Abstract
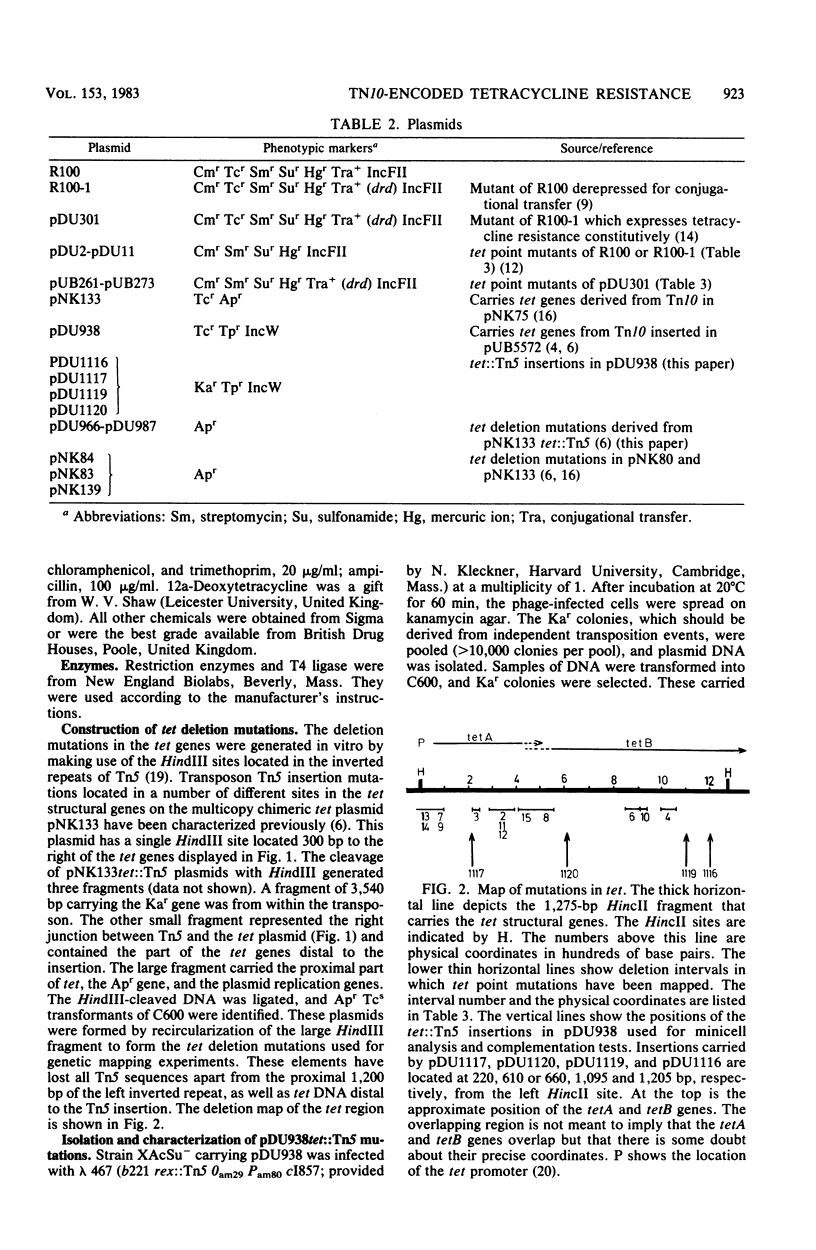
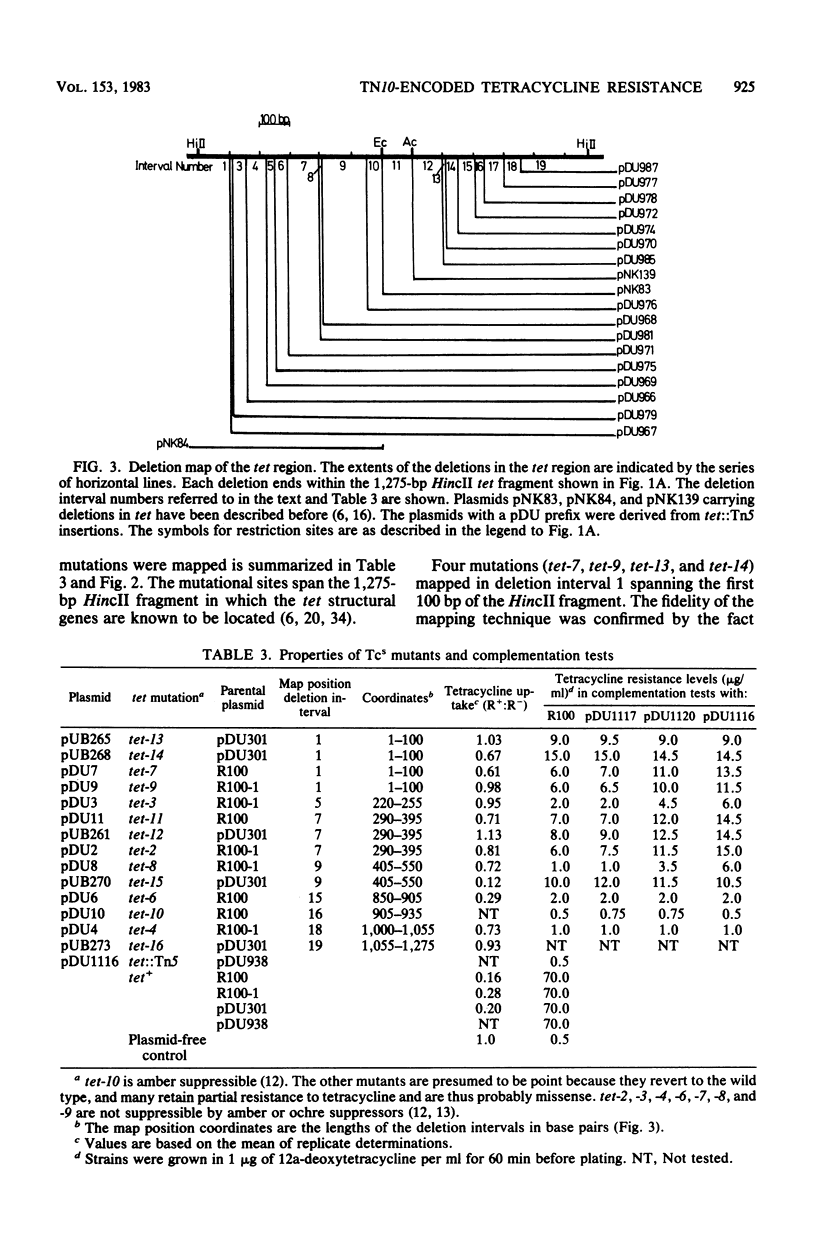
Deletions in the tet genes derived from Tn10 were formed from different tet::Tn5 insertion mutations by removing DNA sequences located between a HindIII site in Tn5 and a HindIII site adjacent to the tet genes. Tetracycline-sensitive point mutations were mapped in recombination tests with the deletions and were thus aligned with the genetic and physical map of the tet region. Plasmids carrying point mutations were tested for complementation with derivatives of pDU938, a plasmid carrying cloned tet genes derived from Tn10 which had been inactivated by Tn5 insertions. Complementation occurred between promoter-proximal tet point mutations and distal tet::Tn5 insertions, suggesting the existence of two structural genes, tetA and tetB. These results, together with the analysis of polypeptides in minicells harboring pDU938tet::Tn5 mutants, suggested that tetA and tetB are expressed coordinately in an operon. The tetB gene encodes the previously characterized 36,000-dalton cytoplasmic membrane TET protein, but the product of tetA was not identified. Point mutations in either tetA or tetB led to the defective expression of the resistance mechanism involving tetracycline efflux. It is suggested that the tetA and tetB products interact cooperatively in the membrane to express resistance.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Chopra I., Howe T. G., Linton A. H., Linton K. B., Richmond M. H., Speller D. C. The tetracyclines: prospects at the beginning of the 1980s. J Antimicrob Chemother. 1981 Jul;8(1):5–21. doi: 10.1093/jac/8.1.5. