Abstract
Using delayed-rectifier potassium channels as examples, we have designed two specific blockers by generating specific antipeptide antibodies to epitopes in the external vestibules of two channel proteins, Kv1.2 and Kv3.1. These antibodies reduced whole-cell Kv1.2 or Kv3.1 currents in transfected cells and the effect was blocked by the corresponding peptide antigen, but not by control peptides. A control antibody had little effect on Kv1.2 currents and the Kv1.2 blocker antibody had limited effect on other related potassium currents. Furthermore, the Kv1.2 blocking antibody inhibited dendrotoxin binding to Kv1.2 channel proteins in transfected cells. Moreover, using the Kv1.2 blocker antibody, we determined the presence and relative contribution of endogenous Kv1.2 to the overall endogenous K+ currents in NG108 neuronal cells. This guided design of specific channel blockers will facilitate future physiological studies on ion channel functions.
Keywords: ion channels, antibody, inhibitor, neurotoxin
introduction
Ion channels are ubiquitous integral membrane proteins that serve numerous physiological functions in excitable and nonexcitable cells (Catterall, 1988; Lester, 1991; Jan and Jan, 1992). They transmit electrical signals to generate physiological cell responses. With electrophysiological recording techniques, a variety of ionic currents in many kinds of cells have been observed (Catterall, 1988; Lester, 1991; Jan and Jan, 1992). The importance of these ionic currents has been demonstrated by pharmacological approaches using either naturally existing ion channel toxins or inorganic and organic ion channel blockers (such as local anesthetics). The essential physiological roles of ion channels in normal cellular functions have been further strengthened by the link of many pathological diseases to defects in ion channel genes (Catterall, 1988; Lester, 1991; Jan and Jan, 1992).
Over the past few years, molecular biological studies have revealed a large number of ion channel genes that could be responsible for the observed diverse ionic currents. For example, there are >20 genes that have been cloned coding for voltage-gated potassium channels (Pongs, 1992; Perney and Kaczmarek, 1993; Chandy and Gutman, 1995; Deal et al., 1996). Just within the Kv1 subfamily of the voltage-gated K+ channels, there are at least seven members and most of them (except Kv1.4) generate similar delayed-rectifier K+ currents. Moreover, different potassium channel subunits can coassemble to form heteromultimeric channels (Isacoff et al., 1990; Ruppersberg et al., 1990; Christie et al., 1990; Sheng et al., 1993; Wang et al., 1993). Finally, the native complex of voltage-gated K+ channels is also composed of accessory β subunits and these β subunits can convert the delayed-rectifier currents into rapidly inactivating A-type K+ currents (Rettig et al., 1994).
The challenge now is to identify the underlying entities (ion channel proteins) responsible for the observed ionic currents in native cells and to define their physiological functions. Although genetic manipulation with targeted deletion of ion channel genes would be helpful, the interpretation of results could be complicated by functional redundancy and developmental abnormalities. Some ion channel blockers are available, but they usually affect a group of ion channels and thus lack specificity towards one specific channel protein. These blockers were found empirically rather than by rational design. Here we develop a method to use antibodies as blockers for specific ion channels. Using two specific antibodies against the external vestibules of two potassium channel proteins, Kv1.2 and Kv3.1, we present evidence for the specific blocking of these channels, but not other related potassium channels, by these antibodies. The efficacy of these antibody blockers is similar to neurotoxins. Furthermore, we have used the Kv1.2 blocker to determine the presence and relative contribution of endogenous Kv1.2 to the overall endogenous K+ currents in NG108 neuronal cells. This immunoelectrophysiological approach should yield specific ion channel blockers.
materials and methods
Affinity-purified Polyclonal Antibodies and Immunoblot
Kv1.2-BA, Kv1-NA, and Kv3.1-BA rabbit polyclonal antibodies were made and affinity purified through a contracted manufacturer (Genemed Biotechnologies, Inc., South San Francisco, CA). A cysteine residue was added to the carboxyl end of the peptide FAEADERDSQFPSIP or the amino end of the peptide DPLR-NEYFFDRNRPS or the carboxyl end of the peptide GAQPNDP-SASEHTH for keyhole limpet hemocyanin conjugation. Rabbit antiserum was purified with the peptide-affinity matrix. The specificity of these antibodies was confirmed by competition experiments with different peptides during immunoblotting. Immunoblot of membrane proteins (30 μg per lane) from HEK and transfected HEK cells was done as previously described with some modifications (Langhans-Rajasekaran et al., 1995; Wan et al., 1996). Boiling lysis solution (1% Triton X-100; 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.5% NP-40, 0.1% SDS, 1% Na-deoxycholate) was used to resuspend membrane proteins.
