Abstract
Annexins are proteins that bind lipids in the presence of calcium. Though multiple functions have been proposed for annexins, there is no general agreement on what annexins do or how they do it. We have used the well-studied conductance probes nonactin, alamethicin, and tetraphenylborate to investigate how annexins alter the functional properties of planar lipid bilayers. We found that annexin XII reduces the nonactin-induced conductance to ∼30% of its original value. Both negative lipid and ∼30 μM Ca2+ are required for the conductance reduction. The mutant annexin XIIs, E105K and E105K/K68A, do not reduce the nonactin conductance even though both bind to the membrane just as wild-type does. Thus, subtle changes in the interaction of annexins with the membrane seem to be important. Annexin V also reduces nonactin conductance in nearly the same manner as annexin XII. Pronase in the absence of annexin had no effect on the nonactin conductance. But when added to the side of the bilayer opposite that to which annexin was added, pronase increased the nonactin-induced conductance toward its pre-annexin value. Annexins also dramatically alter the conductance induced by a radically different probe, alamethicin. When added to the same side of the bilayer as alamethicin, annexin has virtually no effect, but when added trans to the alamethicin, annexin dramatically reduces the asymmetry of the I-V curve and greatly slows the kinetics of one branch of the curve without altering those of the other. Annexin also reduces the rate at which the hydrophobic anion, tetraphenylborate, crosses the bilayer. These results suggest that annexin greatly reduces the ability of small molecules to cross the membrane without altering the surface potential and that at least some fraction of the active annexin is accessible to pronase digestion from the opposite side of the membrane.
Keywords: ion channel, annexin, nonactin, alamethicin, tetraphenylborate
INTRODUCTION
Annexins are a family of calcium-dependent proteins that preferentially bind negatively charged lipids (Moss 1992; Swairjo et al. 1994; Swairjo and Seaton 1994). Annexins have been implicated in a number of membrane-associated biological processes, including vesicle fusion, vesicle trafficking, and ion channel formation, but their biological function remains unknown ( Pollard and Rojas 1988; Creutz 1992; Berendes et al. 1993).
High resolution x-ray crystal structures have been solved for several soluble annexins and some insight into the sites that mediate Ca2+ -dependent binding to bilayers has been obtained ( Swairjo et al. 1995). Annexin XII, originally purified from hydra (Schlaepfer et al. 1992), crystallized as a homo-hexamer (Luecke et al. 1995). Hexamer formation could be induced in solution in the presence of supraphysiological concentrations of Ca2+ (Mailliard et al. 1997), but hexamers were not detected on bilayers (Langen et al. 1998b). On bilayers, annexin XII ( Langen et al. 1998b) and annexin V ( Brisson et al. 1991; Huber et al. 1992) form trimers that correspond to half of the annexin XII hexamer and these trimers appear to sit on the surface of the bilayer. Annexins might well act by altering the properties of the lipid bilayer itself and thus alter the functional properties of proteins within the bilayer.
To test whether annexins could alter functional properties in a model system, we used the well-studied conductance probe nonactin. Nonactin is a hydrophobic cyclic molecule that chelates alkali cations and in effect converts them to hydrophobic cations that permeate the lipid bilayer much more easily than bare ions. This results in a very large increase in the conductance of the lipid bilayer. The carrier mechanism by which nonactin transports ions across the membrane has been extensively studied ( Lauger 1969, Lauger 1972; Lauger and Stark 1970; Szabo et al. 1972; Eisenman 1976) and is sufficiently well understood that the nonactin-induced conductance can be used as a probe for examining how a variety of molecules interact with the lipid bilayer (McLaughlin et al. 1970; Eisenman et al. 1973). In brief, nonactin adsorbed to the surface of the membrane rapidly complexes with cations in solution such that the surface concentration of nonactin–cation complex is proportional to the product of the cation concentration in solution and the membrane-surface concentration of nonactin. The rates of complex translocation across the bilayer are voltage dependent so that the current–voltage curve in symmetric solutions is a hyperbolic sine curve. The shape of this curve and the magnitude of the conductance (at constant nonactin concentration) are sensitive to surface charge, lipid fluidity, dipole moment, and other parameters of the lipid bilayer ( Latorre and Hall 1976). Thus the conductance induced by nonactin provides a sensitive measure of the functional properties of the lipid bilayer.
Alamethicin acts by a very different mechanism than nonactin. Consequently, the effects of annexin on the alamethicin conductance might reveal a different aspect of the action of annexin on the lipid bilayer than nonactin experiments, and combining the results of the two types of experiments might provide a clearer picture of the action of annexin than either alamethicin or nonactin experiments alone. Alamethicin is a 20 amino acid peptide that induces channels in planar lipid bilayers in a very voltage-dependent manner (Gordon and Haydon 1972; Boheim 1974; Sakmann and Boheim 1979; Hall and Cahalan 1982; Vodyanoy et al. 1983, Vodyanoy et al. 1988; Hall et al. 1984; Vodyanoy and Hall 1984; Menestrina et al. 1986; Eisenberg et al. 1997). It acts by inducing channels in the membrane rather than by a carrier mechanism such as nonactin and is very sensitive to changes in surface potential. Finally, we used the hydrophobic anion, tetraphenylborate, which induces capacitative currents in lipid bilayers (Melnik et al. 1977b; Andersen et al. 1978). By using both an anion and a cation probe, we can test whether a given effect is electrostatic or not. Combining the results using all three probes allows development of a consistent model for the action of annexins on the lipid bilayer.
METHODS
Bilayers were formed at room temperature by the union of two monolayers formed from a mixture (1:1 wt:wt) of phosphatidyl choline (840051, l -α PC egg; Avanti Polar Lipids) and phosphatidyl serine (840035, dioleolyl PS; Avanti Polar Lipids) as described elsewhere ( Zampighi et al. 1985; Ehring et al. 1992). In brief, lipid monolayers were opposed over a hole ∼100–200 μm in diameter in a 5-μm-thick Teflon partition dividing the two aqueous phases. The hole, punched by electric spark, was precoated with a 2.5% solution of squalane in n-pentane. Salt solutions were 100 mM NaCl, buffered by 10 mM HEPES-NaOH to pH 7.4, unless other wise noted. Solutions were stirred continuously with magnetic stirring bars, except that stirring was occasionally interrupted to record an I-V curve if stirring-induced noise was too objectionable. Bilayer formation was monitored by measuring capacitance. Silver/silver-chloride wires were used as electrodes to apply voltages and record currents across the bilayer. The rear-chamber potential was taken as ground and the additions of annexin were made to the front chamber (if not otherwise specified). For measurements of membrane conductance, a ramp protocol (−150 to +150 mV, 60 mV/s**) was used. Voltages were generated and currents digitized at a resolution of 12 bits by an AD Lab ADC/DAC board running software written in the lab. Currents were measured by an Axopatch 200A amplifier (Axon Instruments) connected to the AD Lab board as described above.
Alamethicin was purchased from Sigma Chemicals Co., and used without further purification (this is the same material originally provided by Upjohn Co.). Stock solutions were prepared as described.
Wild-type annexin XII was expressed in recombinant bacteria and purified as previously described (Mailliard et al. 1997). Three mutants of annexin XII (E105K, E105K/K68A, and K132E) were prepared by site-directed mutagenesis (Mailliard et al. 1997) and purified by the same procedure. All of the mutants bound phospholipid vesicles with similar Ca2+ dependence to wild-type annexin XII and had identical CD spectra to wild type.