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S. W., Ward J. M., Wallace L. J. Reduced expression of Tn 10-mediated tetracycline resistance in Escherichia coli containing more than one copy of the transposon. J Gen Microbiol. 1981 Sep;126(1):45–54. doi: 10.1099/00221287-126-1-45. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S., Ball P. Tetracycline resistance determinants from groups A to D vary in their ability to confer decreased accumulation of tetracycline derivatives by Escherichia coli. J Gen Microbiol. 1982 Apr;128(4):689–692. doi: 10.1099/00221287-128-4-689. [DOI] [PubMed] [Google Scholar]
- Coleman D. C., Foster T. J. Analysis of the reduction in expression of tetracycline resistance determined by transposon Tn10 in the multicopy state. Mol Gen Genet. 1981;182(1):171–177. doi: 10.1007/BF00422786. [DOI] [PubMed] [Google Scholar]
- Curiale M. S., Levy S. B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982 Jul;151(1):209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Ferrazza D., Levy S. B. Biochemical and immunological characterization of an R plasmid-encoded protein with properties resembling those of major cellular outer membrane proteins. J Bacteriol. 1980 Oct;144(1):149–158. doi: 10.1128/jb.144.1.149-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Davis M. A., Roberts D. E., Takeshita K., Kleckner N. Genetic organization of transposon Tn10. Cell. 1981 Jan;23(1):201–213. doi: 10.1016/0092-8674(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G., Richmond K. M. Translocation of the tetracycline resistance determinant from R100-1 to the Escherichia coli K-12 chromosome. J Bacteriol. 1975 Dec;124(3):1153–1158. doi: 10.1128/jb.124.3.1153-1158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Insertion of the tetracycline resistance translocation unit Tn10 in the lac operon of Escherichia coli K12. Mol Gen Genet. 1977 Sep 9;154(3):305–309. doi: 10.1007/BF00571287. [DOI] [PubMed] [Google Scholar]