Electrophysiological Recordings with HEK-293 Cells
Maintenance, growth, and stable transfection of HEK-293 cells were performed as described before (Huang et al., 1993; Langhans-Rajasekaran et al., 1995). At each passage, some cells were plated onto 2.5-cm culture dishes with small cover slips for electrophysiological measurements. Electrophysiological recordings were made on the same day. Whole-cell patch-clamp recordings were performed as previously described with some modifications (Huang et al., 1993). Recordings were done at room temperature with pipettes pulled from micro-hematocrit capillary tubes with resistance of 2–4 MΩ. The pipette solution contained 180 mM K-Asp, 5 mM NaCl, 5 mM Na-HEPES, 5 mM EGTA, 0.28 mM CaCl2, 0.8 mM MgCl2, 1.5 mM ATP, 0.1 mM GTP, pH adjusted to 7.2. The bath solution contained 118 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 5 mM Na-HEPES, 23 mM glucose, 54 mM sucrose, pH adjusted to 7.4. Whole-cell currents were elicited by voltage pulses of 300-ms duration from a holding potential of −70 mV. Current was measured in the range from −40 to 60 mV, varying the voltage in 10-mV steps. Series resistance values after seal formation were <3 MΩ and were electronically compensated. The current signals were low pass filtered at 5 kHz and leak subtracted. The data were collected by using Axopatch 200A via pCLAMP6. To investigate the effects of antibodies on membrane potassium currents, cells were patched for 5 min to obtain a stable baseline before antibodies were added to the recording chamber.
Electrophysiological Recordings with Neuronal Cells
Maintenance and growth of neuronal NG108-15 cells are as described (Han et al., 1991; Huang et al., 1993). At each passage, some cells were plated onto 2.5-cm collagen-coated culture dishes with small cover slips for electrophysiological measurements. Electrophysiological recordings were made ∼3–7 d after differentiation. Whole-cell patch-clamp recordings of potassium currents were performed as described (Wilk-Blaszczak et al., 1994). Recordings were done at room temperature using 2–4 MΩ resistance electrode. The pipette solution contained 115 mM KCl, 0.1 mM MgCl2, 40 mM HEPES, 3 mM ATP, 0.1 mM GTP, pH adjusted to 7.3 with KOH. The bath solution contained 125 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, 5 mM glucose, pH adjusted to 7.4 with NaOH. Recording protocol, data collection, and analysis were done as with HEK-293 cells.
Dendrotoxin Binding Assay
Binding assay was performed as previously described for agonist binding to muscarinic receptors (Wan et al., 1996, 1997; Bence et al., 1997). Briefly, HEK293 cells stably expressing Kv1.2 channel proteins were grown in 12-well dishes. After being washed two times with HBSS (Hank's balanced salt solution) plus 10 mM HEPES (pH 7.4), 300 μl binding solution was added to each well. The binding solution contains: HBSS plus 10 mM HEPES (pH 7.4), 1 mg/ml BSA, 1 mg/ml bacitracin, 1 mM PMSF, 10 nM 125I-α-dendrotoxin (290 Ci/mmol) (Amersham Corp., Arlington Heights, IL; or Alomone Labs, Jerusalem, Israel), and various concentrations of Kv1.2-BA antibody. After incubation for 4 h at 4°C, cells were washed two times with HBSS plus 10 mM HEPES (pH 7.4) to remove unbound 125I-dendrotoxin. Cells were then lysed with 1 ml of 0.4 N NaOH and transferred to borosilicate tubes. The bound 125I-dendrotoxin was counted with a gamma counter. Nonspecific binding of 125I-dendrotoxin to cells was determined in the presence of excess (10 μM) unlabeled dendrotoxin. Nonspecific inhibition of antibody on dendrotoxin binding was examined in the presence of 500 nM of preimmune IgG with <10% inhibition.
Data Analysis
Data for multisite inhibition can be expressed in terms of a Hill equation (Segel, 1993): log[I/I o/(1 − I/I o)] = −nlog[antibody] + logK′, where I o is the current amplitude before the addition of the antibody; I is that after the addition of the antibody. [antibody] is the concentration of the antibody. n indicates the number of binding sites of the antibody on the channel. IC50 is related to K′ as IC50, n = K′. A Hill plot for the inhibition by both Kv1.2-BA and Kv3.1-BA antibodies gave the slope of the Hill plot −0.75, indicating only one antibody binding to one channel complex. Thus, the Hill equation can be simplified as: I/I o = 1/ (1 + [antibody]/K′). All curve fittings were performed using this equation.
