and K+ was further reduced to 0.09 ± 0.03 (n = 5), but omission of these gluconate salts did not change values for other anions (not shown).
Stoichiometry of AE1 E699Q-mediated Exchange of Intracellular SO4 2− for Extracellular Cl−
To estimate the stoichiometry of AE1 E699Q-mediated SO4 2−/Cl− exchange, it was necessary to measure Cl− influx. Though AE1 E699Q-mediated 36Cl− influx was minimal or absent two days after cRNA injection (Fig. 1 A), 36Cl− influx and 35SO4 2− efflux were both detectable 5–7 d after injection of cRNA (Fig. 6). A second requirement was knowledge of the intracellular [SO4 2−]. Cytosolic fractions isolated from ovarian fragments were subjected to sulfate analysis by barium precipitation (Greenberg et al., 1980). This assay indicated an ovarian cytosolic [SO4 2−] of 1.1 ± 0.1 mM (n = 5 frogs). On this basis, oocyte cytosol [SO4 2−] was assumed to be 1 mM after injection of a 50 nl volume into a nominal 450-nl water space.
Figure 6.
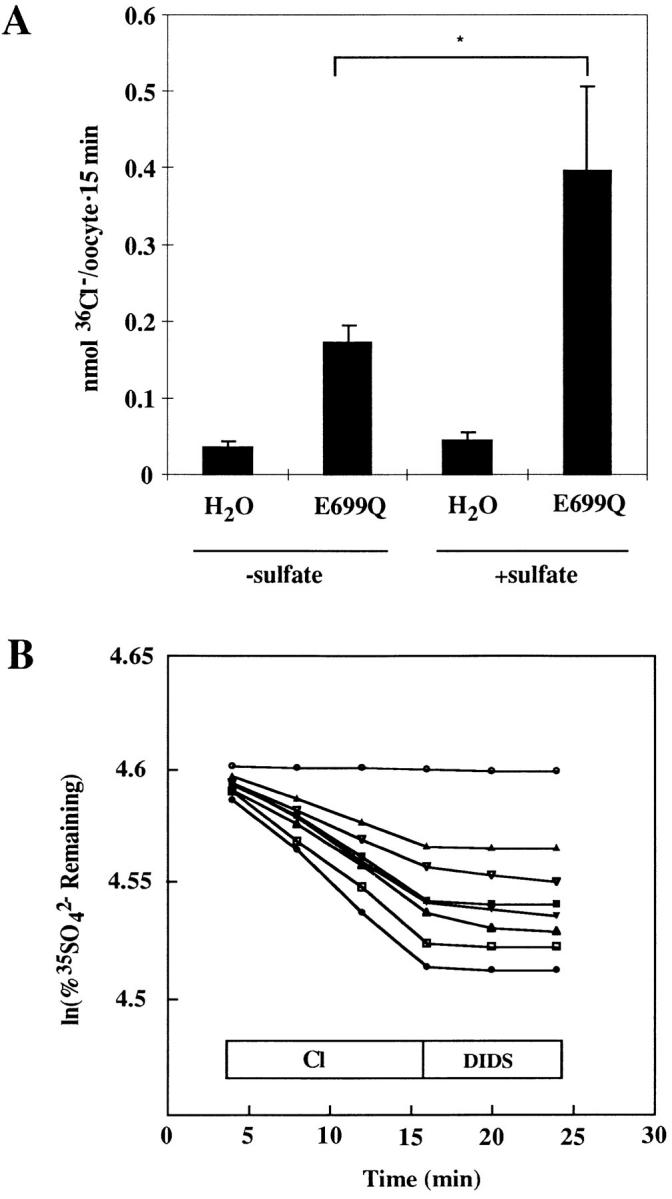
Experimental format for measurement of stoichiometry of influx vs. efflux. (A) 36Cl− influx into acutely SO4 2−-loaded or native oocytes injected 13 d earlier with water or with AE1 E699Q cRNA (*P < 0.01). Representative of five similar, paired experiments. AE1 E699Q-mediated 36Cl− influx was measurable in native oocytes from 8 of 10 frogs tested. Influx was measurable in SO4 2−-loaded oocytes from 9 of 10 frogs tested. (B) Oocytes from the frog of panel A injected 13 d earlier with water (upper trace) or with cRNA (seven lower traces) were acutely injected with 35SO4 2− to a final intracellular [SO4 2−] of ∼14 mM, then subjected to the efflux assay. Note that fractional efflux into Cl− medium was less than observed in Fig. 2 B, in which intracellular [SO4 2−] was ∼1 mM.
The stoichiometry of SO4 2−/Cl− exchange by AE1 E699Q was examined ≥5 d after cRNA injection. AE1 E699Q-mediated SO4 2− efflux into extracellular Cl− was 0.31 ± 0.05 pmol cell−1 s−1 (n = 59 oocytes, 10 frogs). In 96 oocytes from 10 frogs, AE1 E699Q-mediated Cl− influx was 0.25 ± 0.06 pmol cell−1 s−1. The ratio of the mean SO4 2− efflux over the mean Cl− influx was 1.24. In two experiments in which both measurements were performed on oocytes from the same frog, the mean of the two flux ratios was 0.94. Thus, exchange of intracellular endogenous SO4 2− for extracellular Cl− by AE1 E699Q was consistent with a stoichiometry of 1:1.
To maximize AE1 E699Q-mediated rates of SO4 2−/ Cl− exchange stoichiometry experiments were performed with oocytes acutely injected with 50 nl of solution containing 130 mM Na2SO4 buffered with Na HEPES, pH 7.40, to yield a total estimated intracellular [SO4 2−] of 14 mM. Such intracellular injection of SO4 2− produced a 2.2 ± 0.5-fold increase in AE1 E699Q-mediated 36Cl− influx (Fig. 6 A, P < 0.01). This increase presumably reflected increased saturation of the intracellular binding site for sulfate on AE1 E699Q. 36Cl− influx assays were performed with AE1 E699Q-expressing oocytes isolated from the same frog and previously injected on the same day 5–15 d earlier with water or with cRNA encoding AE1 E699Q. 10 min before initiation of 36Cl− uptake, these oocytes were injected with 50 nl of 130 mM Na2SO4 without tracer, to maximize and equalize intra-oocyte sulfate concentrations. For 35SO4 2− efflux experiments, the injected Na2SO4 included carrier-free tracer. The paired influx and efflux experiments carried out with sulfate-loaded oocytes were conducted on the same days (Table I).
Table I.
| Estimated [SO4 2−]i | Expt. | Chloride influx | Sulfate efflux | Sulfate efflux | ||||
|---|---|---|---|---|---|---|---|---|
| Chloride influx | ||||||||
| pmol cell −1 s−1 | ||||||||
| 14.0 mM | 1 | 0.21 ± 0.05 | 0.46 ± 0.11 | 2.19 | ||||
| 2 | 1.10 ± 0.02 | 0.19 ± 0.02 | 0.17 | |||||
| 3 | 0.27 ± 0.05 | 0.24 ± 0.03 | 0.89 | |||||
| 4 | 0.40 ± 0.03 | 0.44 ± 0.03 | 1.10 | |||||
| 5 | 0.39 ± 0.13 | 0.52 ± 0.05 | 1.33 | |||||
| 6 | 0.30 ± 0.05 | 0.32 ± 0.02 | 1.09 | |||||
| 7 | 0.35 ± 0.01 | 0.41 ± 0.07 | 1.17 | |||||
| mean | 0.43 ± 0.11 | 0.37 ± 0.04 | 0.86/1.13 ± 0.23 | |||||
The stoichiometry of Cl− influx and SO4 2− efflux in sulfate-loaded oocytes expressing AE1 E699Q is 1:1. Within each experiment, Cl− influx and SO4 2− efflux were measured on the same day with separate groups of otherwise identically treated oocytes from the same frog. Mean flux ratios are presented as ratios of the mean fluxes and as means ± SEM of the individual experimental flux ratios.
