Abstract
A distinctive feature of the voltage-dependent chloride channels ClC-0 (the Torpedo electroplaque chloride channel) and ClC-1 (the major skeletal muscle chloride channel) is that chloride acts as a ligand to its own channel, regulating channel opening and so controlling the permeation of its own species. We have now studied the permeation of a number of foreign anions through ClC-1 using voltage-clamp techniques on Xenopus oocytes and Sf9 cells expressing human (hClC-1) or rat (rClC-1) isoforms, respectively. From their effect on channel gating, the anions presented in this paper can be divided into three groups: impermeant or poorly permeant anions that can not replace Cl− as a channel opener and do not block the channel appreciably (glutamate, gluconate, HCO3 −, BrO3 −); impermeant anions that can open the channel and show significant block (methanesulfonate, cyclamate); and permeant anions that replace Cl− at the regulatory binding site but impair Cl− passage through the channel pore (Br−, NO3 −, ClO3 −, I−, ClO4 −, SCN−). The permeability sequence for rClC-1, SCN− ∼ ClO4 − > Cl− > Br− > NO3 − ∼ ClO3 − > I− >> BrO3 − > HCO3 − >> methanesulfonate ∼ cyclamate ∼ glutamate, was different from the sequence determined for blocking potency and ability to shift the P open curve, SCN− ∼ ClO4 − > I− > NO3 − ∼ ClO3 − ∼ methanesulfonate > Br− > cyclamate > BrO3 − > HCO3 − > glutamate, implying that the regulatory binding site that opens the channel is different from the selectivity center and situated closer to the external side. Channel block by foreign anions is voltage dependent and can be entirely accounted for by reduction in single channel conductance. Minimum pore diameter was estimated to be ∼4.5 Å. Anomalous mole-fraction effects found for permeability ratios and conductance in mixtures of Cl− and SCN− or ClO4 − suggest a multi-ion pore. Hydrophobic interactions with the wall of the channel pore may explain discrepancies between the measured permeabilities of some anions and their size.
Keywords: gating, multi-ion pore, Sf9 cells, thiocyanate, methanesulfonate
introduction
In voltage-dependent sodium, potassium, and calcium channels, the voltage dependence of gating has usually been attributed to that intrinsic part of the protein molecule known as the voltage sensor (S4 region), although a number of very recent studies have reported some dependence on the presence and nature of the permeating cation (Yellen, 1997). For example, lower than normal concentrations of external potassium substantially reduce the open probability of some K channels. By comparison, cloning and sequencing of the voltage-dependent ClC family of Cl− channels has failed to reveal an S4 type of voltage sensor and experiments on the Torpedo Cl− channel, ClC-0, indicate that there is little or no contribution to gating from intrinsic protein charge movement (Chen and Miller, 1996). Indeed, for two family members, ClC-0, and the skeletal muscle Cl− channel, ClC-1, a number of experiments in which Cl− has been replaced by glucose or by other anions suggest that activation of these channels is mainly controlled by the presence and concentration of external Cl− (Pusch et al., 1995a ; Rychkov et al., 1996). These authors submit that the permeant anion also serves as a ligand that regulates channel opening when it binds to a specific intra-pore site. For a constant external Cl− concentration, the availability of extracellularly derived Cl− at the regulatory site depends on the transmembrane electric field and this confers the experimentally observed voltage dependence upon gating, no intrinsic voltage sensing component of the channel protein being required.
Similar experiments using methanesulfonate as the impermeant Cl− substitute along with the effect of a mutation in ClC-1 (D136G), meanwhile, led Fahlke et al. (1995, 1996) to conclude that D136 is part of a voltage sensor that controls gating, this voltage sensor possibly being subject to electrostatic perturbation by anion binding within the channel. Mutations in ClC-0 (Ludewig et al., 1997) and ClC-1 (Fahlke et al., 1997c) at very different positions, however, cause the same inwardly rectifying gating as D136G, making it unlikely that this residue serves as a voltage sensor. In their latest interpretation of gating, Fahlke et al. (1997b) endorse the functional linkage between ion permeation and gating, but maintain their different viewpoint with respect to the involvement of a voltage sensor. They argue that the voltage responsiveness resulting from voltage-dependent conformational changes can be modulated by permeant ions, and hence be modified by the presence of foreign anions.
Methanesulfonate, which has often been used as the preferred impermeant Cl− substitute because of its negligible permeability and apparent lack of interaction with Cl− in mole fraction substitution studies of membrane conductance in the rat diaphragm muscle (Palade and Barchi, 1977). Other anions (Br−, I−, SO4 2−, and methylsulphate) tested by these authors, appeared to interact to varying degrees with Cl−, displaying nonlinear and anomalous mole fraction effects on membrane conductance when substituted for Cl−. Similar effects of foreign anion substitution on fish skeletal muscle had been demonstrated previously (Hagiwara and Takahashi, 1974).
Early work on frog muscle with halide and NO3 substitution defined the substantial specificity of skeletal muscle anion channels for Cl− over other anions (for review see Bretag, 1987). Woodbury and Miles (1973) tested an extensive range of foreign anions on frog muscle and found that they could be separated into two groups according to how they affected the pH dependence of membrane conductance. For Cl− itself and “chloride-like” anions, membrane conductance decreased at low pH, while, for “benzoate-like” anions, it increased. More recently, the effects of foreign anions on Cl− channels of tissues other than muscle have been analyzed at the single channel level (Bormann et al., 1987; Halm and Frizzell, 1992; Linsdell et al., 1997a ; Tabcharani et al., 1997) and, based on the extensive kinetic information obtained, substantial models of multi-ion pores have been developed (Bormann et al., 1987; Halm and Frizzell, 1992; Linsdell et al., 1997b ).
The skeletal muscle Cl− channel, ClC-1, has a conductance that is too small to allow observation of single channel currents (Pusch et al., 1994). Nevertheless, investigation of ensemble currents can provide valuable insight into the gating and permeation characteristics of this channel (Steinmeyer et al. 1991, 1994; Pusch et al. 1995b ; Astill et al., 1996; Fahlke et al., 1996; Rychkov et al., 1996; Fahlke et al., 1997a , 1997b ).
In the present studies, we have investigated the influence of foreign anions on current kinetics, apparent P open permeability, and conductance of ClC-1 channels that allow a number of predictions to be made about the nature of the channel pore.
methods
Rat ClC-1 (rClC-1) was expressed in Sf9 (a Spondoptera frugiperda insect cell line) cells as described in detail previously (Astill et al., 1996). Cultured Sf9 cells were infected with baculovirus BVDA6.3 containing rClC-1 cDNA and incubated for 28–30 h at 28°C in air. After incubation, infected cells were seeded onto glass coverslips and maintained at room temperature until required. Whole-cell patch-clamp experiments were performed on Sf9 cells between 28 and 34 h postinfection using an EPC7 patch-clamp amplifier and associated standard equipment (List Electronic, Darmstadt, Germany). The usual bath solution contained (mM): 170 NaCl, 2 MgSO4, 2 Ca-gluconate, and 10 HEPES, adjusted to pH 7.5 with NaOH. Lower concentrations of Cl− in the bath solution were achieved by equimolar substitution of 5, 10, 25, 50, 75, 90, or 100% of external Cl− by a particular anion; for impermeant anions the highest concentration in the bath solution was 95%. Electrodes were made of borosilicate glass and had a resistance of 1–3 MΩ when filled with a normal internal solution containing (mM): 40 KCl, 120 K-glutamate, 10 EGTA-Na, and 10 HEPES, adjusted to pH 7.2 with NaOH. In some experiments, the concentration of NaCl in the bath solution was raised to 340 mM, in which case the concentrations of KCl and K-glutamate in the internal solution were doubled. When HCO3 − was used to substitute for Cl−, bath HEPES concentration was increased to 20 mM and the solution was used within 20 min. To reduce possible acidification of the internal medium due to CO2 diffusion into the cell, 120 mM K-glutamate in the internal solution was replaced with equimolar Tris-glutamate, a substitution that, of itself, had no effect on ClC-1 channel properties.
