Abstract
Bile acids have been reported to produce relaxation of smooth muscle both in vitro and in vivo. The cellular mechanisms underlying bile acid–induced relaxation are largely unknown. Here we demonstrate, using patch-clamp techniques, that natural bile acids and synthetic analogues reversibly increase BKCa channel activity in rabbit mesenteric artery smooth muscle cells. In excised inside-out patches bile acid–induced increases in channel activity are characterized by a parallel leftward shift in the activity-voltage relationship. This increase in BKCa channel activity is not due to Ca2+-dependent mechanism(s) or changes in freely diffusible messengers, but to a direct action of the bile acid on the channel protein itself or some closely associated component in the cell membrane. For naturally occurring bile acids, the magnitude of bile acid–induced increase in BKCa channel activity is inversely related to the number of hydroxyl groups in the bile acid molecule. By using synthetic analogues, we demonstrate that such increase in activity is not affected by several chemical modifications in the lateral chain of the molecule, but is markedly favored by polar groups in the side of the steroid rings opposite to the side where the methyl groups are located, which stresses the importance of the planar polarity of the molecule. Bile acid–induced increases in BKCa channel activity are also observed in smooth muscle cells freshly dissociated from rabbit main pulmonary artery and gallbladder, raising the possibility that a direct activation of BKCa channels by these planar steroids is a widespread phenomenon in many smooth muscle cell types. Bile acid concentrations that increase BKCa channel activity in mesenteric artery smooth muscle cells are found in the systemic circulation under a variety of human pathophysiological conditions, and their ability to enhance BKCa channel activity may explain their relaxing effect on smooth muscle.
Keywords: bile salts, Maxi-K channels, smooth muscle, liver disease
INTRODUCTION
Bile acids are physiologically important steroidal anions that are necessary for the hydrolysis and absorption of dietary and biliary lipids in the intestine. They show a wide spectrum of actions on biological systems depending on their concentration. At concentrations greater than their critical micellar concentration (CMC),* bile acids can solubilize membrane components and disrupt the selective permeability of the cell membrane (Coleman et al., 1980; Oelberg et al., 1990). In the micromolar to low millimolar range (i.e., below their CMC), bile acid monomers produce splanchnic vasodilation and may cause systemic hypotension, the latter due to a decrease in peripheral vascular resistance (Kvietys et al., 1981; Thomas et al., 1991; Pak and Lee, 1993). They produce relaxation of smooth muscle in vitro in a wide variety of preparations (for review see Bomzon and Ljubuncic, 1995). However, the cellular and molecular mechanisms underlying these actions are poorly understood.
The activity of large conductance, Ca2+-activated K+ (maxi-K or BKCa) channels is a major factor limiting the degree of depolarization and contraction in vascular smooth muscle. Activation of these channels generates outward currents that, in turn, tend to drive the smooth muscle cell membrane potential in a negative direction and, thus, counteract contraction (Brayden and Nelson, 1992; Asano et al., 1993, Jaggar et al., 2000). Previously, the activity of these channels was found to be increased in vascular smooth muscle by fatty acids (Bregestovski et al., 1989; Kirber et al., 1992; Dopico et al., 1994a; Clarke et al., 1995), which, like bile acids, are lipidic anions. In the present study, we investigated the effects of different bile acids on BKCa channel activity in single smooth muscle cells freshly isolated from rabbit mesenteric artery using whole-cell and single-channel patch-clamp recording techniques.
We demonstrate here that all of the naturally occurring bile acids (cholic, deoxycholic, lithocholic, and taurolithocholic acids) and some of the synthetic analogues tested, markedly and reversibly increase BKCa channel activity, at concentrations similar to those required to relax smooth muscle. This bile acid–induced activation, which appears to result from a direct interaction of the bile acid with the channel complex itself or a closely associated membrane component, was also observed in smooth muscle cells dissociated from rabbit main pulmonary artery and gallbladder, raising the possibility that a direct activation of BKCa channels by bile acids is a common phenomenon for several types of smooth muscle cells. Furthermore, these results suggest that increasing BKCa channel activity is, at least, one of the mechanisms by which bile acids may affect peripheral vascular resistance. Preliminary reports of some of these results have appeared in abstract form (Dopico et al., 1994b, 2001).
MATERIALS AND METHODS
Data Acquisition and Analysis
Both whole-cell and single-channel current recordings were obtained using standard patch-clamp techniques. Currents were recorded using a patch-clamp amplifier (model EPC7; List Electronics) at a bandwidth of 3 kHz, and stored on videotape using a pulse code modulator (model PCM-501ES; Sony). For lithocholic versus (3β,5β)-3-hydroxycholan-24-oic acid (epilithocholic acid) 333-μM paired patch experiments and for the construction of concentration-response curves for deoxycholic versus (3β,5β,12α)-3,12-dihydroxycholan-24-oic acid (“epideoxycholic acid”), currents were recorded using a patch-clamp amplifier (model EPC8; List Electronics), low-pass filtered at 1 kHz with an 8-pole Bessel filter (model 900C; Frequency Devices), digitized at a sampling rate of 10 kHz with an interfase (model 1320; Axon Instruments, Inc.), and stored on a computer hard drive. A Ag/AgCl electrode pellet was used as a ground electrode. For single-channel recordings, patch pipettes were wax-covered and their tips heat-polished; tip resistances ranged between 6 and 10 MΩ. For whole-cell recordings, currents were recorded as described above. Patch pipette tips were heat-polished, but pipettes were not wax-covered; tip resistances ranged between 4 and 6 MΩ. All experiments were performed at room temperature (25°C).
For quantitative comparisons among agents (see Figs. 3, 5, and 7 B), data were later played back from videotape, low-pass filtered at 1 kHz with an 8-pole Bessel filter, sampled at 5 kHz with an IBM compatible computer and a Tecmar Labmaster A/D converter, and analyzed using pClamp6 (Axon Instruments, Inc.) and the Erwin suite of programs, a gift from Dr. Michel Vivaudou (Laboratoire de Biophysique Moleculaire et Cellulaire, UMR CNRS/USF/CEA, Grenoble, France). When data were played back from the computer hard drive for comparing 333 μM lithocholic versus epilitholic acid, or deoxycholic versus “epideoxycholic” acid, they were analyzed using pClamp6. As a measure of channel activity, we used the product of the number of channels in the patch (N, unknown) and the probability that a particular channel is open (Po). NPo (the average number of channels open) and open channel slope conductance (γ) were obtained from all-points amplitude histograms as previously described (Dopico et al., 1998). Unless otherwise indicated, data are shown as mean ± SEM.
Figure 3.
