Abstract
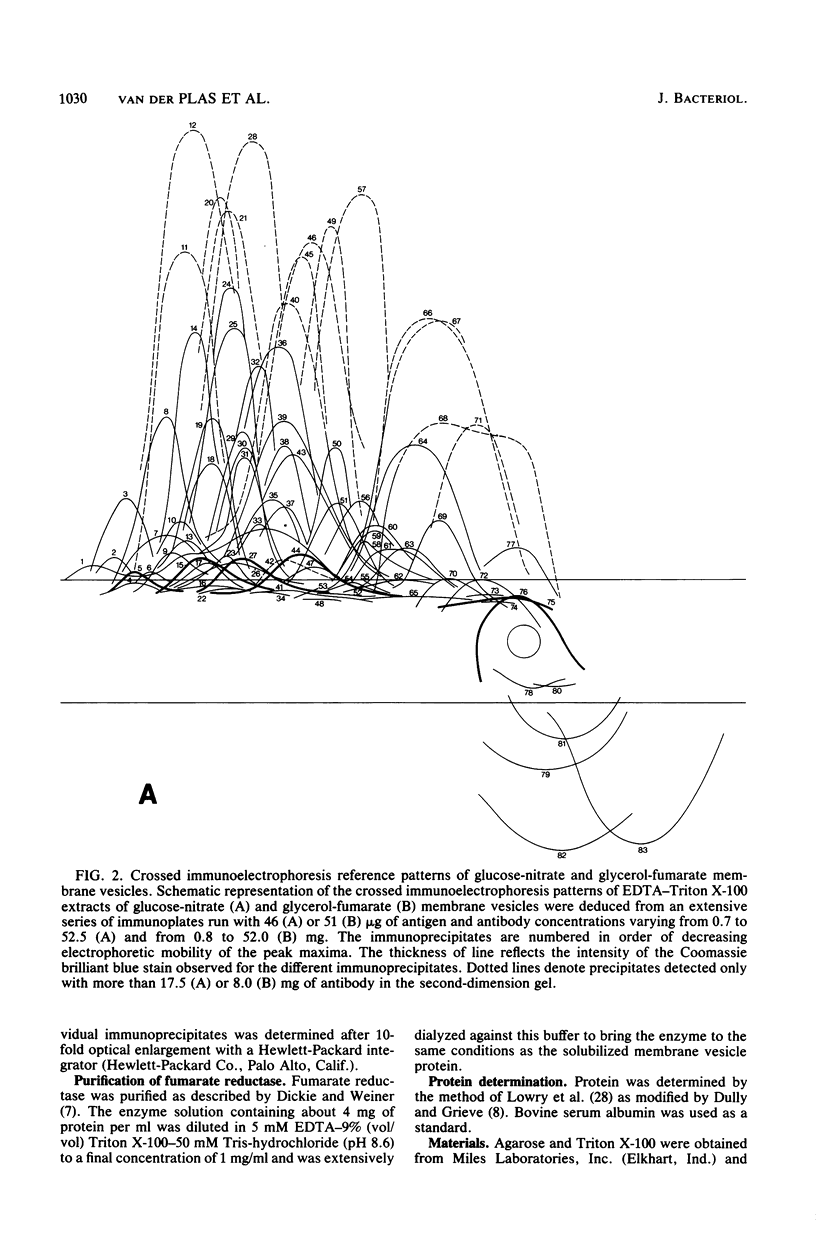
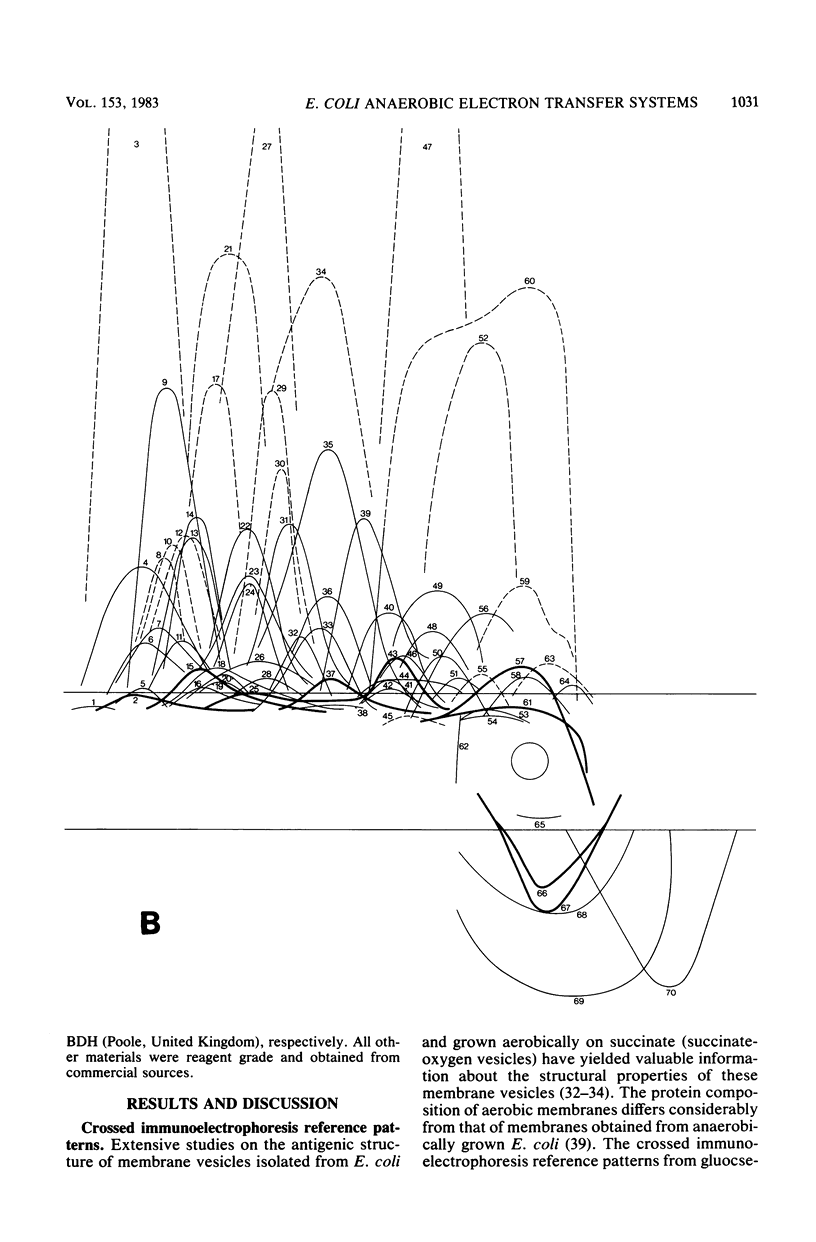
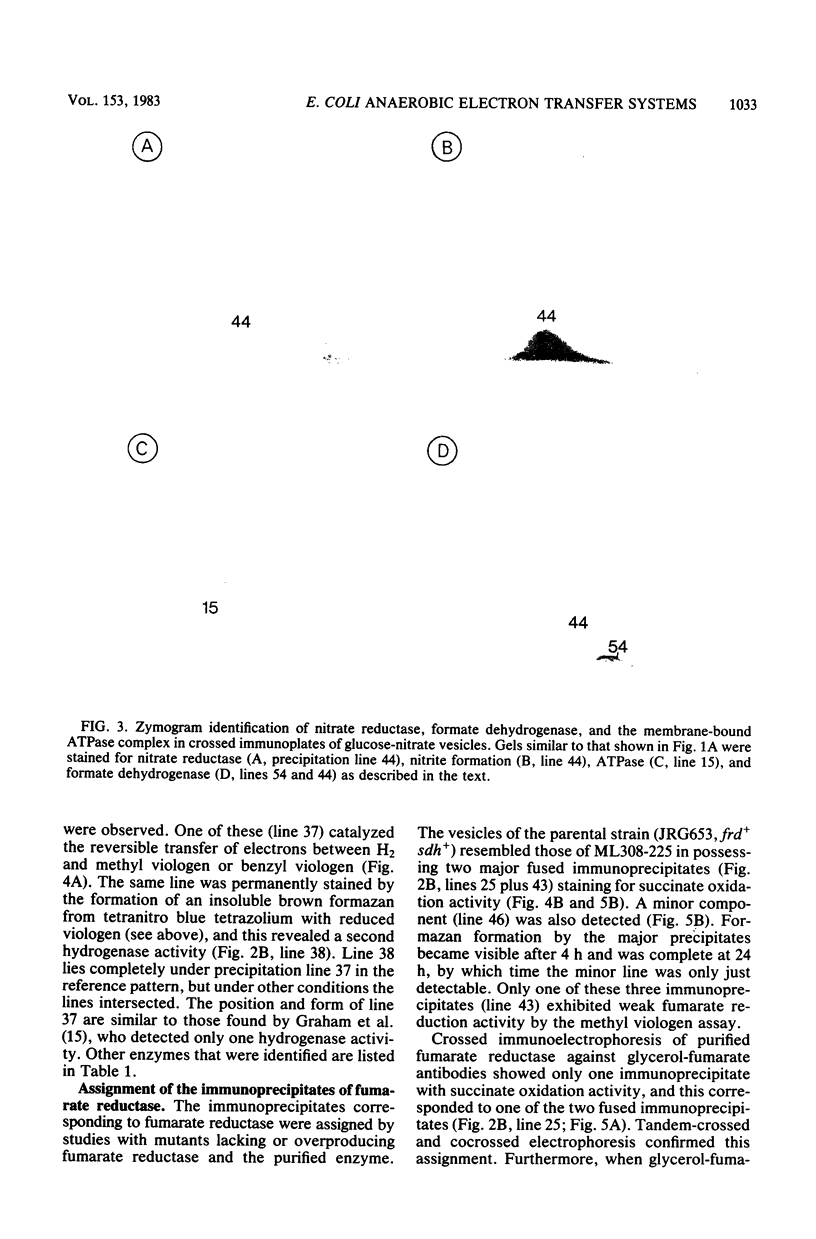
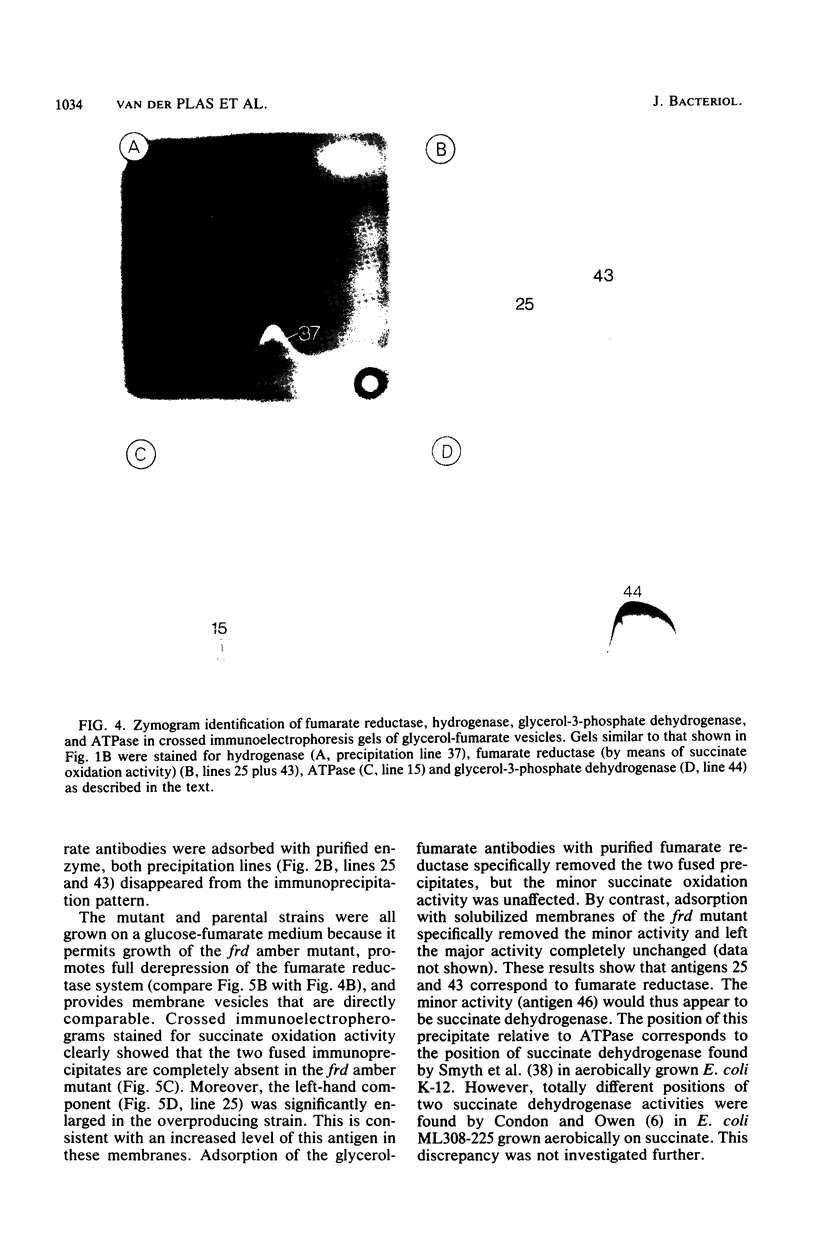
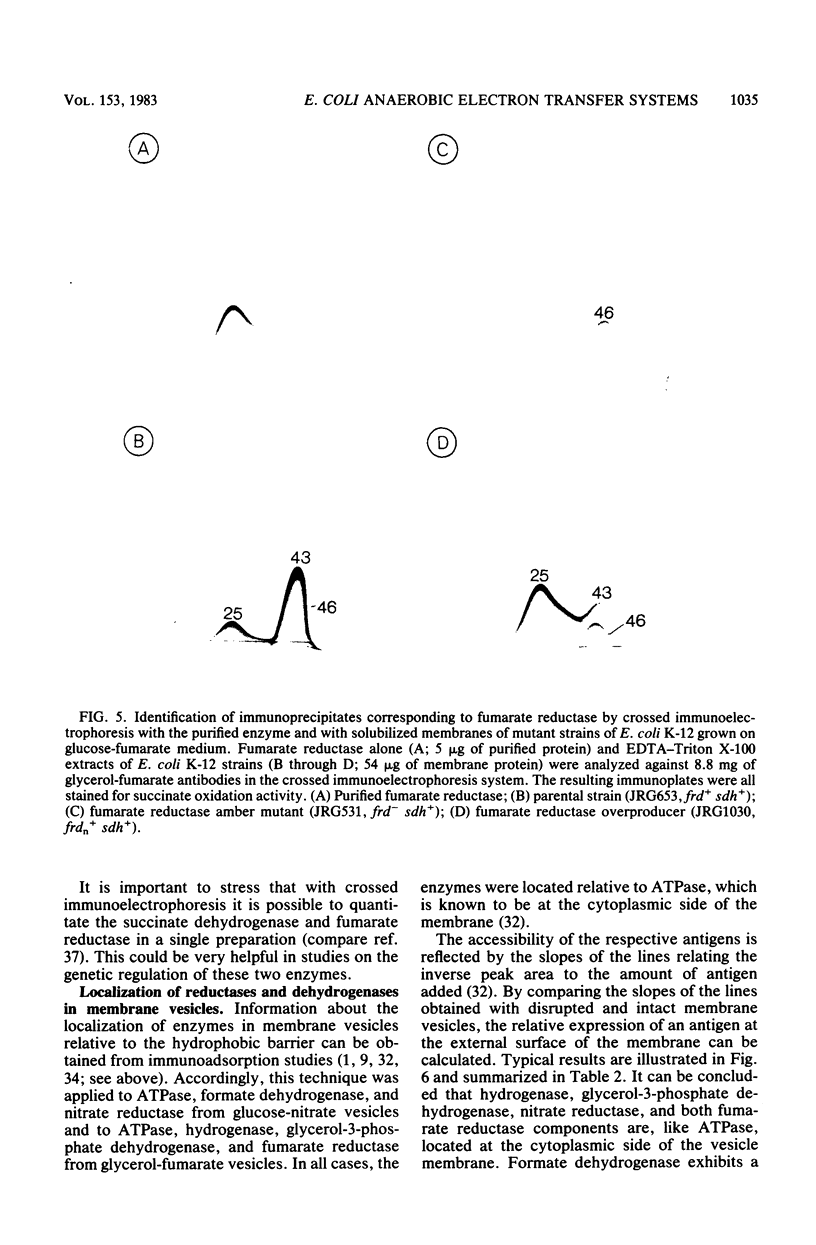
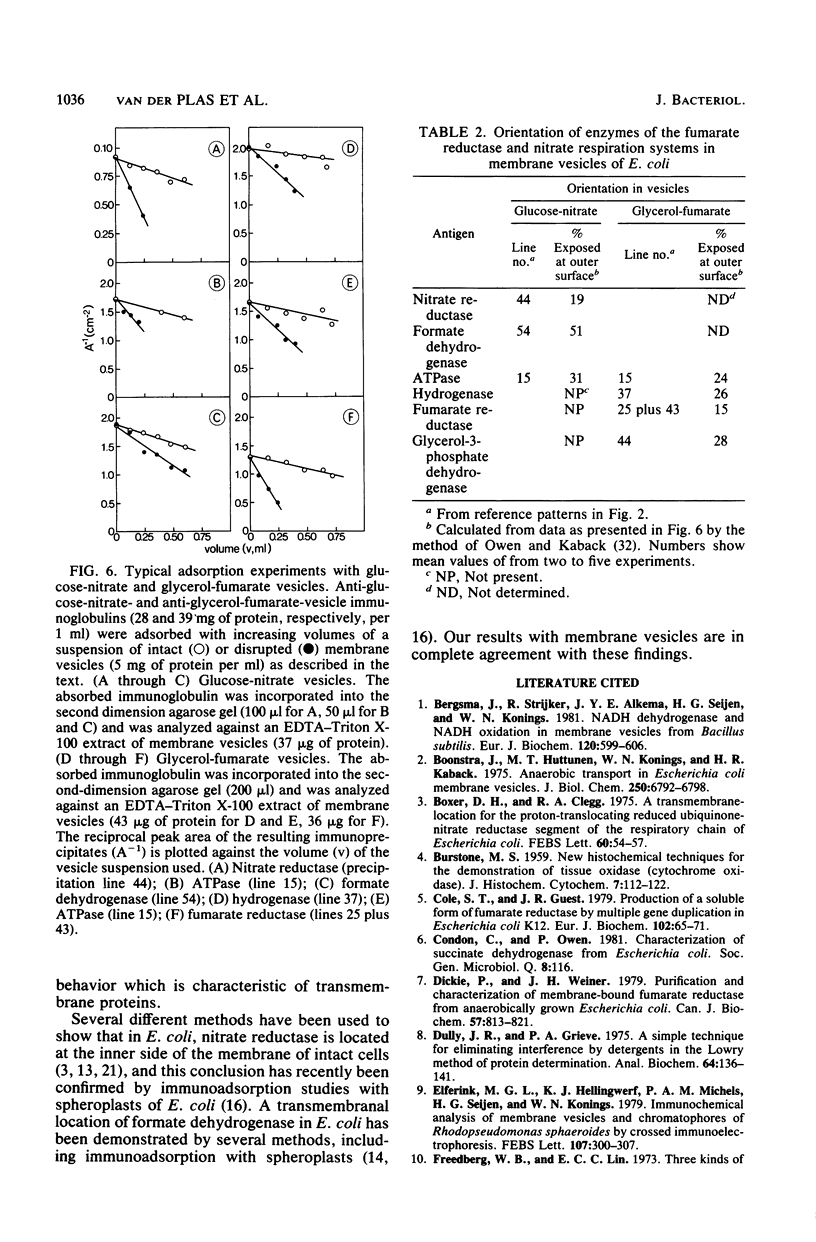
Crossed immunoelectrophoresis was used to analyze the components of membrane vesicles of anaerobically grown Escherichia coli. The number of precipitation lines in the crossed immunoelectrophoresis patterns of membrane vesicles isolated from E. coli grown anaerobically on glucose plus nitrate and on glycerol plus fumarate were 83 and 70, respectively. Zymogram staining techniques were used to identify immunoprecipitates corresponding to nitrate reductase, formate dehydrogenase, fumarate reductase, and glycerol-3-phosphate dehydrogenase in crossed immunoelectrophoresis reference patterns. The identification of fumarate reductase by its succinate oxidizing activity was confirmed with purified enzyme and with mutants lacking or overproducing this enzyme. In addition, precipitation lines were found for hydrogenase, cytochrome oxidase, the membrane-bound ATPase, and the dehydrogenases for succinate, malate, dihydroorotate, D-lactate, 