When necessary, pentobarbitone (0.5 mM), added to the bath solution, was used to block native anion channels in Sf9 cells (Birnir et al., 1992). Data were collected, filtered at 3 or 10 kHz, and analyzed on an IBM-compatible PC using pCLAMP v6.0 software (Axon Instruments, Foster City, CA). Liquid junction potentials between the bath and electrode solutions were estimated by using JPCalc (Barry, 1994) and corrected where specified. Experiments were conducted at a room temperature of 24 ± 1°C.
To obtain permeability ratios, membrane potentials for at least seven different concentrations of each foreign anion were measured in current clamp mode. Shifts in potential (relative to control Cl− solution) were plotted against mole fraction of the anion and fitted with a Goldman-Hodgkin-Katz equation of the form:
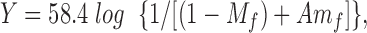 |
1 |
where Y is the shift of the membrane potential, M f (mole fraction) is the concentration of foreign anion, X, relative to the control Cl− concentration (M f =[X−]/[X−] + [Cl−]), and A = (P X/ PCl) is the permeability ratio for this anion relative to Cl− .
To determine relative conductances in the presence of external foreign anions, outward conductance was estimated as a chord conductance (approximating the slope conductance) from the outward current interpolated at a point 20 mV to the right of the reversal potential on the current–voltage (I-V) plot. We constructed these I-V plots from steady state currents obtained at the end of 100-ms test voltage pulses.
Tail currents for voltage steps to −100 mV after 150-ms test pulses in the range from −160 to +100 mV were extrapolated back to the beginning of the pulse by fitting with two exponentials plus a constant (Rychkov et al., 1996). Instantaneous currents so obtained were used to produce apparent P open curves by fitting with a Boltzmann distribution of the form:
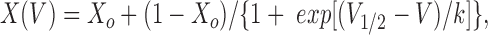 |
2 |
where X o is an offset, V is the membrane potential, V 1/2 is the potential at which X = (1 + X o)/2, and k is the slope factor. The slope factor k was used to calculate the apparent gating valence z, (z = 25.4/k).
Noise analysis was performed as described previously (Pusch et al., 1994) on some outside-out patches on Xenopus oocytes expressing hClC-1. In these experiments, the bath solution contained (mM): 100 NaX (X = Cl−, Br−, etc., or 50 Cl− + 50 I−), 2 MgCl2, 5 HEPES, adjusted to pH 7.3 with NaOH, and the pipette solution contained (mM): 100 NaCl, 2 MgCl2, 5 EGTA, 5 HEPES, adjusted to pH 7.3 with NaOH. Inside-out patches containing hClC-1 were also produced from Xenopus oocytes. In this case, the pipettes were filled with control bath solution containing (mM): 100 NaCl, 2 MgCl2, 5 HEPES, adjusted to pH 7.3 with NaOH, and the patch was exposed to (mM): 100 NaCl, 2 MgCl2, 5 EGTA, 5 HEPES, adjusted to pH 7.3 with NaOH. Where specified, various fractions of the 100 mM NaCl were replaced by equimolar amounts of NaBr or NaI.
results
Various foreign anion substitutions were first tested against the effects of NaCl replacement by glucose. Our presumptions were that permeation through ClC-1 would not be influenced by glucose, that glucose would not interact with any regulatory site or any process modulating ClC-1, and that anion permeation through ClC-1 could be satisfactorily monitored without interference from any other permeation pathways through the Sf9 cell membrane.
When NaCl in the external solution was replaced by glucose, the slope conductance of ClC-1, estimated near the equilibrium potential, showed saturation at physiological external Cl− concentrations, only becoming dependent on external Cl− at low concentrations (Fig. 1). Almost identical results were obtained when glutamate, gluconate, glucuronate, and diatrizoate were substituted for Cl− (Fig. 1, only glutamate is shown). Relative permeabilities for these anions, obtained from the fit of the Goldman-Hodgkin-Katz equation to shifts in membrane potential, were <1% and could not be considered different from zero. Substitution of Cl− by glutamate or glucose influenced current kinetics, increasing the rate of deactivation of inward currents, and shifted the apparent P open curve in the depolarizing direction, as we reported previously (Rychkov et al., 1996). Both of these were dependent on the extent to which Cl− had been replaced. When other anions in this group were substituted for Cl−, they also made current deactivation faster and shifted the apparent P open curve to more positive potentials to the same extent as glutamate (Fig. 2 B).
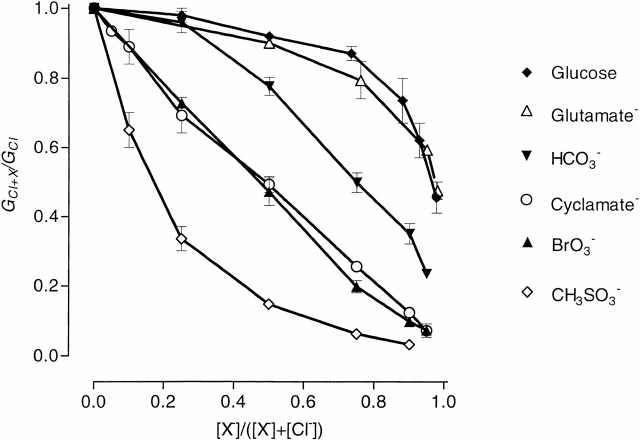
Figure 1.
Membrane conductance of Sf9 cells expressing rClC-1 when impermeant or poorly permeant anions replace Cl− in the external solution. Relative conductance, GX + Cl/GCl, is plotted against mole fraction, [X−]/([X−]+[Cl−]), of the foreign anion. Conductance was estimated as explained in methods.
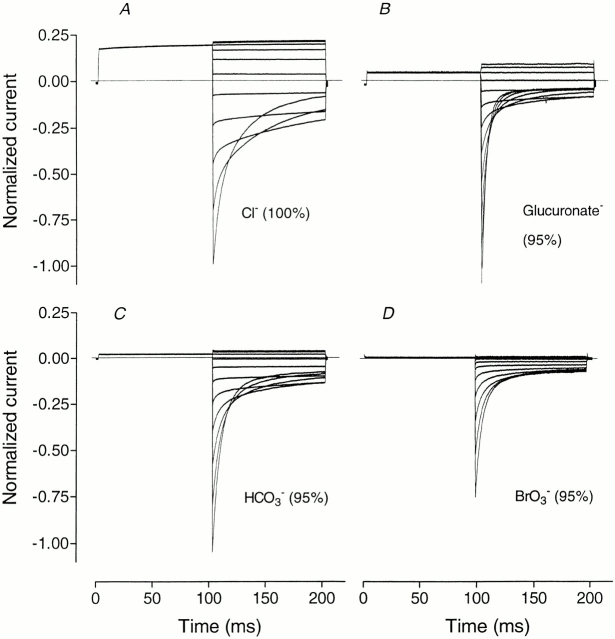
Figure 2.
Kinetics of whole-cell currents in Sf9 cells expressing rClC-1 when impermeant or poorly permeant anions replace 95% of Cl− in the external solution. Currents were elicited by the standard activation protocol from a holding potential of −30 mV with a prepulse to +40 mV followed by steps from −120 to +80 mV in 20-mV increments. Since experiments were performed on different cells, currents have been normalized to the peak inward current in Cl− solution at −120 mV. Peak inward currents from cell to cell ranged between −5 and −10 nA.
