Abstract
Previous studies have shown that genistein increased cystic fibrosis transmembrane conductance regulator (CFTR) channel activity in the presence of saturating concentrations of forskolin and calyculin A in intact cells. Possible molecular mechanisms for genistein's action include inhibition of tyrosine kinases, inhibition of serine/threonine protein phosphatases, or direct binding of genistein to CFTR. Since genistein inhibits several enzymes that hydrolyze ATP, and ATP hydrolysis is an intrinsic property of CFTR, we examined the effect of genistein on CFTR gating in excised inside-out patches from Hi-5 insect cells and NIH3T3 cells expressing recombinant CFTR. Genistein (50 μM) did not open phosphorylated CFTR channels by itself, but increased the ATP- induced CFTR channel current by approximately twofold. A similar magnitude of enhancement was observed when genistein was applied with PKI, a specific inhibitor of protein kinase A, or vanadate, a tyrosine phosphatase inhibitor, suggesting that inhibition of protein phosphatases or tyrosine kinases does not account for genistein's effects. The enhancement of channel current increased with increasing concentrations of genistein and reached a maximum at 35 μM genistein. At higher concentrations of genistein concentration, CFTR channel current decreased, resulting in a bell-shaped dose–response relationship. In the absence of genistein, both open- and closed-time histograms could be fitted with a single exponential function, yielding a mean open time (τO) of 0.302 ± 0.002 s, and a mean closed time (τC) of 0.406 ± 0.003 s. In the presence of 50 μM genistein, the open time histogram could be fitted with a double exponential function with τO1 = 0.429 ± 0.003 s and τO2 = 2.033 ± 0.173 s. Thus, genistein induced a prolonged open state, an effect that mimics that of nonhydrolyzable ATP analogs. Closed time analysis showed that 50 μM genistein caused a prolonged closed state with a time constant of 2.410 ± 0.035 s. We thus conclude that (a) the effects of genistein are likely caused by a direct binding of the drug to the CFTR protein, and (b) at least two binding sites are required to explain the effects of genistein: a high affinity site that decreases the closing rate and a low affinity site that reduces the opening rate.
Keywords: phosphorylation, cystic fibrosis, ATP hydrolysis, dephosphorylation
introduction
Cystic fibrosis is caused by mutations in the single gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR).1 The predicted structure of CFTR contains five parts: two repeats of six membrane-spanning segments, two nucleotide binding folds (NBF1 and NBF2), and a regulatory (R) domain with several consensus serine residues for cAMP-dependent protein kinase (PKA) phosphorylation (Riordan et al., 1989). Its predicted topology places CFTR in the ATP binding cassette transporter superfamily (Riordan et al., 1989; Hyde et al., 1990; Ames et al., 1992). It is believed that members of this family harvest free energy from ATP hydrolysis to pump substrates across the cell membrane, but CFTR is an ohmic chloride channel with a single channel conductance of 8 to ∼13 pS.
The major mechanism for regulation of CFTR activity involves the classical cAMP-PKA pathway. PKA phosphorylation of the R domain is essential for CFTR function (Anderson et al., 1991; Tabcharani et al., 1991; Berger et al., 1991; Rich et al., 1991; Cheng et al., 1991). However, phosphorylation by PKA is not adequate to open the CFTR channel. After phosphorylation, ATP binding and hydrolysis at NBF1 and NBF2 are coupled to the opening and closing of the channel. Previous work on phosphorylation regulation and ATP gating of CFTR chloride channels has established a preliminary model in which ATP binding and hydrolysis at NBF1 controls channel opening while ATP binding and hydrolysis at NBF2 controls channel closing (review by Gadsby et al., 1995). This model proposes that ATP hydrolysis provides the free energy for protein conformational changes in gating transitions.
Genistein, an isoflavonoid found mainly in legumes, has been shown to inhibit tyrosine kinase (Akiyama et al., 1987), topoisomerase (Markovits et al., 1989), and histidine kinase (Huang et al., 1992) activity. The effect of genistein on wild-type CFTR channels was first reported by Illek et al. (1995). In their studies, genistein was shown to activate CFTR Cl− channels in NIH3T3 cells and its effect could be abolished by vanadate (VO4), a tyrosine phosphatase inhibitor. They therefore proposed that, in addition to the cAMP-dependent pathway, CFTR is regulated by a cAMP-independent mechanism that might involve tyrosine phosphorylation. However, more recent reports (Illek et al., 1996; Reenstra et al., 1996; Yang et al., 1997) suggest that genistein may act via inhibition of protein phosphatases. Evidence supporting this latter theory includes the fact that in situ CFTR phosphorylation is increased after treatment with genistein (Reenstra et al., 1996). The importance of genistein or similar compounds is underscored by the demonstration that genistein potentiates cAMP-dependent channel activity from the most common disease-associated mutant CFTR; i.e., deletion of phenylalanine 508 (ΔF508). The open probability (P o) of this mutant channel in cell-attached patches is lower than that of wild-type CFTR in the presence of cAMP stimulation (Dalemans et al., 1991; Haws et al., 1996; Hwang et al., 1997). However, our previous work has demonstrated that the P o in the presence of genistein is almost identical to that of wild-type CFTR (Hwang et al., 1997). Although this latest report also provides preliminary evidence for a direct binding of genistein to CFTR molecules (see Weinreich et al., 1997), kinetic analysis at the single channel level is still lacking. Since genistein has been shown to act on ATP binding sites (Akiyama et al., 1987) and ATP hydrolysis is coupled to CFTR gating (Gadsby et al., 1995), more detailed studies could provide mechanistic information on how genistein modulates CFTR function.
In this study, genistein's effects on CFTR gating were examined in Hi-5 insect cells and NIH3T3 cells expressing wild-type CFTR. In cell-attached mode, low micromolar concentrations of genistein increased CFTR channel currents induced by saturating concentrations of forskolin and calyculin A, a membrane permeant phosphatase inhibitor (Suganuma et al., 1991). After activation of CFTR in cell-attached patches with forskolin and calyculin A, genistein did not open CFTR channels by itself in excised inside-out patches, but increased ATP-elicited CFTR channel currents. Neither PKI, a specific PKA inhibitor, nor VO4, a tyrosine phosphatase inhibitor, affected the ability of genistein to enhance CFTR channel current. Notably, the ability of genistein to enhance CFTR channel activity appears to depend on the phosphorylation level of CFTR. Although genistein increases the activity of both partially phosphorylated and fully phosphorylated CFTR, the level of enhancement is greater on partially phosphorylated channels. At low micromolar concentrations, genistein increased the P o of ATP-stimulated CFTR. However, an inhibitory effect was observed at genistein concentrations higher than 35 μM. Kinetic analysis indicated that the enhancement of CFTR activity resulted from prolongation of the open time, whereas the inhibitory effect was through prolongation of the closed time. The two opposite effects of genistein resulted in a bell-shaped dose–response curve. These results may be explained by a model in which there are two binding sites for genistein: a high affinity site responsible for the enhancement effect, and a low affinity site for the inhibitory effect.
materials and methods
Cell Culture and Electrophysiology
Wild-type CFTR Cl− channels were expressed in Hi-5 insect cells (Invitrogen Corp., San Diego, CA) by infecting the cells with baculovirus containing recombinant CFTR cDNA (Yang et al., 1997), while NIH3T3 cells were stably transfected with wild-type CFTR cDNA (Berger et al., 1991). Insect cells were passaged and grown onto small cover glass chips in 35-mm tissue culture dishes with TNM-FH insect medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 50 mg/liter gentamicin, and 4 mM sodium bicarbonate at 27°C. 2–3 d before experiments, an aliquot of the baculovirus (∼5 μl) was added to the Hi-5 cells. CFTR-containing NIH3T3 cells were grown at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 2 mM glutamine. Immediately before recording, cover glass chips containing either cell type were transferred to a continuously perfused chamber located on the stage of an inverted microscope (Olympus Corp., Tokyo, Japan).