results
Suppression of Kv1.2 Current by Kv1.2-BA Antibody
To test whether antibodies specific for peptides around the pore regions of individual ion channels can be used as channel blockers, we have first examined the blocking ability of an affinity-purified polyclonal antibody generated against a 15 amino acid peptide of a delayed-rectifier potassium channel Kv1.2 (Fig. 1 A). This sequence of amino acids (FAEADERDSQFPSIP), located between the S5 transmembrane and the pore-forming region, is very likely part of the external vestibule or the outer mouth of the channel protein (Fig. 1 B) (Lu and Miller, 1995; Hidalgo and MacKinnon, 1995; Aiyar et al., 1995). Kv1.2 was stably expressed in a mammalian cell line, HEK-293 cells (Huang et al., 1993). The affinity-purified polyclonal antibody (Kv1.2-BA) detected Kv1.2 protein in plasma membranes of Kv1.2-transfected cells (Fig. 1 C). Whole-cell patch-clamp recording revealed large outward potassium currents, elicited by depolarizing voltages, that were absent in untransfected HEK-293 cells (Fig. 2 A). Addition of 250 nM of the affinity-purified polyclonal antibody (Kv1.2-BA) to the external solution blocked ∼70% of the Kv1.2 currents (Fig. 2). The current amplitude decline, seen only after addition of Kv1.2-BA, occurred within 1 min and reached ∼70% suppression in ∼7–15 min (Fig. 2 B). The Kv1.2 current is relatively stable in the absence of antibodies (Fig. 2 B). The current was decreased by the antibody Kv1.2-BA for all voltages ≥0 mV (Fig. 2 C). Also, the suppression was antibody concentration dependent: with a rise in concentration of Kv1.2-BA, the inhibition of Kv1.2 currents increased (Fig. 2 D). The data for the channel inhibition by the blocking antibody could be well fitted by an equation for 1:1 binding between the antibody and the Kv1.2 channel with an IC50 of 54 nM (see Data Analysis in materials and methods). This IC50 is similar to the K d of most potassium channel neurotoxins. Thus, the antibody Kv1.2-BA can suppress the Kv1.2 current in Kv1.2-transfected cells.
Figure 1.
The S5–S6 linker region of Kv1.2, Kv1.3, and Kv3.1 potassium channels. (A) The amino acid sequence alignment of rKv1.2, hKv1.3, and rKv3.1 shows the S5 and S6 transmembrane domains, the pore-forming region (P region) and indicates the peptides used for generating the blocking antibodies (underlined). Dashes represent same amino acids as rKv1.2. (B) Schematic model showing the proposed arrangement of the external vestibule of Kv1.2 (drawn after Aiyar et al., 1995). The peptide used for generating the blocking antibody is in bold and shaded. Only the flanking S5 and S6 domains and two of the four subunits of the channel are illustrated. (C and D) Characterization of Kv1.2-BA and Kv1-NA antibodies. Membrane preparations from HEK-293 cells, untransfected (lane 1), transfected with Kv1.2 (lanes 2 and 4), or transfected with Kv1.3 (lane 3) were subjected to 10% SDS-PAGE and were Western blotted with Kv1.2-BA antibody (C) or Kv1-NA antibody (D). Lane 4 was probed with Kv1.2-BA that had been preincubated with the peptide used to generate Kv1.2-BA (C), or probed with Kv1-NA that had been preincubated with the peptide used to generate Kv1-NA (D). Estimated molecular masses of Kv1.2 and Kv1.3 were 70–80 kD. Bands representing endogenous Kv1 proteins could be seen upon longer exposure. (E) Characterization of Kv3.1-BA antibody. Membrane preparations from HEK-293 cells, untransfected (lane 1), transfected with Kv3.1 (lanes 2 and 4), or transfected with Kv1.2 (lane 3) were subjected to SDS-PAGE and were Western blotted with Kv3.1-BA antibody. Lane 4 was probed with Kv3.1-BA that had been preincubated with the peptide used to generate Kv3.1-BA. Estimated molecular mass of Kv3.1 was 100 kD. Positions of prestained molecular mass markers in kilodaltons are indicated on the left.
Figure 2.
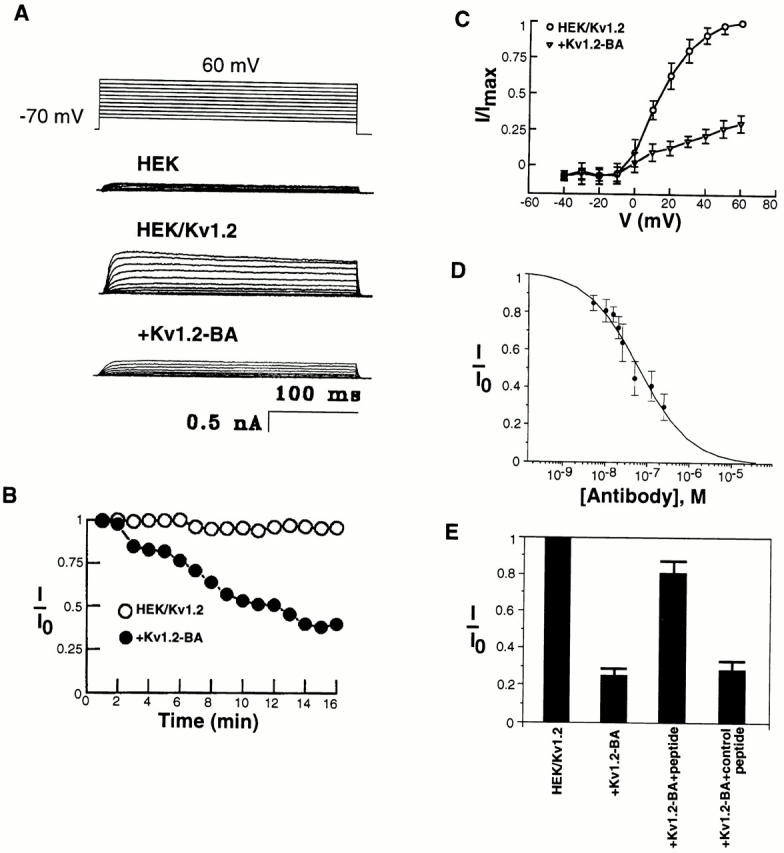