Two much smaller anions, HCO3 − and BrO3 −, although having a measurable permeability relative to Cl− (Table I) had the same (Fig. 2 C) or a similar (Fig. 2 D) effect on current kinetics as the large impermeant organic anions from the previous group. They also shifted the apparent P open curves to more positive potentials without changing the gating charge (Table I), but unlike glutamate and other anions from that group they appeared to interact with Cl− inside the channel to some extent, since the relationship between conductance and concentration was different from that for glucose (Fig. 1). Bicarbonate was less permeant than BrO3 − (Table I) and its effects were closer to those of glutamate: at 95% concentration, HCO3 − shifted V 1/2 by ∼+60 mV, while BrO3 −, at the same concentration, shifted V 1/2 rather less, by ∼+50 mV (compare with the shift of ∼+70 mV for glutamate; Table I). Bromate showed stronger interaction with Cl− inside the channel than HCO3 − since conductance of the channel in the outward direction in the presence of BrO3 − was smaller than in the presence of HCO3 − (Figs. 1 and 2, C and D). At the same time, BrO3 − weakly blocked inward current, while HCO3 − did not show any block (compare the relative amplitudes of inward current in the presence of BrO3 − and HCO3 −; Fig. 2).
Table I.
Relative Permeabilities for Different Foreign Anions and Their Effect on the Parameters of the Popen Curves
| P X/P Cl | V 1/2 * at indicated percent chloride replacement | z | n ‡ | |||||
|---|---|---|---|---|---|---|---|---|
| mV | ||||||||
| Cl− | 1 | −88.2 ± 0.6 | 1.19 ± 0.03 | 23 | ||||
| Br− | 0.37 ± 0.01 | −90.5 ± 2.3 (100%) | 1.12 ± 0.09 | 3 | ||||
| NO3 − | 0.24 ± 0.02 | −117.5 ± 3.3 (50%) −117.0 ± 3.2 (100%) | 0.96 ± 0.11 0.62 ± 0.10 | 4 | ||||
| ClO3 − | 0.24 ± 0.02 | −109.4 ± 1.8 (50%) −114.6 ± 2.5 (100%) | 1.04 ± 0.05 0.58 ± 0.14 | 3 | ||||
| I− | 0.22 ± 0.02 | −117.1 ± 3.3 (10%) −150.3 ± 2.0 (50%) | 1.05 ± 0.05 0.57 ± 0.12 | 4 | ||||
| ClO4 − | anomalous mole-fraction effect 1.95 ± 0.15 (100% Cl− replacement) | −130.2 ± 2.0 (5%) | 1.06 ± 0.11 | 4 | ||||
| SCN− | anomalous mole-fraction effect 1.61 ± 0.18 (100% Cl− replacement) | −137.7 ± 1.0 (5%) −166.2 ± 1.6 (10%) | 1.05 ± 0.05 0.70 ± 0.10 | 5 | ||||
| HCO3 − | 0.027 ± 0.005 | −28.5 ± 3.0 (95%) | 0.97 ± 0.13 | 3 | ||||
| BrO3 − | 0.065 ± 0.006 | −41.3 ± 2.0 (95%) | 1.05 ± 0.07 | 3 | ||||
| Glutamate | 0.008 ± 0.003 | −16.0 ± 1.1 (95%) | 1.07 ± 0.04 | 6 | ||||
| Methanesulfonate | 0.007 ± 0.004 | −113.5 ± 3.5 (50%) −134.9 ± 2.0 (95%) | 0.94 ± 0.11 0.66 ± 0.04 | 3 | ||||
| Cyclamate | 0.008 ± 0.003 | −77.0 ± 2.0 (50%) −60.5 ± 1.5 (95%) | 1.07 ± 0.07 0.62 ± 0.07 | 4 |
Voltages have been corrected for liquid junction potentials (see methods).
The same number of experiments was performed with a particular anion for each different percent chloride replacement.
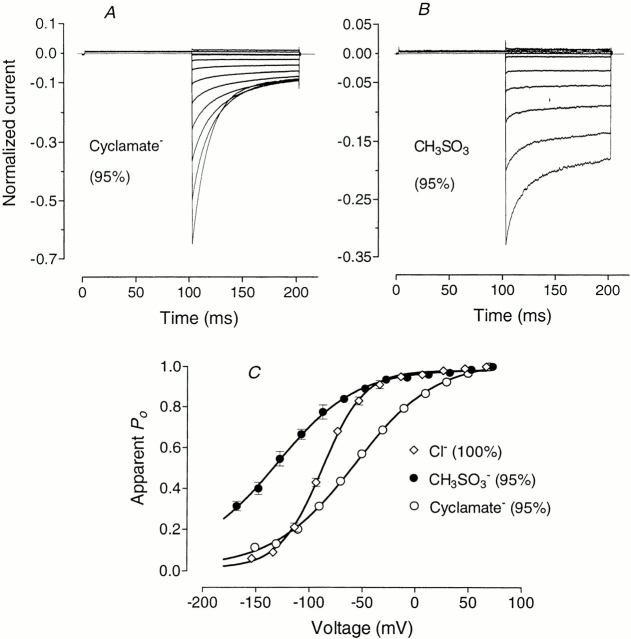
Cyclamate and methanesulfonate were as impermeant as the other large organic anions, but they changed currents through ClC-1 very differently from glutamate (Fig. 3). Cyclamate (when substituting 95% of Cl−) blocked ∼35% of inward current, shifted V 1/2 by +28 mV (compared with ∼+70 mV for glutamate; Table I), and increased the slope coefficient, k, of the P open curve, thus reducing the apparent gating charge, z, to ∼0.63. Methanesulfonate, at the same concentration, blocked up to 70% of inward current and substantially modified the kinetics of current deactivation. Furthermore, the apparent P open curve was shifted in the opposite direction, to more negative potentials, by ∼45 mV, and the apparent gating charge was decreased (Fig. 3; Table I).
Figure 3.
Effect of cyclamate and methanesulfonate on current kinetics and apparent P open curves. Currents were elicited by the standard activation protocol (as in Fig. 2) and 95% of external Cl− was replaced by (A) cyclamate or (B) methanesulfonate. Apparent P open curves (C) are plotted for control (⋄), methanesulfonate (•) and cyclamate (○). Voltages are corrected for liquid junction potentials (see methods).
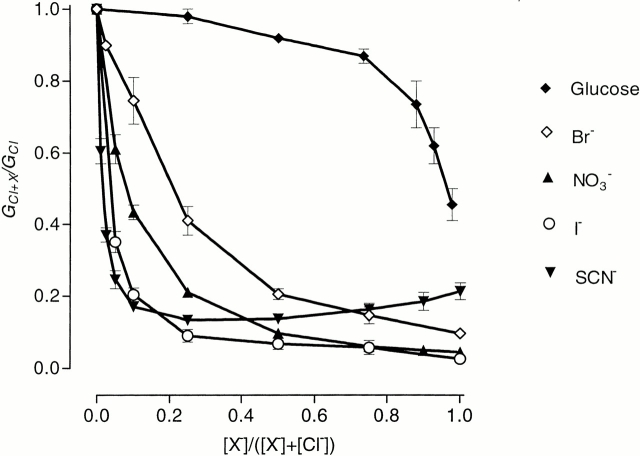
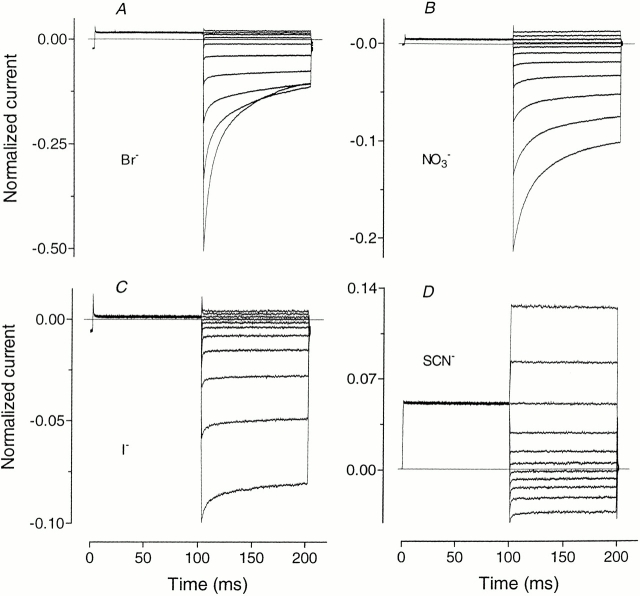
Bromide, NO3 −, ClO3 −, I−, ClO4 −, and SCN− fall into a separate category of foreign anions in regard to their action on ClC-1. With increasing amounts of Br− replacing Cl− in the external solution, the slope conductance, calculated for outward current near the reversal potential, decreased until just 15% of its initial value remained when 100% Br− substituted for Cl− (Fig. 4). At the same time, inward current amplitude (at −120 mV) was reduced by 50%, indicating that Br− blocks the ClC-1 channel (Fig. 5). The relative permeability for Br−, again obtained using the Goldman-Hodgkin-Katz equation and data for different mole fractions of Br−, was 0.37 ± 0.01. There was no evidence of an anomalous mole-fraction effect on permeability for Br−. Kinetics of current deactivation were not substantially altered, nor was there any significant shift in the voltage dependence of the apparent P open, as indicated by V 1/2 (Table I). The main difference was an increased proportion of steady state current at negative potentials (compare Figs. 2 and 5).