6-phosphogluconate, and NADH. Adsorption experiments with intact and solubilized membrane vesicles showed that fumarate reductase, hydrogenase, glycerol-3-phosphate dehydrogenase, nitrate reductase, and ATPase are located at the inner surface of the cytoplasmic membrane; on the other hand, the results suggest that formate dehydrogenase is a transmembrane protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. New histochemical techniques for the demonstration of tissue oxidase (cytochrome oxidase). J Histochem Cytochem. 1959 Mar;7(2):112–122. doi: 10.1177/7.2.112. [DOI] [PubMed] [Google Scholar]
- Bergsma J., Strijker R., Alkema J. Y., Seijen H. G., Konings W. N. NADH dehydrogenase and NADH oxidation in membrane vesicle from Bacillus subtilis. Eur J Biochem. 1981 Dec;120(3):599–606. doi: 10.1111/j.1432-1033.1981.tb05742.x. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Huttunen M. T., Konings W. N. Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem. 1975 Sep 10;250(17):6792–6798. [PubMed] [Google Scholar]
- Boxer D. H., Clegg R. A. A transmembrane location for the proton-translocating reduced ubiquinone leads to nitrate reductase segment of the respiration chain of Escherichia coli. FEBS Lett. 1975 Dec 1;60(1):54–57. doi: 10.1016/0014-5793(75)80417-0. