Abstract
Cardiac muscle fails to relax upon replacement of extracellular Ca2+ with Ba2+. Among the manifold consequences of this intervention, one major possibility is that Na+-Ba2+ exchange is inadequate to support normal relaxation. This could occur due to reduced transport rates of Na+-Ba2+ exchange and/or by failure of Ba2+ to activate the exchanger molecule at the high affinity regulatory Ca2+ binding site. In this study, we examined transport and regulatory properties for Na+-Ca2+ and Na+-Ba2+ exchange. Inward and outward Na+-Ca2+ or Na+-Ba2+ exchange currents were examined at 30°C in giant membrane patches excised from Xenopus oocytes expressing the cloned cardiac Na+-Ca2+ exchanger, NCX1. When excised patches were exposed to either cytoplasmic Ca2+ or Ba2+, robust inward Na+-Ca2+ exchange currents were observed, whereas Na+-Ba2+ currents were absent or barely detectable. Similarly, outward currents were greatly reduced when pipette solutions contained Ba2+ rather than Ca2+. However, when solution temperature was elevated from 30°C to 37°C, a substantial increase in outward Na+-Ba2+ exchange currents was observed, but not so for inward currents. We also compared the relative abilities of Ca2+ and Ba2+ to activate outward Na+-Ca2+ exchange currents at the high affinity regulatory Ca2+ binding site. While Ba2+ was capable of activating the exchanger, it did so with a much lower affinity (K D ∼ 10 μM) compared with Ca2+ (K D ∼ 0.3 μM). Moreover, the efficiency of Ba2+ regulation of Na+-Ca2+ exchange is also diminished relative to Ca2+, supporting ∼60% of maximal currents obtainable with Ca2+. Ba2+ is also much less effective at alleviating Na+ i-induced inactivation of NCX1. These results indicate that the reduced ability of NCX1 to adequately exchange Na+ and Ba2+ contributes to failure of the relaxation process in cardiac muscle.
Keywords: sodium-calcium exchange, transport, regulation, calcium, barium
introduction
Na+-Ca2+ exchange plays a major role in Ca2+ homeostasis in cardiac muscle. Removal of myoplasmic Ca2+ by this mechanism is essential for physiological cardiac relaxation (Bers, 1991). In general, removal of Ca2+ by Na+-Ca2+ exchange is equivalent to Ca2+ entry through L-type Ca2+ channels on a beat-to-beat basis (Bridge et al., 1990). Na+-Ca2+ exchange may also serve an important role as a Ca2+ entry mechanism during cardiac excitation. Several studies have demonstrated that this “reverse mode” of Na+-Ca2+ exchange can trigger sarcoplasmic reticulum Ca2+ release (LeBlanc and Hume, 1990; Kohmoto et al., 1994; Levi et al., 1994; Vornanen et al., 1994; Wasserstrom and Vites, 1996). Consequently, alterations in Na+-Ca2+ exchange function, exemplified by digitalis treatment (Lee and Dagastino, 1982) or alterations in the intracellular Na+ concentration (Harrison and Boyett, 1995), produce major effects on cardiac contractility.
Recently, several regulatory properties have been characterized for the cardiac Na+-Ca2+ exchanger, NCX1. Detailed characterization of these regulatory mechanisms has been accomplished for the native and cloned cardiac Na+-Ca2+ exchanger using the giant excised patch technique. Regarding ionic regulation, both Na+ and Ca2+ regulate exchange activity in addition to serving as the transport substrates (Hilgemann, 1990). Examination of outward (reverse) Na+-Ca2+ exchange currents reveals a complex waveform. The application of Na+ i induces an outward current which undergoes a time-dependent inactivation. The extent of this inactivation is governed by both cytoplasmic Na+ and Ca2+ levels. However, in the presence of a constant level of cytoplasmic Ca2+ (e.g., 1 μM), both outward currents and the extent of inactivation increase as Na+ i levels are increased. This behavior is referred to as Na+ i-induced or I1 inactivation (Hilgemann et al., 1992b ).
Cytoplasmic Ca2+ levels regulate exchange activity by influencing the extent of Na+ i-induced inactivation and through an apparent direct activation of the exchange molecule (Hilgemann et al., 1992a , b ). This direct pathway is referred to as I2 inactivation where removal of cytosolic Ca2+ favors entry into an inactive (I2) state. Both forward and reverse modes of Na+-Ca2+ exchange are regulated by cytoplasmic Ca2+ (Matsuoka et al., 1995), and the high affinity regulatory Ca2+ binding site has been identified for the cardiac exchanger, NCX1 (Levitsky et al., 1994; Matsuoka et al., 1995). The ability of other divalent cations to substitute for Ca2+ at the regulatory site has not been examined in detail.
Na+-Ca2+ exchange has a strict specificity for Na+ as the transported monovalent cation (Philipson and Nicoll, 1993). Less stringency is observed for transport of other divalent cations with Ba2+ and Sr2+ being transported to varying degrees. Earlier studies of this nature have used cardiac sarcolemmal vesicles to examine radioisotope fluxes for different divalent cations. From these reports, it appeared that Ca2+ and Sr2+ were transported at nearly equal rates, whereas Ba2+ transport was ∼20 times slower and exhibited a two- to threefold reduction in affinity for the exchanger (Trosper and Philipson, 1983; Tibbits and Philipson, 1985). More recent electrophysiological and fluorescence measurements yield conflicting results. In one instance, outward Na+-Ba2+ exchange currents were not detectable from whole cell patch clamp experiments using guinea-pig ventricular myocytes (Kimura et al., 1987). However, Ba2+ uptake through the exchanger was readily detected in CHO cells expressing the bovine cardiac Na+-Ca2+ exchanger (Chernaya et al., 1996). An experimental limitation of all the above studies is the difficulty in discriminating between transport and regulatory consequences for this ionic substitution. However, this limitation can be circumvented by using the giant excised patch clamp. In this study, we have compared the effects of Ca2+ and Ba2+ on properties of the cloned, cardiac Na+-Ca2+ exchanger, NCX1. The specific goals were to examine how Ba2+ substitution alters the transport and regulatory properties of NCX1 and to determine if these differences can provide a reasonable account for why cardiac muscle fails to relax in Ba2+-containing media.
methods
Myocyte Preparation and Shortening Measurements
Canine ventricular myocytes were provided by Dr. A . Lukas (University of Manitoba, Winnipeg, Canada) and were prepared as described previously (Lukas and Antzelowitch, 1993). Myocytes were superfused with (in mM): 136 NaCl, 10 HEPES, 8.33 NaH2PO4, 5.4 KCl, 1 MgCl2, 1 CaCl2 or BaCl2, pH 7.4 (using NaOH) at 30°C. Field stimulation of myocytes at 0.5 Hz (1.1–1.5 times threshold) was performed using a Grass SD-9 Stimulator (Grass Instrument Co., Warwick, RI). Shortening was monitored with a video edge detection system (Crescent Electronics, Sandy, UT) and recorded using Axon Instruments (Foster City, CA) hardware and software as described previously (Hryshko et al., 1989).