Figure 4.
Membrane conductance of Sf9 cells expressing rClC-1 when various permeant anions replace Cl− in the external solution. Relative conductance has been plotted against mole fraction of foreign anion as for Fig. 1.
Figure 5.
Kinetics of whole-cell currents in Sf9 cells expressing rClC-1 when various permeant anions replace 100% of Cl− in the external solution. Currents were elicited by the standard activation protocol as for Fig. 2.
Nitrate and ClO3 − had very low relative conductances (e.g., Fig. 4) and, with relative permeabilities of ∼0.25, were somewhat less permeant than Br−. In addition, they blocked up to 80% of the inward current at −120 mV when substituting 100% of the Cl− (Fig. 5, ClO3 − is not shown). Apparent P open in the presence of either of these anions at 50% concentration was shifted to more negative potentials without significant change in the slope of the curve, but if the concentration of NO3 − or ClO3 − was increased up to 100%, the slope factor of the apparent P open curve was increased (Table I), implying a reduction in gating charge to ∼0.6 (normally ∼1.2). Time constants of the deactivating components of the inward current were not appreciably changed by these anions, but the relative proportion of steady state current at −120 mV increased from ∼7 to ∼50% (compare Figs. 2 and 5).
Although I− was quite permeant, with a relative permeability of 0.22 ± 0.02, conductance of I− ions through ClC-1 was negligibly small and not readily distinguishable from leakage. Inward current was blocked by 90% at −120 mV when all Cl− was replaced with I− and current showed little deactivation at negative potentials (Fig. 5). Apparent P open curves obtained at lower mole fractions of I−, where current deactivation at negative potentials was more obvious, showed that the voltage dependence of channel gating was shifted to more negative potentials by I− and the apparent gating charge decreased (Table I).
Thiocyanate and ClO4 − at low concentrations (up to 10%) had effects very similar to I−, they blocked inward current (by ∼90% in the case of 10% SCN−), reduced current deactivation at negative potentials, shifted V 1/2 to more negative potentials, decreased the slope of apparent P open curves (Table I), and shifted membrane potentials towards more positive values. When the concentration of SCN− (or ClO4 −) was raised above 10%, inward current was blocked almost completely, but the amplitude of the outward current started to increase and reached its maximum when all Cl− in the external solution was replaced with SCN− (Fig. 5). Under these conditions, the usual inward rectification was replaced by outwardly rectifying currents (Fig. 5), and SCN− was more permeant than Cl− since membrane potentials were shifted to values more negative than the Cl− equilibrium potential (Fig. 6).
Figure 6.
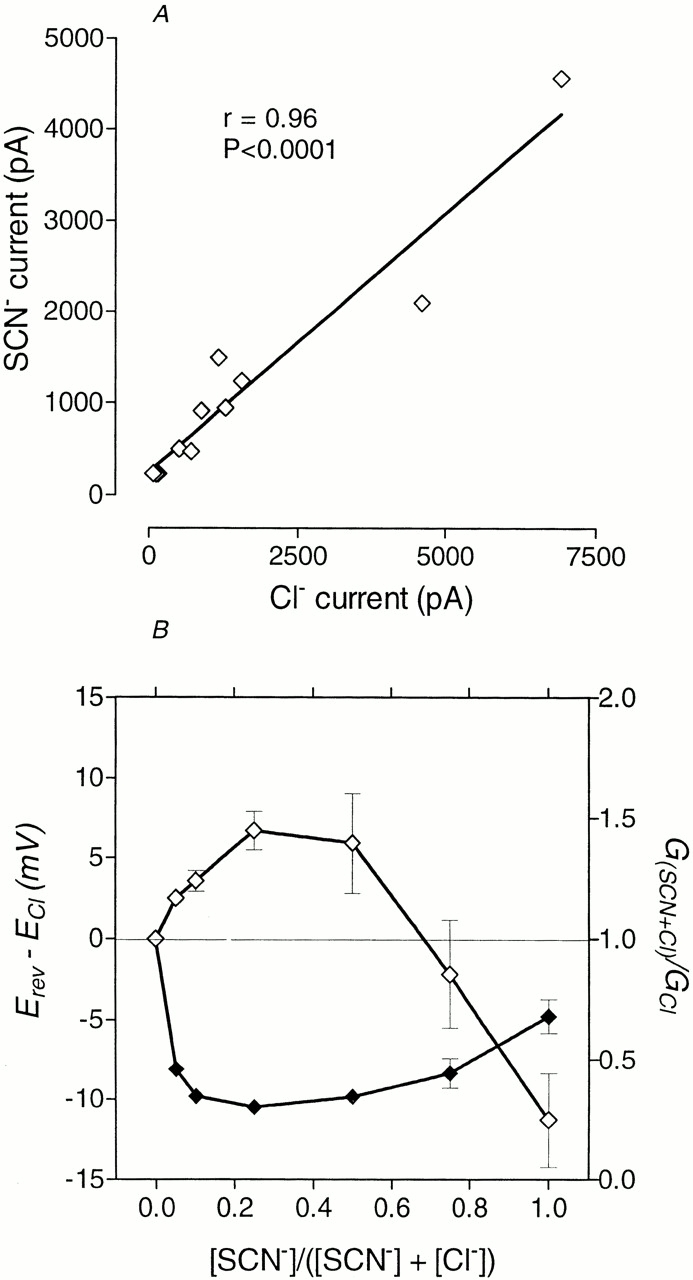
Effects of replacing Cl− with SCN− in the external solution. Amplitudes of outward current in Cl− solution and in SCN− solution were found to be closely correlated (A). Anomalous mole fraction effects were apparent for both permeability and conductance (B). Permeability changes are indicated by the shift in reversal potential (⋄). Relative conductance (♦) in this case was determined from the chord conductance measured at +80 mV. Both permeability and relative conductance are plotted against mole fraction of SCN−.
Endogenous volume-regulated Cl− channels in Sf9 cells were more permeable to SCN− than to Cl− and, furthermore, control cells showed outwardly rectifying currents in the presence of SCN− qualitatively similar to the currents recorded under the same conditions from the cells expressing ClC-1 (not shown). To make sure that the anomalous mole-fraction effect and outward rectification in solutions containing SCN− were not the result of increased activity of the native Cl− channels, concomitant with complete block of ClC-1 channels, experiments with SCN− substitution for Cl− were carried out on cells with different levels of expression of ClC-1. If currents recorded in the presence of SCN− were passing only through native channels, their amplitudes should not be much different from cell to cell and should not depend on the Cl− current amplitude through ClC-1. In reality, there was a high level of correlation between the amplitudes of outward currents, measured in cells expressing ClC-1, bathed in control Cl− solution, and when SCN− replaced Cl− (Fig. 6). We can, therefore, have confidence that ClC-1 is permeable to SCN− (and, likewise, to ClO4 −) and that outward currents recorded in the presence of SCN− are mainly due to conductance through ClC-1.