Blockage of Kv1.2 currents by Kv1.2-BA antibody. (A) Kv1.2 currents in Kv1.2 stably transfected HEK-293 cells in the absence of Kv1.2-BA (HEK/Kv1.2) or in the presence of Kv1.2-BA (+Kv1.2-BA). Untransfected HEK-293 cells have very small endogenous currents (HEK). Addition of 250 nM Kv1.2-BA led to an ∼70% suppression of Kv1.2 currents. Whole-cell currents were elicited by voltage pulses of 300-ms duration from a holding potential of −70 mV. Current was measured in the range from −40 to 60 mV, varying the voltage in 10-mV steps. (B) Time course of the blockage of Kv1.2 currents by Kv1.2-BA (+Kv1.2-BA). In the absence of Kv1.2-BA, the Kv1.2 current is relatively stable for over 15 min (HEK/ Kv1.2). Peak currents (I) at +60 mV at each time point are compared with the currents (I o) before the addition of the antibody. (C) Current– voltage relationship of whole-cell currents. All currents (I) are expressed as a fraction of the peak current (I max) at +60 mV in the absence of the antibody. (D) Kv1.2-BA blockage is concentration dependent. Data points were taken at 15 min after the addition of antibody. The data are mean ± SD of five to seven (n) experiments. (E) Whole-cell potassium currents from Kv1.2-transfected HEK-293 cells in the absence (HEK/Kv1.2) or in the presence (+Kv1.2-BA) of the blocking antibody Kv1.2-BA. Kv1.2-BA (250 nM) reduced the whole-cell K+ currents by ∼70%. Preincubation of Kv1.2-BA with excess of the immunogenic peptide (Kv1.2-BA + peptide) reduced the suppression to the residual effect of ∼25%. Preincubation of Kv1.2-BA with excess of a control peptide (the Kv1-NA immunogenic peptide) had no effects on Kv1.2-BA suppression of Kv1.2 currents.
To ensure that the Kv1.2-BA antibody does indeed bind to the external region of Kv1.2 channel protein where the immunogenic peptide was made and that the blocking effect is due to the binding of the Kv1.2-BA antibody to the channel protein, we performed experiments in which the Kv1.2-BA antibody was preincubated with the immunogenic peptide that was used to generate Kv1.2-BA. If blocking is due to binding of the antibody to the peptide sequence in the external vestibule of the channel protein, preincubation with the peptide should prevent the inhibition. As shown in Fig. 2 E, addition of 250 nM Kv1.2-BA after preincubation with the immunogenic peptide only produced ∼25% inhibition. Preincubation with a control peptide did not inhibit the Kv1.2-BA-induced suppression of Kv1.2 currents (Fig. 2 E). Thus, the majority of the inhibition by Kv1.2-BA on Kv1.2 currents is due to specific binding of Kv1.2 to the particular peptide sequence around the pore region of Kv1.2 channels. The residual ∼25% inhibition is likely due to either nonspecific blocking of the channel by high concentrations of proteins in the bath solution or the presence of other antiserum in the polyclonal antibody preparation that fortuitously recognize sequences of the channel protein. Nonetheless, Kv1.2-BA can significantly block Kv1.2 currents in Kv1.2-transfected cells.
Effect on Kv1.2 Current by a Control Antibody Kv1-NA
To further exclude the possibility that the Kv1.2-BA blocking effects were nonspecific and that any antibody added outside the cells somehow interferes with the channel function, we examined the effects on the Kv1.2 currents by another affinity-purified polyclonal antibody (Kv1-NA) (Fig. 3, A and B). Kv1-NA was generated against a peptide (NEYFFDRNRPS) from the NH2 terminus of Kv1.2, which is identical in all members of the Kv1 family (Fig. 1 D). Addition of this control antibody Kv1-NA had no effect at low concentrations (<20 nM) and very small effects at high concentrations (>100 nM) on the Kv1.2 currents (Fig. 3 B). These results indicate that the effect of Kv1.2-BA is not due to a nonspecific antibody–protein interaction.
Figure 3.
Demonstration of the specificity of the blockage by Kv1.2-BA. (A and B) A control antibody, Kv1-NA (250 nM), has limited effect on Kv1.2 currents. (C and D) Kv1.2-BA (250 nM) has limited effect on Kv1.3 currents in Kv1.3 stably transfected HEK-293 cells. (E and F) Kv1.2-BA (250 nM) has no significant effects on Kv3.1 currents in Kv3.1 stably transfected HEK-293 cells. The data are mean ± SD of five to seven experiments.