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Production of a soluble form of fumarate reductase by multiple gene duplication in Escherichia coli K12. Eur J Biochem. 1979 Dec;102(1):65–71. doi: 10.1111/j.1432-1033.1979.tb06263.x. [DOI] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Elferink M. G., Hellingwerf K. J., Michels P. A., Seÿen H. G., Konings W. N. Immunochemical analysis of membrane vesicles and chromatophoresis of Rhodopseudomonas sphaeroides by crossed immunoelectrophoresis. FEBS Lett. 1979 Nov 15;107(2):300–307. doi: 10.1016/0014-5793(79)80395-6. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Lin E. C. Three kinds of controls affecting the expression of the glp regulon in Escherichia coli. J Bacteriol. 1973 Sep;115(3):816–823. doi: 10.1128/jb.115.3.816-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Quayle J. R. Phenazine ethosulfate as a preferred electron acceptor to phenazine methosulfate in dye-linked enzyme assays. Anal Biochem. 1979 Oct 15;99(1):112–117. doi: 10.1016/0003-2697(79)90050-2. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H., Haddock B. A., Mandrand-Berthelot A. M., Jones R. W. Immunochemical analysis of the membrane-bound hydrogenase of Escherichia coli. FEBS Lett. 1980 May 5;113(2):167–172. doi: 10.1016/0014-5793(80)80584-9. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Immunochemical localization of nitrate reductase in Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(6):1210–1211. doi: 10.1042/bst0061210. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The organization of formate dehydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Jun 1;195(3):627–637. doi: 10.1042/bj1950627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Effects of dicyclohexylcarbodi-imide on proton translocation coupled to fumarate reduction in anaerobically grown cells of Escherichia coli K-12. Biochem J. 1976 Dec 15;160(3):813–816. doi: 10.1042/bj1600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellingwerf K. J., Bolscher J. G., Konings W. N. The electrochemical proton gradient generated by the fumarate-reductase system in Escherichia coli and its bioenergetic implications. Eur J Biochem. 1981 Jan;113(2):369–374. doi: 10.1111/j.1432-1033.1981.tb05075.x. