Abstract
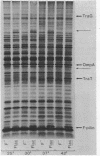
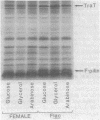
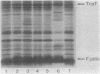
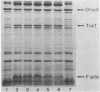
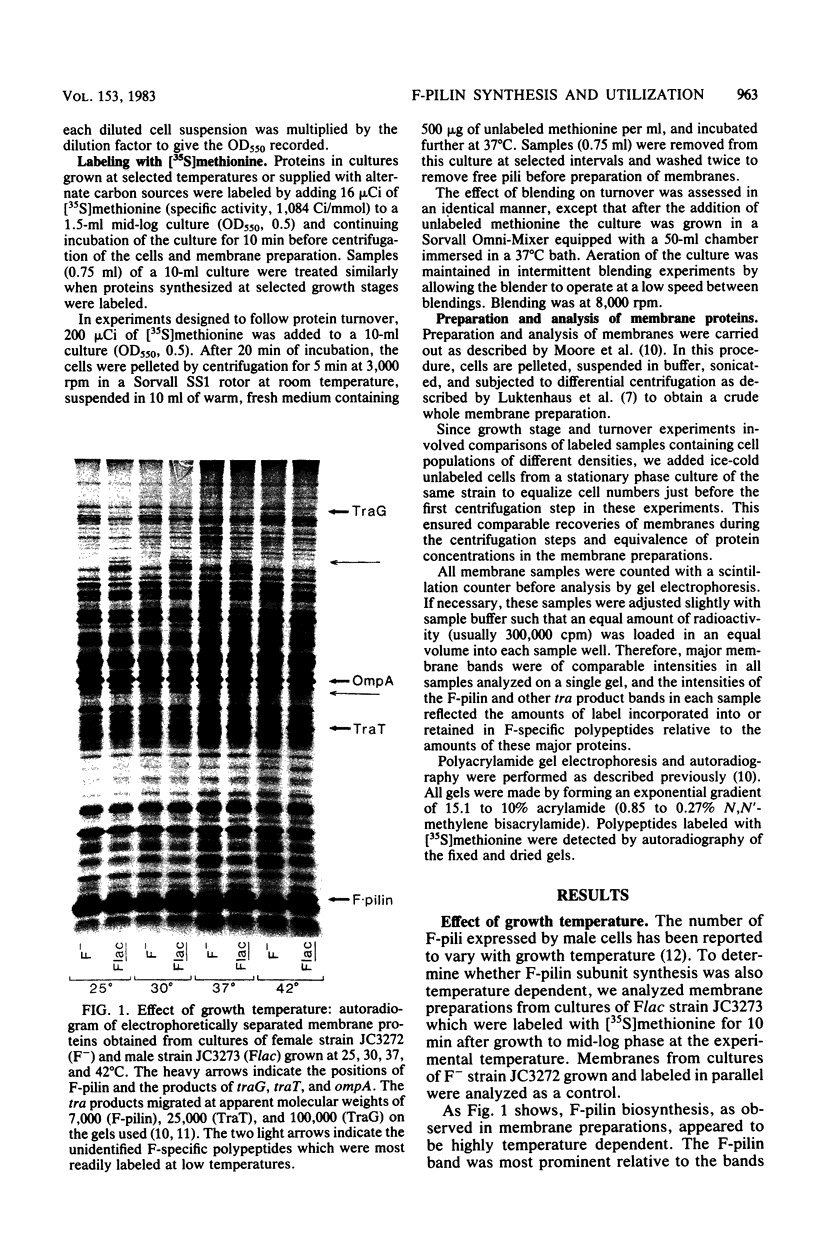
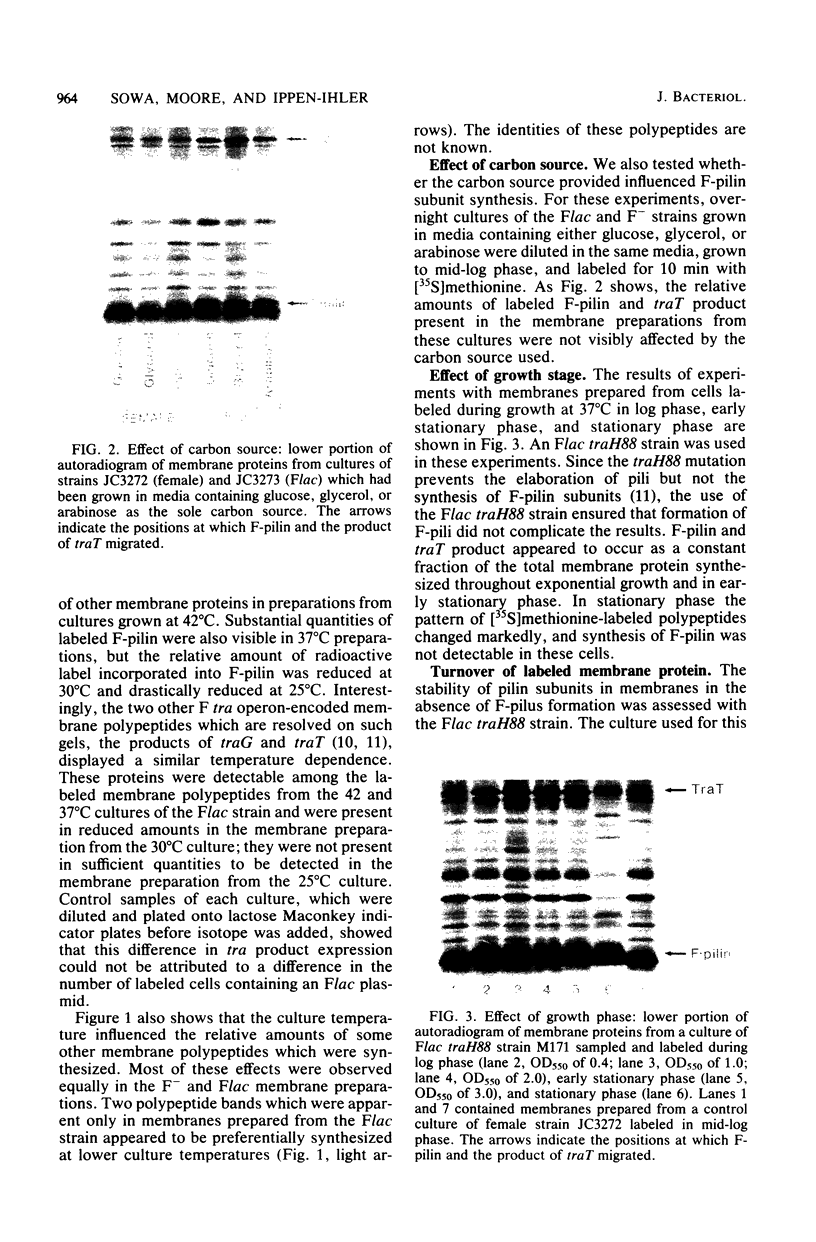
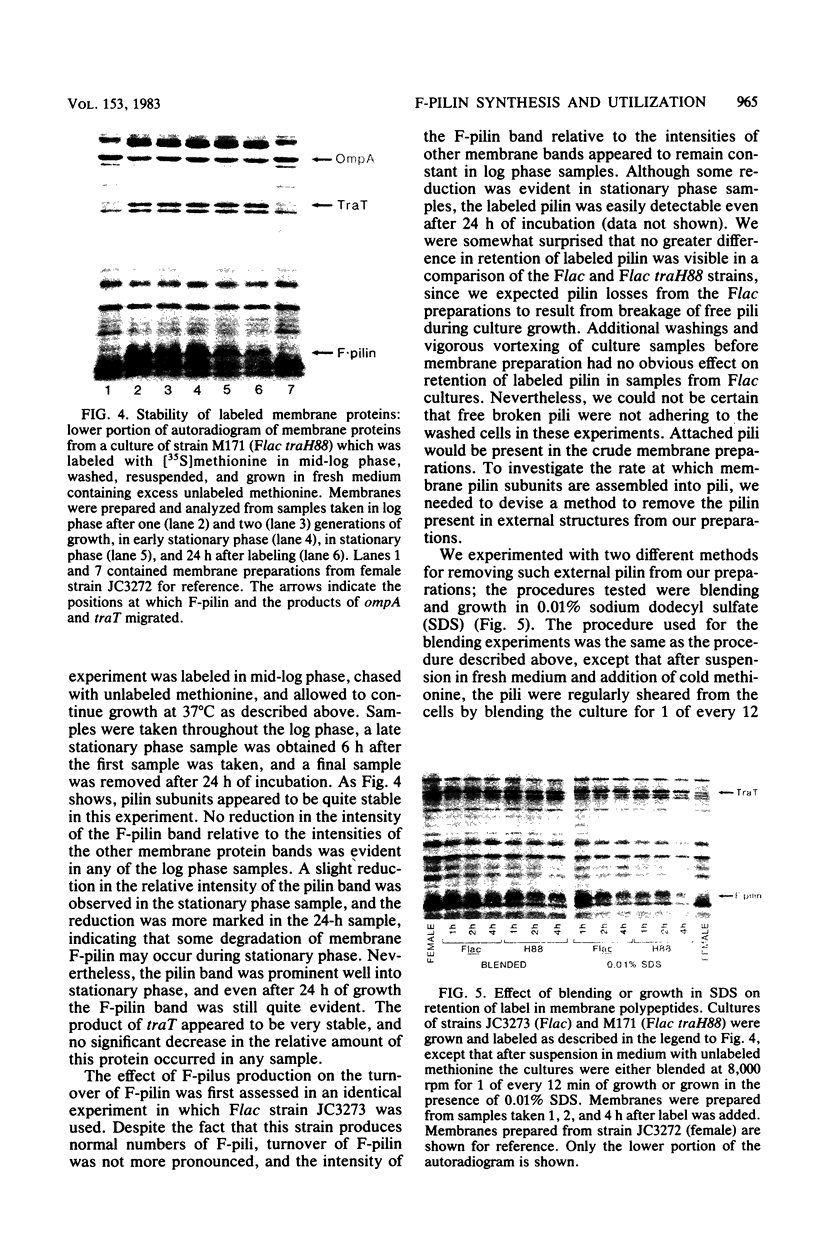
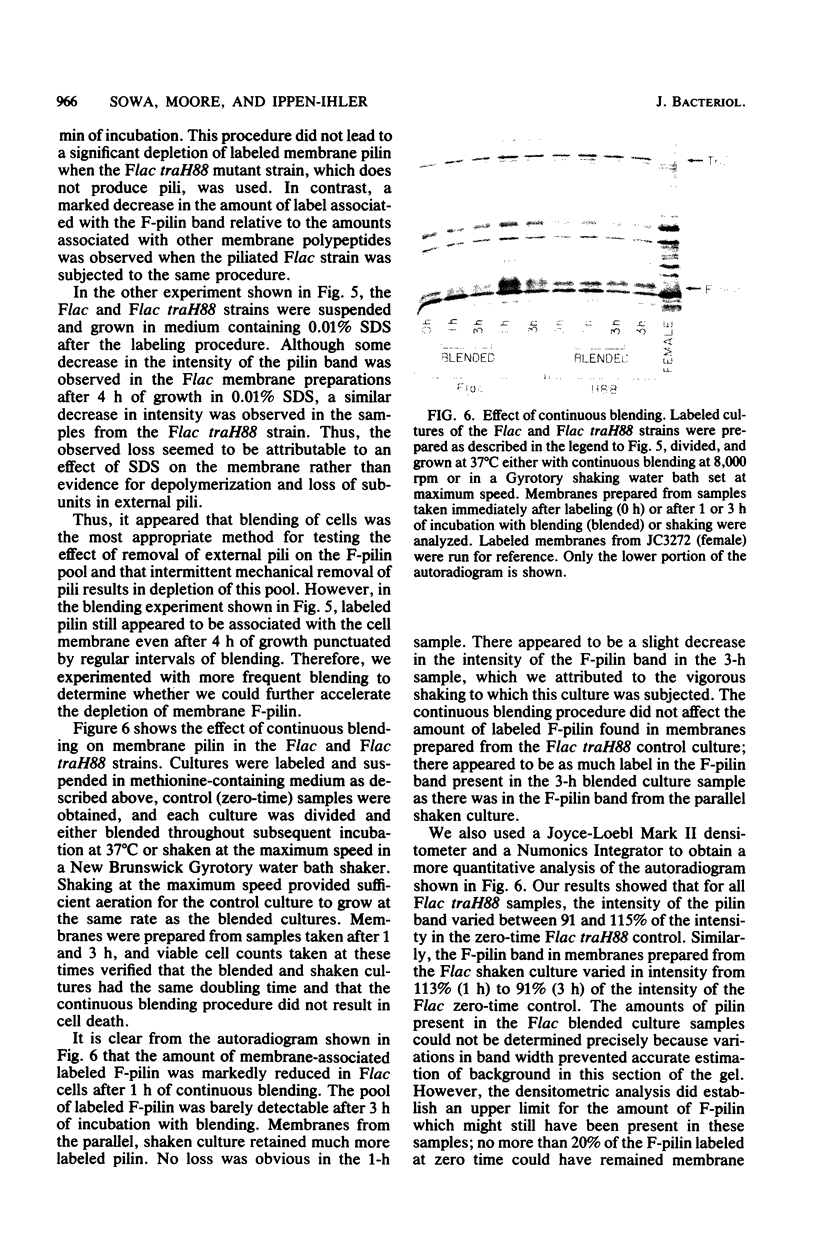
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to study the synthesis and turnover of F-pilin in membrane preparations of Escherichia coli K-12 under conditions which have been reported to affect the production of F-pili. Incorporation of [35S]methionine into membrane F-pilin by cells in log phase was barely detectable at 25°C, but increased with temperature. The labeled pilin band was prominent in membranes from 37°C cultures and even more prominent if the growth temperature was raised to 42°C. The appearance of other tra products in the membranes was similarly temperature dependent. In cultures grown in glucose minimal medium at 37°C, the relative amount of membrane pilin and traT product synthesis remained unchanged from early log phase through early stationary phase; provision of glycerol or arabinose as