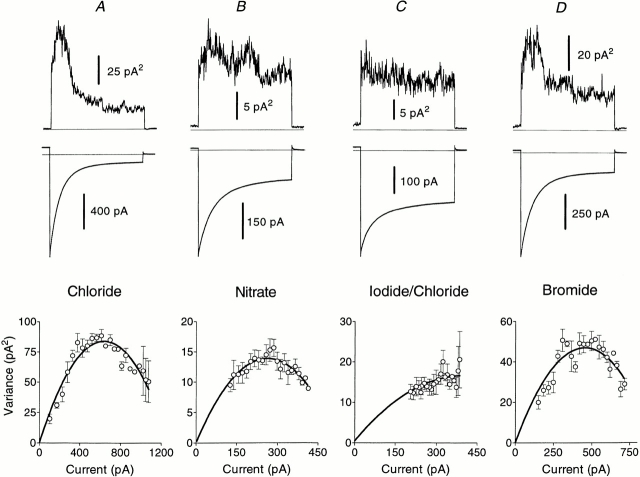
External substitution of Br−, NO3 −, and I− on hClC-1 expressed in Xenopus oocytes had very similar effects to those described above for rClC-1, although block by Br−, I−, and methanesulfonate may have been slightly weaker (Table II). Noise analysis (Fig. 7), performed on outside-out patches containing hClC-1 at −140 mV to gain information about the mechanism of this block, show (Table II) that single channel current amplitude is reduced by foreign anion substitution in the same proportion as whole-cell current.
Table II.
Foreign Anion Block of Macroscopic Currents, I, from Whole-Cell Studies and of Single Channel Currents, i, Estimated by Noise Analysis
| Anion X* | I X/I Cl (hClC-1) | I X/I Cl (rClC-1) | i X (hClC-1) (pA) | i X/i Cl (hClC-1) | ||||
|---|---|---|---|---|---|---|---|---|
| Cl− | 1 | 1 | 0.24 ± 0.04 (n = 11) | 1 | ||||
| Br−‡ | 0.80 ± 0.06 (n = 3) | 0.64 ± 0.07 (n = 3) | 0.19 ± 0.03 (n = 3) | 0.79 ± 0.12 (n = 3) | ||||
| NO3 −‡ | 0.41 ± 0.02 (n = 8) | 0.44 ± 0.05 (n = 4) | 0.085 ± 0.022 (n = 9) | 0.35 ± 0.09 (n = 9) | ||||
| I−‡ | 0.26 ± 0.03 (n = 4) | 0.24 ± 0.05 (n = 4) | ||||||
| I− (50 mM) | 0.38 ± 0.02 (n = 9) | 0.079 ± 0.02 (n = 6) | 0.33 ± 0.08 (n = 6) | |||||
| Methanesulfonate‡ | 0.73 ± 0.11 (n = 3) | 0.67 ± 0.10 (n = 4) | 0.20 ± 0.02 (n = 4) | 0.84 ± 0.10 (n = 4) |
For ease of comparison, peak inward currents for a step to −140 mV in the presence of foreign anions have been normalized to the current in external chloride solution for rC1C-1 and hC1C-1 as appropriate.
Currents, I X and i X, are inward currents carried by internal chloride in the presence of external anion X.
These anions were substituted in equimolar amounts for 127.5 mM Cl (75% of external Cl substituted) in experiments on rClC-1 expressed in Sf9 cells and for 100 mM Cl (98% of external Cl substituted) in experiments on hClC-1 expressed in Xenopus oocytes.
Figure 7.
Nonstationary noise analysis performed on outside-out patches from Xenopus oocytes expressing hClC-1 in the presence of Cl−, NO3 −, I−, or Br−. Variances (top) and means (middle) of current records (n = 150) are shown for standard voltage protocols incorporating a prepulse to +60 mV followed by a 70-ms test pulse to −140 mV. (bottom) Variance–mean current plots are fitted with parabolas according to the equation: σ2 = σ0 2 + iI − I 2/n, where σ0 2 is baseline noise variance, i is single channel current, I is mean current, and n is number of channels. The results shown were obtained from a single patch. Fitted values for the parabolas shown are: (A) for chloride, i = 0.26 pA; (B) for nitrate, i = 0.1 pA; (C) for 50% iodide/50% chloride, i = 0.077 pA; (D) for bromide, i = 0.21 pA.
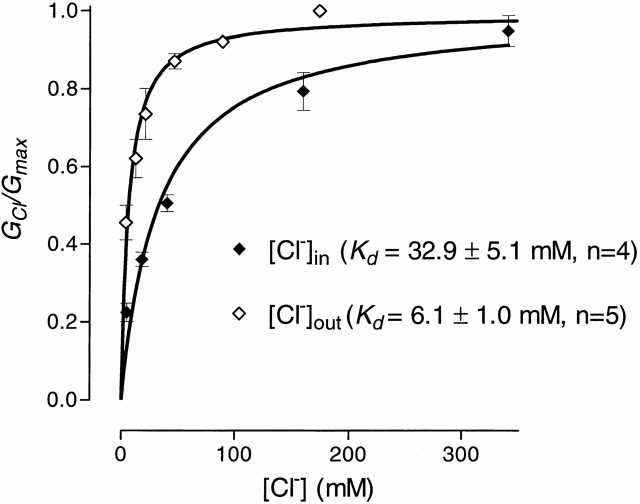
As illustrated in Figs. 1 and 8, conductance to outward currents is saturated at physiological Cl− concentrations with a K d of ∼6 mM. Since it was not possible to change internal Cl− concentration during whole-cell patch-clamp experiments in Sf9 cells, results from separate whole-cell experiments were combined after normalizing in the following way. For different cells, the ratio between chord conductance at −120 mV and current at +80 mV was found to be constant for external Cl− concentrations from 170 to 340 mM and a fixed internal Cl− concentration, presumably reflecting the ratio between single channel currents at −120 and +80 mV. Similarly, ratios were calculated from results obtained using a variety of Cl− concentrations in the pipette, plotted against internal Cl− concentrations and fitted with a one-binding site hyperbola. The fit gave a K d of ∼33 mM. The maximal ratio obtained from the fit was used to normalize the data points, making it possible to present the saturation curves for internal and external Cl− on the one graph (Fig. 8).
Figure 8.
Relationship between Cl− conductance and concentration in rClC-1. In this case, Cl− was replaced by glucose in the external solution but by glutamate in the internal solution. From Fig. 1, it can be seen that the effects of glucose or glutamate replacement in the external solution are almost identical. See text for methods employed in normalization of data.