For recording of CFTR channel current, glass capillary pipette electrodes were made using a two-stage, vertical puller (Narishige, Tokyo, Japan). The pipette tips were fire-polished with a homemade microforge to ∼1 μm external diameter. Pipette resistance was 5–7 MΩ for Hi-5 cells and 3–4 MΩ for NIH3T3 cells in the bath solution. CFTR channel currents were recorded at room temperature with an EPC-9 patch-clamp amplifier (HEKA, Lambrecht, Germany), filtered at 100 Hz with a built-in three-pole Bessel filter (Frequency Devices Inc., Haverhill, MA), and stored on videotapes. Data were subsequently playbacked at a refiltered frequency of 25 Hz with an eight-pole Bessel filter and captured onto a hard disk at a sampling rate of 50 Hz. For spectral analysis, data were filtered at 50 Hz and digitized at 100 Hz. Pipette potential was held at 50 mV in reference to the bath. Downward deflections represent channel openings.
The pipette solution contained (mM): 140 N-methyl-d-glucamine chloride (NMDG-Cl), 2 MgCl2, 5 CaCl2, and 10 HEPES (pH 7.4 with NMDG). The superfusion solution for Hi-5 insect cells contained (mM): 150 NaCl, 2 MgCl2, 1 EGTA, 5 glucose, and 5 HEPES (pH 6.5 with 1 N NaOH). NIH3T3 cells were perfused with (mM): 150 NaCl, 2 MgCl2, 1 EGTA, 5 glucose, and 5 HEPES (pH 7.4 with 1 N NaOH). In excised inside-out patch experiments, the superfusion solution contained (mM): 150 NMDG-Cl, 10 EGTA, 10 HEPES, 8 Tris, and 2 MgCl2 (pH 7.4 with NMDG). After excision, patch pipettes were moved to a small homemade chamber where a complete solution change could be accomplished within 1 s.
Forskolin was purchased from Calbiochem Corp. (San Diego, CA). Calyculin A and genistein were purchased from LC Laboratories (Woburn, MA). Sodium vanadate was purchased from Aldrich Chemical Co. (Milwaukee, WI). MgATP, all salts and buffers were purchased from Sigma Chemical Co. Genistein, forskolin, and calyculin A were dissolved in dimethylsulfoxide at a stock concentration of 100 mM, 20 mM, and 100 μM, respectively. PKI, MgATP, and orthovanadate were dissolved in distilled water as stock solutions. Orthovanadate (VO4) was boiled for 15–20 min before dilution into superfusion solution immediately before use.
Kinetic Analysis
In most of our experiments in the inside-out configuration, channels were first activated with forskolin plus calyculin A in the cell-attached mode. After excision, variable rundown, presumably due to dephosphorylation by membrane-associated protein phosphatases, was observed. For kinetic analysis, recordings with minimal rundown were selected. In experiments for estimation of genistein's effects, 0.5 mM ATP was applied before and after addition of genistein. This bracketing helps in detection of any time-dependent changes in CFTR channel current. Potential effects of rundown were then corrected accordingly. In most cases, the period over which the measurement was made was short; therefore, rundown was negligible.
Macroscopic kinetic analysis.
The mean current amplitude was calculated with the Igor software (Wavemetrics, Lake Oswego, OR). Curve fitting of the dwell time histogram was performed with the Igor software. The power spectrum density was constructed and analyzed to compare the current fluctuations of CFTR Cl− channels activated by ATP and ATP plus genistein. The data were fast Fourier transformed to generate noise spectrum, and then further analysis was performed by the curve fitting using the Lorentzian function: S = S 0/[1 + (f/f c)2], where f c and S 0 are the corner frequency and the zero frequency asymptotes, respectively.
All-point amplitude histograms were generated with the Igor software to determine the single channel amplitude. A Gaussian function was used to fit the histogram data. The single channel amplitude was then obtained by measuring the difference between the two peaks of the histogram representing the amplitude of channel openings and closings, respectively.
Single-channel kinetic analysis.
Dwell time analysis was performed using Igor software. After measuring the open time for each single channel open event, the data were sorted on a basis of duration from shortest to longest and plotted against the upper cumulative probability that a particular opening event will last at least time (t) (see Figs. 5, 8, and 11). Therefore, the probability of an event lasting for at least 60 ms, the shortest defined t for an open state, is 1. This method is similar to that used by Baukrowitz et al. (1994). All open duration data could be fitted with either a single exponential or double exponential function, yielding either one or two time constants, respectively. The same method was applied to analyze the single channel closed events for the closed time constant(s).
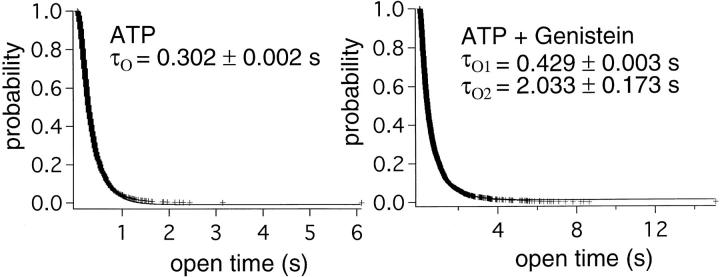
Figure 5.
Effects of genistein on the open time distribution. A distribution of the open times in the presence of 0.5 mM ATP alone were distributed in a single exponential manner, yielding a mean open time of 0.302 s. In the presence of 0.5 mM ATP plus 50 μM genistein, two open time constants of 0.429 and 2.033 s could be derived by fitting the distribution with a double exponential function.
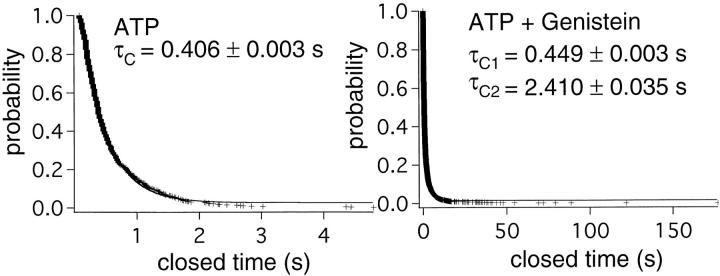
Figure 8.
Effects of genistein on the closed-time distribution. A distribution of the closed times in the presence of 0.5 mM ATP alone were distributed in a single exponential manner yielding a closed time constant of 0.406 s. In the presence of 0.5 mM ATP plus 50 μM genistein, two closed-time constants of 0.449 and 2.410 s could be derived by fitting the distribution with a double exponential function.
For multiple-channel recordings, it was more difficult to obtain open and closed time constants. With a multiple-channel current, it is impossible to pair an opening with a closing of a single channel, thus making an estimation of mean open and closed times more complex. An estimation of the mean channel open and closed times was obtained by examining the average gating behavior of the channels. The mean open time for individual channels in multiple-channel patches can be estimated using the following formula: tn = Σj · tj /n, where tj is the time that j channels open at the same time and n is the total number of transitions from open to close. Therefore, the multiple-channel open event is transferred to n single-channel open events with open time tn for each event. This method, originally described by Fenwick et al. (1982) has been used in similar types of analysis of muscarinic K+ channels (Hosoya et al., 1996). Then the open time constant can be obtained using the same method for the single channel recordings. The closed time constant τC can be obtained through the following formula: P o = τO/(τO + τC), where τO is the open time constant and P o is the single channel open probability, obtained through the ratio of total amount of open time and total time divided by the total number of functional channels.