Molecular Biological Techniques
Oocytes were obtained from Xenopus laevis as described previously (Hryshko et al., 1996). Oocytes were treated with collagenase (20 mg/ml) for 1 h, washed in Barth's solution, treated with 100 mM K2HPO4 for 15 min, washed, and stored overnight in fresh Barth's solution. NCX1 cRNA was prepared using T3 mMessage mMachine (Ambion Inc., Austin, TX) according to the manufacturer's instructions. Oocytes were injected with cRNA (∼5 ng/oocyte), and activity was measured 3–6 d later.
Electrophysiological Techniques
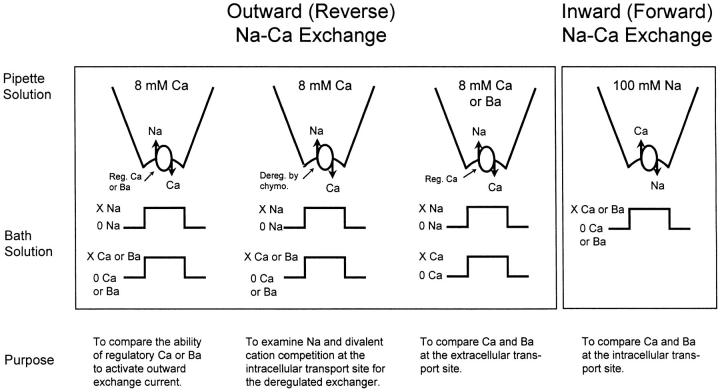
Na+-Ca2+ exchange activity was measured using the giant excised patch clamp technique of Hilgemann (1989) as described previously (Matsuoka et al., 1995; Hryshko et al., 1996). Pipettes were pulled from borosilicate glass and polished to a final diameter of 20–40 μm. Pipettes were coated with a parafilm/mineral oil mixture to enhance patch stability and reduce electrical noise. For seal formation, oocytes were placed in a solution containing (in mM): 100 KOH, 100 MES, 20 HEPES, 5 EGTA, 5 MgCl2, pH 7.0 with MES. Gigaohm seals were formed by gentle suction and patches were excised by progressive movements of the pipette tip. Excised patches were in the inside-out configuration. For outward Na+-Ca2+ exchange current measurements, pipettes were filled with (in mM): 100 NMG-MES, 30 HEPES, 30 TEA-OH, 16 sulfamic acid, 8 CaCO3, 6 KOH, 0.25 ouabain, 0.1 niflumic acid, 0.1 flufenamic acid, pH 7.0 (using MES). Outward Na+-Ca2+ exchange currents were elicited by switching from a Li+ to Na+-based superfusate containing (in mM): 100 Na- or Li-aspartate, 20 MOPS, 20 TEA-OH, 20 CsOH, 10 EGTA, 0–2.30 CaCO3 or 0–7.55 Ba(OH)2, 1–1.5 Mg(OH)2, pH 7.0 (using MES or LiOH). The amounts of Ca2+ and Mg2+ were adjusted to yield free Mg2+ concentrations of 1 mM and various free Ca2+ concentrations as indicated. MAXC software was used to calculate free Ca2+ and Mg2+ concentrations (Bers et al., 1994). For inward current measurements, pipettes contained (in mM): 100 Na-MES, 20 TEA-MES, 20 Cs-MES, 10 HEPES, 10 EGTA, 4 Mg(OH)2, 0.2 ouabain, 0.1 niflumic acid, 0.1 flufenamic acid, 0.002 verapamil, pH 7.0. Inward Na+-Ca2+ exchange currents were activated by switching to the Li+-based Ca2+ containing superfusates described above for outward current measurements. Current data were acquired and analyzed using Axon Instruments (Foster City, CA) hardware and software. Solution changes (∼200 ms) were accomplished using a custom-built 20-channel computer-controlled solution switcher. All experiments were conducted at 30 ± 1°C unless indicated otherwise. The rationale and design of the different types of experiments are summarized in Fig. 1.
Figure 1.
The underlying rationale and different experimental conditions used in the present study are shown. For outward current measurements, extracellular solutions (pipette) contained 8 mM Ca2+ or Ba2+, and currents were activated by switching from Li+-based to Na+-based intracellular (bath) solutions. For inward current measurements, extracellular (pipette) solutions contained 100 mM Na+ and currents were activated by switching to intracellular solutions with various concentrations of either Ca2+ or Ba2+.