In AE1 E699Q-expressing, Na2SO4-loaded oocytes (n = 7 frogs, 73 oocytes), AE1-mediated 36Cl− influx was 0.43 ± 0.11 (SEM) pmol cell−1 s−1. In identically handled AE1 E699Q-expressing, sulfate-loaded oocytes (n = 40) from the same seven frogs, AE1-mediated 35SO4 2− efflux was 0.37 ± 0.04 pmol cell −1 s−1. The ratio of the mean sulfate efflux to the mean chloride influx was 0.86. The mean of the seven individual experimentally determined ratios of sulfate efflux over chloride influx was 1.13 ± 0.23 (Table I). These values were consistent with a 1:1 stoichiometry of SO4 2−/Cl− exchange mediated by AE1 E699Q in Na2SO4-loaded oocytes.2
The SO4 2−/SO4 2− exchange stoichiometry of AE1 E699Q was examined 5 or more days after cRNA injection in the absence of exogenous SO4 2− loading. AE1 E699Q-mediated SO4 2− efflux into 64 mM extracellular SO4 2− was 0.091 ± 0.012 pmol cell−1 s−1 (n = 4 frogs, 20 oocytes). 35SO4 2− influx from 64 mM extracellular SO4 2− into AE1 E699Q-expressing oocytes was 0.079 ± 0.012 pmol cell−1 s−1 (1 frog, 10 oocytes) These values were statistically indistinguishable (P > 0.5), and yielded a flux ratio of 1.15. Thus, under the nonequilibrium conditions used, AE1 E699Q also mediated SO4 2−/SO4 2− exchange with a stoichiometry consistent with 1:1 exchange.
Effect of AE1 E699Q on Membrane Potential
In WRK-BH4-modified, SO4 2−-loaded red cells treated with gramicidin, the acceleration of 86Rb+ efflux by addition of 5 mM extracellular Cl− suggested that the modified AE1 mediated electrogenic anion exchange (Jennings, 1995). Since the 1:1 stoichiometry of AE1 E699Q-mediated SO4 2−/Cl− exchange in oocytes resembled that of WRK-BH4-modified red cells, the effects of substrate anions on membrane potential were directly examined in oocytes expressing either wt or mutant AE1 polypeptides.
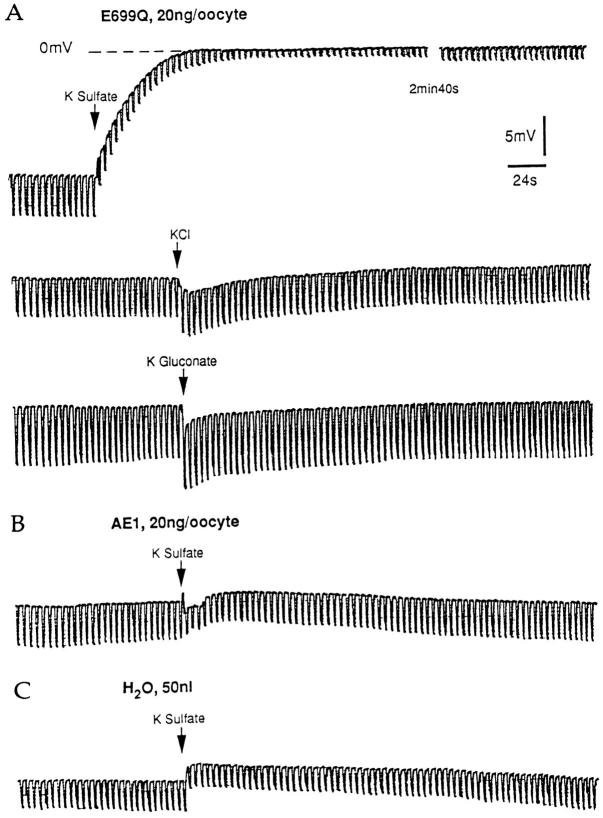
Upon pressure injection of ∼50 nl 87 mM K sulfate, E699Q-expressing oocytes underwent a depolarization of 17 ± 3 mV that was maintained during 30 min of observation and was accompanied by a 66% decrease in membrane resistance (Fig. 7). Estimated intracellular [SO4 2−] concentration was 9–10 mM. Injection of equal volumes of KCl or of K gluconate (87 mM) led to transient 3-mV hyperpolarizations accompanied by small increases in membrane resistance. Wt AE1-expressing oocytes, in contrast, displayed minimal change in membrane potential in response to injection of either K sulfate or K gluconate. Similarly, water-injected oocytes showed no voltage response to injection of K sulfate (Fig. 7, Table II). The depolarization and decrease in membrane resistance which characterized SO4 2− injection into AE1 E699Q-expressing oocytes suggested outward electrogenic flow of negative charge, and supported the hypothesis of electrogenic SO4 2−/Cl− exchange.
Figure 7.
Intracellular anion dependence of electrogenic anion exchange. (A) Oocytes injected 72 h previously with cRNA encoding AE1 E699Q were acutely injected (arrow) with 50 nl of 87 mM K salts of SO4 2−, Cl−, or gluconate as indicated while membrane potential and resistance were recorded. Regular spaced vertical displacements in potential are in response to 10-nA current pulses. (B) An oocyte injected 72 h previously with cRNA encoding wt AE1 was injected with 50 nl 87 mM K2SO4. (C) A water-injected oocyte was injected with 50 nl 87 mM K2SO4.
Table II.
| Injected with | ||||||
|---|---|---|---|---|---|---|
| Oocyte | sulfate | gluconate | chloride | |||
| expressing: E699Q | ||||||
| ΔVm | +17 ± 3 mV | −3 ± 1.6 mV | −3 ± 0.5 mV | |||
| (n) | (9) | (5) | (2) | |||
| ΔRm | −66 ± 1% | +17 ± 5% | +10% | |||
| (n) | (9) | (5) | (2) | |||
| wt AE1 | ||||||
| ΔVm | +0.3 ± 1.7 mV | 0 mV | ND | |||
| (n) | (6) | (2) | ||||
| ΔRm | −9.0 ± 2.1% | — | ND | |||
| (n) | (6) | |||||
| Water | ||||||
| ΔVm | +1.0 ± 0.6 mV | ND | ND | |||
| (n) | (4) | |||||
| ΔRm | −4.0 ± 2.6% | ND | ND | |||
| (n) | (4) | |||||
Changes in Xenopus oocyte membrane potential and transmembrane resistance in response to acute injection of K2SO4 2−, KCl, and K gluconate. Values are means ± SEM.