Annexin-phospholipid binding studies were carried out in mock bilayer experiments using a standard bilayer chamber filled with the same solution used for bilayer experiments and spread with the same amount of phospholipid solution in pentane on the water surface as in bilayer experiments. These mock experiments were identical to standard bilayer experiments except that conductance was not measured. Known aliquots of annexin were added to the salt solution in increasing increments from 50 nM to 3 μM and allowed to equilibrate for 10 min with continuous stirring. Samples of solution were taken for quantitative analysis. Annexin XII was bound to Immobilon (Millipore Corp.) using a slot blot and stained with Coomassie Blue. The stained Immobilon was scanned using a Personal Densitometer running ImageQuant 3.22 (Molecular Dynamics, Inc.), and the amount of annexin XII was determined by comparison to a standard curve.
For pronase digestion experiments, pronase (P5147; Sigma Chemical Co.) was equilibrated with buffer (20 mM HEPES, pH 7.4, containing 100 mM NaCl) by dialysis. This step was crucial because pronase as purchased contained a dialyzable component that altered the nonactin-induced conductance. Preliminary experiments using annexin XII bound to phospholipid vesicles showed that 0.5 U of pronase degraded 5 μg of annexin XII to low molecular weight products within 10 min at room temperature. In the experiments presented in Fig. 6 and Fig. 7B and Fig. C, 10 U of dialyzed pronase (in 50 μl HEPES buffer) were added to the bilayer chamber to the side indicated.
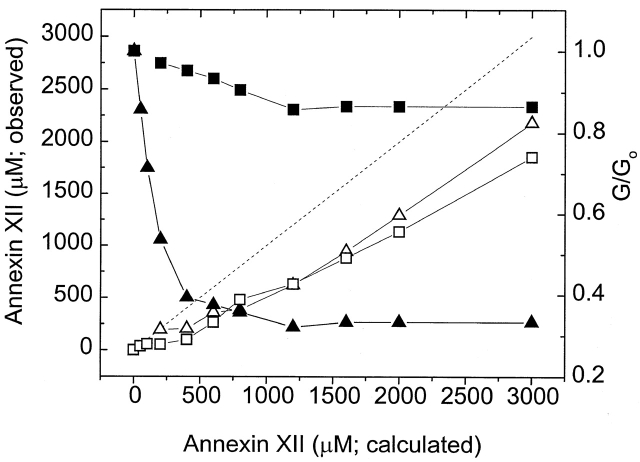
Figure 6.
Binding of annexin XII to lipid bilayers occurs at the same concentration as the reduction of nonactin-induced conductance. The free annexin XII concentration, measured as described in the text by quantitative Coomassie blue densitometry [wild type (▵), E105K (□), left-hand y axis], is plotted against the concentration of annexin XII calculated from the amount of annexin added to the bilayer chamber divided by the chamber volume. The dashed straight line shows what the concentration in solution would be if there were no adsorption of annexin to the bilayer. Thus, the difference between the actual concentration and this curve is proportional to the amount of annexin adsorbed to the bilayer. The plot of annexin XII reduction of the nonactin-induced conductance [wild type (▴), E105K (▪), right-hand y axis] shows that conductance reduction occurs in parallel with annexin adsorption to the lipid bilayer. (Adsorption measurements were done in dummy experiments in standard bilayer chambers with the same lipid solutions as in the conductance experiments.)
Figure 7.
Effect of pronase on the annexin XII reduction of nonactin-induced conductance. This plot shows nonactin-induced conductance as a function of time. First annexin XII was added to one side of lipid bilayer (cis side) and the conductance was allowed to stabilize at its steady state value. The addition of boiled pronase to the trans side of the bilayer had no effect on the conductance; however, the addition active pronase to the trans side resulted in an elevation of the conductance. The addition of pronase to the cis side resulted in a further increase in the conductance. Control experiments showed that pronase added in the absence of annexin had no effect on the nonactin-induced conductance. Note the expanded scale.
RESULTS
Annexin Reduces the Nonactin-induced Conductance
After addition of nonactin to an aqueous phase concentration of 5 × 10−7 M to both sides of the lipid bilayer, we observed a slow increase in conductance that reached steady state level in 30–60 min. In our experiments, we used Na+ as the current-carrying ion (rather than K+, which is conducted more efficiently by nonactin) to reduce the time taken to reach steady state conductance. This required a higher concentration of nonactin than necessary in KCl to achieve a sufficiently high conductance level.
Fig. 1 shows a series of I-V curves illustrating the effect of wild-type annexin XII on the conductance of a nonactin-containing bilayer in the presence of 1 mM Ca2+. The conductance decreased gradually with the addition of annexin XII to one side of the lipid bilayer. This effect of unilateral addition reached saturation between ∼0.8 and 1.2 μM annexin and reduced the conductance to about one third of the original steady state value. Once saturation was achieved by unilateral annexin addition, the addition of 1.2 μM annexin XII to the opposite side of bilayer caused an additional decrease in the conductance.
Figure 1.
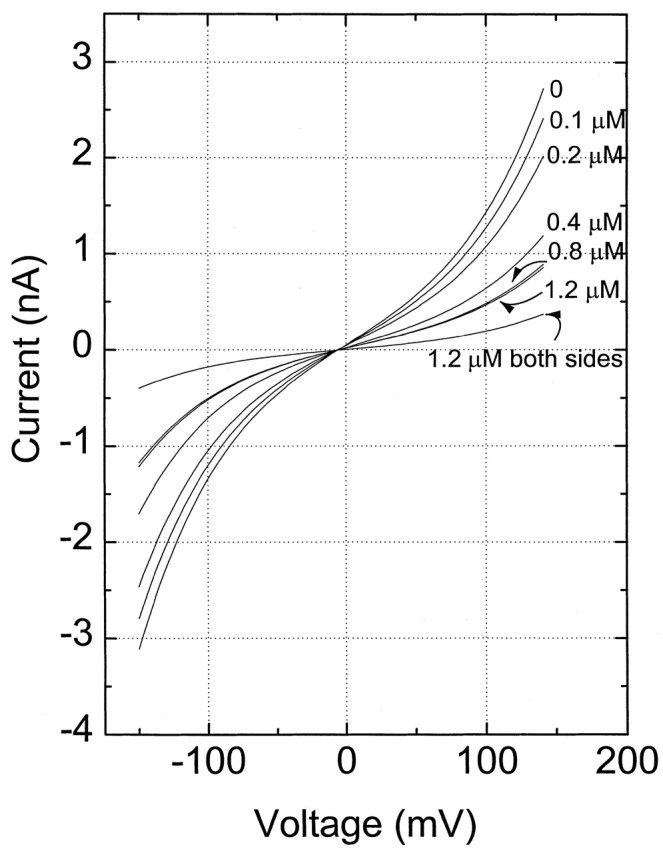
Example of nonactin current–voltage curves with increasing concentrations of annexin XII added to one side of the bilayer (except were noted). The aqueous solution on both sides of the bilayer was 100 mM NaCl, with a nonactin concentration of 5 × 10−7 M. Note that the I-V curves are very nearly symmetric.
Under our standard experimental conditions, pH 7.4, 100 mM NaCl, annexin XII alone in the absence of nonactin did not alter the membrane conductance in any detectable manner. However, at pH 6.5 and below, we do see weakly cation-selective ion channels induced by annexin XII. Thus, low pH can induce formation of ion channels by annexin XII. This finding is consistent with the finding of Langen et al. 1998a, who showed that annexin XII inserts into bilayers at low pH. The detailed characterization of these channels is not the subject of this paper, but we include a small sample of records obtained at pH 7.4 and 6.0 in Fig. 2. The long record taken at pH 7.4 is a sample of a much longer recording from a membrane that showed no channels in the presence of 400 nM annexin XII for 40 min. The trace taken at pH 6.0 shows the appearance of cation-selective channels (data on selectivity not shown) in a relatively short recording.
Figure 2.