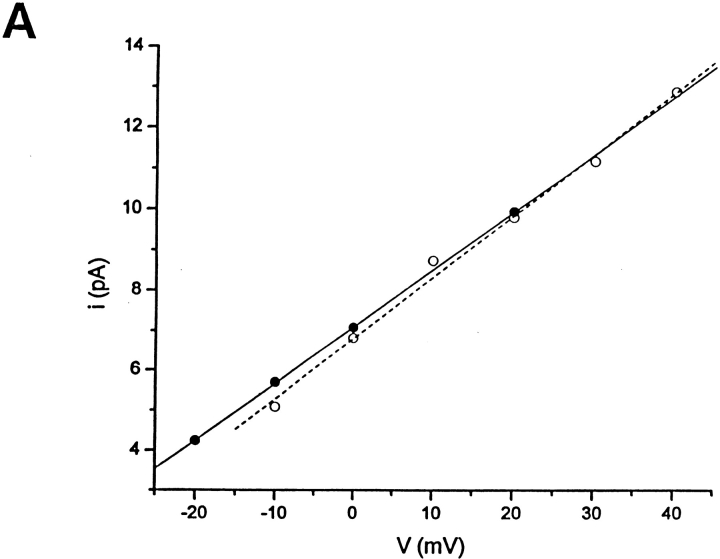
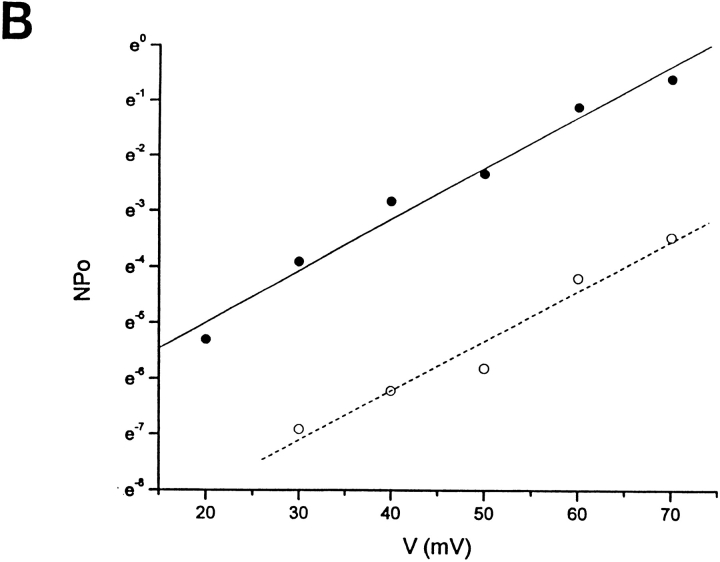
Bile acids increased BKCa channel activity without changing the unitary conductance (A) or the voltage dependence of channel gating (B). (A) Unitary current-voltage relationship obtained in the presence and absence of 100 μM deoxycholic acid recorded in 3 mM [K+]o/130 mM [K+]i from the same inside-out patch. Unitary conductance values are 163 and 167 pS, respectively. Results from three independent patches show no statistically significant difference in slope conductances between controls and in the presence of deoxycholic acid (156 ± 3 vs. 159 ± 8 pS; paired two tailed t test). (B) Over the range of potentials used, the voltage activation of BKCa channels is described by a Boltzmann relationship with a plot of the ln NPo as a function of voltage being linear at low values of Po. Under these conditions, the reciprocal of the slope, a measure of the voltage dependence of gating, is the potential needed to produce an e-fold change in NPo: 11.3 (r = 0.98) and 10.8 (r = 0.99) in the absence and presence, respectively, of 100 μM deoxycholic acid in the application pipette. Records were obtained from an inside-out patch. Similar results were obtained with 100 μM lithocholic acid and 333 μM deoxycholic acid (see results). Segments of the channel record used to calculate NPo and i at each potential were typically 30 s in duration. In both A and B, closed circles (linear regression, continuous line) correspond to data obtained in the presence of 100 μM deoxycholic acid, whereas open circles correspond to data obtained in its absence (linear regression, dotted line).
Figure 5.
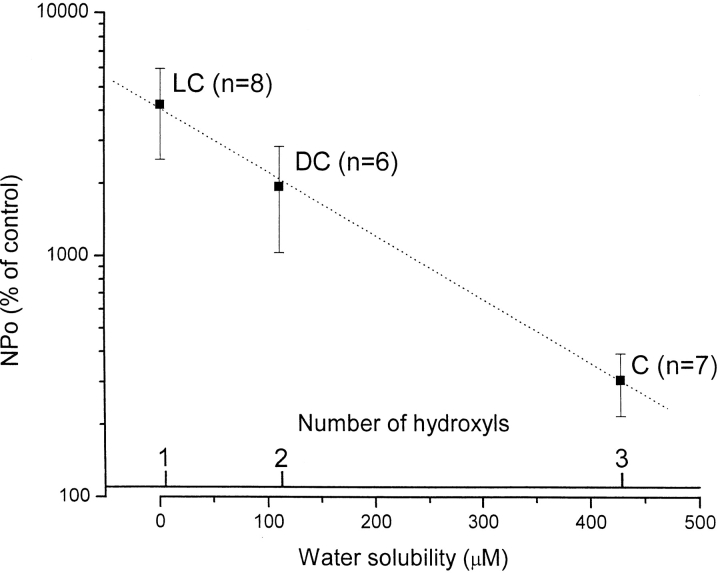
The magnitude of the increase in BKCa channel activity by natural bile acids is inversely related to the number of α-hydroxyl groups attached to the steroid nucleus, and, thus, directly related to the hydrophobicity of the bile acid nucleus. Water solubility values (at 20°C), shown in the outer abscissa, were taken from Igimi and Carey (1980). The number of hydroxyl groups attached to the ring structure is indicated in the inner abscissa. Increases in NPo are expressed as percentages of control values (i.e., before application of bile acid). In all cases, the bile acid (100 μM in the application pipette) was applied to the cytosolic surface of inside-out patches. Since bile acids increase NPo by a constant, multiplicative factor within a wide range of membrane potentials (−40–70 mV; Fig. 3 B and results), increases in NPo obtained at different potentials (range, 0–50 mV) were pooled. Segments of the channel record used to calculate NPo ranged from 30 to 90 s, but were typically 90 s in duration. The specific patches used for the effects of lithocholic acid were randomly chosen from the group of patches exposed to this agent. Only those patches where there was a response to cholic acid (seven out of nine) were used to determine the percent increase in mean NPo caused by this agent. Data are expressed as mean ± SEM; n = number of patches (in parentheses). Mean values were fitted by linear regression (dotted line; r = 0.99). LC, lithocholic acid; DC, deoxycholic acid; C, cholic acid.