results
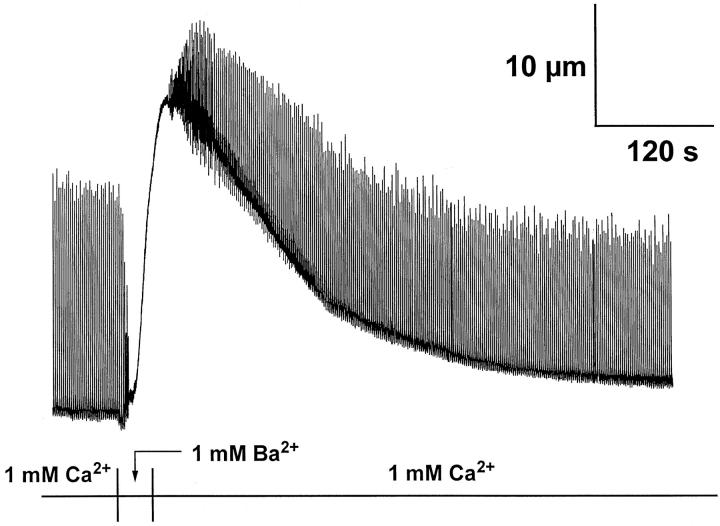
Fig. 2 illustrates the effects of replacing 1 mM extracellular Ca2+ with 1 mM Ba2+ on electrically evoked shortening in a canine ventricular myocyte. Almost immediately, contraction fails and a contracture develops. Upon restoration of Ca2+ to the superfusate, the contracture gradually wanes and near normal resting length and contractions resume. We observed this pattern of contracture in ten cells from three different myocyte preparations. Initial shortening averaged 10.9 ± 0.8% (mean ± SD, n = 10) in the Ca2+-containing superfusate. After recovery from the Ba2+-induced contracture, shortening averaged 8.1 ± 1.0%. While numerous cellular processes are affected by this intervention, our specific goal was to determine how Ba2+ substitution affects the operation of the Na+-Ca2+ exchanger. In particular, we sought to distinguish between the effects of Ba2+ substitution on transport vs. regulatory aspects of Na+-Ca2+ exchange function.
Figure 2.
The effects of replacing 1 mM extracellular Ca2+ with 1 mM Ba2+ on electrically stimulated shortening for a canine ventricular myocyte are shown. Upon substituting Ba2+ for Ca2+, shortening rapidly fails, and a sustained contracture develops. Restoration of extracellular Ca2+ leads to near full recovery of resting length and shortening.
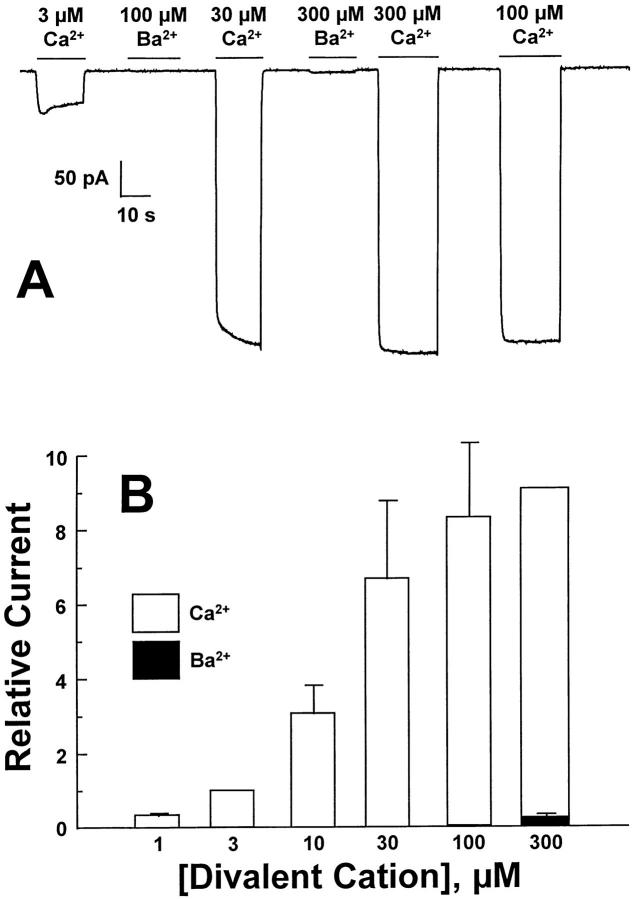
Fig. 3 A shows inward Na+-Ca2+ and Na+-Ba2+ exchange currents from a single excised patch. Physiologically, this “forward” mode of transport corresponds to Ca2+ extrusion. Currents were activated by the application of Li+-based Ca2+- or Ba2+-containing solutions to the cytoplasmic surface of the patch at the concentrations indicated. The pipette solution contained 100 mM Na+, and Ca2+- and Ba2+-containing solutions were applied in a random order. For Ca2+-activation, note the progressive increase of inward currents observed up to concentrations between 30–300 μM. In contrast, at Ba2+ concentrations up to 300 μM, inward currents were barely detectable. In eight patches showing robust Ca2+-activated inward currents at 30°C, Ba2+-activated currents were absent or barely detectable. Three patches were also examined at 37°C and did not exhibit substantial Ba2+-activated inward exchange currents, whereas Ca2+-activated currents were readily observed (not shown). We did not routinely examine higher Ba2+ concentrations as these led to a small outward current at concentrations of 1 mM and above. This small outward current was also observed using pipette solutions containing Li+ rather than Na+ and may represent residual Ca2+ activated Cl− conductance (see discussion).
Figure 3.
(A) Illustrates typical inward currents activated by the application of cytoplasmic Ca2+ or Ba2+. The pipette solution contained 100 mM Na+. Pooled results from three to nine patches (normalized to the current value obtained at 3 μM Ca2+ i in nine patches) (means ± SE) are shown in B.
Fig. 3 B shows pooled data from nine patches for inward current measurements activated by Ca2+ or Ba2+. Each point represents the average from between three and nine determinations with data normalized to the inward current obtained at 3 μM Ca2+. K D values are not reported as not all divalent concentrations could be examined in a single patch. For comparative purposes, currents obtained at 3 μM Ca2+ (n = 9) exceeded currents obtained with 100 μM Ba2+ (n = 4) by 17-fold and 300 μM Ba2+ (n = 6) by 10-fold. As shown below (see Fig. 5), the absence of inward current is not a consequence of failure to activate the exchanger with Ba2+.
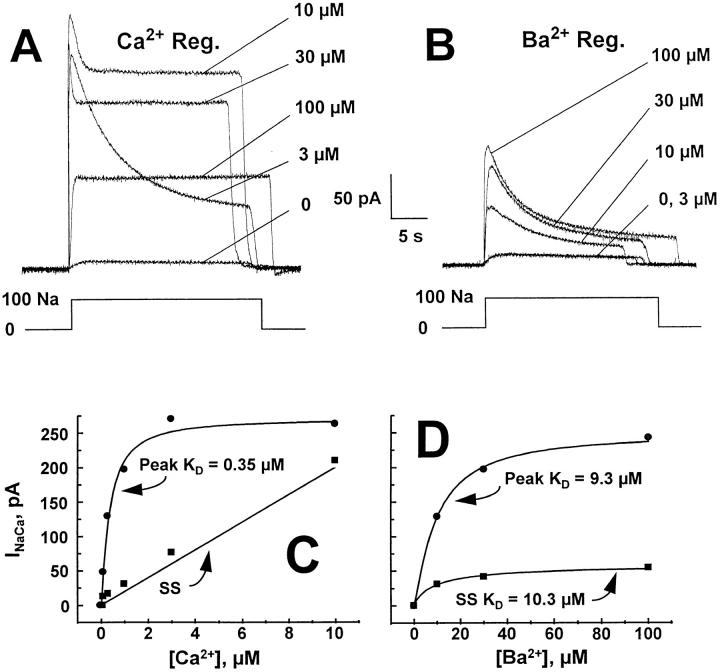
Figure 5.