Example of formation of cation-selective channels at pH 6.0. Solutions were 100 mM NaCl buffered to pH 7.4 with 10 mM HEPES, or 100 mM NaCl buffered to pH 6.0 with 10 mM HEPES. The recording at pH 7.5 is a sample of a membrane with 400 nM of annexin XII that exhibited no channel activity for 40 min. The recording at pH 6.0 was taken ∼10 min after addition of annexin XII to a concentration of 100 nM.
Ca2+ Alters Annexin Binding and the Ability to Reduce Nonactin-induced Conductance
Ca2+ plays an important role in the binding of annexin to phospholipids (Zaks and Creutz 1991; Swairjo et al. 1994, Swairjo et al. 1995; Swairjo and Seaton 1994). In a second series of experiments, we studied the effect of Ca2+ on the ability of annexin XII to reduce the conductance of a nonactin-containing bilayer. The values of G 0, the conductance in the absence of annexin, and G, the conductance in the presence of various concentrations of annexin, were measured as the chord conductance at +150 mV. A family of curves of G/G 0 as a function of annexin concentration taken at different Ca 2+ concentrations is shown in Fig. 3 . At high Ca2+ concentrations, 0.5 μM annexin XII greatly reduced the nonactin-induced conductance, and maximum conductance reduction occurred at annexin concentrations of 0.8–1 μM. Ca 2+ greatly potentiates the effect at annexin concentrations between 30 and 100 μM. But in the absence of Ca2+, annexin XII has no effect on nonactin conductance.
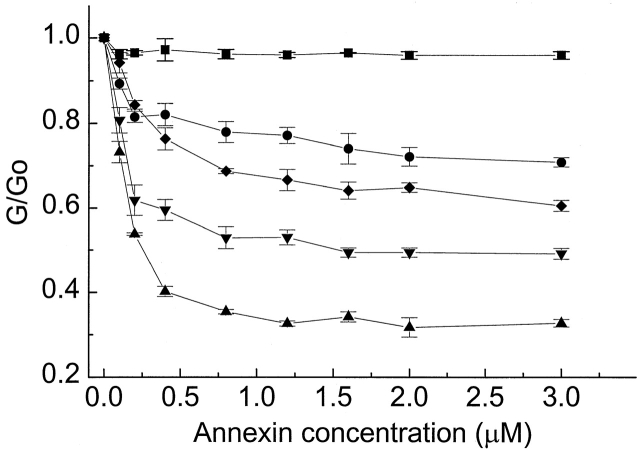
Figure 3.
The effect of calcium concentration on the ability of annexin XII to reduce the nonactin conductance. G/G0 is the ratio of the nonactin-induced conductance at the given annexin concentration to that at zero annexin concentration, both values being measured in the indicated medium. Solutions are 100 mM NaCl with the following calcium concentrations: 30 μM (•), 50 μM (♦), 0.1 mM (▾), and 1.0 mM (▴). In the absence of calcium (0.2 mM EDTA, ▪), annexin XII has no effect on the nonactin-induced conductance. But in the presence of 1 mM Ca2+, 3 μM annexin XII reduces the nonactin concentration to ∼40% that in 100 NaCl, 1mM Ca2+ with no annexin XII. Each point represents at least three measurements from three different bilayers.
Ability of Annexin XII to Reduce Nonactin Conductance Is Not Strongly Dependent on Ionic Strength
The ability of annexin XII to reduce the nonactin-induced conductance did not depend significantly on the concentration of NaCl. Fig. 4 compares the reduction of nonactin-induced annexin XII in solutions of 10, 30, and 100 mM NaCl. All solutions contained 10 mM HEPES and 1 mM Ca2+.
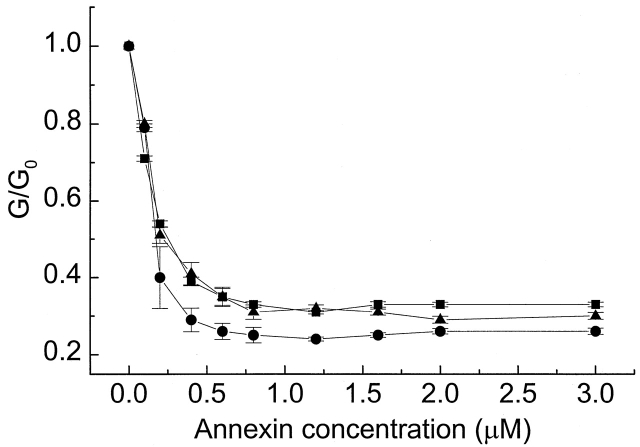
Figure 4.
The effect of salt concentration on the annexin XII reduction of nonactin-induced conductance. Curves were taken in 10 (•), 30 (▴), and 100 (▪) mM NaCl, each solution containing 10 mM HEPES and 1 mM Ca2+. Note that there is little effect of ionic strength on the reduction of nonactin conductance by annexin XII. Each point represents at least three measurements from three different bilayers.
Annexin XII did not significantly reduce the nonactin conductance in solutions containing Mg2+ instead of Ca2+. Also, the addition of 10 mM of Mg2+ to the annexin-containing side of the bilayer after the conductance decrease reached a saturation (in the presence of 1 mM Ca2+) caused a small but reproducible increase in G/G0 (data not shown). In control experiments in which free Ca2+ was eliminated by EDTA (Fig. 2 , top) or phosphatidyl ethanolamine was substituted for the phosphatidyl serine in the bilayer (data not shown), annexin had no effect on the nonactin-induced conductance. Annexin V reduces G/G0 to ∼0.2 in a manner very similar to that of annexin XII (Fig. 5.) Thus, the effects we are observing are not an idiosyncrasy of annexin XII, and may be a general property of annexins.
Figure 5.
The effect of annexin variants on the nonactin-induced conductance. G/G 0 is the ratio of the nonactin-induced conductance at the given annexin concentration divided by the conductance at zero annexin concentration. Note that the mutants E105K (▾) and E105K/K68A (▴) are both ineffective in reducing the nonactin conductance, but that wild-type annexin V (♦) and the mutant K132E (▪) are both approximately as effective as wild-type annexin XII (•). Because mutant annexin XII and wild type bind nearly identically to lipid bilayers [Fig. 6 shows this for our planar bilayers, Mailliard et al. 1997 for vesicles)], this result shows that conductance reduction is not simply a trivial effect of binding. The Ca 2+ concentration for all curves was 1 mM. Each point represents at least three measurements from three different bilayers.
The E105K Annexin Mutant Is Ineffective in Reducing Nonactin Conductance
Previous studies showed that annexin XII forms a hexamer in crystals ( Luecke et al. 1995) and in solution in the presence of very high Ca2+ concentrations (Mailliard et al. 1997). The hexamer is stabilized by a specific interaction of lys 68 with three peptide carbonyls in the opposing trimer and by an intermolecular Ca 2+ binding site formed, in part, by glu 105. Site-directed mutations at these sites (E105K and E105K/K68A) disrupt hexamer formation in solution without changing either the CD spectrum or Ca2+ -dependent binding to phospholipid vesicles ( Mailliard et al. 1997; W. Mailliard and H. Haigler, unpublished results). However, recent site-directed spin labeling studies of annexin XII did not detect hexamer formation on bilayers ( Langen et al. 1998b). These studies showed that >98% of the membrane-bound annexin XII formed a trimer corresponding to half of the hexamer, but the sensitivity of the method could not rule out the possibility that the hexameric form was populated by a very small percentage of the protein. Although the exact roles played by residues glu 105 and lys 68 in membrane-bound annexin XII are not clearly defined, these residues may be keys to understanding the biological function of annexins. They are two of the very few residues that are conserved in all of the many known sequences of mammalian annexins and thus deserve close scrutiny.