Figure 7.
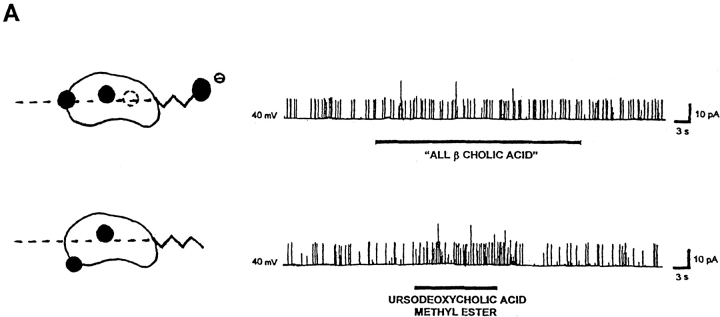
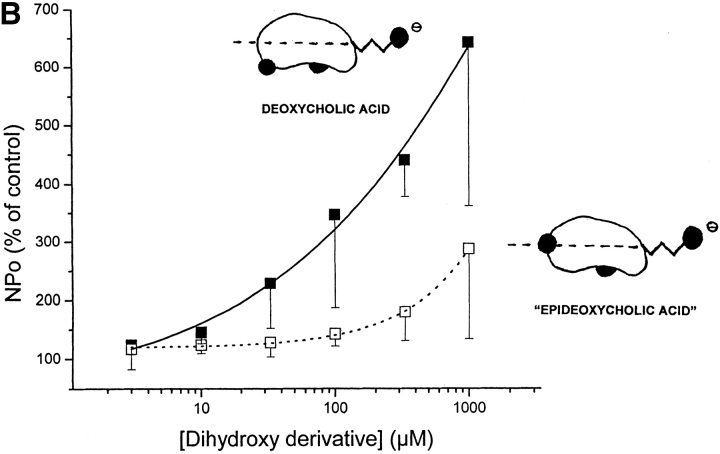
Both planar polarity (given by the location of polar groups in one hemisphere of the bile acid molecule) and hydrophobicity are structural features that favor the activation of BKCa channels by bile acids. (A) A very mild, if any, increase in BKCa channel activity is induced by either “all β cholic acid,” a hydrophilic trihydroxyderivative with all of its hydroxyl groups in the equatorial plane (top trace) or ursodeoxycholic acid methyl ester, a dihydroxyderivative with each of its two hydroxyl groups located on opposite sides of the steroid plane (bottom trace). These two analogues have a diminished planar polarity compared with corresponding “all α” compounds, that is, cholic and deoxycholic acid (Figs. 1, 2, and 7 B, and discussion). The patch potential was set to +40 mV in both cases. (B) Concentration-response curves of channel activation after exposure to deoxycholic acid (closed squares, solid line) versus “epideoxycholic acid” (open squares, dotted line), recorded from inside-out patches. These two dihydroxyderivatives have rather similar hydrophobicity but markedly different “planar polarity”: in deoxycholic acid, both axial hydroxyl groups (C3 and C12) are attached to the side of the molecule opposite to the side where methyl groups are attached, resulting in a hydrophilic (concave) and a hydrophobic (convex) hemisphere; in “epideoxycholic acid,” one axial hydroxyl (C12) is located in the side of the molecule opposite to the side where the methyl groups are attached, but the other (C3) is equatorial (i.e., in the plane of the rings), which results in an overall reduction in “planar polarity” (see shorthand representations). Deoxycholic acid, having similar hydrophobicity but increased planar polarity, is more efficient in increasing BKCa channel activity. For a description of construction of the concentration-response curve see materials and methods. The patch potential was set to 0 to +50 mV in all cases. Channel activity (NPo) was continuously recorded for periods of 20–60 s of exposure to either agent or control solution (DMSO-ethanol), and NPo values were computed from all-points amplitude histograms. Increases in NPo are expressed as percentages of control values (i.e., under control perfusion, recorded immediately before application of bile acid analogue). Data are shown as mean ± SEM (n = 4–6). Mean values were fitted by sigmoidal functions (r2 = 0.99 for both fitting functions).
Cell Isolation, Solutions, and Compound Application
Single smooth muscle cells were isolated from New Zealand rabbit superior mesenteric artery, pulmonary artery, and gallbladder based on a procedure described previously (Dopico et al., 1994a). All solutions were made with ultrapure distilled water (18 MΩ resistance) and with high grade salts. For experiments performed with cell-attached and excised, inside-out patches, the patch pipette solution contained the following (in mM): 127 Na+, 3 K+, 2 Mg2+, 116.5 Cl−, 5 EGTA, and 10 HEPES, pH 7.4. The bathing solution contained the following (in mM): 130 K+, 2 Mg2+, 116.5 Cl−, 5 EGTA, 10 HEPES, and 10 glucose, pH 7.4. This high [K+] bathing solution was used to set the cell membrane potential close to 0 mV for cell-attached patch recordings. For whole-cell and excised, outside-out patch recordings, the electrodes were filled with the same bathing solution described above for cell-attached and inside-out patches, except that glucose was omitted. The bathing solution for these experiments had the following composition (in mM): 110 Na+, 20 K+, 2 Mg2+, 116.5 Cl−, 5 EGTA, 10 HEPES, and 10 glucose, pH 7.4. For the experiments performed in hyperosmotic bathing solutions, the NaCl concentration was increased by 200 mM.
Solutions containing bile acids and related compounds were prepared from a concentrated stock solution of the agent (333 mM) in DMSO and sonicated for ∼10–15 min. This solution was diluted 1/10 in 95% ethanol, and then further diluted into the appropriate aqueous salt solution. In each case, the pH of the solution to be applied to cells/patches was readjusted after the solubilization of the bile acid. The mixture DMSO (<0.03%, final concentration)-ethanol (<0.3%, final concentration) in the bathing solution was used as a control for many of the patches and had no major effect on channel activity (see Table I, legend). Patches where there was a significant effect of the control solution were rejected. For those patches where a particular compound failed to increase channel activity, the control was a compound that consistently did. Both control and agent-containing solutions were applied to the cell by pressure ejection from a micropipette (“puffer pipette”; Dopico et al., 1994a). Concentrations given are those in the puffer pipette. Since compounds were diluted in the bathing solution as they exited the puffer pipette, the concentration given represents the maximum possible, being lower in the vicinity of the patch due to some dilution by the continuous perfusion of the cell chamber with bathing solution. For experiments with patches, bile acids were applied to the extracellular surface of excised outside-out patches, to the cytosolic surface of excised inside-out patches, or to the extracellular surface of the cell membrane not part of the patch (the extra-patch membrane) of cell-attached patches. Sometimes the same patch was used in both cell-attached and excised inside-out configurations.