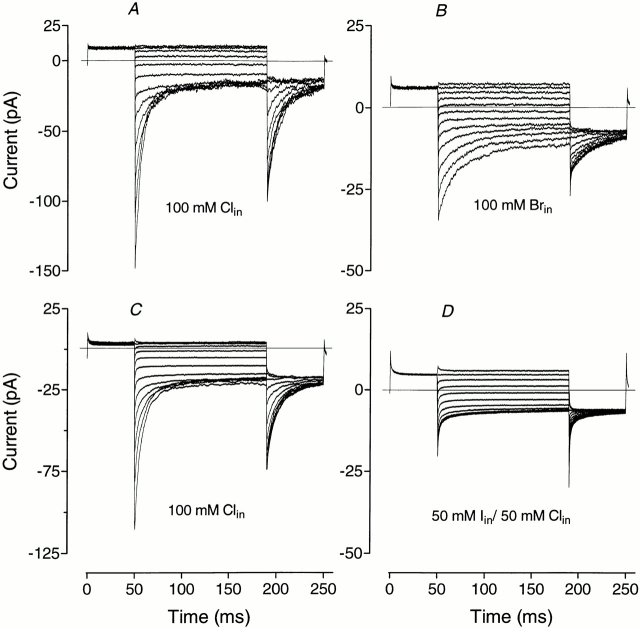
Similar to its external application, Br− applied internally had little effect on apparent P open curves (Table III). But unlike external Br−, which did not affect inward current kinetics, internal Br− slowed down current deactivation and appeared to have a stronger blocking action (Table II indicates 20% block of hClC-1 and 40% block of rClC-1 from the outside, while Fig. 9 shows 70% block of hClC-1 from the inside). These estimates of block from attenuation of inward currents are not strictly comparable as Br− applied from inside blocks inward current to a greater extent than outward current, whereas the opposite is true for block by externally applied Br−. In each case, the block is voltage dependent.
Table III.
Parameters of Apparent Popen Curves from Inside-Out Patches of Xenopus Oocytes after Internal Application of Foreign Anions
| V 1/2 | z | n | ||||
|---|---|---|---|---|---|---|
| mV | ||||||
| Cl− (100%) | −48.5 ± 2.5 | 1.08 ± 0.02 | 5 | |||
| Br− (100%) | −50.8 ± 1.1 | 0.84 ± 0.02 | 4 | |||
| I− (10%) | −48.2 ± 1.1 | 1.03 ± 0.04 | 4 | |||
| (50%) | −37.3 ± 1.0 | 0.52 ± 0.04 | 4 |
Figure 9.
Kinetics of currents in inside-out patches from Xenopus oocytes expressing hClC-1 when Br− and I− are substituted for Cl− in the internal solution. A shows control currents in the patch to which 100% Br− was then applied (B), while C shows control currents in the patch to which 50% I− was later applied (D). Voltage protocol: after a 50-ms prepulse to +60 mV, the membrane potential was stepped for 150 ms from −140 to +80 mV in 20-mV increments, followed by a step to −100 mV for 50 ms.
Intracellular I− had an even stronger blocking effect on inward current (Fig. 9) than Br−, just as occurs when they are applied from the outside. In 100 mM intracellular I− (leaving 4 mM internal Cl−), inward currents through hClC-1 were almost completely blocked and it was impossible to extract parameters describing the voltage dependence. Outward currents were not appreciably affected. Partial replacement of Cl− by I− led to a slight shift of V 1/2 to more positive values and to a decrease in the apparent gating charge, z. The shift of V 1/2 is opposite to the effect of external I−, whereas both external and internal I− led to a reduction in gating charge (Table III).
discussion
In our receptor-operated model of gating in the ClC-1 channel, open probability is controlled by a binding site for Cl−, or certain other anions, which is accessible only from outside and which must be correctly occupied for opening to occur (Rychkov et al., 1996). Consistent with this model, anions we have studied can be divided into three groups according to their ability to open the channel and to permeate it: impermeant anions that are unable to open the channel, anions that can open the channel but cannot permeate it, and anions that can both open the channel and permeate it. Large anions such as glutamate, gluconate, and glucuronate belong to the first group. All of these cause a shift of the deactivation curve to positive potentials similar to that caused by glucose, which provides evidence that the shift in voltage dependence of the channel is due to the absence of Cl− rather than to the presence of the foreign anion.
Since glucose, presumably, does not interact with binding sites regulating the behavior of ClC-1, any difference between those relative conductances obtained when glucose substitutes for NaCl and those where a foreign anion substitutes for Cl−, reflects interaction between Cl− and that anion in the pore. Impermeant anions that do not interfere with the gating, such as glutamate and glucuronate, also do not change relative Cl− conductance. By contrast, HCO3 −, which has just 3% of Cl− permeability, clearly reduces Cl− conductance (Fig. 1) and shifts the apparent P open curve to less positive potentials than the impermeant glutamate. The slightly more permeant BrO3 − has more pronounced effects on channel conductance and gating. That gating is intrinsically linked to permeation and appears to be supported by the behavior of these anions. In the present work, however, we have found other anions, such as cyclamate and methanesulfonate, that can have a substantial effect on gating without being permeant. For example, cyclamate and BrO3 − have much the same effect on conductance and current kinetics and the amount of block of the inward current is also very similar, but while BrO3 − is permeant, cyclamate is impermeant and, in addition, it reduces the gating charge to ∼0.62. These results suggest that cyclamate can bind (more readily than bicarbonate or BrO3 −) to the regulatory binding site that opens the channel and that this site lies external to the selectivity filter, which cyclamate cannot pass. Thus, gating is not always coupled to permeation, probably because specificity of the pore selectivity filter differs from that of the site within the pore that regulates channel opening. It could be that when blocking anions, such as methanesulfonate, are bound to the latter site, they hinder Cl− passage through the pore. Alternatively, although less likely (see below), block could be due to binding at some second site (perhaps the selectivity filter), which must also be deep inside the pore, as indicated by diminished inward current block at negative potentials. In these ways, ClC-1 appears to differ from ClC-0, not because of any profound difference in their mechanisms of fast gating, but, probably, because the selectivity filter and regulatory binding site have very similar specificity in ClC-0, which allows channel activation to be closely linked to permeation, whereas these specificities are different in ClC-1 with the consequences that this entails.
Small inorganic anions all showed potential-dependent block at low concentrations in the sequence: Br− < NO3 − < ClO3 − < I− < ClO4 − < SCN−, which coincides with the ability to shift the voltage dependence of gating, to modify current kinetics, and also coincides with the lyotropic series. For Br−, NO3 −, I−, and methanesulfonate, we have good evidence from noise analysis that all reduction in current amplitude is due to a reduction in single channel current. In the lyotropic series, anions are arranged in order according to their ability to bind or adsorb to proteins, to potentiate the strength of a muscle twitch, and to unwind macromolecules. It is believed that, for binding to follow the lyotropic series, a site requires the combination of two molecular attributes: appropriate anion-attracting groups and neighboring hydrophobic groups (Dani et al., 1983).
If conductance is limited by binding inside the pore, then, the stronger the binding, the smaller the conductance. This seems to be the case when SCN− concentration is low, where strong binding to only one of several intrapore binding sites is sufficient to block the channel. Possibly, as concentration is increased, all intrapore binding sites are occupied by SCN−, and electrostatic repulsion between these ions accelerates the rate-limiting exit steps. When all Cl− outside is replaced by SCN−, positive potentials applied from the inside reduce the probability of internal Cl− entering the channel, so the channel starts to rectify outward just as it also rectifies inward at strongly negative potentials when all SCN− is likely to be displaced from the channels. As is typical of the anomalous mole fraction effects seen in pores with multiple intrapore binding sites, the lowest conductance occurs when a mixture of ions is present in the channel. An anomalous mole-fraction effect for mixtures of Cl− and SCN− is not unique for ClC-1. Some other Cl− channels, such as the γ-aminobutyric acid (GABA) and glycine receptor channels (Bormann et al., 1987) and the cystic fibrosis transmembrane conductance regulator (Tabcharani et al., 1993), have similar properties.