- Foster T. J. R factor-mediated tetracycline resistance in Escherichia coli K12. Dominance of some tetracycline sensitive mutants and relief of dominance by deletion. Mol Gen Genet. 1976 Feb 2;143(3):339–344. doi: 10.1007/BF00269413. [DOI] [PubMed] [Google Scholar]
- Foster T. J. Tetracycline-sensitive mutants of the F-like R factors R100 and R100-1. Mol Gen Genet. 1975;137(1):85–88. doi: 10.1007/BF00332542. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Willetts N. S. Genetic analysis of deletions of R100-1 that are both transfer-deficient and tetracycline-sensitive. J Gen Microbiol. 1976 Mar;93(1):133–140. doi: 10.1099/00221287-93-1-133. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shales S. W., Chopra I., Ball P. R. Evidence for more than one mechanism of plasmid-determined tetracycline resistance in Escherichia coli. J Gen Microbiol. 1980 Nov;121(1):221–229. doi: 10.1099/00221287-121-1-221. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Grinsted J. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid. 1982 May;7(3):239–250. doi: 10.1016/0147-619x(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Wray L. V., Jr, Jorgensen R. A., Reznikoff W. S. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J Bacteriol. 1981 Aug;147(2):297–304. doi: 10.1128/jb.147.2.297-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic T. J., King S. R., Pogue-Geile K. L., Jaskunas S. R. Identification of a second tetracycline-inducible polypeptide encoded by Tn10. J Bacteriol. 1980 Oct;144(1):346–355. doi: 10.1128/jb.144.1.346-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]