The effects of different concentrations of regulatory Ca2+ or Ba2+ on outward Na+-Ca2+ exchange currents are shown for a single patch in A and B. The pipette solution contained 8 mM Ca2+. The different concentrations of regulatory Ca2+ (A) or Ba2+ (B) were present before and during the application of 100 mM Na+ to activate the current. Typical concentration dependencies of peak and steady-state (SS) outward currents are shown for regulation by Ca2+ (C) and Ba2+ (D) from two separate patches. Note the difference in concentration range between these graphs.
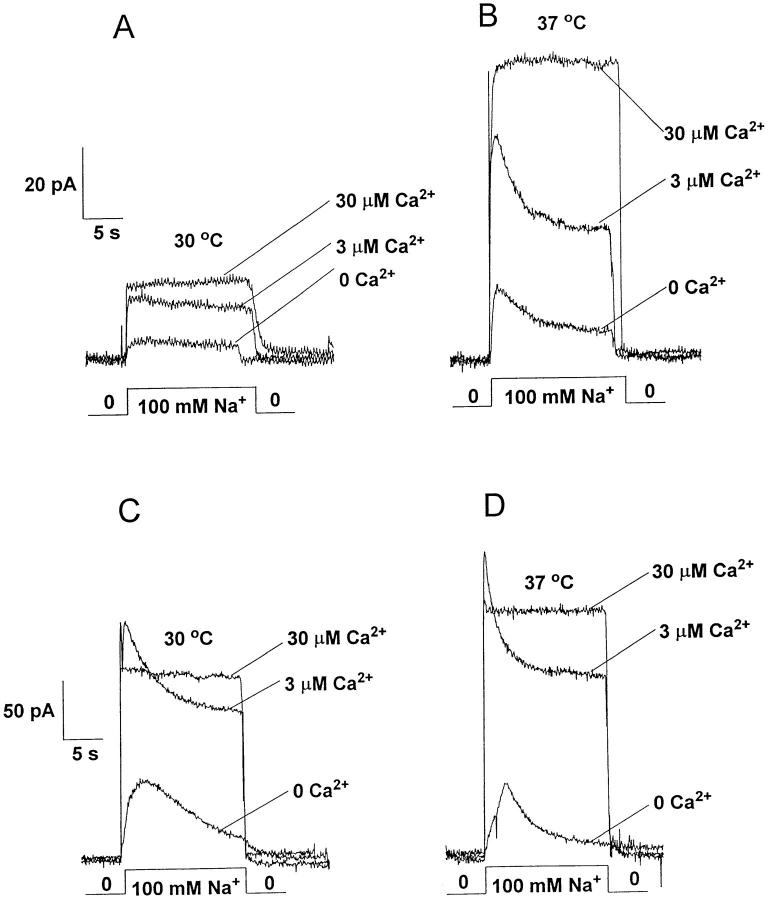
Fig. 4 illustrates outward currents activated by the application of 100 mM Na+ to the cytoplasmic surface of the patch. Physiologically, this “reverse” mode of transport corresponds to Ca2+ entry. Different concentrations of regulatory Ca2+ were present before and during Na+ application, as indicated. Pipettes contained either 8 mM Ba2+ (Fig. 4, traces A and B) or Ca2+ (traces C and D). Several features are noteworthy. For Ba2+-containing pipettes, we observed very small currents at 30°C (<20 pA in six separate patches). Although regulated by Ca2+, the extent of I2 regulation appeared blunted and Na+ i-induced (I1) inactivation was not observed. At 37°C, six of six patches exhibited Na+-Ba2+ exchange activity with regulatory properties more typical of those observed with Ca2+-containing pipette solutions. In three patches for which both temperatures could be examined, a three to fourfold increase in current magnitude was observed for this 7°C increase in temperature, as shown in Fig. 4, A and B. Recall that this striking increase in outward Na+-Ba2+ exchange currents was not evident for inward currents at 37°C. Comparatively, for Ca2+-containing pipettes (Fig. 4, C and D), normal I1 and I2 regulation were observed at both temperatures. Typical for three patches where currents were obtained at both temperatures, Fig. 4, C and D, shows less augmentation of current (30–50% increase) at 37°C than that observed for Na+-Ba2+ exchange. For both Ba2+- and Ca2+- containing pipettes, it is likely that we have underestimated the true temperature sensitivity as current rundown likely occurs during the 5–10 min period required to increase perfusate temperature.
Figure 4.
Typical outward Na+-Ba2+ (A and B) and Na+-Ca2+ (C and D) exchange currents examined at 30°C (left) and 37°C (right). The pipette solution contained 8 mM Ba2+ or Ca2+ and currents were activated by the application of 100 mM Na+ at the indicated concentrations of regulatory Ca2+ i. Data were obtained by making measurements at 30°C followed by increasing bath temperature and repeating measurements at 37°C (5–7 min later).
The activity of Na+-Ca2+ exchangers is regulated by the occupancy status of a high affinity Ca2+ binding site on the cytoplasmic surface of the molecule (Levitsky et al., 1994; Matsuoka et al., 1995; Hryshko et al., 1996). Consequently, alterations in the activation of Na+-Ca2+ exchange activity by Ba2+ could also contribute to the reduced ability of NCX1 to transport Ba2+. To investigate this possibility, we compared regulation of outward Na+-Ca2+ exchange currents by Ca2+ and Ba2+. Fig. 5, A and B, shows current traces obtained from a single patch at different concentrations of regulatory Ca2+ or Ba2+, respectively. At each concentration indicated, regulatory Ca2+ or Ba2+ was present before and during the application of 100 mM Na+ to activate the outward current. Pipette Ca2+ was constant at 8 mM. Note that Ba2+ was considerably less effective at activating Na+-Ca2+ exchange compared to Ca2+. For example, virtually no current is observed at 3 μM Ba2+ whereas this level of Ca2+ leads to near maximal currents for Ca2+. In addition, the progressive loss of I1 inactivation with increasing Ca2+ is much less apparent for Ba2+-regulated currents.