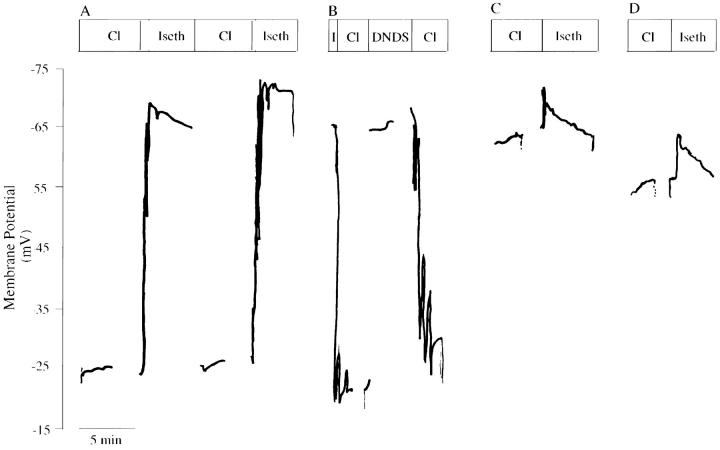
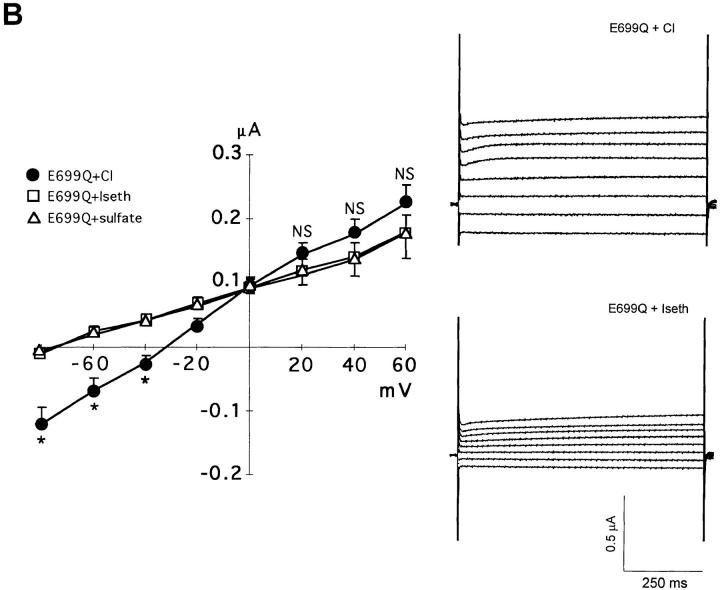
To distinguish between AE1 E699Q-mediated electrodiffusive transport and electrogenic exchange, the extracellular anion selectivity of membrane potential changes was investigated in SO4 2−-loaded oocytes (Fig. 8, Table III). Whereas resting potentials in Cl− medium were −58 ± 4 mV and −55 ± 2 mV in water-injected (n = 11) and wt AE1-expressing oocytes (n = 20), respectively, oocytes expressing AE1 E699Q displayed a depolarized resting potential of −38 ± 2 mV (n = 60). Substitution of extracellular Cl− by isethionate produced no change in membrane potential in wt AE1-expressing and water-injected oocytes, but caused AE1 E699Q-expressing oocytes to hyperpolarize by 28 ± 2 mV (n = 60). In contrast, replacement of extracellular isethionate by SO4 2−, produced little or no change in membrane potential (Table III). Addition of DNDS in the continued presence of extracellular Cl− hyperpolarized membrane potential in AE1 E699Q-expressing oocytes (Fig. 8 B) by 24 ± 2 mV (n = 25, 4 frogs). Taken together with the isotopic flux data, these results suggest that AE1 E699Q mediated asymmetric, electrogenic 1:1 SO4 2−/Cl− exchange and electroneutral 1:1 SO4 2−/ SO4 2− exchange.
Figure 8.
Membrane potential changes in Na2SO4-loaded oocytes in response to varied extracellular conditions. (A) Membrane potential of a Na2SO4-loaded E699Q-expressing oocyte during two sequential transitions from extracellular Cl− to isethio-nate. (B) Membrane potential of a Na2SO4-loaded E699Q-expressing oocyte in extracellular Cl− medium during introduction and removal of DNDS. (C) Membrane potential of a Na2SO4-loaded wt AE1-expressing oocyte during transition from extracellular Cl− to isethionate. (D) Membrane potential of a Na2SO4-loaded oocyte, previously injected with water, during transition from extracellular Cl− to isethionate. Gaps in the voltage records represent application of stepped voltage protocols (see Fig. 9).
Table III.
| Chloride | Isethionate | Δ Iseth-Cl | Sulfate | Δ Iseth-Sulfate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A1 E699Q | −38 ± 1.6 | −67 ± 1.2 | +28 ± 1.9 | −62 ± 2.2 | +4.6 ± 2.5 | |||||
| (60) | (51) | (20) | (15) | (15) | ||||||
| AE1 wt | −55 ± 1.9 | −56 ± 2.1 | +0.6 ± 1.1 | −48 ± 4.1 | +3.5 ± 4.6 | |||||
| (20) | (20) | (20) | (9) | (9) | ||||||
| Water | −58 ± 2.3 | −56 ± 2.7 | −2 | −52 ± 4.1 | +5.6 ± 9.8 | |||||
| (11) | (10) | (6) | (3) | (3) |
Resting membrane potential (mV) of Na2SO4-injected oocytes in the presence of different extracellular anions. Values are means ± SEM. AE-expressing oocytes were from 8 frogs; water-injected oocytes were from 2 frogs. Δ values (mV) represent means ± SEM of indicated number of individual differences.
Stoichiometry of SO4 2− Efflux and Current Mediated by AE1 E699Q
The hypothesis of 1:1 electrogenic SO4 2−/Cl− exchange predicts that AE1 E699Q should mediate outward flow of negative charge from oocytes with properties identical to and of magnitude equal to AE1 E699Q-mediated SO4 2− efflux from oocytes. Therefore, SO4 2−-loaded oocytes expressing wt and mutant AE1 cRNAs were subjected to two-electrode voltage clamp in order to characterize and to quantitate current flow mediated by the AE1 E699Q polypeptide.