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Lamont A., Garland P. B. The mechanism of proton translocation driven by the respiratory nitrate reductase complex of Escherichia coli. Biochem J. 1980 Jul 15;190(1):79–94. doi: 10.1042/bj1900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Kaback H. R. Anaerobic transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3376–3381. doi: 10.1073/pnas.70.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lund K., DeMoss J. A. Association-dissociation behavior and subunit structure of heat-released nitrate reductase from Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2207–2216. [PubMed] [Google Scholar]
- Miki K., Lin E. C. Enzyme complex which couples glycerol-3-phosphate dehydrogenation to fumarate reduction in Escherichia coli. J Bacteriol. 1973 May;114(2):767–771. doi: 10.1128/jb.114.2.767-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Wilson T. H. Proton translocation associated with anaerobic transhydrogenation from glycerol 3-phosphate to fumarate in Escherichia coli. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1570–1575. doi: 10.1016/0006-291x(78)91400-6. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Immunochemical analysis of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1413–1422. doi: 10.1021/bi00575a004. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovbjerg H. Immunoelectrophoretic studies on human small-intestinal brush-border proteins. Biochem J. 1981 Mar 1;193(3):887–890. doi: 10.1042/bj1930887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: changes in composition associated with anaerobic growth and fumarate reductase amber mutation. J Bacteriol. 1974 Mar;117(3):954–959. doi: 10.1128/jb.117.3.954-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Crispín J. A., Dubourdieu M., Chippaux M. Localization and characterization of cytochromes from membrane vesicles of Escherichia coli K-12 grown in anaerobiosis with nitrate. Biochim Biophys Acta. 1979 Aug 14;547(2):198–210. doi: 10.1016/0005-2728(79)90003-3. [DOI] [PubMed] [Google Scholar]
- Ten Brink B., Konings W. N. Generation of an electrochemical proton gradient by lactate efflux in membrane vesicles of Escherichia coli. Eur J Biochem. 1980 Oct;111(1):59–66. doi: 10.1111/j.1432-1033.1980.tb06074.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Dickie P. Fumarate reductase of Escherichia coli. Elucidation of the covalent-flavin component. J Biol Chem. 1979 Sep 10;254(17):8590–8593. [PubMed] [Google Scholar]