TABLE I.
Increases in BKCa Channel Activity by Different Natural Bile Acids and Synthetic Analogues in Smooth Muscle Cells Freshly Isolated from Rabbit Superior Mesenteric Artery, Recorded in Cell-attached (C-A), Inside-out (I-O), or Outside-out (O-O) Patches
| C-A | I-O | O-O | |
|---|---|---|---|
| Unconjugated bile acids | |||
| Cholic acid | 5/5 | 7/9 | 2/2 |
| Deoxycholic acid | 2/2 | 10/10 | 1/1 |
| Lithocholic acid | 5/5 | 14/14 | 7/7 |
| Modifications in the lateral chain | |||
| Tauroconjugated bile acid: | |||
| Taurolithocholic acid | 0/5 | 2/2 | 3/3 |
| Neutral compounds: | |||
| Cholic acid methyl ester | 5/6 | 4/4 | n/t |
| “Cholic alcohol” | 5/5 | 3/3 | n/t |
| Modifications of groups directly attached to the steroid rings | |||
| Compounds with polar groups on the side of the molecule opposite to the methyl groups: |
|||
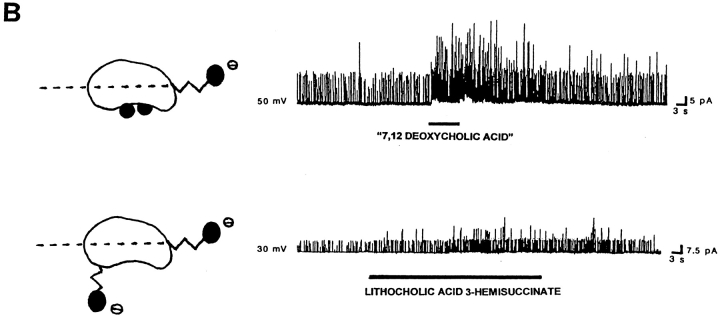
| “7,12 deoxycholic acid” | 4/4 | 4/4 | n/t |
| lithocholic acid 3-hemisuccinate | 5/5 | 3/3 | n/t |
| ursodeoxycholic acid methyl ester | 1/1 | 2/2 | n/t |
| “epideoxycholic acid” | n/t | 4/4 | n/t |
| Compounds without polar groups on the side of the molecule opposite to the methyl groups: |
|||
| ursocholanic acid | 0/3 | 1/6 | 4/7 |
| epilithocholic acid | 0/2 | 0/4 | n/t |
| “all β cholic acid” | 1/7 | 2/7 | 2/2 |
Results are expressed as the ratio of the number of positive results (i.e., reversible increase in channel NPo) to the number of patches tested. All compounds were applied at 100-μM concentration in the puffer pipette; n/t: not tested. Lower concentrations also were used for some compounds: lithocholic acid, 3 μM (C-A, 2/2; I-O, 1/2), and 30 μM (I-O, 3/4); deoxycholic acid, 3 μM (I-O, 5/5), 10 μM (I-O, 5/5), and 33 μM (C-A, 1/4; I-O, 12/15); “epideoxycholic acid,” 3 μM (I-O, 6/6), 10 μM (I-O, 6/6), and 33 μM (I-O, 4/4); cholic acid, 33 μM (I-O, 1/1); and cholic acid methyl ester, 33 μM (I-O, 4/5). Compounds were prepared from stock solutions and diluted in bath solution to the final concentration (materials and methods). Solvents used to prepare the stock solutions were equally diluted and applied onto the patches (controls). The DMSO-ethanol dilution mixture did not noticeably modify channel activity (C-A, 0/11; I-O, 0/18; O-O, 0/9). Using O-O patches, we also found that the increase in BKCa channel activity by either taurolithocholic acid (1/1) or ursocholanic acid (1/1) when applied to the extracellular surface of the patch in 310 mM NaCl (“hyperosmotic solution”) was similar to that evoked by these compounds in a normosmotic solution, hyperosmotic control solution itself having no noticeable effect on channel activity (n = 2). These recordings were obtained using conditions identical to those described in Fig. 2, except that NaCl was added to the extracellular solution to obtain a high ionic strength solution, which should shield surface charge and, thus, reduce its possible action on channel activity. The number of patches in the outside-out configuration are usually lower than those in the cell-attached or inside-out configurations because of the technical difficulties associated with obtaining outside-out patches in our preparation. The fact that some of the bile acid analogues did not work in some or all of the inside-out patches at the concentration used may be a reflection of the sensitivity of the putative binding site for a particular feature of the structure or the position of the patch in the pipette. For cell-attached patches, the lack of an effect may reflect the additional requirement for the bile acid or bile acid analogue to flip across the bilayer or, less likely, to travel laterally in the bilayer to get to the putative binding site.
For paired experiments in which the effects of 333 μM epilithocholic and lithocholic acids were quantitatively compared in the same inside-out patch, the intracellular side of the patch was alternatively placed into the mouth of three larger pipettes (∼1 mm diam), each containing either one of the analogues or the appropriate control of DMSO-ethanol. This gravity-fed delivery system avoids the dilution of agent-containing solutions that occurs when applied by puffer pipette as discussed in the previous paragraph (Dopico et al., 1998). For these experiments, control perfusion had essentially no effect on channel activity (n = 13) when compared with bathing solution in the chamber.
For the construction of concentration-response curves to deoxycholic versus “epideoxycholic” acid, the cytosolic surface of inside-out patches were alternatively exposed to a given concentration of either agent, and channel activity was continuously recorded before, during, and after exposure. We did not consider data where irreversible changes in channel activity occurred during analogue perfusion. Channel activity in the presence of either dihydroxy derivative was compared with control activity obtained under perfusion with control solution and recorded immediately before either analogue application. Both control bath (containing DMSO-ethanol, as explained above) and bile acid–containing solutions were applied to the intracellular surface of the patch for 20–60 s, using an automated, pressurized, DAD-12 superfusion system (ALA Scientific).