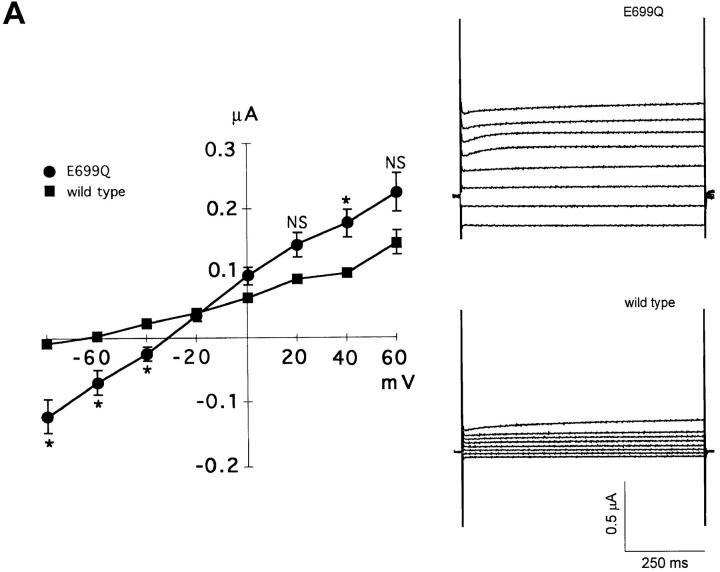
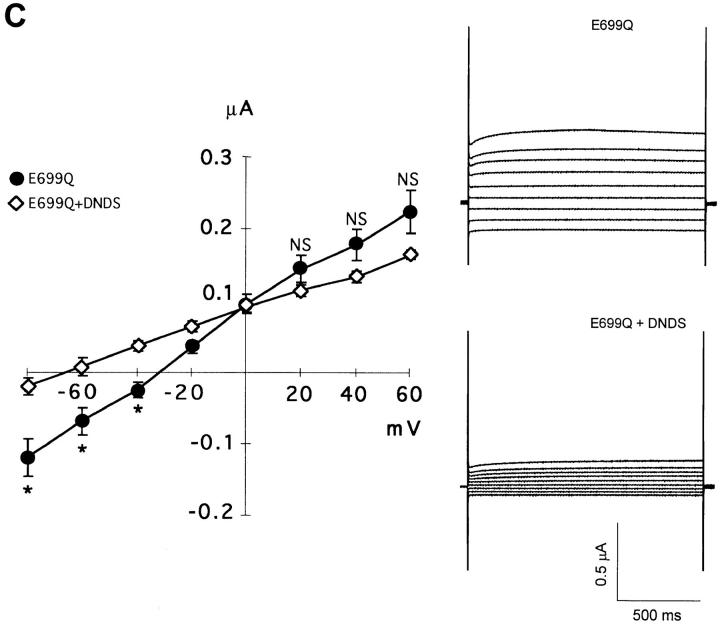
Fig. 9 A compares current-voltage relationships of SO4 2−-loaded oocytes expressing wt AE1 and AE1 E699Q. The ∼1 μS conductance measured in oocytes expressing wt mouse AE1 was similar to that of 0.7 μS measured by Fievet et al. (1995). The oocytes expressing AE1 E699Q exhibited a small but significant linear inward current not seen in oocytes expressing wt AE1 at membrane potentials more positive than −80 mV (Fig. 9). The current-voltage relationship remained linear to test potentials as negative as −150 mV (not shown). The current in wt AE1-expressing oocytes displayed a reversal potential of −64 mV (Fig. 9 A; 20 oocytes from 6 frogs) similar to the value of −69 mV measured in water-injected oocytes (not shown; 8 oocytes from 6 frogs). Oocytes expressing AE1 E699Q exhibited substantially increased conductance, with a depolarized reversal potential of −33 mV (Fig. 9 A; 54 oocytes from 8 frogs). Substitution of extracellular Cl− by isethionate greatly reduced inward current in Na2SO4-loaded oocytes expressing AE1 E699Q, and restored oocyte reversal potential to the more hyperpolarized value of −74 mV (Fig. 9 B; 44 oocytes from 7 frogs). Substitution of extracellular NaCl by isosmotic Na2SO4 reduced inward current and hyperpolarized oocyte reversal to the same relative extents (Fig. 9 B; 5 oocytes). Exposure of Na2SO4-loaded oocytes in extracellular chloride to DNDS (200 μM) similarly inhibited AE1 E699Q-associated inward current, and similarly hyperpolarized the reversal potential to −68 mV (Fig. 9 C; 24 oocytes from 4 frogs). Thus, the conductance of AE1 E699Q-expressing oocytes was restored to that typical of wt AE1-expressing oocytes either by substitution of extracellular chloride by isethionate or by sulfate, or by addition of the anion exchange inhibitor, DNDS. Taken together, these data are consistent with the presence of AE1 E699Q-mediated electrogenic sulfate efflux at inside-negative oocyte resting potential.
Figure 9.
AE1 E699Q-associated currents. (A) Mean I-V curves and representative current traces from Na2SO4-loaded oocytes in extracellular Cl− medium expressing wt AE1 (filled squares, n = 20 oocytes from 6 frogs) and AE1 E699Q (filled circles, n = 54 oocytes from 8 frogs). *P < 0.04; NS, P > 0.05. (B) Comparison of E699Q-mediated currents in Na2SO4-loaded oocytes in the presence of extracellular Cl− (filled circles), extracellular isethionate (open squares, n = 44 oocytes from 7 frogs), and extracellular sulfate (open triangles, n = 5 oocytes from 1 frog). *P < 0.002. (C) Comparison of E699Q-mediated currents in Na2SO4-loaded oocytes in extracellular chloride in the absence (filled circles) and presence of 200 μM DNDS (open diamonds, n = 24 oocytes from 4 frogs) *P < 0.03. Voltage was stepped from a holding potential of −50 mV to test potentials between −80 and +60 mV in 20-mV increments. At potentials ≥+80 mV, endogenous outward currents were activated in all groups of oocytes. Data recorded in extracellular chloride from AE1 E699Q-expressing oocytes is replicated in all three panels.
To evaluate whether inward current carried by AE1 E699Q sufficed to account for AE1 E699Q-mediated SO4 2− efflux, oocyte current at the resting potential in extracellular Cl− medium was interpolated from the measured I-V relationships. These values were compared to AE1 E699Q-mediated SO4 2− efflux into Cl− medium (Table IV). In 12 experiments examining 104 Na2SO4-loaded oocytes, AE1 E699Q-mediated SO4 2− efflux into extracellular Cl− was 0.48 + 0.15 pmol cell−1 s−1. SO4 2− efflux did not differ significantly (P > 0.5, two-tailed t test) from AE1 E699Q-mediated inward currents calculated by any of three criteria: 0.58 ± 0.15 pmol charge cell−1 s−1 (for current in extracellular Cl− minus that in extracellular isethionate); 0.37 ± 0.08 pmol charge cell−1 s−1 (for current in E699Q-expressing oocytes minus that in wt AE1-expressing oocytes); and 0.43 ± 0.05 pmol charge cell−1 s−1 (for current in absence of DNDS minus that in the presence of DNDS). Thus, the outflow of charge mediated by AE1 E699Q in sulfate-loaded oocytes at resting membrane potential sufficed to account for SO4 2− efflux from similarly treated oocytes.
Table IV.
| AE1 E699Q-mediated sulfate efflux (sulfate ions) | 0.48 ± 0.15 pmol cell −1 s−1 (n = 12) | |
| AE1 E699Q-mediated inward current (elementary charges) | 0.58 ± 0.15 pmol cell −1 s−1 (Cl-Iseth, n = 6) | |
| 0.37 ± 0.08 pmol cell−1 s−1 (E699Q-WT, n = 6) | ||
| 0.43 ± 0.05 pmol cell−1 s−1 (E699Q-DNDS, n = 4) |
The magnitude of AE1 E699Q-mediated inward current at resting potential sufficed to account for all measured AE1 E699Q-mediated sulfate efflux. n is number of separate experiments, each with 6–12 oocytes. The 12 experiments in which sulfate efflux was measured include the 7 presented in Table I plus 5 additional experiments, not included in Table I because they were not paired with measurements of Cl− influx.