To further investigate the highly conserved glu 105 and lys 68 residues on annexin XII, we measured the effects of site-directed mutations at these residues on nonactin conductance. Fig. 5 shows that both E105K and E105K/K68A annexin XII decreased G/G 0 to only ∼0.8 of its initial steady state value, while wild-type annexin XII decreased it to ∼0.3. The dependence of G/G 0 on annexin concentration was fit to an adsorption isotherm of the form:
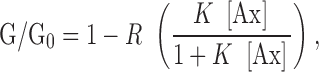 |
1 |
where R is the fraction by which the conductance is reduced at very high annexin concentration, [Ax] is the annexin concentration, and K is the association constant. Wild-type annexin XII concentration dependence was well fit by this adsorption isotherm with an apparent pK of 6.4, while the apparent pK for the mutants, E105K and E105K/K68A, was very approximately 4.3 (pK = −log K.) A site-directed mutant of annexin XII in which lysine 132 was mutated to glu (K132E) was also tested and found to be very similar to wild-type annexin XII in its ability to reduce nonactin conductance (Fig. 5). We also tested human annexin V and found the apparent pK of the isotherm was ∼5.7.
Although the E105K and E105K/K68A mutants did not reduce nonactin-mediated conductance as effectively as wild-type annexin XII, they bound equally effectively to phospholipid vesicles in the presence of Ca2+ (Mailliard et al. 1997). This implies that the mechanism of decreased nonactin conductance involves more than simple binding. To rule out the trivial possibility that the binding to planar lipid bilayers was significantly different than that to vesicles, we compared the E105K and wild-type annexin XII binding to phospholipid bilayers in the exact set-up used for the nonactin experiments. Increasing amounts of either E105K or wild-type annexin XII were added to bilayer chambers in the presence of 1 mM Ca2+ under conditions identical to those used in the nonactin experiments. Then the concentration of free annexin XII in small aliquots withdrawn from the chamber was measured by Coomassie staining and densitometry. For both wild-type and E105K annexin XII, there was no free annexin XII in solution after additions that were calculated to give a free concentration of 500 nM (Fig. 6). As higher amounts of protein were added, the measured protein concentration increased approximately in proportion to the amount added. Our interpretation of these data is that all of the protein in the initial additions became bound to the phospholipid and protein began to appear in solution only after the binding capacity of the phospholipid was saturated. The reduction of G/G 0 by wild-type annexin XII began to saturate at the same point as free annexin XII began to appear in solution, thereby implying that wild-type annexin XII is exerting its effect while bound to the bilayer. E105K and wild-type annexin XII had very similar binding curves ( Fig. 6), even though they had strikingly different effects on G/G0. The mechanistic implications of these data are considered in the discussion.
Trans Pronase Reduces the Annexin Reduction of Nonactin-induced Conductance
In an attempt to determine whether annexin XII added to one side of the membrane exerts its effect on the nonactin conductance via membrane-spanning structures, we added pronase to the trans compartment ( Fig. 7). (The cis compartment being that to which annexin XII is added and the trans compartment being the compartment on the opposite side of the membrane). After steady state conductance was achieved in the presence of 400 nM annexin XII (added to the cis side at point A, Fig. 7), the addition of pronase to the trans compartment (at point C) caused an increase in G/G0. Note that the addition of boiled pronase trans (at point B) before the addition of active pronase had no effect. The addition of pronase to the cis compartment (at point D, Fig. 7) resulted in a slight further increase in G/G0 (Fig. 7).
Fig. 7 shows an ideal experiment with control boiled pronase and active pronase added to the same membrane. This experiment was “ideal” in the sense that it proved possible to perform all the controls and the experimental addition of active pronase on one single bilayer. Additional experiments where only active pronase or only boiled pronase were added to the membrane supported the conclusions illustrated in Fig. 7. Though the magnitude of the conductance increase induced by trans pronase varied from experiment to experiment, it was always at least 20% of the decrease induced by addition of annexin XII (in four separate experiments). In control experiments, pronase had no effect on the conductance of a nonactin-containing bilayer in the absence of annexin, and pronase inactivated by boiling had no effect on the annexin reduction of the nonactin conductance when added to either the cis or trans side. Thus, pronase added to the trans side of the membrane reliably reduced the effect of annexin XII added to the cis side.
Cis and trans Annexin Act Very Differently on the Alamethicin Conductance
Alamethicin acts by a very different mechanism than nonactin. Consequently, the effects of annexin on the alamethicin conductance might reveal a different aspect of the action of annexin on the lipid bilayer than nonactin experiments. Combining the results of the two types of experiments might provide a clearer picture of the action of annexin than either alamethicin or nonactin experiments alone. Cis annexin XII has virtually no effect on the alamethicin-induced I-V curve (Fig. 8 A). The small shifts observed after annexin addition are similar to the shifts seen in the normal time course of an experiment in which no annexin was added. Fig. 8 A shows two I-V curves made by sweeping the voltage from −150 to +50 mV. One trace is a control in the presence of 10 × 10−7 M alamethicin added cis, and the second trace was taken after adding 1.2 μM annexin XII to the cis side. The voltage at which 0.5 μA of alamethicin current was obtained was 77.5 ± 3.3 mV (n = 10) before the addition of annexin, and 74.7 ± 2.1 mV (n = 9) after the addition of annexin XII cis in the particular experiment shown. Similar results were obtained in five additional experiments when annexin was added to the cis side of the membrane.
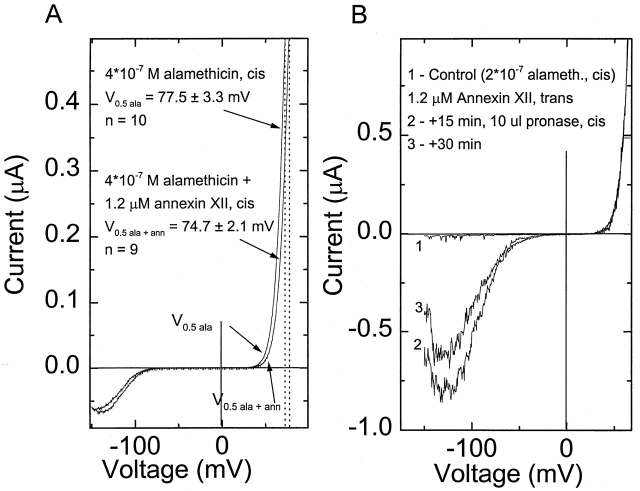
Figure 8.
Effects of annexin XII and pronase on alamethicin-induced conductance of bilayer. (A) I-V curves obtained when 4 × 10−7 of alamethicin and 1.2 μM of annexin XII were added to the cis side of the bilayer. Cis and trans solutions contained 100 mM NaCl, pH 7.4. (B, 1) I-V curve after addition of 2 × 10−7 M of alamethicin to the cis side of the bilayer, (2) I-V curve after 1.2 μM of annexin XII was added at trans side of bilayer, and (3) I-V curve taken after pronase was added to the cis side of bilayer. Ionic conditions are the same as in A.
Addition of annexin XII to the trans side of the membrane, opposite the addition of alamethicin, produced a quite different effect. The negative branch of the I-V curve is shifted dramatically to lower voltages, and the kinetics of the negative branch, but not the positive branch, are greatly slowed (data not shown). There is no shift of the positive branch of the I-V curve (Fig. 8 B, 1 and 2).
As in the case of the nonactin conductance, the effects of the E105K mutant on alamethicin conductance are much smaller than those of wild type (data not shown).
Cis Pronase Reduces the Trans–Annexin-induced Shift of the Negative Branch of the Alamethicin I-V Curve
The effect of trans annexin on the alamethicin conductance is reduced by pronase-added trans to the annexin (but cis to the alamethicin). Fig. 8 B shows I-V curves made by sweeping the voltage from −150 to +50 mV. Trace 1 was obtained after addition of 2 × 10−7 M alamethicin to the cis side of the bilayer. Trace 2 was obtained after the addition of 1.2 μM annexin XII to the trans side of the bilayer. Annexin addition increases the current of the negative branch and shifts it toward smaller magnitudes of applied voltage. Trace 3 shows the I-V curve after the addition of pronase on the cis side of the bilayer. This addition of pronase reduces the current of the negative branch of the I-V curve. Thus, pronase partly restores the character of the I-V curve to that which prevailed before the addition of annexin.