With a K d of 6 mM, “outward” Cl− conductance for outward currents through ClC-1 is saturated at physiological external Cl− concentrations. Saturation is presumed to arise when the binding–unbinding steps of permeation become rate limiting (rate of ion entry approaches the maximum rate for the unbinding steps). For inward currents, the K d is ∼33 mM, which suggests that there is a deeper energy well for Cl− ions passing through the channel from the external side than for those passing from the inside. This can be interpreted in terms of the rate limiting binding site being closer to the external side. When positive potential is applied from inside, this binding site is occupied by Cl−, but other intrapore sites will be free. Conductance is then very low due to strong binding of Cl− to this site. When Cl− is moving out under the influence of a positive potential on the outside, all binding sites in the channel will be occupied. Electrostatic repulsion between Cl− ions can then accelerate the rate-limiting exit step and increase conductance (Hille, 1992). In this way, open channel rectification and the different K ds on either side of the membrane may be explained. The effects of foreign anions applied to the inside are also different from their effects on the outside. From inside, Br− and I− block inward current without affecting outward current, whereas, from outside, both currents are blocked. Also, from the inside, I− shifts the apparent P open curve in the opposite direction, to more positive potentials. These results are all consistent with and extend the view of ClC-1 as a channel with multiple internal binding sites having different characteristics and, especially, they support the proposal that there is at least one inner and one outer binding site inside the channel that are quite distinct from each other (see Fahlke et al., 1997b ).
The complete permeability sequence determined from our study was SCN− ∼ ClO4 − > Cl− > Br− > NO3 − ∼ ClO3 − > I− > BrO3 − > HCO3 − ∼ (F−), while just for halides it was Cl− > Br− > I− > F−. This sequence corresponds to a cationic site of moderately strong field strength in the membrane (Eisenman sequence 4; Eisenman, 1965). However, not only electrostatic but also hydrophobic interactions are involved in anion permeation through ClC-1, and so the permeability sequence need not be directly related to the electrostatic field strength of the relevant binding site. From blocking ability when applied externally, the binding sequence is SCN− > ClO4 − > I− > NO3 − > Br− > BrO3 − > HCO3 − ∼ (F−), which corresponds to the low field strength Eisenman sequence 1 (Eisenman, 1965). These results agree with early studies of foreign anion permeability (Woodbury and Miles, 1973) and of block of 36Cl− efflux (Harris, 1958) by foreign anions in frog muscle where the same sequence difference occurred. This would indicate that the site accessible from outside, at which block occurs, is different from the site governing selectivity and because blocking ability corresponds to opening ability, that block probably occurs at the regulatory binding site.
The effects of some of the anions studied in this work on rat ClC-1 have been investigated in human ClC-1 by Fahlke et al. (1997a), who obtained a similar sequence for relative permeability: Cl− ∼ SCN− > Br− > NO3 − > I− > methanesulfonate. Permeability ratios calculated by Fahlke et al. (1997a) were somewhat higher than in our work, which can be explained by differences in internal Cl− concentration. In a multi-ion pore, the permeability ratios will depend on the absolute concentrations. Although for halides the differences were relatively small, methanesulfonate was found to be impermeant in rat ClC-1, while in human ClC-1, P metsulf/P Cl was ∼0.2. With impermeant anion substitution, we have found that the Sf9 cells cannot for long sustain membrane potentials as highly positive as ∼+80 mV with 140 mM of Cl− inside and only 8 mM of Cl− remaining outside. As external Cl− was replaced with impermeant anion to achieve this condition, membrane potential increased to approximately the predicted +80 mV, but soon dropped to less positive values due to increased leakage. We therefore generally used a lower concentration of Cl− (40 mM) in the internal solution. The difference in results might also be accounted for by Cl− accumulation near the external mouth of the channel, which could be higher at higher internal Cl− concentration. It is also possible that the inconsistencies could be due to a real difference between rat and human ClC-1. Some results, such as nondeactivating currents at negative potentials in the presence of I−, are similar in both studies but explained differently. Fahlke et al. (1997b) sug-gest that inherently voltage-dependent conformational changes are modulated by I−, which locks the channel in a nondeactivating state. While we do not dispute that I− might lock the channel in the open state, evidence is lacking for the existence of conformational changes dependent upon an intrinsic voltage sensor in ClC channels (see introduction). Our interpretation is, instead, based on a mechanism (Pusch et al. 1995a ; Rychkov et al., 1996) that is more akin to gating in receptor-operated channels. Anion concentration at a specific intrapore binding site regulates channel opening. Hence, a binding site that has a higher affinity for I− than for Cl− will maintain channel opening in the presence of I−, achievable negative potentials being unable to displace I− from the site.
Estimates of the dimensions of a number of cation and anion channels have been made using a simple cylindrical pore model (Hille, 1975; Bormann et al., 1987) that allows the permeation of ions smaller than the diameter of the cylinder. Relative permeabilities can be described by the equation:
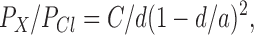 |
3 |
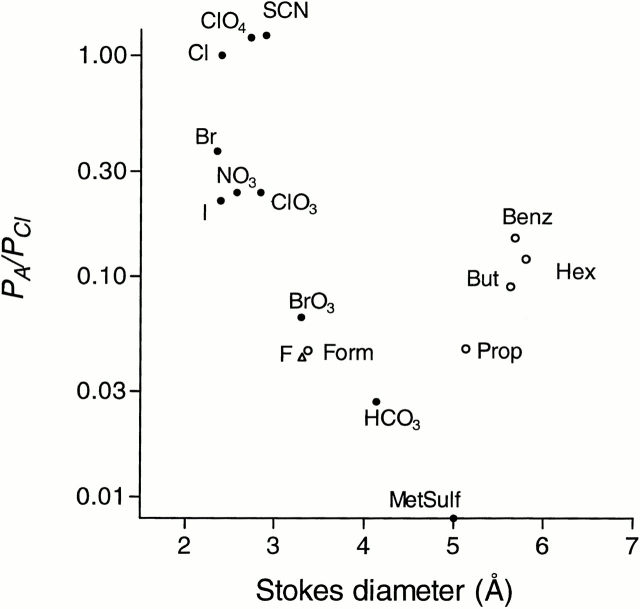
where C is a constant, a is the pore diameter, and d is the Stokes diameter for the particular ion. Although for the ClC-1 channel this equation could not reliably be fitted to the experimental points, the relative permeability of anions included in the present study clearly decreased with increasing size of the anion (Fig. 10). Since HCO3 −, with a Stokes diameter of ∼4.2 Å, is still measurably permeant and methanesulfonate (∼5 Å) is not, this might place an upper limit on the pore diameter. For comparison, the relative permeabilities of several hydrophobic anions are included on the graph. Stokes diameters of the permeant hydrophobic anions is significantly bigger, up to ∼6 Å for hexanoate. However, if those anions are approximated by a cylinder, their cylindrical diameter is ∼4.3 Å (Halm and Frizzell, 1992). Still, they are much more permeant than HCO3 − and their relative permeability increases along with the size of the hydrophobic part of the molecule, suggesting the likelihood of significant hydrophobic interactions with the wall of the pore (see Woodbury and Miles, 1973, for similar results on frog muscle). As well as having an anomalous relationship between permeability and size, these hydrophobic anions have complicated effects on ClC-1 channel gating, which will be described in detail elsewhere. It may well be that the aromatic monocarboxylate blockers (Bretag, 1987; Astill et al., 1996; Rychkov et al., 1997) have their effect due to their ability to enter the pore because of their charge but interact very strongly with the pore wall.
Figure 10.
Dependence of relative permeability of rClC-1 expressed in Sf9 cells on the apparent ionic diameter of various anions. Apparent ionic diameters, d, were calculated from the Einstein-Stokes relation, d = 183.6/λ°, where λ° is the limiting conductance for the ion (Robinson and Stokes, 1959). Relative permeability for F− was determined from the biionic potential with 160 mM of F− in the internal solution and 170 mM of Cl− in the external solution.
For several anion channels that were studied at the single channel level, multiple binding site models have been developed based on extensive kinetic data (Bormann et al., 1987; Halm and Frizzell, 1992; Linsdell et al., 1997b ). It is apparent that very similar models of anion permeation could account for the characteristics of ClC-1, but that a detailed quantitative description of such a model must await more information at the single channel level.