Estimate of AE1 E699Q-mediated H+ Transport during SO4 2−/Cl− Exchange
WRK-BH4-treated red cells mediate SO4 2−/Cl− exchange without evidence for the H+/SO4 2− cotransport (Jennings and Al-Rhaiyel, 1988) that typifies wt AE1 (Milanick and Gunn, 1982, 1984). The electrogenicity of 1:1 SO4 2−/Cl− exchange mediated by AE1 E699Q also suggested the absence of H+ cotransport with SO4 2−. Therefore, H+ efflux from SO4 2−-loaded oocytes was estimated from the time-dependent change in BCECF fluorescence ratio of minimally buffered extracellular medium (Jaisser et al., 1993). Water-injected oocytes did not detectably acidify the extracellular medium (n = 3). dpHo/dt in the droplets surrounding AE1 E699Q-expressing oocytes previously injected with Na2SO4 was −0.011 ± 0.003 min−1 (n = 5), corresponding to outward JH + of 0.049 pmol cell−1 s−1. With the simplifying assumption of no proton backleak, H+ efflux associated with expression of AE1 E699Q was ∼10% of the level of AE1 E699Q-mediated SO4 2− efflux. However, JH + had the same value in AE1 E699Q-expressing oocytes not previously injected with Na2SO4 (n = 6), suggesting that the proton efflux was not coupled to SO4 2− transport. Thus, SO4 2− transport by AE1 E699Q was largely unaccompanied by protons.
discussion
The experiments presented above demonstrate a complex phenotype of anion exchange in Xenopus oocytes expressing mouse AE1 polypeptide with the E699Q missense mutation. The AE1 E699Q polypeptide was processed and delivered to the surface of the oocyte (Fig. 3). There it exhibited both a loss-of-function phenotype with respect to exchange of intracellular Cl− for extracellular anion (Fig. 1) and a gain-of-function phenotype with respect to exchange of intracellular SO4 2− for extracellular anion (Figs. 2, 5, and 6). Thus, SO4 2−/ Cl− exchange was asymmetric: whereas exchange of intracellular SO4 2− for extracellular Cl− was increased, exchange of extracellular SO4 2− for intracellular Cl− was undetectable. Though the sensitivity of anion exchange to inhibition by DNDS was unchanged (Fig. 4) and the rank-order of exchange rates with various extracellular anions was little changed from the corresponding wt values (Fig. 5), the mechanism of SO4 2−/ Cl− exchange differed dramatically from that of wt AE1. The Xenopus oocyte expression system allowed unambiguous discrimination between two possible mechanisms of SO4 2−/Cl− exchange in the absence of proton cotransport (Jennings, 1995), electroneutral exchange of 2 Cl− for 1 SO4 2− and electrogenic exchange of 1 Cl− for 1 SO4 2−.
Electrogenicity of Anion Exchange by AE1 E699Q
Murine AE1 E699Q-mediated exchange of intracellular SO4 2− for extracellular Cl− with a stoichiometry of 1:1 in Xenopus oocytes (Table I). This exchange was unaccompanied by stoichiometric proton transport and was electrogenic (Tables II and III, Figs. 7 and 8). In SO4 2−-loaded Xenopus oocytes at inside-negative membrane potentials, AE1 E699Q mediated an inward current that required the presence of intracellular SO4 2− and extracellular Cl− and was inhibited by DNDS (Fig. 9). AE1 E699Q-mediated inward current measured at the resting membrane potential did not differ detectably in magnitude from E699Q-mediated efflux of 35SO4 2−, and so could account entirely for SO4 2− efflux (Table IV).
The mutation of glutamate to glutamine at position 699 converted the obligate electroneutrality of wt AE1-mediated anion exchange to a more flexible mechanism, in which exchange of intracellular SO4 2− for extracellular Cl− by AE1 E699Q was electrogenic, but AE1 E699Q-mediated SO4 2−/SO4 2− exchange was electroneutral (Fig. 9 B). The change in charge of this single amino acid altered the capacity of AE1 to effect transmembrane transport of net charge, without alteration of the wt stoichiometry of 1:1 anion exchange. Electrogenic anion transport by wt mouse AE1 was not observed in Xenopus oocytes (Fig. 9 A, Tables II and III), in agreement with earlier observations of Grygorczyk et al. (1987) and Fievet et al. (1995). In human red cells in which membrane potential was clamped with gramicidin and imposed K+ gradients, Jennings et al. (1990) also found that neither Cl−/Cl− exchange nor Cl−/ HCO3 − exchange displayed detectable potential dependence.
Wt AE1 in red cells cotransports H+ with SO4 2− in electroneutral exchange for Cl−. This cotransport is reflected in stimulation of AE1-mediated SO4 2− transport at acid pH (Milanick and Gunn, 1982, 1984). WRK-BH4 modification of red cells (Jennings, 1995) led to AE1-mediated SO4 2− transport that was active at neutral pH, not further stimulated at acid pH, and was unaccompanied by H+ cotransport. In the present study, the electrogenicity of 1:1 SO4 2−/Cl− exchange by AE1 E699Q suggested the absence of H+ cotransport; indeed, AE1 E699Q-mediated SO4 2− efflux in Xenopus oocytes was unaccompanied by stoichiometric H+ efflux (results). Moreover, neither SO4 2−/Cl− exchange nor SO4 2− / SO4 2− exchange were accelerated by intracellular or by extracellular protons (Chernova and Alper, manuscript in preparation).
Asymmetry of Sulfate Transport by AE1 E699Q
SO4 2−/Cl− exchange in WRK-BH4-modified human red cells was vectorially asymmetric: exchange of intracellular SO4 2− for extracellular Cl− was 10-fold faster than was exchange of intracellular Cl− for extracellular SO4 2−, though both rates were increased (Jennings, 1995). Xenopus oocytes expressing AE1 E699Q displayed a qualitatively similar asymmetry. Whereas exchange of intracellular SO4 2− for extracellular SO4 2− or Cl− was easily detected (Figs. 2, 5, and 6), exchange of intracellular Cl− for extracellular SO4 2− or Cl− was undetectable not only at 48 h (Fig. 1 B) but also 15 d after cRNA injection (not shown).
These differences in the relative rates of oppositely directed anion hetero-exchange in WRK-BH4-modified red cells and in AE1 E699Q-expressing oocytes compared to wt AE1 function may arise from differences in rate constants for both inward and outward translocation. The contribution of the outward translocation step can be more easily estimated, since both wt and mutant proteins transport extracellular Cl− at operationally maximal rates when measured in the presence of appropriate intracellular anions. In extracellular Cl−, rate constants for SO4 2− efflux reflect the contribution of the E699 negative charge to a local structure in wt AE1 that maintains a low energy barrier for outward translocation of the Cli −-carrier complex and a much higher energy barrier for outward translocation of the (H+/SO4 2−)i-carrier complex. Neutralization of the E699 charge by chemical modification or by mutation likely elevates the energy barrier for outward translocation of the Cli −-carrier complex. In contrast, this neutralization appears to lower the energy barrier for outward translocation of the SO4 2− i-carrier complex, despite the movement of a unit charge across the membrane electric field associated with the mutant transport cycle. The absence of evident H+/SO4 2− cotransport by AE1 E699Q is either due to a greatly elevated energy barrier for outward translocation of the (H+/SO4 2−)i -carrier complex, or simply to loss of the H+ binding site, with failure to form the (H+/SO4 2−)i - carrier complex.
Despite the minimal changes in ID50 for DNDS (Fig. 4) and in the rank order for most extracellular anions (Fig. 5) produced by the E699Q mutation, it is possible that the inward translocation step also contributes to accelerated SO4 2− i/Cl− o exchange by AE1 E699Q. A role for acceleration of the inward translocation step is more evident in SO4 2− i/ SO4 2− o exchange, which occurs at close to maximal (SO4 2− i/Cl− o) rates in AE1 E699Q-expressing oocytes. This contrasts with undetectable rates of SO4 2− efflux in oocytes expressing wt AE1, whether in exchange for intracellular Cl− or SO4 2−.