results
Enhancement of CFTR Activity by Genistein
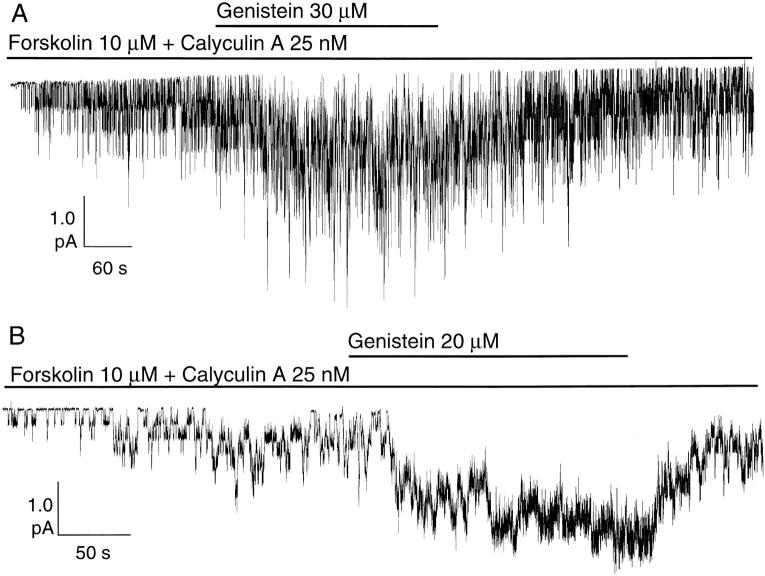
Our previous studies (Yang et al., 1997) have shown that, in Hi-5 insect cells transiently expressing wild-type CFTR, genistein further increased CFTR channel currents activated with forskolin and calyculin A, a membrane-permeant inhibitor of phosphatases 1 and 2A. Fig. 1 A shows a representative experiment from a cell-attached patch in Hi-5 cells. Macroscopic CFTR currents were elicited by bath application of saturating concentrations of forskolin (10 μM) and calyculin A (25 nM). Application of 30 μM genistein in the presence of forskolin and calyculin A significantly increased CFTR channel activity by 3.20 ± 1.14-fold (n = 7). A similar magnitude (3.16 ± 0.57-fold, n = 6) of enhancement of forskolin- and calyculin A-induced CFTR currents by genistein was observed in NIH3T3 cells (Fig. 1 B). Upon removal of genistein, this enhancement of cAMP-dependent CFTR channel activity was readily reversible. Thus, genistein increases CFTR activity even with maximal cAMP stimulation and inhibition of phosphatase 1 and 2A. These results and the fact that genistein does not activate CFTR in the absence of cAMP stimulation (Yang et al., 1997) suggest that genistein only potentiates the activity of CFTR channels that have been phosphorylated by PKA.
Figure 1.
Potentiation of cAMP-dependent CFTR channel activity by genistein in cell-attached patches. (A) Reversible activation of CFTR by genistein (30 μM) in the presence of saturating concentration of forskolin (10 μM) and calyculin A (25 nM) in Hi-5 insect cells. Steady state current was 0.46 pA before application of genistein and 1.47 pA in the presence of genistein. (B) Potentiation of forskolin- and calyculin A-activated CFTR activity by genistein in NIH3T3 cells. The steady state mean current amplitude was 0.66 pA before application of genistein and 2.25 pA in the presence of genistein.
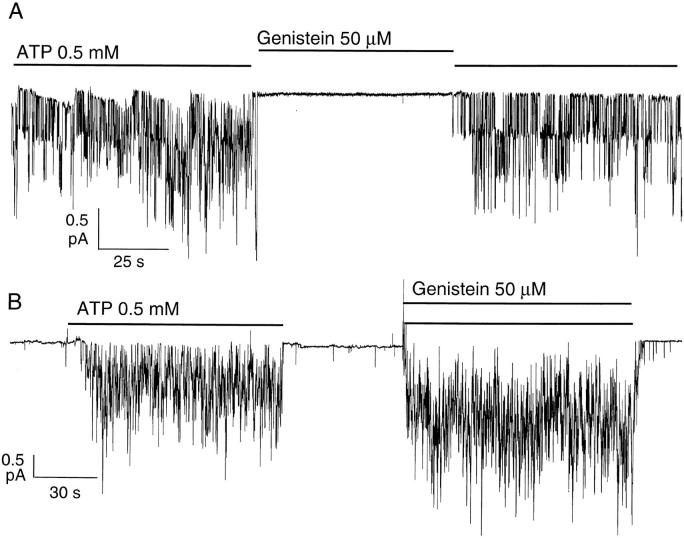
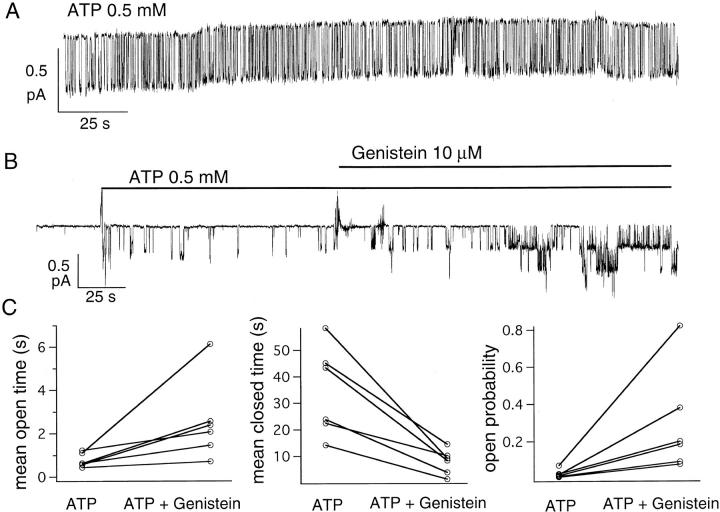
Since it has been demonstrated that genistein can bind to the ATP binding site in tyrosine kinases, we first tested whether genistein can mimic ATP to open phosphorylated CFTR channels. Channels were first activated with 10 μM forskolin plus 25 nM calyculin A in cell-attached patches from Hi-5 insect cells. Gating of CFTR by ATP was then examined in cell-free, excised inside-out patches. Fig. 2 A shows that opening of phosphorylated CFTR depends on the presence of ATP and that 50 μM genistein alone, in the absence of ATP, did not open CFTR channels. However, when 50 μM genistein was applied together with 0.5 mM ATP, the elicited CFTR current was higher than that in the presence of ATP alone (Fig. 2 B).
Figure 2.
Enhancement of ATP-dependent CFTR activity in excised inside-out patches. CFTR channels were activated with 10 μM forskolin plus 25 nM calyculin A in cell-attached patches before excision into an inside-out configuration. (A) Genistein alone fails to open phosphorylated CFTR. Channel activity in excised inside-out patches was only seen in the presence of ATP. (B) Genistein could enhance channel activity in the presence of ATP. The steady state mean current amplitudes were 0.88 pA with ATP alone and 1.62 pA with ATP plus genistein.
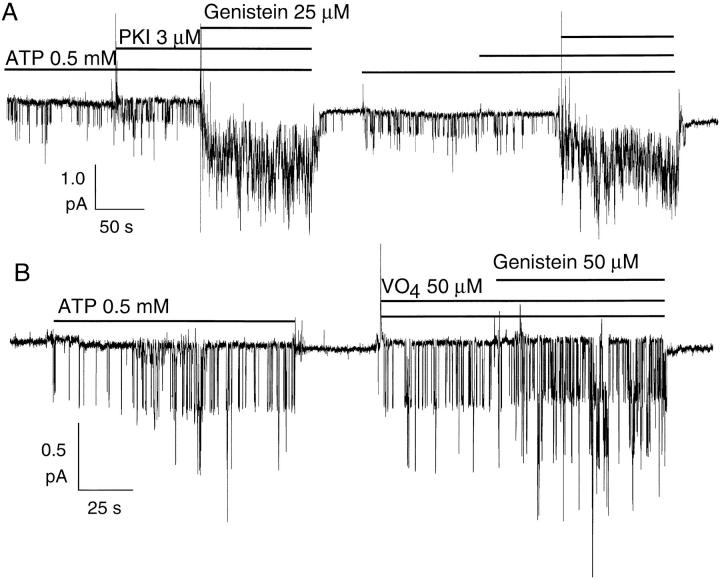
The simplest explanation for these results is that genistein affects CFTR gating through direct binding to the CFTR protein. However, two other possibilities may be considered. First, it is likely that a local network of PKA and phosphatases exists in excised membrane patches. Based on this scenario, besides opening CFTR channels, ATP would also be used by PKA to phosphorylate CFTR. Membrane-bound phosphatases acting in concert with PKA would establish an equilibrium between phosphorylation and dephosphorylation. Thus, when ATP was applied alone, the fraction of phosphorylated channels in the patch would be less than unity. If genistein acts via inhibition of phosphatases, then its application with ATP will shift the phosphorylation/dephosphorylation equilibrium in the direction of a higher phosphorylation, thereby increasing the current. This model predicts that application of the PKA inhibitor, PKI, should decrease ATP-induced CFTR channel current in excised patches and that PKI should abolish genistein's effects. However, 3 μM PKI (a saturating concentration; Cheng et al., 1986) did not decrease channel activity induced by 0.5 mM ATP, and CFTR channel activity was still increased after application of genistein in the presence of ATP and PKI (Fig. 3 A).