Fig. 5, C and D, shows typical concentration dependencies for Ca2+ and Ba2+ regulation, respectively, for outward exchange currents in two separate patches. The concentration ranges examined were selected to determine K D and illustrate differences between Ca2+ and Ba2+ on steady-state current properties. Current levels were similar in these two different patches. The K D for Ca2+ regulated peak INaCa was 0.35 μM. The complex relationship of regulatory Ca2+ with I1 and I2 inactivation leads to a near linear relation for steady state currents up to 10 μM Ca2+, followed by a progressive decline as Ca2+ begins to compete with cytoplasmic Na+ (as seen in Fig. 5 A). Ba2+ regulated peak INaCa and steady-state INaCa exhibited K D's of 9.3 and 10.3 μM, respectively, and I1 inactivation was not dramatically changed (as seen in Fig. 5 B). We observed similar differences in regulation of outward currents between Ca2+ vs. Ba2+ in 12 different patches.
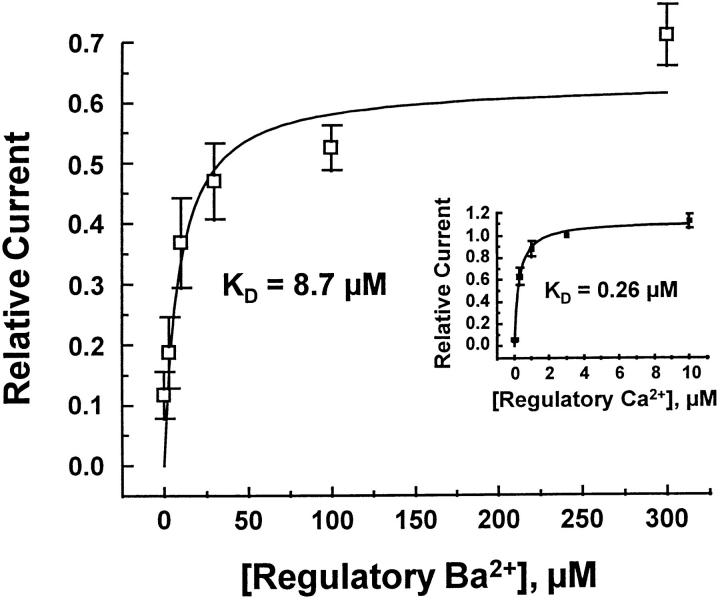
Pooled data from 9 patches is shown in Fig. 6 for peak outward currents regulated by Ba2+ or Ca2+. Current values were normalized to that obtained for peak outward current in the presence of 3 μM regulatory Ca2+, allowing direct comparison of all data. Two features are obvious from this graph. First, the affinity of regulatory Ba2+ (K D = 8.7 μM) is ∼30 times lower than that for Ca2+ (K D = 260 nM). Second, the efficiency of Ba2+ regulation is substantially lower than that observed with Ca2+. Even at the highest Ba2+ concentrations examined, peak current was substantially lower than that observed for Ca2+.
Figure 6.
Regulation of outward Na+-Ca2+ exchange currents by Ba2+ and Ca2+ (inset). Pooled results (mean ± SD) from three to nine determinations in nine separate patches. Currents were normalized to the value of current obtained at 3 μM regulatory Ca2+ i (in all 9 patches).
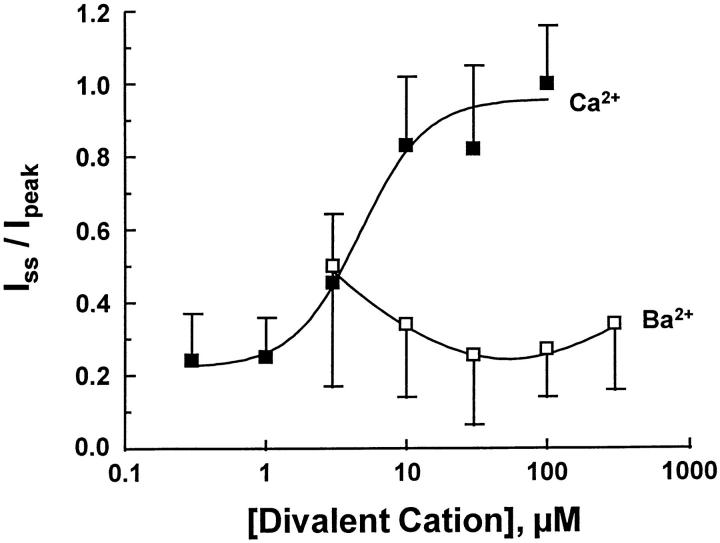
Ba2+ appeared to be much less effective at alleviating Na+-induced inactivation compared with Ca2+. This behavior is illustrated in Fig. 7 for pooled results from nine patches (3–9 determinations at each concentration). As regulatory Ca2+ was progressively increased, steady-state current approached the same level as peak current. In contrast, this behavior was not observed when Ba2+ served as the regulatory ion. In two patches examined at 1 mM regulatory Ba2+ (not shown), a small reduction in peak to steady state ratio was observed.
Figure 7.
The relationship between peak and steady-state outward Na+-Ca2+ exchange currents regulated by Ca2+ i (filled squares) and Ba2+ i (open squares) is shown for pooled results from three to nine patches (mean ± SD). For Ca2+ i regulation, steady-state current approaches peak current levels reflecting the progressive reduction in I1 inactivation. In contrast, I1 inactivation is not attenuated by regulatory Ba2+ i.