Annexin Slows the Kinetics of Tetraphenylborate Translocation
Annexin also alters the conductance induced by the hydrophobic anion tetraphenylborate. Tetraphenylborate adsorbs strongly to the bilayer surface and distributes across the membrane under the influence of the applied potential. When a voltage pulse is applied, tetraphenylborate moves from the adsorption layer on one side of the membrane to that on the other side, producing a capacitive sort of current whose time course is a measure of the rate at which tetraphenylborate crosses the membrane. In the presence of either unilaterally added annexin XII, the rate at which tetraphenylborate crosses the membrane is reduced. Bilateral addition of annexin XII slows the rate even more. Annexin also reduces the amount of tetraphenylborate adsorbed to the membrane (data not shown).
Fig. 9 A shows the current induced by tetraphenylborate (10−6 M) added to both sides of the lipid bilayer. The heavy solid curves show the currents induced by voltage pulses of ±200 mV in the absence of annexin. The heavy dotted curves show the tetraphenylborate current induced by the same voltage after the addition of 1.2 μM annexin cis. Note that both the amplitude and time constant are reduced. The heavy dashed curves show the tetraphenylborate current responses to pulses of the same voltages after the addition of annexin XII to both sides of the membrane at a concentration of 1.2 μM. All of these curves were fit to single exponentials (the calculated fitting curves are shown as lighter traces and are invisible where they overlap the data.). The time constants of the fits are shown in Fig. 9 B. Note that the time constant is progressively longer as the annexin is added first to one side and then to both sides. Note also that the effect of annexin on the time constants is nearly symmetrical for the positive and negative voltage pulses. Thu, s annexin, even added unilaterally, does not induce any intrinsic potential bias in the interior of the membrane.
Figure 9.
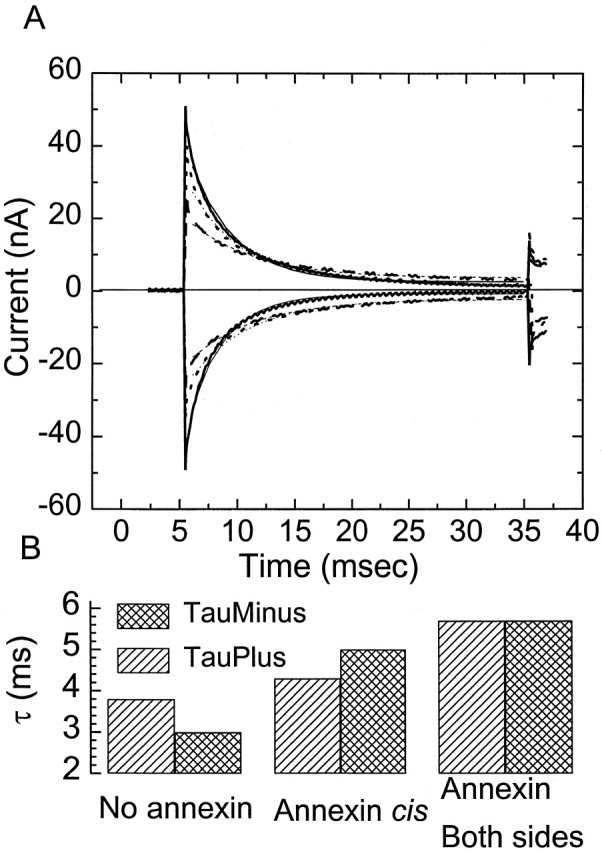
Effect of annexin XII on tetraphenylborate-induced currents. Cis and trans solutions contained 100 mM NaCl, pH 7.4. Tetraphenylborate was added to both sides of the bilayer at a concentration of 10−6 M. (A) Current traces induced by voltage pulses of ±200 mV: heavy solid lines plot currents in the absence of annexin, heavy dotted lines plot currents after the addition of 1.2 μM of annexin XII to the cis side, heavy dashed curves plot currents after addition of 1.2 μM to both sides of the bilayer. The calculated single exponential fits to each curve are shown as lighter traces with the same line character as the data generating the fit. (B) The time constants of the fits obtained from the data in A. Note that both unilateral and bilateral annexin increase the time constant; that is, reduce the rate of translocation of tetraphenylborate.
DISCUSSION
Although the exact physiological roles of annexins are unknown, there are a number of proposed functions, including vesicle transport, exocytosis, and channel formation (Rojas et al. 1990; Berendes et al. 1993; Burger et al. 1994, Burger et al. 1996; Demange et al. 1994; Arispe et al. 1996). In this paper, we show that annexin XII and annexin V have a profound effect on the functional properties of a planar lipid bilayer (Fig. 1 and Fig. 3 Fig. 4 Fig. 5). In fact, in 1 mM Ca2+, they reduce the nonactin-induced conductance by >60%, in parallel with their ability to bind to negatively charged membranes in the presence of calcium ( Fig. 1 and Fig. 3 Fig. 4 Fig. 5 ). In addition, annexins alter the characteristic properties of alamethicin-induced conductance differently, depending on which side of the membrane they are added to. Thus, annexins alter important global properties of the lipid bilayer in such a way as to change the properties of resident conductance mechanisms. Peripheral binding to the membrane cannot account for all of the effects. The E105K mutant binds just as well as wild type, but has little effect on the nonactin-induced conductance. Our results allow us to characterize partly the annexin structures that alter the properties of the lipid bilayer. We believe we can construct a consistent picture of the action of annexin XII on the lipid bilayer making use of our conductance probe data as well as spin label experiments published elsewhere (Langen et al. 1998a, Langen et al. 1998b). Although most of our results were obtained with annexin XII, annexin V acts in qualitatively the same manner on both nonactin and alamethicin. Thus, the ability to alter the functional properties of the lipid bilayer may be a general property of annexins.
Before discussing how annexins alter the functional properties of the lipid bilayer, we consider two peripheral issues: formation of ion channels by annexins and evidence that annexins cross or span the lipid bilayer.
Annexin XII Does Not Form Channels under Our Experimental Conditions
Our results indicate that a substantial amount of annexin XII is bound to the membrane. Yet annexin XII per se does not increase the conductance of the membrane under these conditions. Indeed, part of our motivation for these studies was our inability to observe ion channels induced by annexin at neutral pH. We wondered if annexin was in fact interacting with our bilayers in any way. These experiments, combined with our failure to observe channels induced by annexin alone under the conditions of our experiments, allow us to provide an upper limit on the probability of an adsorbed annexin molecule forming an ion channel under these conditions.
To estimate the amount of membrane-bound annexin, we must necessarily assume a relation between the amount of bound annexin to the effect of annexin on the nonactin-induced conductance. It seems to us that a reasonable assumption is that, at concentrations of annexin above ∼1 μM, the membrane surface is nearly covered with annexin. This would be in agreement with its ability to inhibit phospholipase A2 by denying the enzyme access to substrate (Haigler et al. 1987). Our planar bilayers are ∼100 μm in diameter, so taking the area of the annexin molecule as ∼104 square angstroms (something of an overestimate) would give us ∼3 × 108 annexin molecules bound to the surface at saturation.