Figure 3.
Effects of PKI or vanadate on genistein-induced potentiation of ATP-dependent CFTR channel currents. CFTR channels were first activated in a cell-attached mode as described in Fig. 2. (A) PKI does not diminish potentiation of CFTR channel activity by genistein. (B) Vanadate fails to abolish enhancement by genistein.
We next consider the possibility that both tyrosine kinases and phosphatases are present in the membrane patches since tyrosine kinases and tyrosine phosphatases both exist as intrinsic membrane proteins and as membrane-associated proteins (Lee and Pilch, 1994; Tonks and Neel, 1996). Based on this scenario, tyrosine phosphorylation of CFTR or a regulatory substrate would inhibit CFTR activity, and application of genistein would increase CFTR current via inhibition of tyrosine kinases. Application of genistein with ATP should shift the equilibrium of tyrosine phosphorylation/dephosphorylation such that the steady state level of tyrosine-phosphorylated substrate would be decreased by the actions of endogenous tyrosine phosphatases. This model predicts that application of orthovanadate (VO4), a nonspecific tyrosine phosphatase inhibitor, should decrease ATP-induced CFTR channel current in excised patches and that VO4 should abolish genistein's effects. However, addition of 50 μM VO4 did not decrease the ATP-dependent CFTR channel activity. Moreover, 50 μM genistein still increased the ATP-induced CFTR channel current by 2.2 ± 0.3-fold (n = 5) in the presence of 50 μM VO4. Thus, though other, more complicated, mechanisms might explain our results, the simplest interpretation is that genistein binds to CFTR and thereby affects CFTR gating.
Genistein Prolongs the Channel Open Time
To further understand how genistein affects CFTR gating, the following experiments and analysis were performed. In excised patches containing multiple CFTR channels, the macroscopic current amplitude is determined by three factors: the total number of phosphorylated channels, n, the single channel amplitude, i, and the single channel open probability, P o. Since CFTR channels had been activated (and therefore phosphorylated) in cell-attached patches, and PKI had no effect on ATP-dependent CFTR currents, n is relatively constant during the short period the experiments were performed. Only an increase in i or P o can account for the increase of the macroscopic current by genistein.
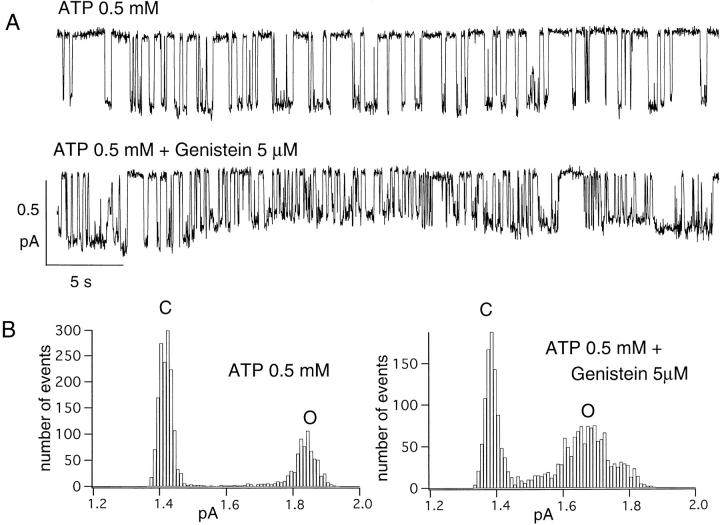
Fig. 4 A shows a current trace in an excised inside-out patch containing a single channel from a Hi-5 insect cell. In the presence of ATP alone (Fig. 4 A, top), both openings and closings are in the range from several hundred milliseconds to seconds. In the presence of genistein, prolonged openings are clearly discernible (Fig. 4 A, bottom). Genistein also changed the single-channel current amplitude in such a way that there were more subconductance states with variable single-channel amplitudes compared with that in the presence of ATP alone. The corresponding amplitude histograms for ATP alone and ATP plus genistein are shown in Fig. 4 B. The single channel amplitude (the distance between the two peaks of the histogram) is smaller in the presence of genistein. The peak representing the open state in the presence of genistein is also wider than the peak representing the open state with ATP alone. This is due to the multiple subconductance states of the channel in the presence of genistein because full conductance openings can still be seen. The single channel amplitude, calculated from amplitude histograms, is 13 ± 3% smaller (n = 4) in the presence of genistein plus ATP than in the presence of ATP alone. Therefore, enhancement of ATP-induced macroscopic CFTR channel current by genistein is due to an increase in P o.
Figure 4.
Effects of genistein on single channel open time and amplitude. All traces are 43 s in length. CFTR channels were first activated in a cell-attached mode as described in Fig. 2. (A) Prolongation of CFTR open time by genistein in Hi-5 insect cells. (top) Single CFTR channel activity in the presence of 0.5 mM ATP, and (bottom) in the presence of 0.5 mM ATP plus 5 μM genistein. (B) Effects of genistein on single channel amplitude. The amplitude histogram of the above record clearly shows a decrease in single channel amplitude in the presence of genistein. Gaussian fits of the amplitude histogram yielded amplitudes of OATP = 0.422 pA and OATP + Genistein = 0.291 pA (baseline subtracted). Note the increase in the width of the OATP + Genistein peak, indicating that more conductance states are present in the presence of genistein.
To collect a large enough number of events for dwell-time analysis, we pooled all the open and closed times from multiple single channel recordings. Fig. 5 shows the resulting open-time histogram (see Fig. 8 A for the closed-time histogram). In the presence of ATP alone, the open-time histogram was fitted with a single exponential function with the open time constant τO = 0.302 ± 0.002 s. In the presence of ATP and genistein, the open-time histogram was fitted with a double exponential function with two open time constants: τO1 = 0.429 ± 0.003 s and τO2 = 2.033 ± 0.173 s. These results suggest that genistein induces a prolonged open state, an effect that mimics that of nonhydrolyzable ATP analogues (Gunderson and Kopito, 1994; Hwang et al., 1994). A similar prolongation of the open time by genistein was observed by French et al. (1997). Support for the conclusion made from the single-channel kinetic analysis was obtained from spectral analysis of macroscopic noise. Time-domain macroscopic currents were transformed to power-frequency spectra using fast Fourier transformation. The resulting spectra were fitted with a Lorentzian function. The average corner frequency obtained for CFTR activity activated by ATP alone (f c = 1.135 ± 0.164 Hz, n = 10) was significantly higher than that by ATP plus genistein (f c = 0.640 ± 0.047 Hz, n = 10).
High Concentrations of Genistein Inhibit ATP-dependent CFTR Gating
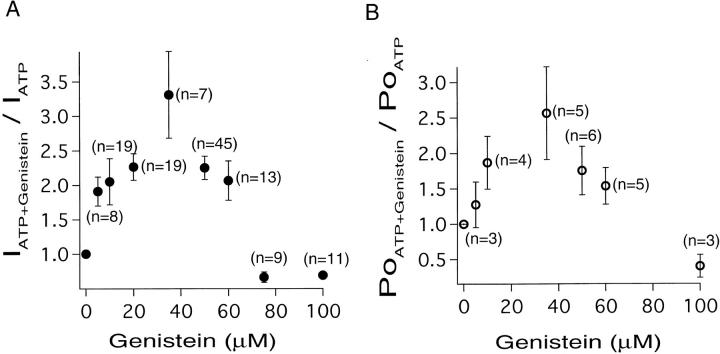
The enhancement of macroscopic CFTR channel current by genistein increased with increasing concentrations of the drug, reaching a maximum of 3.31 ± 0.62-fold (n = 7) at 35 μM genistein. Surprisingly, at concentrations of genistein higher than 35 μM, the enhancement of macroscopic CFTR current was decreased (Fig. 6 A). Notably, at concentrations of genistein higher than 75 μM, channel current was lower than that obtained with ATP alone. The bell-shaped dose–response relationship suggests two opposite effects of genistein in excised patches, enhancement at lower concentrations and inhibitory at higher concentrations.