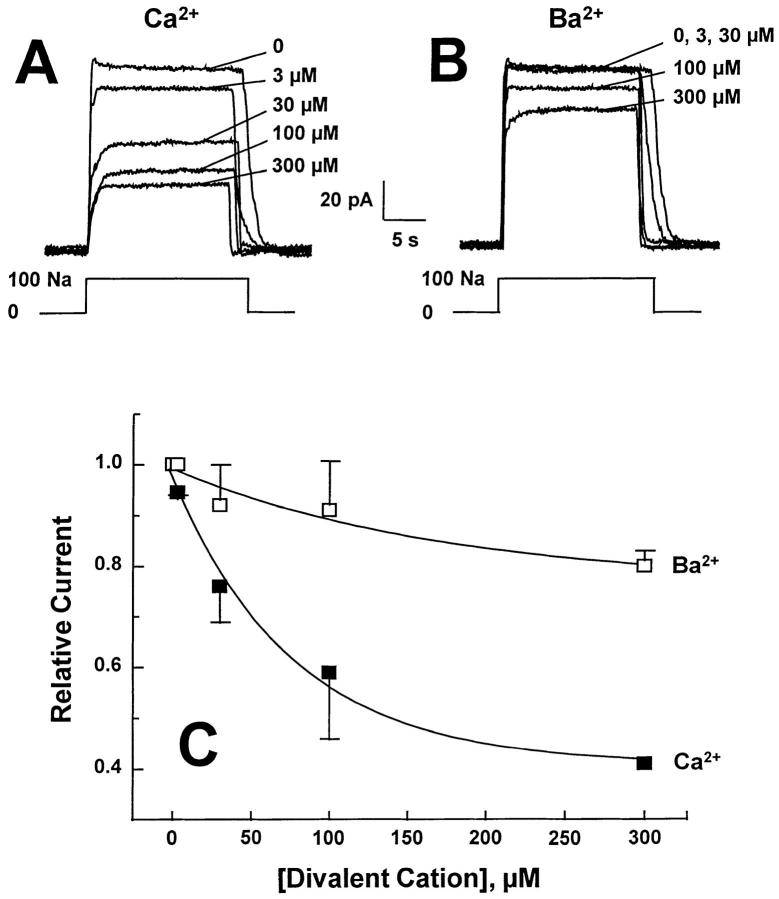
Finally, we compared the ability of Ca2+ i and Ba2+ i to compete for Na+ i at the intracellular transport site. To accomplish this, outward Na+-Ca2+ exchange currents were examined in excised patches after treatment with 1–2 mg/ml α-chymotrypsin for 1–2 min. After this treatment, both I1 inactivation and divalent regulatory effects (I2 inactivation) were eliminated. Thus, competition between Na+ i and divalent cations could be observed by examining outward current with different concentrations of divalents present. Divalent vs. Na+ i competition appears as a reduction of outward current due primarily to: (a) increasing electroneutral Ca2+-Ca2+ or Ca2+-Ba2+ exchange, (b) simple competition between Na+ and divalent cation occupancy of the transport site, and/or (c) the progressive reduction in driving force for the exchange reaction. Typical and pooled results (mean ± SD, n = 4) are shown in Fig. 8. Outward currents were activated by 100 mM Na+, and pipette Ca2+ was constant at 8 mM. For Ca2+ competition, substantial inhibition of outward exchange current is evident at concentrations greater than 3 μM Ca2+. In contrast, the inhibitory effects of Ba2+ are greatly reduced and only become evident between 100 and 300 μM Ba2+.
Figure 8.
Typical deregulated outward Na+-Ca2+ exchange currents from a single patch. The pipette contained 8 mM Ca2+ and currents were activated by the application of 100 mM Na+. Both I1 and I2 regulation are absent after deregulation and the inhibitory effects of different concentrations of Ca2+ and Ba2+ are evident. Pooled results from four patches (mean ± SD) are shown in B. Currents were normalized to the value of current obtained in the absence of regulatory divalent cations.
discussion
We examined the ability of Ba2+ to substitute for Ca2+ on several aspects of Na+-Ca2+ exchange function. We show that Na+-Ba2+ exchange is substantially reduced in the forward direction (i.e., Na+ o-Ba2+ i exchange), appears to be reduced in the reverse direction (Na+ i-Ba2+ o exchange) and is considerably less effective as an activator of the exchanger at the high affinity regulatory Ca2+ binding site. These alterations in Na+-Ca2+ exchange function are likely to contribute to the failure of cardiac relaxation during superfusion with Ba2+-containing media.
For inward current measurements, substantial Na+-Ca2+ exchange currents were observed at Ca2+ concentrations of 1 μM and above. In contrast, inward currents due to Na+-Ba2+ exchange were barely detectable even at 100 and 300 μM Ba2+ (Fig. 3). As both Na+-Ca2+ and Na+-Ba2+ currents were measured in the same patches, our results indicate that Na+-Ba2+ exchange is orders of magnitude less effective than Na+-Ca2+ exchange. Our data indicate that both the affinity for transport and the efficiency of transport are greatly reduced for Na+-Ba2+ exchange. For example, less inward current was observed at 300 μM Ba2+ than was produced by 3 μM Ca2+. Comparatively, with equal concentrations of Ca2+ and Ba2+ (e.g., 100 μM), inward Na+-Ca2+ exchange current exceeded Na+-Ba2+ exchange by nearly 300-fold.
A K D for Ba2+-activation of inward Na+-Ba2+ exchange was not determined as higher concentrations of Ba2+ (e.g., 1 mM) invariably led to the appearance of a small outward current. Although the induction of this unidentified outward current may partially mask inward currents at lower Ba2+ concentrations (e.g., 100– 300 μM), it seems unlikely that we have grossly under-estimated Na+-Ba2+ exchange. One possibility is that the outward current represents an endogenous Ca2+-activated Cl− conductance in oocyte membranes. Despite using Cl−-free pipette and perfusing solution, residual Cl− from the sealing solution invariably contaminates the pipette. However, if present, inward Na+-Ca2+ exchange currents would also be underestimated, presumably by a similar or greater amount. In addition, we observed this pattern of large Na+-Ca2+ vs. small Na+-Ba2+ exchange currents during several long recordings (>10 min) in single patches. Over this time course, nearly complete run-down of the Ca2+-activated Cl− conductance occurs due to diffusion of Cl− from the pipette tip and genuine current rundown.