Inward current associated with AE1 E699Q-mediated exchange of intracellular SO4 2− for extracellular Cl− could arise from outward movement of substrate-associated negative charge during the SO4 2− efflux step or from inward movement of protein-associated positive charge during the Cl− influx step. The oocyte experiments did not allow discrimination between these possible mechanisms of charge movement. The experiments of Jennings (1995) with WRK-BH4–modified red cells assessed the efflux of SO4 2− at varying membrane potentials and varying extracellular Cl− concentrations. Kinetic analysis with assumption of a ping-pong transport mechanism suggested that the principal charge-carrying limb of the anion transport cycle of WRK-BH4–modified AE1 was the influx of Cl− rather than the efflux of divalent SO4 2−. The resulting model of anion translocation by wt AE1 proposed outward transfer through the transbilayer electric field of a neutral complex of two protein-associated positive charges with the two negative charges of transported SO4 2−. In contrast, during the inward flux of monovalent Cl−, one of the protein-associated positive charges (which in wt AE1 may be paired with and neutralized by the negative charge of E699) remains unpaired. Muller-Berger et al. (1995b) have proposed H752 as a candidate residue to ion pair with E699, based on the indirect evidence of similar changes in the apparent pK for AE1-mediated Cl− efflux produced by the independent mouse AE1 mutations H752S and E699D. Discovery of intragenic second site revertants within AE1 E699 mutants will test more stringently this and other possible charge pairs.
Consequences of the E699Q Mutation to Anion Selectivity and Stilbene Sensitivity
Despite human E681's putative location at the COOH terminus of a stretch of nearly 25 hydrophobic amino acids extending from the exofacial terminus of AE1's putative transmembrane span 8, its WRK-reactivity in red cells suggested its accessibility to the extracellular space (Jennings and Smith, 1992). However, in contrast to the dramatic changes in outward anion translocation rates produced by the E699Q mutation in mouse AE1, changes in inward translocation rates in the presence of elevated intracellular sulfate concentration were minimal for tested anions other than sulfate. The rates of SO4 2− efflux in exchange for extracellular gluconate and isethionate were higher than expected from red cell studies, but similar to those measured in oocytes expressing wt AE1 and AE2. It remains unclear why isethionate and gluconate are not impermeant anions with respect to anion exchange in Xenopus oocytes.3 The phenomenon could reflect altered conformation of the anion translocation pathway of either wt or mutant AE1 expressed in oocytes compared to red cells, or activation of endogenous anion transport pathways of the oocyte by expression of heterologous AE polypeptides. The presence of substantial endogenous oocyte permeabilities to extracellular gluconate and isethionate has been suggested by Costa et al. (1989).
The approximately equipotent inhibition by DNDS of AE1 E699Q-mediated SO4 2−/Cl− exchange and of wt AE1-mediated Cl−/Cl− exchange suggests preservation in the mutant of those exofacial structures of AE1 which interact with stilbene inhibitors.
Influence of the Host Cell
The origins of the differences between the activities of AE1 E699Q in Xenopus oocytes and of WRK-BH4-modified red cell AE1 likely reside in the chemical and steric differences at position 699 between the amide group of glutamine and the hydroxyl group of hydroxynorvaline, in addition to differences in host cell environments. A role for charge-independent steric factors in the interaction between E699 and transported anions was suggested by the loss of SO4 2−/SO4 2− exchange in microsomes from 293 cells expressing AE1 E699D, in contrast to the increased activity observed in microsomes from cells expressing either E699Q or E699K (Sekler et al., 1995). However, expression of these mutant AE1 polypeptides in Xenopus oocytes had different consequences. AE1 E699K expressed in Xenopus oocytes did not confer measurable SO4 2−/SO4 2− exchange either 2 d (Fig. 2) or 2 wk after cRNA injection (data not shown). In addition, AE1 E699D expressed in Xenopus oocytes retained ∼40% of wt levels of 36Cl− influx activity (Muller-Berger et al., 1995a ). Thus, the consequences of selected mutations in AE1 E699 differed in different host cell expression systems. Since the WRK-BH4 protocols optimized for the human red cell (Jennings, 1995) did not inhibit wt AE1-mediated Cl−/Cl− exchange in Xenopus oocytes (Chernova and Alper, unpublished observations), direct comparison of WRK-BH4-modified AE1 in red cells and in oocytes was not possible.
Relationship to Chloride Conductance Attributed to Wt AE1
The DIDS-inhibitable portion of human red cell Cl− conductance has been attributed to conductive anion “tunneling” through the AE1 protein (Frohlich, 1988). Jennings (1995) noted that WRK-BH4-modification of human red cells, in addition to its effects on anion exchange, increased Cl− conductance 8–10-fold. In addition to the absence of trans-anion dependence, a property distinguishing red cell Cl− conductance from anion exchange is the insensitivity of Cl− conductance to concentrations of phloretin that inhibit anion exchange (Frohlich, 1988). However, 200 μM phloretin inhibits both SO4 2−/Cl− exchange (n = 6) and inward current (n = 4, not shown) mediated by AE1 E699Q. Together with the demonstrated trans-anion dependence, this result suggests that anion tunneling contributed minimally to AE1 E699Q-mediated electrogenic anion transport in oocytes.
Expression in oocytes of wt trout AE1 cRNA was reported to confer on oocytes a Cl− conductance (Fievet et al., 1995). This conductance differed in at least three ways from mouse AE1 E699Q-mediated electrogenic exchange of intracellular SO4 2− for extracellular Cl−. First, trout AE1-mediated Cl− conductance took place in the presence of the conserved glutamate at the position corresponding to mouse E699. Second, Cl− conductance and Cl− exchange by trout AE1 displayed very different stilbene sensitivities, whereas electrogenic and electroneutral variants of anion exchange by mouse AE1 E699Q exhibited similar stilbene sensitivities. Third, trout AE1 expressed in Xenopus oocytes exhibited maximal anion exchange activity comparable in magnitude to that of mouse AE1 but exhibited a Cl− conductance ∼50-fold larger than the AE1 E699Q-mediated currents in the present study. Interestingly, however, trout AE1 expression at levels lower than 15% of maximal anion exchange rates was unassociated with Cl− current, whereas large currents accompanied higher levels of AE1 expression (Fievet et al., 1995). Though the mechanistic relationship between trout AE1-associated Cl− conductance and AE1 E699Q-mediated electrogenic anion exchange is unclear, each may require for expression of electrogenic transport some minimal level of electroneutral exchange activity.
Trout AE1 expression in oocytes was also associated with increased taurine transport (Fievet et al., 1995). Similarly, skate erythrocyte AE1 has been proposed to mediate taurine transport (Musch et al., 1994). However, expression in oocytes of neither wt mouse AE1 nor of AE1 E699Q was associated with increased 3H-taurine efflux, whether measured in isotonic or in hypotonic chloride medium (Chernova and Alper, unpublished results).
Relationship to Other Sulfate Transporters
AE1 is thought to be the principal sulfate transporter of red cells. In addition to decreased red cell SO4 2− transport noted in the setting of heterozygous AE1 loss-of-function mutations in hereditary spherocytosis (Jarolim et al., 1994; Tanner, 1993), increased red cell SO4 2− transport has been associated with the human AE1 mutation P868L in hereditary acanthocytosis (Bruce et al., 1993). These data, along with the broad spectrum of anions transported by AE1 in erythrocytes, has encouraged speculation that nonerythroid AE proteins serve as physiologically important transporters of anions other than Cl− and HCO3 −, including SO4 2−. Other Na+-independent SO4 2− transporters and related proteins from mammalian tissues cloned by functional expression (Bissig et al., 1994), by differential expression (Silberg et al., 1995), or by positional cloning (Hastbacka et al., 1994) comprise a distinct gene family unrelated in sequence to the AE anion exchanger gene family, despite evidence for SO4 2−/HCO3 − exchange mediated by the transporter Sat1 (Bissig et al., 1994). At least one member of this gene family has been implicated in Cl−/HCO3 − exchange by genetic linkage (Hoglund et al., 1996). In contrast, a role for any endogenous AE polypeptide in physiological SO4 2− transport by nonerythroid cells remains to be demonstrated.