Figure 6.
Dose–response relationship between genistein concentration and its effects on ATP-dependent CFTR activity. (A) The extent of genistein's augmentation of ATP-induced CFTR current was estimated by the ratio (IATP + Genistein/IATP) of the steady state current amplitude before and after application of various concentrations of genistein in presence of 0.5 mM ATP. (B) Plot of genistein concentration versus the ratio of P o.
Since genistein decreased single channel amplitude (Fig. 4 B), this dose–response relationship obtained from analysis of macroscopic CFTR channel current does not reliably reflect the effects of genistein on CFTR gating. We thus analyze the effects of genistein on single channel P o using recordings from patches that contained less than four channel steps. In those patches where individual steps of channel opening and closing can be discerned clearly, we were able to estimate P o accurately without any interference from genistein effects on the single channel amplitude. The P o of CFTR exhibited a bell-shaped dose– response relationship to genistein that was very similar to that obtained from measurement of macroscopic channel current (Fig. 6 B).
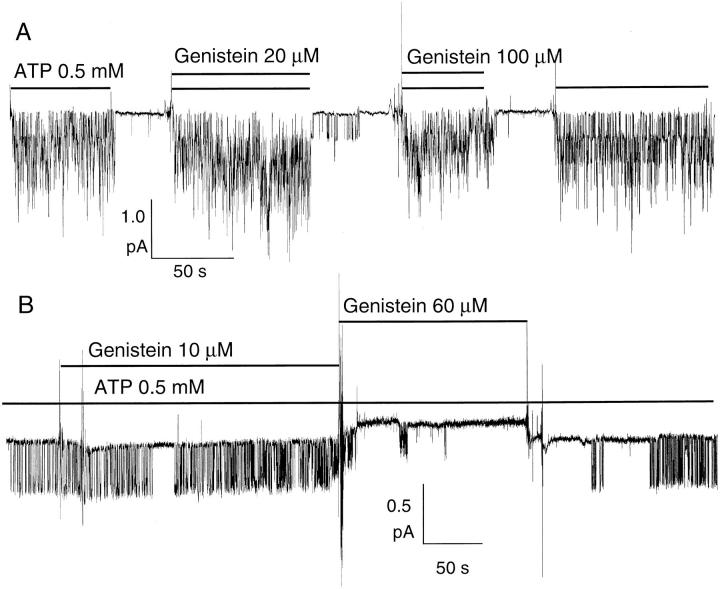
Fig. 7 A shows a representative recording in an excised patch from Hi-5 insect cells. The mean current amplitude in the presence of 20 μM genistein plus 0.5 mM ATP is approximately twofold higher than that with ATP alone. In the same patch, application of 100 μM genistein generated a current that is only ∼93% of the current with ATP. Both enhancement and inhibitory effects were readily reversible upon removal of genistein. In patches containing multiple channels, the net current generated by ATP plus 100 μM genistein resulted from a combination of potentiation and inhibition effects. If genistein binds to two different sites to produce these effects, one might expect that the enhancement effect coexists with the inhibitory effect even at 100 μM genistein. In other words, with multi-channel patches, some channels are potentiated and some are inhibited at any instant of the recording. Therefore, the inhibitory effect is likely underestimated if one simply calculates the percent decrease of the macroscopic current by genistein.
Figure 7.
Inhibition of ATP- dependent CFTR activity by genistein. CFTR channels were first activated in a cell-attached mode as described in Fig. 2. (A) CFTR channel activity in the presence of high concentrations of genistein is smaller than that with ATP alone. 20 μM genistein enhances ATP-induced channel activity (mean current amplitude = 0.96 pA). The channel activity in the presence of ATP alone, both before and after application of genistein (mean current amplitude = 0.55 and 0.59 pA, respectively), is somewhat higher than in the presence of 100 μM genistein and ATP (mean current amplitude = 0.53 pA). (B) Prolongation of channel closed time by 60 μM genistein.
At the single molecule level, the channel may be either potentiated or inhibited. This was substantiated when observing effects of genistein in patches containing a single channel (Fig. 7 B). Prolonged closed events were rarely observed in the presence of 10 μM genistein, but at 60 μM genistein only a few bursts of openings interrupted by long closed events were seen. This result also suggests that the inhibitory effect of genistein was due to a prolonged closed state. Dwell-time analysis of the closed-time distribution revealed a closed time constant τC = 0.406 ± 0.003 s in the presence of ATP alone (Fig. 8). In the presence of genistein, the closed time histogram can be fitted with a double exponential function with τC1 = 0.449 ± 0.003 s and τC2 = 2.410 ± 0.035 s. Thus, genistein can either increase CFTR channel activity through prolongation of the open time (i.e., a decrease of the closing rate) or decrease CFTR channel activity through prolongation of the closed time (i.e., a decrease of the opening rate).
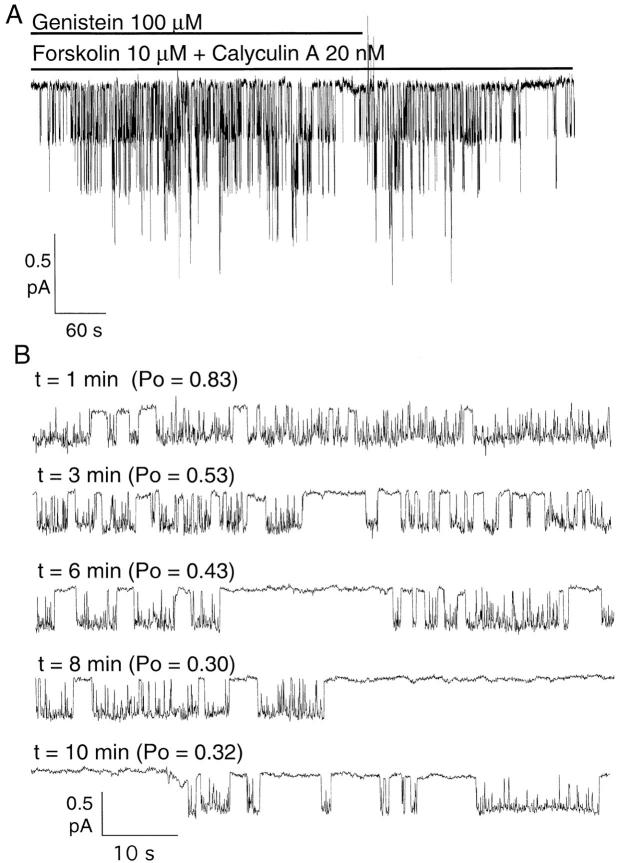
The inhibitory effect of genistein was also observed in cell-attached patches. Fig. 9 A shows a biphasic response to 100 μM genistein in a cell-attached patch from Hi-5 cells. In the presence of saturating concentrations of forskolin (10 μM) and calyculin A (20 nM), application of 100 μM genistein first increased CFTR channel activity. After 3–4 min, the channel activity reached a maximum level, and then declined. The channel activity was transiently recovered upon removal of 100 μM genistein. Fig. 9 B shows both the potentiation and the inhibition of a single channel by 100 μM genistein in a cell-attached patch from a Hi-5 insect cell in the presence of forskolin (10 μM) and calyculin A (20 nM). Note the appearance of relatively long openings (enhancement effect) soon after application of 100 μM genistein. After several minutes of genistein application, prolonged closings (inhibition effect) appear, although the longer openings still remain. The slow time course for inhibition by higher concentrations of genistein could be due to a rate-limiting diffusion of the compound to its site(s) of action. This slow time course may explain why we previously reported only enhancement of CFTR channel activity using 50 μM genistein (Yang et al., 1997).