If we had, on average, three channels under these conditions, the probability of channel formation would be ∼1 in 108. We did not actually see any channels at pH 7.5, even at saturating annexin concentrations. Thus, taking an average of three observed channels under these conditions provides very much an upper limit on the channel-forming probability at neutral pH. If the annexin density were 100× smaller than the above estimate, the channel forming probability for a membrane bound annexin would be 100× larger, or 1 in 106! Nonactin-induced conductance is reduced to less than half its control value at saturating concentrations of annexin. It is hard to see how this could happen if annexin occupied much less than 1% of the membrane area, so the upper and lower estimates above likely bracket the true amount of annexin bound. We never observed any channels at neutral pH. And even by the very generous over estimate described above, which allows that we might have missed three channels, the probability of annexin XII forming ion channels under the conditions of our experiments is very small, somewhere between 1 in 106 and 1 in 108.
But can annexins form channels under any conditions? The answer is clearly yes. We have found that reducing pH to ∼6.5 or lower induces annexin XII to form slightly cation-selective channels, as shown in Fig. 2. Moreover, Langen et al. 1998a, using spin label techniques, have shown that annexin inserts into the lipid bilayer at low pH. But the principal and most dramatic effect of annexins XII and V at neutral pH is to alter the properties of the bilayer, not to form channels, and it is this effect that is the subject of this paper.
An Appreciable Fraction of the Bound Annexin XII Is Accessible to Pronase on the Opposite Side of the Membrane
The effects of pronase in both alamethicin and nonactin experiments show that annexin on one side of the membrane is accessible to digestion from the other side (Fig. 7 and Fig. 8 B). Pronase is well known not to cross cell membranes and has been a useful tool for revealing membrane protein topology and cannot cross the bilayer (see, for example, Bezanilla and Armstrong 1977; Arias and Kyte 1979; Rottem et al. 1979; Wagner and Kelly 1979). Because pronase alone has no effect on either the nonactin- or the alamethicin-induced conductance, its effect in the presence of annexin XII must be mediated by digestion of annexin XII on one side of the membrane and by pronase on the other side. Our experiments are consistent with two possibilities. Either annexin XII adsorbs to the surface of the membrane (or to a single monolayer of the bilayer), but is able to cross the membrane from one side to the other where it can be digested, or annexin XII forms a transmembrane structure accessible to proteolytic digestion from both sides of the bilayer. If the first of these possibilities is true, annexin XII would have to cross the membrane quite rapidly to produce a species of annexin XII on the trans side, which is both accessible to digestion and capable of reducing the nonactin conductance. The spin label finding ( Langen et al. 1998a) that low pH stabilizes a transmembrane form of annexin XII suggests that the most likely possibility is that the transmembrane annexin species seen at acid pH can also exist at neutral pH. But at neutral pH the transmembrane form is too transient, or present in too small a proportion, to form channels or be detected by spin-label methods.
Possible Modes of Annexin Action
But even though annexin does not form channels under the conditions of our experiments, it clearly alters the properties of the lipid bilayer. How might annexin be influencing the conducting properties of nonactin and alamethicin? The first possibility that comes to mind is alteration of the membrane surface charge that would change the concentration of cations near the surface and thus change the concentration of charge carrier. Increased positive surface charge would reduce the nonactin-induced conductance by reducing the cation concentration at the surface of the membrane. This might occur through a binding of negatively charged lipid or addition of charges intrinsic to the annexin molecule itself. Alternatively, annexin XII could reduce the surface area of the membrane available to nonactin, alter the fluidity of the membrane, produce a phase separation of lipids, or change the dipole potential of the membrane ( Hall and Latorre 1976; Latorre and Hall 1976; Melnik et al. 1977a). For example, a plug of annexin inserted into one or both monolayers of the bilayer could decrease the effective lipid area of the membrane and thus reduce the conductance. The effective reduction of the conductance could be disproportionately larger than the area of the annexin molecule itself, owing to propagating effects on the structure of the bilayer. Finally, annexin could alter the fluidity of the bilayer, perhaps by restraining head groups in a particular pattern or bending the bilayer locally counter to its natural curvature so as to reduce the rate of translocation of the nonactin-cation complex and trap alamethicin at the surface. Or a combination of these effects could occur in parallel. Our data do not reveal the detailed mechanism, but definitely show that annexin makes the membrane more resistant to the passage of small molecules, regardless of shape or charge.
Annexin Does Not Act by Simple Surface Charge
Several lines of evidence eliminate the possibility that annexin acts by altering the surface charge of the membrane. First, annexin XII itself is nearly neutral at neutral pH and thus is not likely to be able to appreciably alter the surface charge density by more than a fraction of a charge per 100 Å2. Such a small change in surface charge density cannot account for the large change in conductance observed ( McLaughlin et al. 1970). Moreover, the addition of the divalent cation magnesium (which would be expected to reduce the conductance if surface charge were the cause) actually results in an increase in conductance (data not shown, magnesium reduces the effect of annexin, possibly by interfering with one more of the Ca2+ binding sites). Second, as shown in Fig. 3, there is little difference in the maximum reduction of nonactin-induced conductance in 10 mM NaCl (G/G0 = 0.25) and 100 mM NaCl (G/G 0 = 0.3). If the alteration of conductance were mediated by a change in surface charge, the reduction should be much smaller in 100 mM salt. If the entire reduction in conductance were attributed to surface charge, the calculated change in surface potential from zero annexin to a saturating annexin concentration would be ∼30 mV in 100 mM salt. This predicts that G/G0 should be 0.05 in 10 mM salt at high annexin concentrations. The actual value (Fig. 3) is ∼0.25. Thus, the surface charge component of the conductance reduction, if any, must be small.
In addition, K132E, which is two electronic charges more negative than wild type, has essentially the same ability to lower conductance as wild type. A change of this magnitude in a molecule with many charges might not be significant, but this datum clearly shows that a small change in charge is not sufficient in itself to alter the ability of annexin XII to reduce the nonactin-induced conductance.
Moreover, the nonactin current–voltage curves (Fig. 1) are symmetrical within experimental error. This indicates that there is very little, if any, asymmetry in the surface charge induced by the addition of annexin XII to one side of the membrane. The relatively large reduction in conductance by annexin would be accompanied by a large asymmetry in the I-V curve if a significant portion of the effect were mediated by surface charge ( Hall and Latorre 1976; Latorre and Hall 1976).
Alamethicin is much more sensitive to transmembrane potentials than nonactin, and the affect of both cis and trans annexin XII on the alamethicin conductance is inconsistent with either a surface-charge or a dipole-potential mechanism. The shift of the alamethicin I-V curve seen when annexin is added to the cis side of the membrane is always very small (∼2 mV in Fig. 8 A). If there were a shift in potential large enough to explain the reduction in the nonactin conductance, we would expect to see a shift in the alamethicin I-V curve on the order of 25 mV or so.
Finally, annexin reduces the rate of tetraphenylborate translocation across the bilayer. The nonactin-Na+ (or K+) complex is a cation. Because tetraphenylborate is an anion, any electrostatic effect would have to act oppositely on tetraphenylborate and nonactin, yet annexin slows the rate at which both of these probes cross the membrane and by approximately the same amount.
We conclude that the annexin-induced surface charge plays a negligible role in altering the properties of the lipid bilayer. The above considerations also rule out an annexin-induced dipole potential that would shift the alamethicin conductance voltage curve on addition to either side of the membrane and would act on tetraphenylborate oppositely from nonactin.
Reduction of Nonactin-induced Conductance Is Not a Simple Consequence of Annexin Binding to the Membrane Surface
Binding to the membrane surface alone is not sufficient to explain the effects of annexin on membrane properties because the E105K mutant that binds equally well to phospholipid vesicles does not reduce the nonactin conductance to nearly the same degree as wild type. This is so in spite of the increased positive charge of E105K that would be expected to decrease cation conductance even more if annexin charge were an important factor. This is consistent with the observation that changing the charge in K132E has little effect on the ability of this mutant to reduce the nonactin-induced conductance.