In conclusion, we have shown (a) the multi-ion nature of ClC-1 with demonstrable anomalous mole fraction effects of SCN− and ClO4 −; (b) two of the anion binding sites, an inner and an outer site, are accessible from the cytoplasmic solution and from the external solution, respectively, and have different binding properties even for Cl−; (c) the outer binding site appears to have characteristics that make it the prime candidate for regulation of channel opening and for channel block by external anions, but it is not simultaneously the site that determines channel selectivity; (d) block of macroscopic currents by foreign anions can be accounted for entirely by a reduction in single channel conductance without any requirement for an effect on P open; (e) minimum pore diameter is ∼4.5 Å; and (f) foreign anions can be clearly categorized into three different groups according to their interaction with ClC-1.
Acknowledgments
This work was supported by the Neuromuscular Research Foundation of the Muscular Dystrophy Association of South Australia, the Australian Research Council (A.H. Bretag and M.L. Roberts), the Research Committee of the University of South Australia (A.H. Bretag), the Deutsche Forschungsgemeinschaft, Germany, and the Muscular Dystrophy Association (T.J. Jentsch).
Footnotes
Dr. Pusch's present address is Istituto di Cibernetica e Biofisica, CNR, Via de Marini 6, I-16149 Genova, Italy.
references
- Astill DStJ,, Rychkov GY, Clarke JD, Hughes BP, Roberts ML, Bretag AH. Characteristics of skeletal muscle chloride channel ClC-1 and point mutant R304E expressed in Sf-9 insect cells. Biochim Biophys Acta. 1996;1280:178–186. doi: 10.1016/0005-2736(95)00281-2. [DOI] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Birnir B, Tierney ML, Howitt SM, Cox GB, Gage PW. A combination of human α1 and β1subunits is required for formation of detectable GABA-activated chloride channels in Sf-9 cells. Proc R Soc Lond Ser B Biol Sci. 1992;250:307–312. doi: 10.1098/rspb.1992.0163. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J Physiol (Camb) 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag AH. Muscle chloride channels. Physiol Rev. 1987;67:618–724. doi: 10.1152/physrev.1987.67.2.618. [DOI] [PubMed] [Google Scholar]
- Chen TY, Miller C. Nonequilibrium gating and voltage dependence of the ClC-0 Cl−channel. J Gen Physiol. 1996;108:237–250. doi: 10.1085/jgp.108.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Sanchez JA, Hille B. Lyotropic anions: Na channel gating and Ca electrode response. J Gen Physiol. 1983;81:255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G. Some elementary factors involved in specific ion permeation. Proc Physiol Sci. 1965;4:489–506. [Google Scholar]
- Fahlke C, Beck CL, George AL. A mutation in autosomal dominant myotonia congenita affects pore properties of the muscle chloride channel. Proc Natl Acad Sci USA. 1997a;94:2729–2734. doi: 10.1073/pnas.94.6.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Dürr C, George AL. Mechanism of ion permeation in skeletal muscle chloride channels. J Gen Physiol. 1997b;110:551–564. doi: 10.1085/jgp.110.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Rosenbohm A, Mitrovic N, George AL. Mechanism of voltage-dependent gating in skeletal muscle chloride channels. Biophys J. 1996;71:695–706. doi: 10.1016/S0006-3495(96)79269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Rüdel R, Mitrovic N, Zhou M, George AL. An aspartic acid residue important for voltage-dependent gating of human muscle chloride channels. Neuron. 1995;15:463–472. doi: 10.1016/0896-6273(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Yu HT, Beck CL, Rhodes TH, George AL. Pore-forming segments in voltage-gated chloride channels. Nature. 1997c;390:529–532. doi: 10.1038/37391. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Takahashi K. Mechanism of anion permeation through the muscle fibre membrane of an elasmobranch fish, Taeniura lymma. . J Physiol (Camb) 1974;238:109–127. doi: 10.1113/jphysiol.1974.sp010513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm DR, Frizzell RA. Anion permeation in an apical membrane chloride channel of a secretory epithelial cell. J Gen Physiol. 1992;99:339–366. doi: 10.1085/jgp.99.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EJ. Anion interaction in frog muscle. J Physiol (Camb) 1958;141:351–365. doi: 10.1113/jphysiol.1958.sp005979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels of Excitable Cell Membranes. 2nd Ed. Sinauer Associates, Inc., Sunderland, MA. 378–386.
- Hille, B. 1975. Ionic selectivity of Na and K channels of nerve membranes. In Membranes. Vol. 3. Lipid Bilayers and Biological Membranes: Dynamic Properties. G. Eisenman, editor. Marcel Dekker, New York. 255–323. [PubMed]
- Linsdell P, Tabcharani JA, Rommens JM, Hou Y-X, Chang X-B, Tsui L-C, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997a;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Tabcharani JA, Hanrahan JW. Multi-ion mechanism for ion permeation and block in the cystic fibrosis transmembrane conductance regulator chloride channel. J Gen Physiol. 1997b;110:365–377. doi: 10.1085/jgp.110.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Jentsch TJ, Pusch M. Inward rectification in ClC-0 chloride channels caused by mutations in several protein regions. J Gen Physiol. 1997;110:165–171. doi: 10.1085/jgp.110.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade PT, Barchi RL. Characteristics of the chloride conductance in muscle fibers of the rat diaphragm. J Gen Physiol. 1977;69:325–342. doi: 10.1085/jgp.69.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Rehfeld A, Jentsch TJ. Gating of the voltage-dependent chloride channel ClC-0 by the permeant anion. Nature. 1995a;373:527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- Pusch M, Steinmeyer K, Koch MC, Jentsch TJ. Mutations in dominant human myotonia congenita drastically alter the voltage dependence of the ClC-1 chloride channel. Neuron. 1995b;15:1455–1463. doi: 10.1016/0896-6273(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Pusch M, Steinmeyer K, Jentsch TJ. Low single channel conductance of the major skeletal muscle chloride channel, ClC-1. Biophys J. 1994;66:149–152. doi: 10.1016/S0006-3495(94)80753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, R.A., and R.H. Stokes. 1959. Electrolyte Solutions. Butterworth & Co., London. 463 pp.
- Rychkov GY, Pusch M, Astill DStJ, Roberts ML, Jentsch TJ, Bretag AH. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. J Physiol (Camb) 1996;497:423–435. doi: 10.1113/jphysiol.1996.sp021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov GY, Astill DStJ, Bennetts B, Hughes BP, Bretag AH, Roberts ML. pH-dependent interactions of Cd2+and a carboxylate blocker with the rat ClC-1 chloride channel and its R304E mutant in the Sf-9 insect cell line. J Physiol (Camb) 1997;501:355–362. doi: 10.1111/j.1469-7793.1997.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991;354:301–304. doi: 10.1038/354301a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K, Lorenz C, Pusch M, Koch MC, Jentsch TJ. Multimeric structure of the ClC-1 chloride channel revealed by mutations in dominant myotonia congenita (Thomsen) EMBO (Eur Mol Biol Organ) J. 1994;13:737–743. doi: 10.1002/j.1460-2075.1994.tb06315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Linsdell P, Hanrahan JW. Halide permeation in wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels. J Gen Physiol. 1997;110:341–354. doi: 10.1085/jgp.110.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Rommens JM, Hou Y-X, Chang X-B, Tsui L-C, Riordan JR, Hanrahan JW. Multi-ion pore behaviour in the CFTR chloride channel. Nature. 1993;366:79–82. doi: 10.1038/366079a0. [DOI] [PubMed] [Google Scholar]
- Woodbury JW, Miles PR. Anion conductance of frog muscle membranes: one channel, two kinds of pH dependence. J Gen Physiol. 1973;62:324–353. doi: 10.1085/jgp.62.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single channel seeks permeant ion for brief but intimate relationship. J Gen Physiol. 1997;110:83–85. doi: 10.1085/jgp.110.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]