Figure 9.
Inhibition of forskolin- and calyculin A-stimulated CFTR activity by genistein in cell-attached modes. (A) When first applied to the external bath solution, 100 μM genistein enhanced CFTR activity, but after several minutes of application activity declined, suggesting a rate-limiting diffusion. This decline in CFTR activity was reversible after the start of washout of genistein. (B) Time course of genistein's inhibitory effect. Each 1-min trace is from a continuous recording from a Hi-5 cell prestimulated with 10 μM forskolin and 20 nM calyculin A. Recordings were made at various times after the start of genistein (100 μM) perfusion.
Enhancement by Genistein Depends on the Phosphorylation Level of CFTR
In excised inside-out patches, an average increase of the ATP-induced CFTR channel activity of two- to threefold was observed with 20–50 μM genistein (Fig. 6). However, we noted a relatively large variation in the fold increase. For example, in experiments where the ATP-dependent CFTR activity was relatively low (see materials and methods), genistein produced an apparently greater enhancement (e.g., Fig. 3 A). Interestingly, in these patches the closed time is much longer than 400 ms, a value obtained from patches with apparently higher P o. We hypothesize that the variable CFTR activity in excised patches was due to variable phosphatase activity associated with the membrane patch, resulting in channels from different patches having different levels of phosphorylation and that genistein's effects might depend on the degree of CFTR phosphorylation. In cell-attached patches, different levels of CFTR phosphorylation may be obtained by using different concentrations of forskolin. In excised patches, partial or more complete phosphorylation of CFTR may be obtained by modulating the amount or duration of exogenous PKA application. Since the relationship between forskolin and cAMP concentrations in NIH3T3 cells has been well characterized (Illek et al., 1995), and similar effects of genistein (both qualitatively and quantitatively) were observed in the NIH3T3 expression system (Hwang et al., 1997), experiments were designed using NIH3T3 cells to test our hypothesis.
As a control, Fig. 10 A shows a typical gating behavior in an excised patch from NIH3T3 cells where a single CFTR channel was strongly activated by saturating concentrations of forskolin (10 μM) and calyculin A (50 nM) in the cell-attached configuration. The open and closed time constants (data not shown) from this single-channel recording are consistent with those derived from multiple experiments (Figs. 5 A and 8). These results also suggest that the gating parameters of CFTR in excised inside-out patches from NIH3T3 cells are similar to those from Hi-5 insect cells, although CFTR expressed in these two systems assumes a somewhat different gating pattern in cell-attached patches (Yang et al., 1997).
Figure 10.
The potentiation effect of genistein depends on the phosphorylation level of CFTR. (A) Typical gating behavior of a single, strongly phosphorylated, CFTR channel in an excised inside-out patch from NIH3T3 cells stimulated with forskolin plus calyculin A. (B) ATP-dependent gating of partially phosphorylated CFTR and effects of genistein on partially phosphorylated CFTR. In NIH3T3 cells, channels were activated by 50 nM forskolin to achieve partial phosphorylation. After excision, 10 μM genistein enhanced the ATP-dependent CFTR activity by approximately sevenfold. (C) Paired open times, closed times, and open probabilities from six different experiments in the presence of 0.5 mM ATP and 0.5 mM ATP plus 10 μM genistein. Although there is a large variation from experiment to experiment, a net increase in mean open time, decrease in mean closed time, and increase in P o was seen in all experiments (six of six).
Fig. 10 B shows an experiment where CFTR channels were first activated with 50 nM forskolin (a submaximal concentration; Illek et al., 1995) in a cell-attached configuration. The patch was then excised into an inside-out mode. The P o was ∼0.03 in the presence of 0.5 mM ATP with long closed events. Neither the P o nor the kinetic parameters were changed when [ATP] was increased from 0.5 to 2 mM. However, addition of 10 μM genistein caused an ∼10-fold (9.90 ± 1.65, n = 6) increase of P o. Not only was the mean open time increased with genistein (3.56 ± 0.65 fold, n = 6), but also the mean closed time was only ∼25% (24.5 ± 5.8%, n = 6) of that in the presence of 0.5 mM ATP alone. Similar results were obtained in excised patches where CFTR channels were activated with brief pulses of PKA and ATP (data not shown). Assuming most channels in the patch preactivated with submaximal concentrations of forskolin were phosphorylated incompletely, this result suggests that partially phosphorylated CFTR has a lower opening rate and that genistein increases the opening rate and decreases the closing rate of the partially phosphorylated CFTR. Fig. 10 C summarizes the effects of genistein on presumably partially phosphorylated CFTR in six patches prestimulated with submaximal concentrations of forskolin (50 nM). In contrast to partially phosphorylated channels, when CFTR in excised patches were activated with exogenous PKA (to enhance phosphorylation) plus ATP, only an approximately twofold (2.19 ± 0.33, n = 7) increase of the ATP-dependent CFTR activity by 10 μM genistein was observed.
discussion
Direct Effects of Genistein on CFTR Gating
Coupling of ATP hydrolysis to gating transitions is a hallmark feature of CFTR Cl− channels (Anderson et al., 1991). Several models of CFTR gating have since been proposed (Hwang et al., 1994; Gunderson and Kopito, 1994; Carson et al., 1995). It is generally agreed that hydrolysis of ATP at NBF1 is coupled to (Hwang et al., 1994; Carson et al., 1995) or is required for (Gunderson and Kopito, 1994) the opening of PKA-phosphorylated CFTR, whereas binding and hydrolysis of ATP at NBF2 control the closing. Thus, the rate of ATP hydrolysis at NBF1 modulates how long channels stay in the closed state and the rate of ATP hydrolysis at NBF2 determines how long channels remain in the open state. Our single-channel kinetic analysis shows that for strongly phosphorylated CFTR both the mean open and the mean closed times are in the range of several hundred milliseconds in the presence of 0.5 mM ATP. These values are very close to those reported by Li et al. (1993) for purified CFTR incorporated in black lipid membranes. Measuring ATP hydrolysis from purified CFTR protein, Li et al. (1996) recently suggest that one ATP molecule is hydrolyzed by CFTR per second. Their data are consistent quantitatively with the idea that ATP hydrolysis at NBFs is coupled to the gating transitions that yield time constants of several hundred milliseconds. Short-lived closings within individual openings, observed in several reports (Winter et al., 1994; Gunderson and Kopito, 1994), could represent open channel blockade (e.g., compare Fischer and Machen, 1994, 1996).
Our results show that effects of genistein on highly phosphorylated CFTR are through prolongation of channel open or closed time, depending on the concentration of genistein. Since genistein is a known inhibitor of several enzymes that hydrolyze ATP, the simplest explanation for our observations is that, at low concentrations, genistein decreases the ATP hydrolysis rate at NBF2, whereas, at high concentrations, it reduces the ATP hydrolysis rate at NBF1. The bell-shaped dose–response curve is a result of genistein binding to two sites distinguished by their different affinities and effects on CFTR gating. But where does genistein bind? Considering the fact that the ATP binding motif is the common feature among those enzymes that are inhibited by genistein, we argue that genistein interacts directly with NBFs. However, genistein does not compete with ATP since a similar magnitude of potentiation was observed when [ATP] was increased fourfold (Wang, F., and T.-C. Hwang, unpublished observations). Moreover, genistein had no effect on the ATP dose–response relationship (Weinreich et al., 1997), suggesting genistein- and ATP-binding sites are probably different. Pharmacological results suggest that genistein binds to the ATP-binding site of tyrosine kinases (Akiyama et al., 1987), but genistein and ATP are not likely to share the same binding site in topoisomerases (Markovits et al., 1989). Lack of competition between ATP and genistein in our experiments suggests that genistein binding to CFTR allosterically inhibits ATP hydrolysis. Although we favor a direct action of genistein on CFTR molecules, it is entirely possible that genistein may not bind directly to the NBFs. Alternatively, the molecular targets for genistein could be a protein that is associated with CFTR. Testing genistein on purified CFTR in bilayers could provide direct evidence that genistein interacts with CFTR. Development of high-affinity genistein analogs will hopefully shed light on where genistein binds. Thus, genistein and its analogs could provide a useful chemical probe to study the structure and function of NBFs.