Acknowledgments
We are grateful to J. Zeind for sulfate determinations, G. Jacquet for technical assistance, and to Dr. M. Gola and A. Stuart-Tilley for discussion.
This work was supported by National Institutes of Health grants DK43495 (S.L. Alper), DK34854 (Harvard Digestive Diseases Center to S.L. Alper), RR01032 (Beth Israel Hospital General Clinical Research Center Core Laboratory), NS30591, and DK45628 (K. Strange), DK77726 (The Children's Hospital Renal Training Grant to M. Hand), and the CNRS (M. Crest). S.L. Alper and K. Strange are Established Investigators of the American Heart Association.
Footnotes
Portions of this work were presented at the Forty-ninth annual meeting of the Society of General Physiologists (1995. J. Gen. Physiol. 106: 31a) and at the Twenty-eighth annual meeting of American Society of Nephrology (1995. J. Am. Soc. Nephrol. 6:303a).
Abbreviations used in this paper: AE1, anion exchanger 1; DNDS, 4,4′-dinitrostilbene-2,2′-disulfonic acid; WRK, Woodward's Reagent K (N-ethyl-5-phenylisoxazolium 3′-sulfonate); wt, wild-type.
Exclusion of the “outlier” experiment 2 from the calculations of the flux ratios resulted only in small changes. Such an edited mean of the individual flux ratios, 1.30 ± 0.19, did not differ statistically from the value in Table I. Such an edited ratio of the individual experimental mean flux values, 1.24 ± 0.05, differed slightly from that in Table I, but both values were consistent with a 1:1 stoichiometry of SO4 2− i/Cl− o exchange. Jennings (1995) also observed a ratio of SO4 2− efflux to Cl− influx of 1.21 ± 0.1 (SEM) in WRK-BH4–treated human red cells in the presence of gramicidin (Table 1 of Jennings, 1995).
Though AE1 E699Q-mediated inward current at clamped resting potential was of sufficient magnitude to conclude that all anion exchange flux measured under open circuit conditions was electrogenic (Table IV), extracellular isethionate supported a rate of AE1 E699Q-mediated 35SO4 2− efflux of 0.2 relative to extracellular Cl− (Fig. 5) but supported no greater inward current than did extracellular sulfate. Several factors might account for this discrepancy without invoking an alternate transport mechanism. First, current values of 0.2 relative to those recorded in extracellular Cl− are near or below the threshold of detection against the variable background current of individual oocytes. Second, the two assays were performed under different experimental conditions. Sulfate efflux into extracellular isethionate (Fig. 5) was measured 10 min after injection of oocytes with 50 nl carrier-free Na235SO4 in 50 mM Na HEPES, pH 7.4, whereas membrane potential change in response to extracellular anion substitution (Fig. 8, Table III) was measured in oocytes maintained in isethionate medium for 1–6 h after injection with 50 nl of 130 mM Na2SO4, 50 mM Na HEPES, pH 7.4. Lastly, evaluation of the effect of extracellular isethionate on the membrane potential of AE1 E699Q-expressing oocytes requires comparison with the effect of an extracellular monovalent anion to which the oocyte is “absolutely” or maximally impermeable (at least as judged by 35SO4 2− efflux). Experiments to address this issue are underway.
references
- Alper SL. The band 3-related AE anion exchanger gene family. Cell Physiol Biochem. 1994;4:265–281. [Google Scholar]
- Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Definition of intercalated cell subtypes in rat kidney collecting duct using antibodies against erythroid band 3 and renal vacuolar H+ATPase. Proc Natl Acad Sci USA. 1989;86:5429–5433. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Noy S, Cabantchik ZI. Transport domain of the erythrocyte anion exchange protein. J Membr Biol. 1990;115:217–228. doi: 10.1007/BF01868637. [DOI] [PubMed] [Google Scholar]
- Bartel D, Lepke S, Layh-Schmitt G, Legrum B, Passow H. Anion transport in oocytes of Xenopus laevis induced by expression of mouse erythroid band 3 protein-encoding cRNA and of a cRNA derivative obtained by site-directed mutagenesis at the stilbene disulfonate binding site. EMBO (Eur Mol Biol Organ) J. 1989;8:3601–3609. doi: 10.1002/j.1460-2075.1989.tb08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghout A, Raida M, Lerum B, Passow H. The effects of dansylation on the pH dependence of SO4 2− and Cl− equilibrium exchange and on the H+/SO4 2−cotransport across the red blood cell membrane. Biochim Biophys Acta. 1988;986:75–82. doi: 10.1016/0005-2736(89)90274-5. [DOI] [PubMed] [Google Scholar]
- Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem. 1994;269:3017–3021. [PubMed] [Google Scholar]
- Brosius FC, Alper SL, Garcia A-M, Lodish HF. The major kidney band 3 transcript predicts an amino-terminal truncated band 3 polypeptide. J Biol Chem. 1989;264:7784–7787. [PubMed] [Google Scholar]
- Bruce LJ, Kay MMB, Lawrence C, Tanner MJA. Band 3HT, a human red cell variant associated with acanthocytosis and increased anion transport, carries the mutation Pro-868-\>Leu in the membrane domain of band 3. Biochem J. 1993;293:317–320. doi: 10.1042/bj2930317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova MN, Jarolim P, Palek J, Alper SL. Overexpression of AE1 Prague, but not of AE1 SAO, inhibits wild-type AE1 trafficking in Xenopus oocytes. J Membr Biol. 1995;148:203–210. doi: 10.1007/BF00207276. [DOI] [PubMed] [Google Scholar]
- Costa PF, Emilio MG, Fernandes PL, Ferreira HG, Ferreira KG. Determination of ionic permeability coefficients of the plasma membrane of Xenopus laevis oocytes under voltage clamp. J Physiol (Lond) 1989;413:199–211. doi: 10.1113/jphysiol.1989.sp017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet B, Gabillat N, Borgese F, Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO (Eur Mol Biol Organ) J. 1995;14:5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich O. The “tunneling” mode of biological carrier-mediated transport. J Membr Biol. 1988;101:189–198. doi: 10.1007/BF01872834. [DOI] [PubMed] [Google Scholar]
- Garcia A-M, Lodish HF. Lysine 539 of human band 3 is not essential for anion transport or inhibition by stilbene disulfonates. J Biol Chem. 1989;264:19607–19613. [PubMed] [Google Scholar]
- Greenberg, A.E., J.J. Connor, D. Jenkins, and M.A. Franson. 1980. Sulfate, turbidimetric method #426C. In Standard Methods for the Examination of Water and Waste Water, 15th Ed. The American Public Health Association, Washington, D.C. 439–440.