Effects on Related Kv1.3 or Kv3.1 Currents by Kv1.2-BA Antibody
To additionally assure the specificity, we have tested the Kv1.2-BA antibody on related potassium channels. Kv1.3 belongs to the same subfamily of delayed-rectifier potassium channels as Kv1.2. Kv1.3 has the same sequence around the pore region as Kv1.2, with the exception of five amino acids (Fig. 1 A). When stably expressed in HEK-293 cells, Kv1.3 exhibits C-type slow inactivation (Attali et al., 1992; Panyi et al., 1995) (Fig. 3 C). In contrast to the results obtained with Kv1.2 currents, addition of Kv1.2-BA had limited effect on Kv1.3 currents, only small effect at high concentrations (similar to the residual effect on Kv1.2 currents by Kv1.2-BA preincubated with the immunogenic peptide) (Fig. 3, C and D). Kv3.1 is another delayed-rectifier K+ channel, but belongs to a different subfamily (Yokoyama et al., 1989; Luneau et al., 1991). Although Kv3.1 has high sequence homology with Kv1.2 in transmembrane domains and pore-forming regions, it differs from Kv1.2 in the region where Kv1.2-BA binds (Fig. 1 A). As would be expected, there was only very small change of Kv3.1 currents expressed in HEK-293 cells after addition of high concentrations of Kv1.2-BA (again, similar to the residual effect on Kv1.2 current by Kv1.2-BA preincubated with the immunogenic peptide) (Fig. 3, E and F). In these experiments, we have demonstrated that Kv1.2-BA specifically blocks the Kv1.2 currents without affecting other related K+ currents.
Inhibition of Binding of 125I-Dendrotoxin by Kv1.2-BA
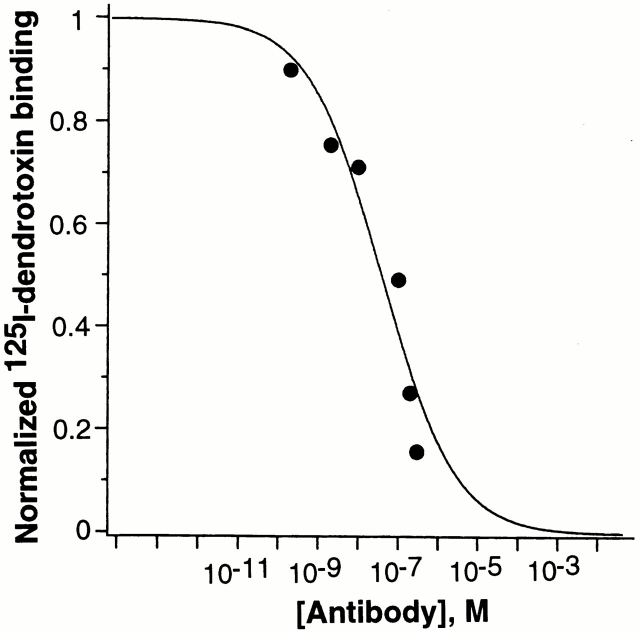
Kv1.2 channel protein is one of the major receptors for dendrotoxin in vivo (Scott et al., 1990). Since α-dendrotoxin also binds to the external vestibule of Kv1.2 channel protein (Hurst et al., 1991), it is expected that binding by α-dendrotoxin and Kv1.2-BA antibody should be mutually exclusive. If Kv1.2 channel protein is first bound with the Kv1.2-BA antibody, then binding of α-dendrotoxin to Kv1.2 channel protein should be decreased compared with the binding in the absence of Kv1.2-BA antibody, due to the occupancy of the channel proteins by the antibody that excludes the binding of α-dendrotoxin. As shown in Fig. 4, increased concentrations of Kv1.2-BA led to the inhibition of 125I-α-dendrotoxin binding in Kv1.2-transfected HEK cells. Thus, this data further demonstrates that Kv1.2-BA blocks Kv1.2 currents by binding to the external vestibule of Kv1.2 channel protein.
Figure 4.
Inhibition of 125I-dendrotoxin binding by Kv1.2-BA antibody. HEK-293 cells with stably transfected Kv1.2 channels were incubated with 125I-labeled α-dendrotoxin (10 nM; 290 Ci/mmol) in the presence of the indicated concentrations of Kv1.2-BA antibody. Preimmune IgG did not have significant effects. At 500 nM, preimmune IgG produced ∼10% inhibition of 125I-dendrotoxin binding. Data are expressed as fractions of dendrotoxin binding compared with controls in which no Kv1.2-BA antibody was added. Data are representative of three similar experiments.
Suppression of Endogenous Kv1.2 Current by Kv1.2-BA Antibody
We also tested the Kv1.2-BA antibody on endogenous currents in neuronal cells. Previously, Kv1.2 had also been cloned by screening a cDNA library made from the neuronal NG108-15 cells, and it has been shown that Kv1.2 is abundantly expressed in NG108-15 cells (Yokoyama et al., 1989). Therefore, we performed whole-cell patch-clamp analysis on NG108-15 cells (Han et al., 1991; Wilk-Blaszczak et al., 1994) (Fig. 5). Addition of the blocking antibody Kv1.2-BA (250 nM) resulted in a 76 ± 12% (n = 6) decrease of the whole-cell potassium currents within 10–15 min, a significantly (P < 0.002, paired t test) higher inhibition than that caused by the control antibody Kv1-NA (25 ± 8% [n = 6] decrease over the same period with 250 nM Kv1-NA). Untreated cells showed an 8 ± 3% (n = 6) decrease. These results demonstrate that Kv1.2-BA blocks endogenous Kv1.2 current as well as heterologously expressed Kv1.2 currents.