Effects of Genistein on Single Channel Conductance
At least three discrete subconductance states have been shown for the CFTR Cl− channel incorporated in bilayers (Tao et al., 1996). Although the mechanism for these subconductance states is unknown, it has been suggested that they might be caused by different subunit aggregation (Tao et al., 1996), or reflect different protein conformations in the ATP hydrolysis cycle (Gunderson and Kopito, 1995). We also have observed discrete subconductance states of CFTR in cell-attached or excised patches from Hi-5 insect cells transiently expressing the wild-type CFTR (Wang et al., 1996). However, the effect of genistein on the single channel current amplitude is unusual. First, genistein seems to promote the appearance of subconductance states. Even in the presence of a high concentration of genistein, full conductance states can be clearly seen (Fig. 4 A, bottom). This observation is inconsistent with the concept of fast blockers, which uniformly decrease single channel amplitude (Hille, 1992). Multiple subconductance states with variable current amplitudes are observed in the presence of genistein (Fig. 4 A, bottom). Second, single channel amplitudes sometimes show a gradual change instead of a discrete transition from one state to the other (Fig. 4 A, bottom).
Although the molecular mechanism for subconductance states of ion channels remains unknown, the structure of the permeation pore is likely altered in different subconductance states. Effects of genistein on the single channel amplitude and perhaps on the phosphorylation status of CFTR (Illek et al., 1996; Reenstra et al., 1996; Yang et al., 1997) may suggest more than two binding sites for genistein. However, if our hypothesis is correct that NBFs are the sole molecular targets for genistein, our results suggest that a conformational change after genistein binding may alter the permeation pore. This raises the interesting possibility that part of NBFs might contribute to the formation of the channel pore. Recent demonstrations that NBF1 has extracellular accessibility as determined by chemical modification with the biotinylation reagent, NHS-biotin (Gruis and Price, 1997), supports this possibility. Considering the close proximity of the channel gate to the channel pore in Shaker K+ channels (Liu et al., 1997) and nicotinic receptor channels (Labarca et al., 1995), one should not be surprised that NBFs might be physically close to the CFTR pore.
Effects of Genistein on Partially Phosphorylated CFTR
The magnitude of genistein's enhancement depends on the phosphorylation status of CFTR. When genistein was tested on partially phosphorylated CFTR, a stronger potentiation effect was observed. Genistein not only prolongs the open time, but also shortens the closed time of partially phosphorylated CFTR, resulting in an ∼10-fold increase of the ATP-dependent CFTR channel activity. A similar inverse relationship between the phosphorylation status and the degree of potentiation by genistein was recently reported (French et al., 1997). These dramatic potentiation effects are observed even at 10 μM genistein. If the binding site for these potentiation effects is also in NBF2, an apparent conclusion from our data is that binding of genistein at NBF2 can affect the function of NBF1 when CFTR is partially phosphorylated. Interactions between different cytoplasmic domains have been implicated in several studies (e.g., Kiser et al., 1996) and preliminary biochemical evidence has been reported recently (Gruis, D.B., and E.M. Price, manuscript submitted for publication). Thus, our results, together with others, strongly suggest that NBF1, NBF2, and the R domain should be considered as a single functional unit.
While studying effects of genistein on partially phosphorylated CFTR, we also uncovered a gating mechanism for partially phosphorylated CFTR. Our previous studies have provided experimental evidence for regulation of CFTR gating by multiple PKA phosphorylation sites (Hwang et al., 1993). The data suggest that when CFTR channels are partially phosphorylated, the channel open time is shortened. Thus, it is proposed that stronger phosphorylation promotes longer channel opening through modulating the function of NBF2 (Hwang et al., 1994). However, those studies do not rule out other mechanisms of CFTR modulation through differential phosphorylation. By using different experimental strategies, we demonstrate that when CFTR channels are partially phosphorylated, the closed time is prolonged. Since the opening rate is determined mainly by ATP hydrolysis at NBF1, this result suggests the presence of phosphorylation site(s) that control the P o through modulating the function of NBF1. Furthermore, this prolonged closed time of partially phosphorylated CFTR is not affected by increasing [ATP] from 0.5 to 2.0 mM. Since [ATP] <2 mM is already a saturating concentration for CFTR gating (Weinreich et al., 1997), the step(s) that are effected by phosphorylation of those sites is likely beyond the initial binding of ATP to the ATP-binding pocket. Our results on partially phosphorylated CFTR also agree with the kinetic studies on mutant CFTR where some of the PKA consensus serines are converted to alanines (Hanrahan, J.W., personal communication). Although regulation of CFTR activity via multiple phosphorylation sites is very complicated, both electrophysiological and biochemical results suggest that phosphorylation in the R domain likely affects the biochemical reactions at both NBFs.
Effects of Genistein on CFTR Phosphorylation/Dephosphorylation
Our results as well as others (Weinreich et al., 1997; French et al., 1997) demonstrate a direct interaction between genistein and CFTR in excised inside-out membrane patches. But can these effects of genistein on CFTR gating quantitatively account for the potentiation action of genistein on cAMP-dependent CFTR channel activity in cell-attached patches? When the wild-type CFTR is strongly phosphorylated with saturating concentrations of forskolin (e.g., 10 μM) in NIH3T3 cells or Calu-3 cells, genistein increases forskolin- dependent CFTR channel currents by approximately threefold (Hwang et al., 1997). Genistein causes an ∼40-fold increase in the forskolin-dependent CFTR channel activity in Hi-5 insect cells transiently expressing wild-type CFTR (Yang et al., 1997). In Hi-5 insect cells, when phosphatases 1 and 2A are inhibited (Fig. 1 A), an approximately threefold enhancement is seen with genistein. In excised patches, however, genistein increases ATP-dependent CFTR activity by 2–10-fold, depending on the phosphorylation status of the channel. In addition to these quantitative discrepancies in results obtained from electrophysiological experiments, it is also notable that genistein increases the steady state phosphorylation level of CFTR (Reenstra et al., 1996). Thus it is likely that genistein also somehow affects the equilibrium of phosphorylation/dephosphorylation of CFTR. Since genistein does not increase cellular [cAMP] (nor does it stimulate PKA), it is not likely that genistein can promote CFTR phosphorylation through PKA. One possibility, first raised in our previous report (Hwang et al., 1997), is that genistein binding to CFTR may inhibit the dephosphorylation reactions in the R domain. It is possible that genistein affects dephosphorylation of CFTR via binding to site(s) other than NBFs. However, an alternative, perhaps more interesting, possibility is that the effects of genistein on CFTR dephosphorylation are secondary to a conformational change caused by binding of genistein at NBFs. For example, promoting the open state through genistein's effects on NBFs may increase the steady state phosphorylation level of CFTR if the open channel conformation has a slower dephosphorylation rate than that of the closed state.
Physiological Significance
Our results provide evidence for a direct action of genistein on CFTR. One would predict that genistein should enhance cAMP-dependent CFTR activity in all systems expressing CFTR if the CFTR protein is the direct target for genistein. Indeed, genistein has been shown to potentiate cAMP-dependent CFTR channel activity in several heterologous expression systems. These include Xenopus oocytes (Weinreich et al., 1997), Hi-5 insect cells (Yang et al., 1997), HEK293 cells (Hwang et al., 1997), and NIH3T3 cells (Illek et al., 1995; Reenstra et al., 1996; Yang et al., 1997). Genistein also enhances cAMP-dependent CFTR channel activity in cells that express endogenous wild-type CFTR, such as Calu-3 cells (Hwang et al., 1997), T-84 cells (Sears et al., 1995), and shark rectal glands (Lehrich and Forrest, 1995). It is therefore likely that genistein would also be effective in epithelial cells in vivo. Furthermore, since genistein, at low concentrations, prolongs channel open time, we predict that genistein may have therapeutic value in that it may enhance channel activity of disease-associated CFTR mutants that exhibit partial function.