Chemicals
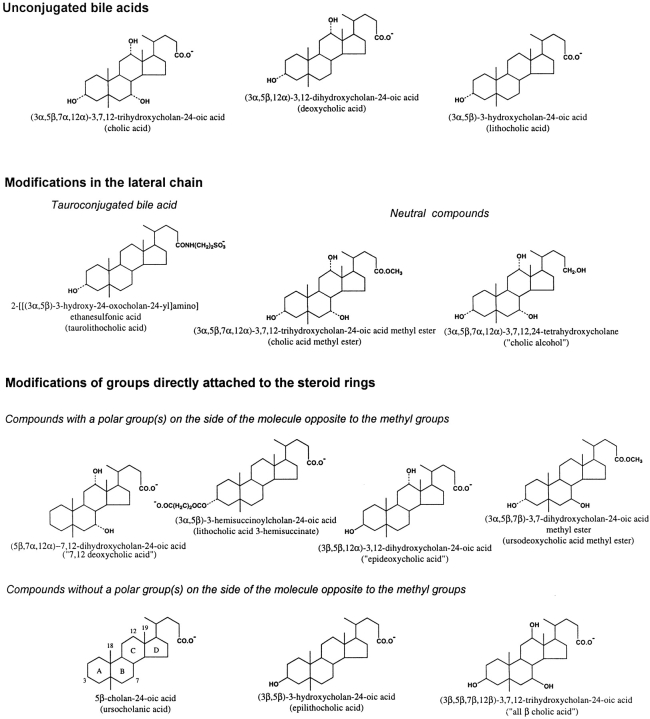
(3α,5β,7α,12α)-3,7,12-trihydroxycholan-24-oic acid (cholic acid); (3α,5β,12α)-3,12-dihydroxycholan-24-oic acid (deoxycholic acid); (3α,5β)-3-hydroxycholan-24-oic acid (lithocholic acid), and (3α,5β,7α,12α)-3,7,12-trihydroxycholan-24-oic acid methyl ester (cholic acid methyl ester) were purchased from Sigma-Aldrich. 2-[[(3α,5β)-3-hydroxy-24-oxocholan-24-yl]amino]ethanesulfonic acid (taurolithocholic acid); (3α,5β,7α,12α)-3,7,12,24-tetrahydroxy cholane (“cholic alcohol”); 5β-cholan-24-oic acid (ursocholanic acid); (5β,7α,12α)-7,12-dihydroxycholan-24-oic acid (“7,12 deoxycholic acid”); (3α,5β)-3-hemisuccinoylcholan-24-oic acid (lithocholic acid 3-hemisuccinate); (3β,5β)-3-hydroxycholan-24-oic acid (epilithocholic acid); (3β,5β,12α)-3,12-dihydroxycholan-24-oic acid (epideoxycholic acid; also referred to as “isodeoxycholic acid” by some authors; Roda et al., 1983), and (3α,5β,7β)-3,7-dihydroxycholan-24-oic acid methyl ester (ursodeoxycholic acid methyl ester) were purchased from Steraloids Inc. (3β,5β,7β,12β)-3,7,12-trihydroxycholan-24-oic acid (“all β cholic acid”) was a gift from Dr. T. Iida (Nihon University College of Engineering, Koriyama, Japan). Common names and those created for the convenience of the reader (the latter in quotation marks) are in parentheses and used throughout. Structures of the bile salts used are provided in Fig. 1 .
Figure 1.
Conventional chemical representation of the molecular structure of bile acids and analogues. The free carboxyl group (pKa ≅ 5) at the end of the lateral chain is shown in its ionized form, which predominates in the experimental conditions used (pH 7.4). The numbers of critical carbon atoms in the steroid nucleus (ring structure), to which chemical groups are attached, are shown in the structure of ursocholanic acid. For substitution groups, a dotted line indicates the α-configuration, whereas a continuous line indicates the β-configuration. Both chemical names and common or “created” names (the latter in parentheses) are provided.
RESULTS
Bile Acids Increase BKCa Channel Activity in Mesenteric Artery Smooth Muscle Cells
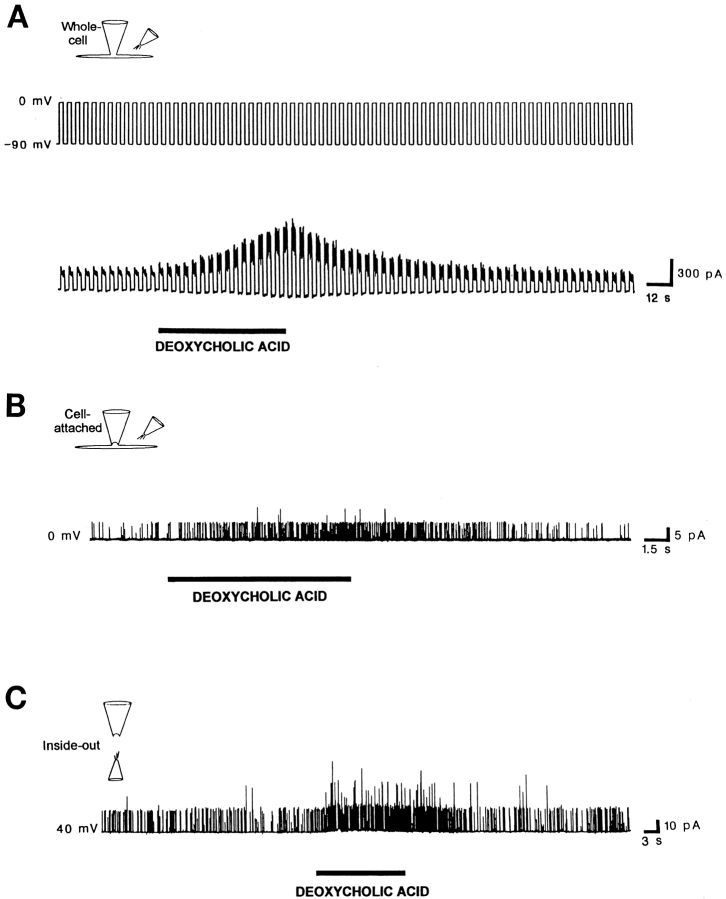
When deoxycholic acid (100 μM in the application pipette) was applied to a rabbit mesenteric artery smooth muscle cell, it rapidly and reversibly increased the amplitude of a voltage-gated, noninactivating current (Fig. 2 A). With the membrane potential continuously stepped between −90 and 0 mV, that is negative and positive to the calculated equilibrium potential for K+ (EK = −48 mV), the application of deoxycholic acid generated an inward current at −90 mV and a much larger outward current at 0 mV. The size of this sustained current, as well as its voltage dependence, strongly suggested that deoxycholic acid was affecting a Ca2+-activated K+ current. We previously have identified at the single-channel level BKCa channel activity that presumably underlies this current (Dopico et al., 1994a).
Figure 2.