Possible Mechanisms of Annexin Action
The above experiments conclusively rule out changes in surface charge and dipole potential as mechanisms by which annexin reduces nonactin conductance, and they demonstrate that the mechanism of action is highly structure specific. All three classes of experiments, on nonactin, alamethicin, and tetraphenylborate, suggest that annexin acts by hindering the ability of each of the probes to cross the membrane, not by altering electrostatics. Such a mechanism is obviously consistent with the reduction of the nonactin conductance and the slowing of tetraphenylborate translocation. But its applicability to the alamethicin experiments is less apparent.
First let us consider the failure of cis annexin to have any effect on the alamethicin I-V curve (Fig. 8 A). In this case, annexin does not alter the partition of alamethicin to the surface of the bilayer and apparently has little effect on the insertion of alamethicin into the membrane under the influence of the electric field. However, when added to the trans side, annexin greatly alters the negative branch of the I-V curve. We suggest this occurs because annexin decreases the rate of alamethicin transfer from the trans membrane–aqueous interface to the trans aqueous phase, causing an increase in the surface concentration of alamethicin on the trans side and a consequent increase in the conductance for a given negative voltage. This mechanism is consistent with the known actions of alamethicin ( Schindler 1979; Hall 1981), the reduction of the nonactin conductance by annexin, and the slowing of the tetraphenylborate translocation rate.
Subtle structural features clearly play a role in altering membrane functional properties. The E105K mutant is much less effective than wild-type annexin XII in altering the conductances induced by both alamethicin and nonactin, even though its crystal structure is remarkably similar to that of wild type (Cartailler, J.-P., H.T. Haigler, and H. Luecke, manuscript in preparation). Our data suggest that the E105K mutant interacts with the lipid bilayer in a radically different manner from wild-type annexin XI. Just how these interactions differ is not clear, but there are interesting differences in the crystal structures of wild-type annexin XII and the E105K mutant. While the wild-type crystallizes in the presence of Ca2+ at pH 7.8, the mutant only crystallizes at low Ca2+ concentrations under mildly acidic conditions (Cartailler, J.-P., H.T. Haigler, and H. Luecke, manuscript in preparation). Comparing and contrasting the effects of these two proteins should provide insight into the mechanisms by which they alter the properties of lipid bilayers.
Our experiments do not completely elucidate the mechanism by which annexin hinders the movement of these probes across the membrane, but they do suggest a few possibilities. Increases in viscosity, lipid phase separation, or changes in lipid curvature are reasonable, but by no means the only, candidates for mechanism of action. Of this short list, curvature may be the most attractive because it offers the possibility of separating effect from binding. For example, curvature could explain the E105K mutant results as the ability to bind but not bend the membrane. Determination of which mechanism or mechanisms annexin actually uses to alter membrane properties will require additional experiments, but our experiments demonstrate clearly that annexin XII and annexin V dramatically increase the resistance of lipid bilayers to the passage of small probe molecules. These results suggest that a possible physiological role of annexins could be to modulate membrane properties in a manner that can be controlled by local concentrations of calcium and protons.
Acknowledgments
We thank Mary Hawley for expert technical assistance.
This work was supported in part by National Institutes of Health grants EY05561 and GM57998 to J.E. Hall, GM55651 to H.T. Haigler, and GM56445 to H. Luecke, and by 5T32CA09054 predoctoral training support for W.S. Mailliard.
References
- Andersen O.S. , Feldberg S. , Nakadomari H. , Levy S. , McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys. J. 1978 ;21:35–70 . doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I.M. , Kyte J. Examination of intramolecular heterogeneity of plasma membrane protein degradation in canine renal tubular epithelial cells and in rat liver. Biochim. Biophys. Acta. 1979 ;557:170–178 . doi: 10.1016/0005-2736(79)90099-3. [DOI] [PubMed] [Google Scholar]
- Arispe N. , Rojas E. , Genge B.R. , Wu L.N.Y. , Wuthier R.E. Similarity in calcium channel activity of annexin V and matrix vesicles in planar lipid bilayers. Biophys. J. 1996 ;71:1764–1775 . doi: 10.1016/S0006-3495(96)79377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendes R. , Voges D. , Demange P. , Huber R. , Burger A. Structure–function analysis of the ion channel selectivity filter in human annexin V. Science. 1993 ;262:427–430 . doi: 10.1126/science.7692599. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. , Armstrong C.M. Inactivation of the sodium channel. I. Sodium current experiments . J. Gen. Physiol. 1977 ;70:549–566 . doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J. Membr. Biol. 1974 ;19:277–303 . doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Brisson A. , Mosser G. , Huber R. Structure of soluble and membrane-bound human annexin V. J. Mol. Biol. 1991 ;220:199–203 . doi: 10.1016/0022-2836(91)90002-n. [DOI] [PubMed] [Google Scholar]
- Burger A. , Berendes R. , Liemann S. , Benz J. , Hofmann A. , Gottig P. , Huber R. , Gerke V. , Thiel C. , Romisch J. , Weber K. The crystal structure and ion channel activity of human annexin II, a peripheral membrane protein. J. Mol. Biol. 1996 ;257:839–847 . doi: 10.1006/jmbi.1996.0205. [DOI] [PubMed] [Google Scholar]
- Burger A. , Voges D. , Demange P. , Perez C.R. , Huber R. , Berendes R. Structural and electrophysiological analysis of annexin V mutants. Mutagenesis of human annexin V, an in vitro voltage-gated calcium channel, provides information about the structural features of the ion pathway, the voltage sensor and the ion selectivity filter. J. Mol. Biol. 1994 ;237:479–499 . doi: 10.1006/jmbi.1994.1249. [DOI] [PubMed] [Google Scholar]
- Creutz C.E. The annexins and exocytosis. Science. 1992 ;258:924–931 . doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Demange P. , Voges D. , Benz J. , Liemann S. , Gottig P. , Berendes R. , Burger A. , Huber R. Annexin Vthe key to understanding ion selectivity and voltage regulation? Trends Biochem. Sci. 1994 ;19:272–276 . doi: 10.1016/0968-0004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Ehring G.R. , Lagos N. , Zampighi G.A. , Hall J.E. Phosphorylation modulates the voltage dependence of channels reconstituted from the major intrinsic protein of lens fiber membranes . J. Membr. Biol. 1992 ;126:75–88 . doi: 10.1007/BF00233462. [DOI] [PubMed] [Google Scholar]
- Eisenberg M. , Hall J.E. , Mead C.A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J. Membr. Biol. 1997 ;14:143–176 . doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Eisenman G. The molecular basis for ion selectivity and its possible bearing on the neurobiology of lithium. Neurosci. Res. Program. Bull. 1976 ;14:154–161 . [PubMed] [Google Scholar]
- Eisenman G. , Szabo G. , McLaughlin S.G. , Ciani S.M. Molecular basis for the action of macrocyclic carriers on passive ionic translocation across lipid bilayer membranes. J. Bioenerg. 1973 ;4:93–148 . doi: 10.1007/BF01516052. [DOI] [PubMed] [Google Scholar]