Acknowledgments
We thank Dr. Elmer M. Price for providing baculovirus containing CFTR cDNA.
This work was supported by the National Institutes of Health (HL-53445 and DK-51767), the Cystic Fibrosis Foundation, and the American Heart Association, Missouri Affiliate. F. Wang is a recipient of the Molecular Biology Postdoctoral Fellowship at the University of Missouri-Columbia.
Footnotes
Luis Reuss served as guest editor on this article.
Abbreviations used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator; NMDG, N-methyl-d-glucamine; R domain, regulatory domain.
references
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinase. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Ames GF, Lecar H. ATP-dependent bacterial transporters and cystic fibrosis: analogy between channels and transporters. FASEB J. 1992;6:2660–2666. doi: 10.1096/fasebj.6.9.1377140. [DOI] [PubMed] [Google Scholar]
- Anderson MP, Berger HA, Rich DR, Gregory RJ, Smith AE, Welsh MJ. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Hwang T-C, Nairn AC, Gadsby DC. Coupling of CFTR Cl−channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Berger HA, Anderson MP, Gregory RJ, Thompson S, Howard PW, Mauer RA, Mulligan R, Smith AE, Welsh MJ. Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel. J Clin Invest. 1991;88:1422–1431. doi: 10.1172/JCI115450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Kemp BE, Pearson RB, Smith AL, Misconi L, Van Patten SC, Walsh DA. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986;25:989–992. [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq J-P, Lazdunski M. Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol (Camb) 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Machen TE. CFTR displays voltage dependence and two gating modes during stimulation. J Gen Physiol. 1994;104:541–566. doi: 10.1085/jgp.104.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Machen TE. The tyrosine kinase p60c-srcregulates the fast gate of the cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J. 1996;71:3073–3082. doi: 10.1016/S0006-3495(96)79501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French PJ, Bijman J, Bot AGM, Boomaars WEM, Scholte BJ, De Jonge HR. Genistein activates CFTR-Cl channels via a tyrosine kinase- and protein phosphatase-independent mechanism. Am J Physiol. 1997;273:C747–C753. doi: 10.1152/ajpcell.1997.273.2.C747. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Nagel G, Hwang T-C. The CFTR chloride channel of mammalian heart. Annu Rev Physiol. 1995;57:387–416. doi: 10.1146/annurev.ph.57.030195.002131. [DOI] [PubMed] [Google Scholar]
- Gruis, D.B., and E.M. Price. 1997. The nucleotide binding folds of the cystic fibrosis transmembrane conductance regulator are extracellularly accessible. Biochemistry. 36:7739–7745. [DOI] [PubMed]
- Gunderson KL, Kopito RR. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J Biol Chem. 1994;269:19349–19353. [PubMed] [Google Scholar]
- Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role of ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- Haws CM, Nepomuceno IB, Krouse ME, Wakelee H, Law T, Xia Y, Nguyen H, Wine JJ. ΔF508-CFTR channels: kinetics, activation by forskolin, and potentiation by xanthines. Am J Physiol. 1996;270:C1544–C1555. doi: 10.1152/ajpcell.1996.270.5.C1544. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic channels of excitable membranes. 2nd ed. Sinauer Associates, Inc., Sunderland, MA. 390–393.
- Hosoya Y, Yamada M, Ito H, Kurachi Y. A functional model for G protein activation of the muscarinic K+channel in guinea pig atrial myocytes. J Gen Physiol. 1996;108:485–495. doi: 10.1085/jgp.108.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Nasr M, Kim Y, Mathews HR. Genistein inhibits protein histidine kinase. J Biol Chem. 1992;267:15511–15515. [PubMed] [Google Scholar]
- Hwang T-C, Horie M, Gadsby DC. Functionally distinct phospho-forms underlie incremental activation of PKA-regulated Cl−conductance in mammalian heart. J Gen Physiol. 1993;101:629–650. doi: 10.1085/jgp.101.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T-C, Nagel G, Nairn AC, Gadsby DC. Regulation of gating of CFTR Cl channel by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci USA. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T-C, Wang F, Yang IC-H, Reenstra W. Potentiation of ΔF508 channel function by genistein binding to CFTR. Am J Physiol. 1997;273:C988–C998. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]
- Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Santos G, Widdicombe JH, Machen TE, Reenstra WW. Cyclic AMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Machen TE. Alternate stimulation of apical CFTR by genistein in epithelia. Am J Physiol. 1996;270:C265–C275. doi: 10.1152/ajpcell.1996.270.1.C265. [DOI] [PubMed] [Google Scholar]
- Kiser GL, Chang X-B, Riordan JR. Two-hybrid analysis of CFTR domain interactions. Pediatric Pulmonology. 1996;S13:213. [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Lee J, Pilch PF. The insulin receptor: structure, function, and signaling. Am J Physiol. 1994;266:C319–C334. doi: 10.1152/ajpcell.1994.266.2.C319. [DOI] [PubMed] [Google Scholar]
- Lehrich RW, Forrest JN., Jr Tyrosine phosphorylation is a novel pathway for regulation of chloride secretion in shark rectal gland. Am J Physiol. 1995;269:F594–F600. doi: 10.1152/ajprenal.1995.269.4.F594. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Reyes E, Jensen T, Chang X, Rommens JM, Bear CE. The cystic fibrosis mutation (ΔF508) does not influence the chloride channel activity of CFTR. Nat Genet. 1993;3:311–316. doi: 10.1038/ng0493-311. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman ME, Yellen G. Gates access to the pore of a voltage-dependent K+channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier J-M, Le Pecq J-B, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- Reenstra WW, Yurko-Mauro K, Dam A, Raman S, Shorten S. CFTR chloride channel activation by genistein: the role of serine/threonine protein phosphatases. Am J Physiol. 1996;271:C650–C657. doi: 10.1152/ajpcell.1996.271.2.C650. [DOI] [PubMed] [Google Scholar]
- Rich DP, Gregory RJ, Anderson MP, Manavalan P, Smith AE, Welsh MJ. Effect of deleting the R domain on CFTR-generated chloride channels. Science. 1991;253:205–207. doi: 10.1126/science.1712985. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B-S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J-L, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Sears CL, Firoozmand F, Mellander A, Chambers FG, Ermar IG, Bot AGM, Scholte B, de Jonge HR, Donowitz M. Genistein and tyrphostin 47 stimulate CFTR-mediated Cl−secretion in T84 cell monolayers. Am J Physiol. 1995;269:G874–G882. doi: 10.1152/ajpgi.1995.269.6.G874. [DOI] [PubMed] [Google Scholar]
- Suganuma M, Fujiki H, Furuya-Suguri H, Yoshizawa S, Yasumoto S, Kato Y, Fusetani N, Sugimura T. Calyculin A, an inhibitor of protein phosphatases, is a potent tumor promotor on CD-1 mouse skin. Cancer Res. 1991;50:3521–3525. [PubMed] [Google Scholar]
- Tabcharani JA, Chang X-B, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl−channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991;352:628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tao T, Xie J, Drumm ML, Zhao J, Davis PB, Ma J. Slow conversions among subconductance states of cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J. 1996;70:743–753. doi: 10.1016/S0006-3495(96)79614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks NK, Neel BG. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- Wang F, Yang IC-H, Price EM, Hwang T-C. Stable sub-conductance states of CFTR chloride channels. Biophys J. 1996;70:A367. [Google Scholar]
- Weinreich F, Wood PG, Riordan JR, Nagel G. Direct action of genistein on CFTR. Pflügers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- Winter MC, Sheppard DN, Carson MR, Welsh MJ. Effect of ATP concentration on CFTR Cl−channels: a kinetic analysis of channel regulation. Biophys J. 1994;66:1398–1403. doi: 10.1016/S0006-3495(94)80930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IC-H, Cheng T-H, Wang F, Price EM, Hwang T-C. Modulation of CFTR chloride channels by calyculin A and genistein. Am J Physiol. 1997;272:C142–C155. doi: 10.1152/ajpcell.1997.272.1.C142. [DOI] [PubMed] [Google Scholar]