Deoxycholic acid (100 μM in the application pipette) reversibly increases BKCa currents and channel activity in smooth muscle cells freshly isolated from rabbit superior mesenteric artery. (A) Deoxycholic acid increases a voltage-dependent, noninactivating whole-cell K+ current. The membrane potential (top trace) was continuously stepped between −90 and 0 mV (i.e., negative and positive to EK [−48 mV]). Transient exposure to deoxycholic acid generated an inward current at −90 mV and a much larger outward current at 0 mV. (B) Deoxycholic acid reversibly increases BKCa channel activity in a cell-attached patch with the patch potential set at 0 mV. Deoxycholic acid was applied to the extracellular side of the extra-patch membrane. (C) Deoxycholic acid reversibly increases BKCa channel activity in an inside-out patch, with the patch potential set at +40 mV. Deoxycholic acid was applied to the cytosolic surface of the patch. Both sides of the excised patch were exposed to a zero Ca2+ solution containing 5 mM EGTA to chelate trace amounts of Ca2+. Lower concentrations of deoxycholic acid also were effective in increasing channel activity (Table I, legend). Similar results were obtained with other unconjugated natural bile acids such as cholic and lithocholic acids (Figs. 5, 6 A, and Table I).
To determine whether deoxycholic and other bile acids may, indeed, increase BKCa channel activity, we examined the effects of this compound and two other naturally occurring bile acids (Fig. 1). As we (Dopico et al., 1994a) and others (Mistry and Garland, 1998) previously have demonstrated, the open channel current-voltage relationship obtained from BKCa channels in inside-out patches showed a large unitary conductance of 156 ± 3 pS in 3 mM [K+]o/130 mM [K+]i (n = 3) with no open channel rectification in the range of potentials applied (−40 to +60 mV; Fig. 3 A). Their activity characteristically increased at more positive membrane potentials: ∼10 mV for an e-fold change in NPo at low Po values (Fig. 3 B) or upon elevating the [Ca2+] at the intracellular surface of the patch (Dopico et al., 1994a; Mistry and Garland, 1998). In both cell-attached and excised inside-out patches, deoxycholic acid (Fig. 2, B and C), as well as cholic and lithocholic acid, reversibly increased the activity of BKCa channels (Dopico et al., 1994b; Table I; see also Fig. 6 A). Results with excised outside-out patches are covered below under a separate subheading. The fact that all naturally occurring bile acids reversibly increased channel activity from patches in the cell-attached configuration, in which the intracellular environment is kept intact, indicates that channel activation by bile acids is not limited to the artificial situations of cell-free membrane patches (inside-out and outside-out configurations) and dilution of the cytosol by the pipette solution (whole-cell configuration).
Figure 6.
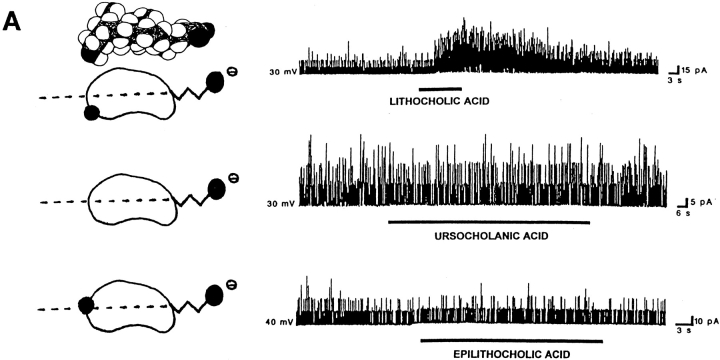
The location of polar groups in the side of the steroid nucleus opposite to where the hydrophobic methyl groups are located favors the activation of BKCa channels by bile acids. For each panel, records of channel activity in inside-out patches in the absence and presence of a given bile acid analogue (100 μM) are shown on the right. A shorthand representation of the analogue applied is on the left. In the representations, the dotted line crossing these bean-shaped molecules illustrates the equatorial plane, and black circles correspond to polar groups (whether hydroxyls or carboxyls). The lateral chain and the methylenes of the hemisuccinate are depicted as zig-zag lines. For lithocholic acid, the Stuart-Briegleb space filling model also is shown at the top of the corresponding shorthand representation. (A) Lithocholic acid rapidly increases BKCa channel activity (top trace), whereas ursocholanic acid, which does not have any hydroxyl groups (middle trace), or the β-isomer of lithocholic acid (bottom trace) fail to do so. The top and middle records were obtained in the same patch, with the patch potential set to +30 mV. The bottom record was obtained with the patch potential set to +40 mV. (B) Either “7,12 deoxycholic acid” (top trace) or lithocholic acid 3-hemisuccinate (bottom trace) reversibly increase BKCa channel activity. These two compounds do not have a hydroxyl in the α configuration at C3, but still possess polar groups (whether hydroxyls or a bulky hemisuccinate) in the axial plane of the molecule, opposite to the hydrophobic side of the steroid plane (see Fig. 1 and models of these analogues on the left of each record). The patch potential was set to +50 and +30 mV.
To determine whether an increase in macroscopic BKCa current evoked by deoxycholic acid could be due to an enhancement in channel unitary conductance for K+, as well as an increase in channel NPo, we studied the effect of bile acids on the single-channel current-voltage relationship obtained from inside-out patches in 3 mM [K+]o/130 mM [K+]i. In the presence of 100 μM deoxycholic acid, the slope conductance of the open channel current-voltage relationship was not significantly different from control obtained in the same patch (Fig. 3 A). This result was repeated in two other patches, providing a mean conductance value of 159 ± 8 pS (n = 3) in the presence of the bile acid. This value is not statistically different from control values (see above), as evaluated with a paired, two-tailed t test (P > 0.05). A similar unitary conductance was obtained in the presence of 100 μM lithocholic acid (165 vs. 163 pS for the same cell in the absence of lithocholic acid) under identical recording conditions. Therefore, any bile acid increase in the macroscopic K+ current due to BKCa channels can be attributed solely to the bile acid–induced enhancement of BKCa channel NPo.