Figure 5.
Inhibition of endogenous Kv1.2 currents by Kv1.2-BA in neuronal cells. (A) Whole-cell potassium currents from NG108-15 cells in the absence (NG108-15) or presence (+Kv1.2-BA) of the blocking antibody Kv1.2-BA. Kv1.2-BA (250 nM) reduced the whole-cell K+ currents by ∼75%. (B) The control antibody Kv1-NA (250 nM) reduced the current by ∼25%. Data are representative of six similar experiments.
Application of the Method to Another Subfamily of K+ Channels
To extend this method to other subfamilies of K+ channels, we have generated another polyclonal antibody against a 14 amino acid peptide (GAQPNDPSASEHTH) from the external vestibule of the Kv3.1 channel protein (Fig. 1, A and E). This affinity-purified polyclonal anti–Kv3.1 antibody (Kv3.1-BA) detected Kv3.1 protein in plasma membranes of Kv3.1-transfected HEK-293 cells (Fig. 1 E). Kv3.1-BA at 250 nM blocked ∼79% of the Kv3.1 currents from Kv3.1-transfected HEK cells (Fig. 6 A). The Kv3.1 current is stable in the absence of antibodies. The suppression is antibody dose dependent (Fig. 6 B). The IC50 is 58 nM. This Kv3.1-BA antibody had limited effects on Kv1.2 currents (Fig. 6, C and D). Thus, the antibody Kv3.1-BA can specifically suppress Kv3.1 currents.
Figure 6.
Blockage of Kv3.1 currents by Kv3.1-BA antibody. (A) Kv3.1 currents in Kv3.1 stably transfected HEK-293 cells in the absence (HEK/Kv3.1) or presence (+Kv3.1-BA) of Kv3.1-BA. Addition of 250 nM of Kv3.1-BA led to an ∼80% suppression of Kv3.1 currents. (B) Kv3.1-BA blockage is concentration dependent. Data points were taken at 15 min after the addition of antibody. (C and D) Kv3.1-BA has limited effects on Kv1.2 currents in Kv1.2 stably transfected HEK-293 cells. The data are mean ± SD of five to seven experiments.
discussion
A large number of ion channel genes have been cloned. These genes underline the extraordinary diversity, heterogeneity, and complexity of ionic currents observed in cells. However, the lack of specific ion channel blockers for each cloned channel has hampered the elucidation of the physiological functions of these channels. In the present study, we have shown that the antibody against the Kv1.2 external vestibule specifically blocks heterologous Kv1.2 currents, without significantly affecting currents recorded from cells transfected with other related K+ channels. The antibody against the Kv3.1 external vestibule specifically blocks Kv3.1 currents. We have also tested these antibodies on endogenous currents. Our data support a model in which this peptide region is indeed exposed at the extracellular surface since the antipeptide antibodies bind extracellularly and interfere with ionic currents.
In practice, like any other methods using antibodies, the concentration used is important and controls are essential. It is useful to test several concentrations of antibodies to obtain appropriate amounts to maximize inhibition and minimize nonspecific background suppression. If the antibody is used at too high a concentration, high background (nonspecific suppression) can result. If the antibody concentration is too low, the inhibition will be too small to be certain of specificity. In general, the optimal concentrations for purified polyclonal antibodies will be in the range of ∼20–60 nM. At concentrations <60 nM, the specific antibodies could reduce the currents significantly (as high as 50%), while nonspecific antibodies produced no suppression other than rundown (<10%). When examining endogenous currents, it is very important to compare the suppression produced by the channel-blocking antibody with a control antibody. Or one can perform the experiments in the presence and absence of the immunogenic peptide used to generate the antibody and compare the differences.
Given the remarkable specificity and selectivity of antibodies for their targets, our method provides an approach to rationally design specific ion channel blockers. A large number of K+ channels expressing similar properties makes it a difficult task to correlate a native K+ current with a cloned K+ channel gene. At present time, the identification of a particular current in cells possessing a broad array of overlapping currents depends on a limited array of pharmacological tools. Approaches targeted at the biosynthesis or assembly of channel proteins such as introducing antisense oligonucleotides or expressing dominant negative mutant channels in native cells have limitations; for example, the dependence on the turnover rate of endogenous channel proteins (Tu et al., 1995; Chung et al., 1995). Since antibody strategy is aimed at the functional membrane channel proteins, it avoids such limitation. Furthermore, antibodies have been raised that detect single amino acid substitutions, or differentiate between phosphorylated and unphosphorylated peptides (Harlow and Lane, 1988). Thus, they are excellent tools to discriminate among structurally related ion channels.