a substitute carbon source had no obvious effect. Turnover of traT product and membrane F-pilin, as assessed in an Flac tra mutant strain which is incapable of elaborating pili, was not rapid. Both traT product and pilin subunits labeled in mid-log phase cells were still apparent in the membranes after growth of the cells to stationary phase. The relative amount of labeled pilin decreased with prolonged incubation in stationary phase, but the relative amount of traT product did not decrease even after the culture was incubated for 24 h. When wild-type Flac piliated cells were used, a similar result was obtained, but in this case, loss of F-pilin from the preparations could be acclerated by blending the cells. Although intermittent blending during culture growth caused a slow depletion of the labeled pilin pool, continuous blending resulted in the rapid disappearance of this pool from our preparations. Loss of other membrane polypeptides was not accelerated by our blending procedure, and blending did not affect the turnover of the pilin pool of the Flac tra mutant. Our data are consistent with a model in which pilin subunits are assembled transiently into pili, conserved by retraction, and made available for subsequent reassembly. Growth in 0.01% sodium dodecyl sulfate did not accelerate loss of pilin from the Flac strain compared with the Flac tra strain, and we suggest that in the presence of sodium dodecyl sulfate at this concentration, F-pili are not elaborated from cell surfaces.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Morelli G., Schwuchow S. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J Bacteriol. 1978 Sep;135(3):1053–1061. doi: 10.1128/jb.135.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Inuzuka M., Tomoeda M. Purification and characterization of F pili from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5579–5585. doi: 10.1021/bi00644a030. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Cell-cell interactions in conjugating Escherichia coli: purification of F pili with biological activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1237–1241. doi: 10.1073/pnas.75.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Moore B. A., Masters M., Donachie W. D. Individual proteins are synthesized continuously throughout the Escherichia coli cell cycle. J Bacteriol. 1979 May;138(2):352–360. doi: 10.1128/jb.138.2.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. Location of an F-pilin pool in the inner membrane. J Bacteriol. 1981 Apr;146(1):251–259. doi: 10.1128/jb.146.1.251-259.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. The effect of tra mutations on the synthesis of the F-pilin membrane polypeptide. Mol Gen Genet. 1981;184(2):260–264. doi: 10.1007/BF00272914. [DOI] [PubMed] [Google Scholar]
- Novotny C. P., Fives-Taylor P. Retraction of F pili. J Bacteriol. 1974 Mar;117(3):1306–1311. doi: 10.1128/jb.117.3.1306-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C. P., Lavin K. Some effects of temperature on the growth of F pili. J Bacteriol. 1971 Sep;107(3):671–682. doi: 10.1128/jb.107.3.671-682.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Date T. Bacterial sex pili. Prog Biophys Mol Biol. 1975;30(1):23–56. doi: 10.1016/0079-6107(76)90004-3. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N. The transcriptional control of fertility in F-like plasmids. J Mol Biol. 1977 May 5;112(1):141–148. doi: 10.1016/s0022-2836(77)80161-7. [DOI] [PubMed] [Google Scholar]