- Gordon L.G. , Haydon D.A. The unit conductance channel of alamethicin. Biochim. Biophys. Acta. 1972 ;255:1014–1018 . doi: 10.1016/0005-2736(72)90415-4. [DOI] [PubMed] [Google Scholar]
- Haigler H.T. , Schlaepfer D.D. , Burgess W.H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J. Biol. Chem. 1987 ;262:6921–6930 . [PubMed] [Google Scholar]
- Hall J.E. Voltage-dependent lipid flip-flop induced by alamethicin. Biophys. J. 1981 ;33:373–381 . doi: 10.1016/S0006-3495(81)84901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E. , Cahalan M.D. Calcium-induced inactivation of alamethicin in asymmetric lipid bilayers. J. Gen. Physiol. 1982 ;79:387–409 . doi: 10.1085/jgp.79.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E. , Latorre R. Nonactin-K+ complex as a probe for membrane asymmetry . Biophys. J. 1976 ;16:99–103 . doi: 10.1016/S0006-3495(76)85667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E. , Vodyanoy I. , Balasubramanian T.M. , Marshall G.R. Alamethicina rich model for channel behavior. Biophys. J. 1984 ;45:233–247 . doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R. , Berendes R. , Burger A. , Schneider M. , Karshikov A. , Luecke H. , Romisch J. , Paques E. Crystal and molecular structure of human annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the annexin family of proteins. J. Mol. Biol. 1992 ;223:683–704 . doi: 10.1016/0022-2836(92)90984-r. [DOI] [PubMed] [Google Scholar]
- Langen R. , Isas J.M. , Hubbell W.L. , Haigler H.T. A transmembrane form of annexin XII detected by site-directed spin labeling Proc. Natl. Acad. Sci. USA. 95 1998 . 14060 14065a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen R. , Isas J.M. , Luecke H. , Haigler H.T. , Hubbell W.L. Membrane-mediated assembly of annexins studied by site-directed spin labeling J. Biol. Chem. 273 1998 . 22453 22457b [DOI] [PubMed] [Google Scholar]
- Latorre R. , Hall J.E. Dipole potential measurements in asymmetric membranes. Nature. 1976 ;264:361–363 . doi: 10.1038/264361a0. [DOI] [PubMed] [Google Scholar]
- Lauger P. Transport phenomena in membranes. Angew. Chem. Int. Ed. Engl. 1969 ;8:42–54 . doi: 10.1002/anie.196900421. [DOI] [PubMed] [Google Scholar]
- Lauger P. Carrier-mediated ion transport. Science. 1972 ;178:24–30 . doi: 10.1126/science.178.4056.24. [DOI] [PubMed] [Google Scholar]
- Lauger P. , Stark G. Kinetics of carrier-mediated ion transport across lipid bilayer membranes. Biochim. Biophys. Acta. 1970 ;211:458–466 . doi: 10.1016/0005-2736(70)90251-8. [DOI] [PubMed] [Google Scholar]
- Luecke H. , Chang B.T. , Mailliard W.S. , Schlaepfer D.D. , Haigler H.T. Crystal structure of the annexin XII hexamer and implicatrions for bilayer insertion. Nature. 1995 ;378:512–515 . doi: 10.1038/378512a0. [DOI] [PubMed] [Google Scholar]
- Mailliard W.S. , Luecke H. , Haigler H.T. Annexin XII forms calcium-dependent hexamers in solution and on phospholipid bilayersa chemical cross-linking study. Biochemistry. 1997 ;36:9045–9050 . doi: 10.1021/bi970749v. [DOI] [PubMed] [Google Scholar]
- McLaughlin S.G. , Szabo G. , Eisenman G. , Ciani S.M. Surface charge and the conductance of phospholipid membranes. Proc. Natl. Acad. Sci. USA. 1970 ;67:1268–1275 . doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik E. , Latorre R. , Hall J.E. , Tosteson D.C. Phloretin-induced changes in ion transport across lipid bilayer membranes J. Gen. Physiol. 69 1977 . 243 257a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnik E. , Latorre R. , Hall J.E. , Tosteson D.C. Phloretin-induced changes in ion transport across lipid bilayer membranes J. Gen. Physiol. 69 1977 . 243 257b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina G. , Voges K.P. , Jung G. , Boheim G. Voltage-dependent channel formation by rods of helical polypeptides . J. Membr. Biol. 1986 ;93:111–132 . doi: 10.1007/BF01870804. [DOI] [PubMed] [Google Scholar]
- Moss S.E. The Annexins 1992 . Portland Press Ltd; London, UK: pp. 173 pp [Google Scholar]
- Pollard H.B. , Rojas E. Ca2+-activated synexin forms highly selective, voltage-gated Ca2+ channels in phosphatidylserine bilayer membranes. Proc. Natl. Acad. Sci. USA. 1988 ;85:2974–2978 . doi: 10.1073/pnas.85.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E. , Pollard H.B. , Haigler H.T. , Parra C. , Burns A.L. Calcium-activated endonexin II forms calcium channels across acidic phospholipid bilayer membranes. J. Biol. Chem. 1990 ;265:21207–21215 . [PubMed] [Google Scholar]
- Rottem S. , Markowitz O. , Hasin M. , Razin S. Outer membrane proteins of smooth and rough strains of Proteus mirabilis . Eur. J. Biochem. 1979 ;97:141–146 . doi: 10.1111/j.1432-1033.1979.tb13095.x. [DOI] [PubMed] [Google Scholar]
- Sakmann B. , Boheim G. Alamethicin-induced single channel conductance fluctuations in biological membranes. Nature. 1979 ;282:336–339 . doi: 10.1038/282336a0. [DOI] [PubMed] [Google Scholar]
- Schindler H. Autocatalytic transport of the peptide antibiotics suzukacillin and alamethicin across lipid membranes. FEBS Lett. 1979 ;104:157–160 . doi: 10.1016/0014-5793(79)81105-9. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D. , Fisher D.A. , Brandt M.E. , Bode H.R. , Jones J.M. , Haigler H.T. Identification of a novel annexin in Hydra vulgaris. Characterization, cDNA cloning, and protein kinase C phosphorylation of annexin XII. J. Biol. Chem. 1992 ;267:9529–9539 . [PubMed] [Google Scholar]
- Swairjo M.A. , Concha N.O. , Kaetzel M.A. , Dedman J.R. , Seaton B.A. Ca(2+)-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 1995 ;2:968–974 . doi: 10.1038/nsb1195-968. [DOI] [PubMed] [Google Scholar]
- Swairjo M.A. , Roberts M.F. , Campos M.B. , Dedman J.R. , Seaton B.A. Annexin V binding to the outer leaflet of small unilamellar vesicles leads to altered inner-leaflet properties31P- and 1H-NMR studies. Biochemistry. 1994 ;33:10944–10950 . doi: 10.1021/bi00202a013. [DOI] [PubMed] [Google Scholar]
- Swairjo M.A. , Seaton B.A. Annexin structure and membrane interactionsa molecular perspective. Annu. Rev. Biophys. Biomol. Struct. 1994 ;23:193–213 . doi: 10.1146/annurev.bb.23.060194.001205. [DOI] [PubMed] [Google Scholar]
- Szabo G. , Eisenman G. , McLaughlin S.G. , Krasne S. Ionic probes of membrane structures. Ann. NY Acad. Sci. 1972 ;195:273–290 . [PubMed] [Google Scholar]
- Vodyanoy I. , Hall J.E. Thickness dependence of monoglyceride bilayer membrane conductance . Biophys. J. 1984 ;46:187–193 . doi: 10.1016/S0006-3495(84)84012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanoy I. , Hall J.E. , Balasubramanian T.M. Alamethicin-induced current-voltage curve asymmetry in lipid bilayers. Biophys. J. 1983 ;42:71–82 . doi: 10.1016/S0006-3495(83)84370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodyanoy I. , Hall J.E. , Vodyanoy V. Alamethicin adsorption to a planar lipid bilayer. Biophys. J. 1988 ;53:649–658 . doi: 10.1016/S0006-3495(88)83145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J.A. , Kelly R.B. Topological organization of proteins in an intracellular secretory organellethe synaptic vesicle. Proc. Natl. Acad. Sci. USA. 1979 ;76:4126–4130 . doi: 10.1073/pnas.76.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaks W.J. , Creutz C.E. Ca(2+)-dependent annexin self-association on membrane surfaces . Biochemistry. 1991 ;30:9607–9615 . doi: 10.1021/bi00104a007. [DOI] [PubMed] [Google Scholar]
- Zampighi G.A. , Hall J.E. , Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc. Natl. Acad. Sci. USA. 1985 ;82:8468–8472 . doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]