Antibodies have previously been used in functional studies of channels. Antipeptide antibodies, made against regions between S5 and S6 transmembrane segments of domains I and IV of the sodium channel α subunit, effectively inhibit the binding of α-scorpion toxin to sodium channels reconstituted in phospholipid vesicles or synaptosomes (Thomsen and Catterall, 1989). It was not shown whether these antibodies could block sodium currents. An antipeptide antibody, by binding to a region in the intracellular loop between domains III and IV, slows sodium channel inactivation (Vassilev et al., 1988). Furthermore, it has been found that antisera from patients with Lambert-Eaton Myasthenic Syndrome (an autoimmune disease of neuromuscular transmission) could inhibit calcium channel currents (Kim and Neher, 1988). Antisera from some patients with Isaacs' Syndrome (acquired neuromyotonia) have antibodies against potassium channels and could increase neuronal excitability, possibly due to blocking of potassium currents (Shillito et al., 1995). One monoclonal antibody that was generated against membrane fragments of the eel electroplax attenuates sodium current (Meiri et al., 1986). Another monoclonal antibody that recognizes the dihydropyridine-binding complex in rabbit muscle transverse tubules inhibits calcium current in a mouse muscle cell line (Morton et al., 1988). However, in all these cases, the binding sites on the channel proteins are not clear.
The biophysical mechanism of the blocking ability of Kv1.2-BA on Kv1.2 currents or Kv3.1-BA on Kv3.1 currents is not yet clear. The target peptide sequence is close to the ion flux pathway, and it is likely that the antibody binds to the peptide and physically blocks the permeation of K+ ions, as is the case with scorpion toxins (MacKinnon and Miller, 1988). Alternatively, antibody binding could cause a conformational change in the channel protein that closes the pore. The blockage could result either from the obstruction of the open channel or by preventing the channel from achieving an open conformation. Questions regarding the effect of the antibody on single-channel conductance and/or open probability remain to be answered with further experiments. Regardless of the mechanism, however, we have presented an approach to identifying a specific ion channel involved in producing a particular endogenous current. This immunoelectrophysiological approach should also be applicable to other ion channels. Also, this method can be used or modified as a rational drug design using ion channels as therapeutic targets. In addition, it should be possible to design channel openers by generating antibodies that force the channel into an open configuration. Furthermore, using the same peptide or other nearby peptides as probes, it is possible to screen for small peptides or oligonucleotides (instead of antibodies) that could be used as ion channel blockers using combinatorial chemistry.
Acknowledgments
We thank Carol Deutsch for the Kv plasmids and Hui-Quan Han for NG108 cells. We are grateful to Larry Palmer, Henry Sackin, and Olaf Andersen for help and discussions. We thank O. Andersen, T. Maack, L. Palmer, E. Piros, and A. Weinstein for reading the manuscript.
Footnotes
Research in our lab was supported by grants from the National Institutes of Health, the National Science Foundation, and the American Heart Association. B.-Y. Zhou is a Charles H. Revson Foundation Fellow in Biomedical Research. X.-Y. Huang is a Beatrice F. Parvin Investigator of the American Heart Association, New York City Affiliate.
references
- Aiyar J, Withka JM, Rizzi JP, Singleton DH, Andrews GC, Lin W, Boyd J, Hanson DC, Simon M, Dethlefs B, et al. Topology of the pore-region of a K+channel revealed by the NMR-derived structures of scorpion toxins. Neuron. 1995;15:1169–1181. doi: 10.1016/0896-6273(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Attali B, Romey G, Honore E, Schmid-Alliana A, Mattei M-G, Lesage F, Ricard P, Barhanin J, Lazdunski M. Cloning, functional expression, and regulation of two K+channels in human T lymphocytes. J Biol Chem. 1992;267:8650–8657. [PubMed] [Google Scholar]
- Bence K, Ma W, Kozasa T, Huang X-Y. Direct stimulation of Bruton's tyrosine kinase by Gq-protein α-subunit. Nature. 1997;389:296–299. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of voltage-sensitive ion channels. Science. 1988;242:50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Chandy, K.G., and G.A. Gutman. 1995. Voltage-gated K+ channel genes. In CRC Handbook of Receptors and Channels. R.A. North, editor. CRC Publications, Boca Raton, FL. 1–71.
- Christie MJ, North RA, Osborne PB, Douglass J, Adelman JP. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990;2:405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- Chung S, Saal DB, Kaczmarek LK. Elimination of potassium channel expression by antisense oligonucleotides in a pituitary cell line. Proc Natl Acad Sci USA. 1995;92:5955–5959. doi: 10.1073/pnas.92.13.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal KK, England SK, Tamkun MM. Molecular physiology of cardiac potassium channels. Physiol Rev. 1996;76:49–67. doi: 10.1152/physrev.1996.76.1.49. [DOI] [PubMed] [Google Scholar]
- Han H-Q, Nichols RA, Rubin MR, Bahler M, Greengard P. Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature. 1991;349:697–700. doi: 10.1038/349697a0. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Antibodies. Cold Spring Harbor, New York. 726 pp.