- Groves JD, Tanner MJA. The effects of glycophorin A on the expression of the human red cell anion transporter (band 3) in Xenopus oocytes. J Membr Biol. 1993;140:81–88. doi: 10.1007/BF00234488. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Schwarz W, Passow H. Potential dependence of the “electrically silent” anion exchange across the plasma membrane of Xenopus oocytes mediated by the band 3 protein of mouse red blood cells. J Membr Biol. 1987;99:127–136. doi: 10.1007/BF01871232. [DOI] [PubMed] [Google Scholar]
- Hanke-Baier P, Raida M, Passow H. Comparison of murine band 3 protein-mediated Cl−transport as measured in mouse red cells and in oocytes of Xenopus laevis. Biochim Biophys Acta. 1988;940:136–140. doi: 10.1016/0005-2736(88)90017-x. [DOI] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, et al. The diastrophic dystrophy gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg M-L, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nature Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol. 1994;266:C1295–C1307. doi: 10.1152/ajpcell.1994.267.5.C1295. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Jiang L, Chernova MN, Alper SL. Hypertonic activation of AE2 anion exchanger in Xenopus oocytes via NHE-mediated intracellular alkalinization. Am J Physiol. 1995;268:C201–C209. doi: 10.1152/ajpcell.1995.268.1.C201. [DOI] [PubMed] [Google Scholar]
- Izuhara K, Okubo K, Hamasaki N. Conformational change of band 3 protein induced by diethyl pyrocarbonate modification in human erythrocyte ghosts. Biochemistry. 1989;28:4725–4728. doi: 10.1021/bi00437a032. [DOI] [PubMed] [Google Scholar]
- Jaisser J, Horisberger J-D, Geering K, Rossier BC. Mechanisms of urinary K+ and H+excretion: primary structure and functional expression of a novel H,K-ATPase. J Cell Biol. 1993;123:1421–1429. doi: 10.1083/jcb.123.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim P, Rubin H, Liu S-C, Cho M, Brabec V, Derrick L, Yi S, Saad S, Alper S, Brugnara C, Golan D, Palek J. Band 3 Prague: a frameshift duplication in the erythroid band 3 gene in a kindred with hereditary spherocytosis with band 3 protein deficiency. J Clin Invest. 1994;93:121–130. doi: 10.1172/JCI116935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML. Reductive methylation of the two 4,4′-diisothiocyano-dihydrostilbene-2,2′-disulfonate-binding lysine residues of band 3, the human erythrocyte anion transport protein. J Biol Chem. 1982;257:7554–7559. [PubMed] [Google Scholar]
- Jennings ML. Rapid electrogenic sulfate-chloride exchange mediated by chemically modified band 3 in human erythrocytes. J Gen Physiol. 1995;105:21–47. doi: 10.1085/jgp.105.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Al-Rhaiyel S. Modification of a carboxyl group that appears to cross the permeability barrier in the red blood cell anion transporter. J Gen Physiol. 1988;92:161–178. doi: 10.1085/jgp.92.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Anderson MP. Chemical modification of glutamate residues at the stilbenedisulfonate site of human red blood cell band 3 protein. J Biol Chem. 1987;262:1691–1697. [PubMed] [Google Scholar]
- Jennings ML, Schulz RK, Allen M. Effects of membrane potential on electrically silent transport: potential-independent translocation and asymmetric potential-dependent substrate binding to the red blood cell anion exchange protein. J Gen Physiol. 1990;96:991–1012. doi: 10.1085/jgp.96.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings ML, Smith JS. Anion-proton cotransport through the human red blood cell band 3 protein. Role of glutamate 681. J Biol Chem. 1992;267:13964–13971. [PubMed] [Google Scholar]
- Julien T, Zaki L. Studies on inactivation of anion transport in human red blood cell membrane by reversible and irreversible acting arginine-specific reagents . J Membr Biol. 1988;102:217–224. doi: 10.1007/BF01925715. [DOI] [PubMed] [Google Scholar]
- Kietz D, Bartel D, Lepke S, Passow H. pH-dependence of inhibition by H2DIDS of mouse erythroid band 3-mediated Cl−transport in Xenopus oocytes. The effect of oligonucleotide-directed replacement of Lys-558 by an Asn residue. Biochim Biophys Acta. 1991;1064:81–88. doi: 10.1016/0005-2736(91)90414-4. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Lodish HF. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature (Lond) 1985;316:234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- Lepke S, Passow H. Inverse effects of dansylation of the red blood cell membrane on band 3 protein-mediated transport of sulphate and chloride. J Physiol (Lond) 1982;328:27–48. doi: 10.1113/jphysiol.1982.sp014251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanick MA, Gunn RB. Proton-sulfate cotransport: mechanism of hydrogen and sulfate addition to the chloride transporter of human red blood cells. J Gen Physiol. 1982;79:87–113. doi: 10.1085/jgp.79.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanick MA, Gunn RB. Proton-sulfate cotransport: external proton activation of sulfate influx into human red blood cells. Am J Physiol. 1984;247:C247–C259. doi: 10.1152/ajpcell.1984.247.3.C247. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Kang D, Aranibar N, Wood PG, Ruterjans H, Passow H. Roles of Histidine 752 and Glutamate 699 in the pH dependence of mouse band 3 protein-mediated anion transport. Biochemistry. 1995a;34:9325–9332. doi: 10.1021/bi00029a007. [DOI] [PubMed] [Google Scholar]
- Muller-Berger S, Karbach D, Konig J, Lepke S, Wood PG, Appelhans H, Passow H. Inhibition of mouse erythroid band 3-mediated chloride transport by site-directed mutagenesis of histidine residues and its reversal by second site mutation of Lys 558, the locus of covalent H2DIDS binding. Biochemistry. 1995b;34:9315–9324. doi: 10.1021/bi00029a006. [DOI] [PubMed] [Google Scholar]
- Musch MW, Davis EM, Goldstein L. Oligomeric forms of skate erythrocyte band 3: effect of volume expansion. J Biol Chem. 1994;269:19683–19686. [PubMed] [Google Scholar]
- Passow H. Molecular aspects of band 3-protein-mediated anion transport across the red blood cell membrane. Rev Physiol Biochem Pharmacol. 1986;103:62–203. doi: 10.1007/3540153330_2. [DOI] [PubMed] [Google Scholar]
- Passow H, Lepke S, Wood PG. Exploration of the mechanism of mouse erythroid band 3-mediated anion exchange by site-directed mutagenesis. Prog Cell Res. 1992;2:85–98. [Google Scholar]
- Schwarz W, Gu Q, Passow H. Potential dependence of mouse band 3-mediated anion exchange in Xenopus oocytes. Prog Cell Res. 1992;2:161–168. [Google Scholar]
- Sekler I, Lo RS, Kopito RR. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J Biol Chem. 1995;270:28751–28758. doi: 10.1074/jbc.270.48.28751. [DOI] [PubMed] [Google Scholar]
- Silberg DG, Wang W, Moseley RH, Traber PG. The Down Regulated in Adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem. 1995;270:11897–11902. doi: 10.1074/jbc.270.20.11897. [DOI] [PubMed] [Google Scholar]
- Tanner MJA. Molecular and cellular biology of the erythrocyte anion exchanger. Semin Hematol. 1993;30:34–57. [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Wieth JO, Andersen OS, Brahm J, Bjerrum PJ, Borders CL., Jr Chloride-bicarbonate exchange in red blood cells. Phil Trans R Soc Lond B Biol Sci. 1982;299:383–399. doi: 10.1098/rstb.1982.0139. [DOI] [PubMed] [Google Scholar]
- Wood PG, Muller H, Sovak M, Passow H. Role of Lys 558 and Lys 869 in substrate and inhibitor binding to the murine band 3 protein: a study of the effects of site-directed mutagenesis of the band 3 protein expressed in Xenopus laevis. J Membr Biol. 1992;127:139–148. doi: 10.1007/BF00233286. [DOI] [PubMed] [Google Scholar]