Both forward and reverse transport modes of Na+-Ca2+ exchange are regulated by cytoplasmic Ca2+ (Matsuoka et al., 1995). Therefore, the possibility exists that reduced inward Na+-Ba2+ exchange is a consequence of reduced exchanger activation by Ba2+. However, the large differences we observed between inward Na+-Ca2+ and Na+-Ba2+ exchange currents cannot be attributed to the failure of Ba2+ to activate the exchanger. As shown in Figs. 5 and 6, 300 μM Ba is sufficient to activate ∼60% of the current obtainable with Ca2+ activation. Therefore, even though Ba2+ activation is less effective than Ca2+, substantial activation of inward Na+-Ba2+ exchange currents would be expected at the concentrations examined. Thus, alterations of Vmax and/or the apparent K D for transport appear to be causal for reduced inward Na+-Ba2+ exchange. The results obtained from patches after deregulation by α-chymotrypsin (Fig. 8) showed relatively weak competition between Ba2+ and Na+, supporting the idea of lower affinity at the intracellular transport site.
It is of interest to compare our results with those obtained from other experimental systems. For example, in cardiac sarcolemmal vesicles, Vmax is reduced by 21-fold and the K m is 2.4-fold larger when Ba2+ is substituted for Ca2+ (Tibbits and Philipson, 1983). As vesicular uptake is now considered to be mediated almost exclusively by inside-out oriented vesicles (Li et al., 1991), this result is analogous to inward current measurements from giant excised patches. Our results show a similar or greater reduction in transport capacity and a much larger shift in affinity for Na+-Ba2+ exchange. As an example, a 100 pA inward current for Na+-Ca2+ exchange would result in a comparatively small Na+-Ba2+ exchange current (i.e., 5 pA). However, at Ba2+ concentrations 30 μM and below, inward currents were never observed. This may be due to our inability to reliably measure currents less than a few picoamps. In CHO cells expressing the bovine cardiac Na+-Ca2+ exchanger, extracellular Na+-dependent 133Ba2+ efflux could be measured, consistent with forward Na+-Ba2+ exchange. However, a reduction in Ba2+ concentration due to forward Na+-Ba2+ exchange was not observed in these same cells based on fura-2 measurements (Condrescu et al., 1997). The reasons for these discrepancies remain unknown but may reflect loss of resolution by the various techniques.
Outward Na+-Ba2+ exchange currents appear to be considerably smaller than those observed using Ca2+ as the transported cation. However, since we cannot measure outward Na+-Ca2+ and Na+-Ba2+ exchange currents in the same patch, this comparison is strictly qualitative. Notwithstanding this limitation, comparison with other published data shows that Ba2+ substitution for Ca2+ can eliminate or greatly diminish currents associated with the exchanger based on whole cell measurements in guinea pig (Kimura et al., 1987) and rabbit ventricular cells (Shimoni and Giles, 1987). In contrast, Na+-Ba2+ exchange activity could be readily measured in CHO cells expressing the cardiac exchanger using either 133Ba2+ or fura-2 measurements under conditions analogous to our outward exchange measurements (Chernaya et al., 1996; Condrescu et al., 1997). Exchange activity was also regulated by cytosolic Ca2+ in this preparation (Chernaya et al., 1996; Condrescu et al., 1997).
One surprising result from the present study is the marked effects of temperature on the appearance of outward Na+-Ba2+ exchange currents (Fig. 4). At 30°C, the small outward currents are regulated by Ca2+ i (I2) but do not exhibit Na+ i-dependent (I1) inactivation. Substantially greater currents are observed at 37°C, with near normal I1 and I2 regulation. This suggests a high energy barrier for Ba2+ translocation. This condition or a greatly reduced affinity for extracellular Ba2+ would reduce the fraction of exchangers with intracellularly oriented ion binding sites and consequently would alleviate I1 inactivation. Such behavior is analogous to lowering extracellular Ca2+ which reduces I1 inactivation (Hilgemann et al., 1992a ). This strong temperature dependence must also be considered when comparing results from other studies examining Na+-Ba2+ exchange.
Finally, we examined the ability of Ba2+ to substitute for Ca2+ as an activator of the exchanger at the high affinity regulatory Ca2+ binding site. Three major differences are observed for Ba2+. First, the affinity for Ba2+ regulation is reduced nearly 30-fold. Second, the efficiency of Ba2+ activation is considerably less than that observed with Ca2+ over the concentration range examined. At 300 μM regulatory Ba2+, maximal outward Na+-Ca2+ exchange currents are only ∼60% of those observed for regulatory Ca2+. Third, unlike Ca2+, Ba2+ does not appear to strongly influence I1 inactivation. Raising cytoplasmic Ca2+ progressively alleviates I1 inactivation (Hilgemann et al., 1992a ) whereas differences in the profile of Ba2+ regulated currents are unremarkable. This feature may prove useful in future studies of the mechanism(s) of I1 and I2 regulation.
In conclusion, we have characterized the effects of substituting Ba2+ for Ca2+ on inward and outward exchange currents and on regulatory properties of the cloned canine cardiac Na+-Ca2+ exchanger, NCX1. By all accounts, the transport and regulatory consequences of this substitution should severely impair the ability of the exchange mechanism to maintain ionic homeostasis. Other contributory factors include the reduction or absence of SR Ba2+ uptake (Palade, 1987; Chernaya et al., 1996) and prolonged depolarization due to effects on L-type Ca2+ channels (Lee et al., 1985) and K+ channels (Imoto et al., 1987). However, while Ba2+ substitution alters numerous other homeostatic processes, the demonstrated changes in Na+-Ca2+ exchange function alone would appear sufficient to prevent cardiac relaxation.
Acknowledgments
The authors thank Dr. Ken Philipson for providing canine cardiac NCX1 cDNA, Dr. Anton Lukas for providing canine ventricular myocytes, and Dr. John P. Reeves for helpful discussions and communication of his results prior to publication.
This work was supported by grants from the Medical Research Council of Canada, the Heart and Stroke Foundation of Canada, and the Manitoba Health Research Council.
references
- Bers, D.M. 1991. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Press, Dordrecht. 71–92.