- Hidalgo P, MacKinnon R. Revealing the architecture of a K+channel pore through mutant cycles with a peptide inhibitor. Science. 1995;268:307–310. doi: 10.1126/science.7716527. [DOI] [PubMed] [Google Scholar]
- Huang X-Y, Morielli AD, Peralta EG. Tyrosine kinase- dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Busch AE, Kavanaugh MP, Osborne PB, North RA, Adelman JP. Identification of amino acid residues involved in dendrotoxin block of rat voltage-dependent potassium channels. Mol Pharmacol. 1991;40:572–576. [PubMed] [Google Scholar]
- Isacoff EY, Jan YN, Jan LY. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990;345:530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Structural elements involved in specific K+channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Kim YI, Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988;239:405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Langhans-Rajasekaran SA, Wan Y, Huang X-Y. Activation of Tsk and Btk tyrosine kinases by G protein βγ subunits. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA. Strategies for studying permeation at voltage-gated ion channels. Annu Rev Physiol. 1991;53:477–496. doi: 10.1146/annurev.ph.53.030191.002401. [DOI] [PubMed] [Google Scholar]
- Lu Q, Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995;268:304–307. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- Luneau CJ, Williams JB, Marshall J, Leviyan ES, Oliva C, Smith JS, Antanavage J, Folander K, Stein RB, Swanson R, et al. Alternative splicing contributes to K+channel diversity in the mammalian central nervous system. Proc Natl Acad Sci USA. 1991;88:3932–3936. doi: 10.1073/pnas.88.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R, Miller C. Mechanism of charybdotoxin block of Ca2+-activated K+channels. J Gen Physiol. 1988;91:335–349. doi: 10.1085/jgp.91.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri H, Goren E, Bergmann H, Zeitoun I, Rosenthal Y, Palti Y. Specific modulation of sodium channels in mammalian nerve by monoclonal antibodies. Proc Natl Acad Sci USA. 1986;83:8385–8389. doi: 10.1073/pnas.83.21.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton ME, Caffrey JM, Brown AM, Froehner SC. Monoclonal antibody to the a1-subunit of the dihydropyridine-binding complex inhibits calcium currents in BC3H1 myocytes. J Biol Chem. 1988;263:613–616. [PubMed] [Google Scholar]
- Panyi G, Sheng Z-F, Tu L-W, Deutsch C. C-type inactivation of a voltage-gated K+channel occurs by a cooperative mechanism. Biophys J. 1995;68:896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perney TM, Kaczmarek LK. Expression and regulation of mammalian K+channel genes. Semin Neurosci. 1993;5:135–145. [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992;72:S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcel DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+channels altered by presence of β-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg JP, Schroter KH, Sakmann B, Stocker M, Sewing S, Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990;345:535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Segel, I.H. 1993. Enzyme kinetics. John Wiley & Sons, Inc., New York. 957 pp.
- Scott VES, Parcej DN, Keen JN, Findlay JBC, Dolly JO. α-Dendrotoxin acceptor from bovine brain is a K+channel protein. J Biol Chem. 1990;265:20094–20097. [PubMed] [Google Scholar]
- Sheng M, Liao YJ, Jan YN, Jan LY. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. . Nature. 1993;365:72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, van den Berg RJ, Plomp JJ, Kempen GT, Chauplannaz G, Wintzen AR. Acquired neuromyotonia: evidence for autoantibodies directed against K+channels of peripheral nerves. Ann Neuol. 1995;38:714–722. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Catterall WA. Localization of the receptor site for α-scorpion toxins by antibody mapping: implications for sodium channel topology. Proc Natl Acad Sci USA. 1989;86:10161–10165. doi: 10.1073/pnas.86.24.10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Santarelli V, Deutsch C. Truncated K+ channel DNA sequences specifically suppress lymphocyte K+channel gene expression. Biophys J. 1995;68:147–156. doi: 10.1016/S0006-3495(95)80169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM, Scheuer T, Catterall WA. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988;241:1658–1661. doi: 10.1126/science.241.4873.1658. [DOI] [PubMed] [Google Scholar]
- Wan Y, Bence K, Hata A, Kurosaki T, Veillette A, Huang X-Y. Genetic evidence for a tyrosine kinase cascade preceding the mitogen-activated protein kinase cascade in vertebrate G protein signaling. J Biol Chem. 1997;272:17209–17215. doi: 10.1074/jbc.272.27.17209. [DOI] [PubMed] [Google Scholar]
- Wan Y, Kurosaki T, Huang X-Y. Tyrosine kinases in activation of the MAP kinase cascade by G-protein-coupled receptors. Nature. 1996;380:541–544. doi: 10.1038/380541a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wilk-Blaszczak MA, Singer WD, Gutowski S, Sternweis PC, Belardetti F. The G protein G13 mediates inhibition of voltage-dependent calcium current by bradykinin. Neuron. 1994;13:1215–1224. doi: 10.1016/0896-6273(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Imoto K, Kawamura T, Higashida H, Iwabe N, Miyata T, Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. FEBS Lett. 1989;259:37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]