Channel Activation by Bile Acids Is Not due to Changes in the Voltage Dependence of Channel Gating, the Local Concentration of Ions, or Freely Diffusible Cytosolic Second Messengers
Since BKCa channel activity increases as the membrane potential is made more positive or with increases in [Ca2+] at the cytosolic side of the membrane, we tested for their possible involvement in the increase in BKCa channel activity caused by bile acids. The voltage dependence of channel activation was studied in inside-out patches in the presence and absence of an effective concentration of bile acid (Fig. 3 B). Over the range of potentials used here, the voltage dependence of activation can be described by a Boltzmann relationship such that a plot of ln NPo as a function of membrane potential is linear at low values of Po. The reciprocal of the slope (”slope factor”), a measure of the voltage dependence of channel gating, is the potential required to produce an e-fold change in Po (Singer and Walsh, 1987). Data from an inside-out patch (Fig. 3 B) show the expected increase in ln NPo as the membrane potential was made more positive and its linearity with potential at low Po (r = 0.98). The slope factor is 11.3 mV, which is consistent with previous values reported for BKCa channels in this (Dopico et al., 1994a; Mistry and Garland, 1998) and other smooth muscle preparations (for review see McManus, 1991). More importantly, Fig. 3 B also shows that, in the same patch, the slope factor in the presence of an effective concentration of bile acid (100 μM deoxycholic acid) is essentially the same (slope factor = 10.8 mV; r = 0.99). Data from this and two other patches indicate that there was no change in slope factor (11.1 ± 0.4 and 11.6 ± 1.0 mV, respectively in the absence and presence of 100 μM deoxycholic acid; a nonsignificant difference, paired, two-tailed t test, n = 3). In two other inside-out patches, similar results were obtained with another bile acid, lithocholic acid (100 μM; slope factor = 10.4 vs. 9.6 mV in its absence and presence), or higher concentrations of deoxycholic acid (333 μM; slope factor = 11.9 vs. 13.6 mV in its absence and presence). These data indicate that bile acids do not modify the voltage dependence of channel gating, but produce, at low Po, a parallel shift in the NPo-voltage relationship toward negative potentials (assuming N remains unchanged).
Bile acids might increase BKCa channel activity through one or more Ca2+-mediated mechanisms. Bile acids have been reported to behave as Ca2+ ionophores in cultured type II pneumonocytes and proteoliposomes as well as a two phase bulk partitioning system (Oelberg et al., 1990; Zimniak et al., 1991), to release Ca2+ from an IP3-sensitive microsomal pool from hepatocytes (Combettes et al., 1989), to increase Ca2+ influx through a receptor-operated Ca2+-channel pathway in hepatocytes (Beuers et al., 1993), and possibly to increase K+ and Cl− conductances via an IP3-mediated release of Ca2+ from intracellular stores in colonic cells (Devor et al., 1993). All three unconjugated natural bile acids tested (i.e., cholic, deoxycholic, or lithocholic acid; 100 μM in the application pipette) readily increased BKCa channel activity in 31 out of 33 inside-out patches (Table I). We recorded the effects of bile acids on channel activity 15 min or more after patch excision with both sides of the patch exposed to a solution with no added Ca2+ and containing 5 mM EGTA to chelate trace amounts of this divalent. Therefore, it is unlikely that the increase in BKCa channel activity by bile acids is mediated by the release of Ca2+ from intracellular stores, transmembrane fluxes of Ca2+ or freely diffusible cytosolic messenger molecules. In addition, since the bile acid–induced increase in BKCa channel activity was recorded from excised-patches using solutions that contained no nucleotides, cellular processes such as phosphorylation, GTP-binding protein modulation, NADPH-dependent metabolism/modulation, etc., are not involved.
We also considered the possibility that the enhancement of channel activity by bile acids might be mediated by bile acid–induced changes in the concentrations of other ions (OH−, H+, etc.,) that have been reported to affect BKCa channel activity. For example, cytoplasmic hydroxyl (OH−) ions are known to increase the activity of BKCa channels in smooth muscle cells (Kume et al., 1990). Bile acids might open channels passing any of these ions, which in the presence of a membrane potential could result in changes in ion concentration in the vicinity of the patch. To test for this possibility, we evaluated the ability of bile acids to increase BKCa channel activity in inside-out patches at membrane potentials above and below 0 mV in symmetric solutions (using the 130-mM K+-containing solution); i.e., with the driving force for a given ion either outward or inward. We found similar increases in channel activity by 100 μM lithocholic acid at +20 mV and −20 mV (n = 2). Thus, it is unlikely that bile acids increase BKCa channel activity by means of bile-acid– induced fluxes of ions in the vicinity of the membrane patch.
Together, these data indicate that the bile acid–induced increase in BKCa channel activity is not secondary to changes in the levels of freely diffusible second messengers or ions. Rather, it appears that the effect of bile acids on BKCa channel activity results from a direct interaction of bile acids with the channel itself or some closely associated membrane component.
The range of concentrations for all of the compounds that effectively increased BKCa channel activity in mesenteric artery smooth muscle cells was 3 μM–1 mM in the application pipette (Table I, legend). This range is below the CMC of the bile acids applied in our studies, which, even for the more hydrophobic, monohydroxylated derivatives, is above 1 mM under the conditions we used (Carey and Small, 1972; Helenius et al., 1979; Roda et al., 1983; Heuman and Bajaj, 1994). This and other considerations (see discussion) led us to suggest that increases in BKCa channel activity by bile acids do not result from a “nonspecific detergent effect” on the membrane produced by bile acid micelles, but rather as a consequence of an interaction between bile acid monomers and a target(s) in the cell membrane patch. To better understand the nature of this interaction, we explored some of the structural features in the bile acid molecule that favor activation of BKCa channels by these compounds.
Several Substitutions on the Lateral Chain of the Bile Acid Molecule Do Not Affect Its Action on BKCa Channel Activity
In our effort to understand the structural features in the bile acid molecule that may be important for bile acid–induced BKCa channel activation, we first examined the effect of various chemical substitutions in the lateral chain of the bile acid molecule on bile acid–induced channel activation. Conjugation with taurine is a physiologically important process in bile acid metabolism (Scharschmidt, 1982). Unconjugated bile acids have a free carboxyl group at the end of the lateral chain, whereas tauroconjugates have an amide-sulphonic acid group instead. The negatively charged sulphonic group (pKa = 1–2) is largely ionized both within the physiological range of pH and under our recording conditions, which makes the lateral chain highly polar and, in general, also decreases the overall hydrophobicity of the molecule (Scharschmidt, 1982; Carey, 1985).