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+buffers. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- Bridge JHB, Smolley JR, Spitzer KW. The relationship between charge movements associated with ICa and INa-Cain cardiac myocytes. Science (Wash DC) 1990;248:376–378. doi: 10.1126/science.2158147. [DOI] [PubMed] [Google Scholar]
- Chernaya G, Vazquez M, Reeves JP. Sodium-calcium exchange and store-dependent calcium influx in transfected Chinese hamster ovary cells expressing the bovine cardiac sodium-calcium exchanger. J Biol Chem. 1996;271:5378–5385. doi: 10.1074/jbc.271.10.5378. [DOI] [PubMed] [Google Scholar]
- Condrescu M, Chernaya G, Kalaria V, Reeves JP. Barium influx mediated by the cardiac sodium-calcium exchanger in transfected chinese hamster ovary cells. J Gen Physiol. 1997;109:41–51. doi: 10.1085/jgp.109.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Boyett MR. The role of the Na+-Ca2+exchanger in the rate-dependent increase in contraction in guinea-pig ventricular myocytes. J Physiol (Camb) 1995;482:555–566. doi: 10.1113/jphysiol.1995.sp020539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW. Giant excised cardiac sarcolemmal membrane patches: sodium and sodium-calcium exchange currents. Pflüg Arch. 1989;415:247–249. doi: 10.1007/BF00370601. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW. Regulation and deregulation of cardiac Na+-Ca2+exchange in giant excised sarcolemmal membrane patches. Nature (Lond) 1990;344:242–245. doi: 10.1038/344242a0. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Collins A, Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange: secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol. 1992a;100:933–961. doi: 10.1085/jgp.100.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann DW, Matsuoka S, Nagel GA, Collins A. Steady-state and dynamic properties of cardiac sodium-calcium exchange: sodium-dependent inactivation. J Gen Physiol. 1992b;100:905–932. doi: 10.1085/jgp.100.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryshko LV, Matsuoka S, Nicoll DA, Weiss JN, Schwarz EM, Benzer S, Philipson KD. Anomalous regulation of the Drosophila Na+-Ca2+exchanger. J Gen Physiol. 1996;108:67–74. doi: 10.1085/jgp.108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryshko LV, Stiffel V, Bers DM. Rapid cooling contractures as an index of sarcoplasmic reticulum calcium content in rabbit ventricular myocytes. Am J Physiol. 1989;257:H1369–H1377. doi: 10.1152/ajpheart.1989.257.5.H1369. [DOI] [PubMed] [Google Scholar]
- Imoto Y, Ehara T, Matsuura H. Voltage- and time-dependent block of IK1 underlying Ba2+-induced ventricular automaticity. 1987. Am J Physiol. 1987;252:H325–H333. doi: 10.1152/ajpheart.1987.252.2.H325. [DOI] [PubMed] [Google Scholar]
- Kimura J, Miyamae S, Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol (Lond) 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmoto O, Levi A, Bridge JHB. Relation between reverse sodium-calcium exchange and sarcoplasmic reticulum release in guinea-pig ventricular cells. Circ Res. 1994;74:550–554. doi: 10.1161/01.res.74.3.550. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Hume JR. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science (Wash DC) 1990;248:372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lee CO, Dagastino M. Effect of strophanthidin on intracellular Na ion activity and twitch tension of constantly driven canine Purkinje fibres. Biophys J. 1982;40:185–198. doi: 10.1016/S0006-3495(82)84474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Marban E, Tsien RW. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol (Lond) 1985;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AJ, Spitzer KW, Kohmoto O, Bridge JHB. Depolarization-induced Ca entry via Na-Ca exchange triggers SR release in guinea pig cardiac myocytes. Am J Physiol. 1994;266:H1422–H1433. doi: 10.1152/ajpheart.1994.266.4.H1422. [DOI] [PubMed] [Google Scholar]
- Levitsky DO, Nicoll DA, Philipson KD. Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+exchanger. J Biol Chem. 1994;269:22847–22852. [PubMed] [Google Scholar]
- Li Z, Nicoll DA, Collins A, Hilgemann DW, Filoteo AG, Penniston JT, Weiss JN, Tomich JM, Philipson KD. Identification of a peptide inhibitor of the cardiac Na+-Ca2+exchanger. J Biol Chem. 1991;266:1014–1020. [PubMed] [Google Scholar]
- Lukas A, Antzelevitch C. Differences in the electrophysiological response of canine ventricular epicardium and endocardium to ischemia. Role of the transient outward current. Circulation. 1993;88:2903–2915. doi: 10.1161/01.cir.88.6.2903. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD. Regulation of the cardiac Na+-Ca2+ exchanger by Ca2+: mutational analysis of the Ca2+-binding domain. J Gen Physiol. 1995;105:403–420. doi: 10.1085/jgp.105.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+release from isolated sarcoplasmic reticulum. J Biol Chem. 1987;262:6135–6141. [PubMed] [Google Scholar]
- Philipson KD, Nicoll DA. Molecular and kinetic aspects of sodium-calcium exchange. Int Rev Cytol. 1993;137C:199–227. [PubMed] [Google Scholar]
- Shimoni Y, Giles W. Separation of Na-Ca exchange and transient inward currents in heart cells. Am J Physiol. 1987;253:H1330–H1333. doi: 10.1152/ajpheart.1987.253.5.H1330. [DOI] [PubMed] [Google Scholar]
- Tibbits GF, Philipson KD. Na+-dependent alkaline earth metal uptake in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1985;817:327–332. doi: 10.1016/0005-2736(85)90035-5. [DOI] [PubMed] [Google Scholar]
- Trosper TL, Philipson KD. Effects of divalent and trivalent cations on Na+-Ca2+exchange in cardiac sarcolemmal vesicles. Biochim Biophys Acta. 1983;731:63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- Vornanen M, Shepherd N, Isenberg G. Tension-voltage relations of single myocytes reflect Ca release triggered by Na/Ca exchange at 35°C but not 23°C. Am J Physiol. 1994;267:C623–C632. doi: 10.1152/ajpcell.1994.267.2.C623. [DOI] [PubMed] [Google Scholar]
- Wasserstrom JA, Vites AM. The role of Na+-Ca2+exchange in activation of excitation-contraction coupling in rat ventricular myocytes. J Physiol (Lond) 1996;493:529–542. doi: 10.1113/jphysiol.1996.sp021401. [DOI] [PMC free article] [PubMed] [Google Scholar]