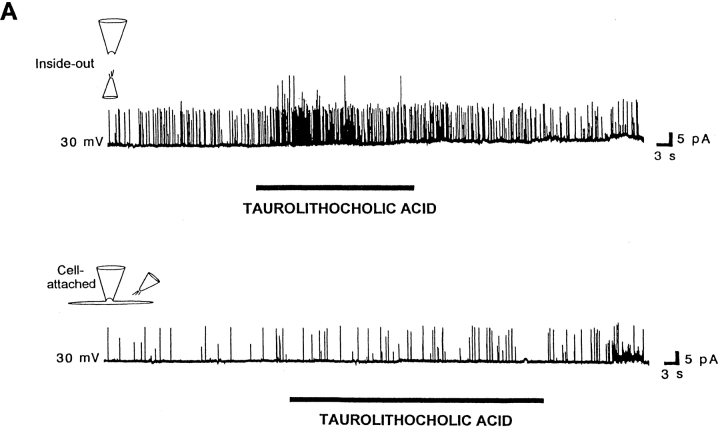
Taurolithocholic acid, an analogue of lithocholic acid that has a sulphonic group at the very end of its lateral chain (Fig. 1) and not a free carboxyl, readily increased BKCa channel activity in inside-out patches (Fig. 4 A, top trace, and Table I) in a manner similar to lithocholic acid (see Fig. 6 A, top trace, and Table I). These data from excised inside-out patches, which indicate that an amide-sulphonic group can substitute for a free carboxyl in the lateral chain of the bile acid molecule for BKCa channel activation, are in line with previous results reporting that alkyl sulphonates increase the activity of K+ channels that are also fatty acid activated (Petrou et al., 1994; Clarke et al., 1995). However, taurolithocholic acid failed to readily increase channel activity in cell-attached patches (Fig. 4 A, bottom trace) when applied to the extrapatch membrane (outside the area of the patch). Some increase in channel activity was observed only after a 10-min application (not shown). This delayed increase in activity is consistent with the poor ability of a tauroconjugated bile acid to “flip” across lipid bilayers or cell membranes (Cabral et al., 1987; Kamp and Hamilton, 1993; Donovan and Jackson, 1997) to gain access to a putative target site.
Figure 4.
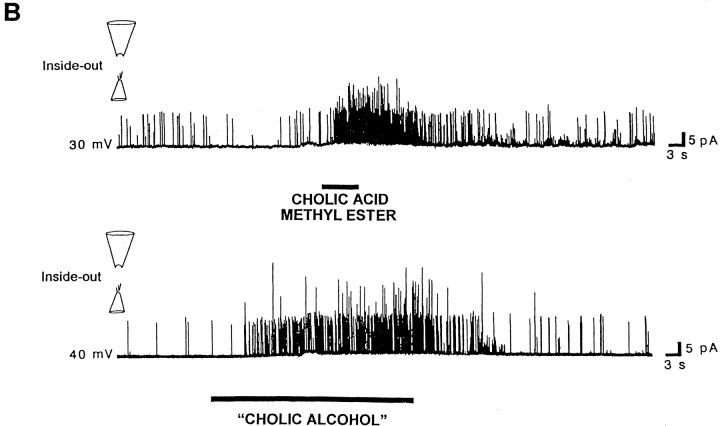
Chemical modifications in the lateral chain of the bile acid molecule do not markedly modify bile acid–induced increases in BKCa channel activity. (A) Taurolithocholic acid, a sulfoconjugate of lithocholic acid having a very polar lateral chain, rapidly increases BKCa channel activity in an inside-out patch (top trace), but fails to do so in a cell-attached patch (bottom trace). Both records were obtained in the same patch with the patch potential set to +30 mV, the bile acid analogue concentration being 100 μM in the application pipette. (B) Synthetic bile acid analogues that lack negatively charged groups in the lateral chain are effective in enhancing BKCa channel activity: cholic acid methyl ester or “cholic alcohol” (bottom trace) rapidly and reversibly increase channel activity, when applied to the cytosolic surface of inside-out patches. The patch potential was set to +30 and +40 mV, respectively. In both cases, the bile acid analogue concentration was 100 μM in the application pipette. Similar results were obtained when these two neutral compounds were applied to cell-attached patches (Table I).
Having shown that taurolithocholic acid mimics the increase in BK channel activity by unconjugated bile acids in excised inside-out patches, we examined whether increases in activity require negatively charged groups in the lateral chain of the molecule, which is attached to the steroid nucleus at C17 (Fig. 1). We used compounds where the free carboxyl group in the bile acid was replaced with a relatively nonpolar methyl ester or with a polar primary hydroxyl. Both of these uncharged compounds, cholic acid methyl ester and “cholic alcohol” (Fig. 1), rapidly and reversibly increased BK channel activity (Fig. 4 B and Table I) in the same way as cholic acid did (see Fig. 5 and Table I). These three compounds have the same steroid nucleus but different lateral chains (Fig. 1). Thus, negative charges on the bile acid molecule are not necessary for increasing BKCa channel activity in mesenteric artery smooth muscle cells. These results contrast with those obtained in smooth muscle cells where the negative charge, indeed, is required for fatty acid activation of two types of K+ channels (including BKCa channels; Petrou et al., 1994; Clarke et al., 1995). Thus, it appears that, when applied to inside-out patches, bile acids act at a site with a structural specificity different from the one affected by fatty acids.
The increases in BKCa channel activity caused by cholic acid methyl ester and taurolithocholic acid (see above), each with extra atoms of carbon on the lateral chain compared with cholic, deoxycholic, or lithocholic acids, indicate that bile acid–induced increases in BKCa channel activity also do not depend on a specific length of the bile acid lateral chain. Moreover, data obtained with these uncharged agents and with taurolithocholic acid demonstrate that several major chemical modifications in the lateral chain of the bile acid molecule do not noticeably modify the increase in BKCa channel activity obtained with bile acids in excised inside-out patches. The fact that the bile acid–induced increases in channel activity remain following changes in the lateral chain which affect the overall polarity of the molecule seems to indicate that the overall hydrophobicity of the bile acid molecule does not play an important role in bile acid–induced increase in BKCa channel activity. The lack of critical role for structural determinants in the lateral chain and overall hydrophobicity of the molecule in modulation of channel activity led us to examine possible structural features of the steroid nucleus that determine enhancement of channel activity.
The General Hydrophobicity of the Steroid Nucleus Favors Channel Activation by Natural Bile Acids: The Increase in Activity Varies Inversely with the Number of Hydroxyl Groups
It is generally accepted that, for the same degree of ionization of the carboxyl group (pKa ≅ 5) and ionic strength of the aqueous medium, bile acid hydrophobicity decreases with an increase in the number of hydroxyl groups attached to the steroid nucleus (Carey, 1985; Cabral et al., 1987; Sung et al., 1993; Heuman and Bajaj, 1994). The solubility of bile acids in water at 20°C can be used as an indicator of the overall hydrophobicity of the molecule (Igimi and Carey, 1980). We found that, in spite of the variability in the response to a given bile acid from patch to patch, lithocholic was more effective than deoxycholic, which, in turn, was more effective than cholic acid in increasing BKCa channel activity in inside-out patches (Dopico et al., 2001; Fig. 5). These data demonstrate that those bile acids that have fewer hydroxyl groups attached to the steroid nucleus and, therefore, increased hydrophobicity (or poorer water solubility; Carey, 1985; Cabral et al., 1987; Sung et al., 1993; Heuman and Bajaj, 1994), are significantly more effective activators of the BKCa channel. Moreover, since the compounds compared here have the same lateral chain (Fig. 1), it appears that the augmentation of channel activity is favored by the general hydrophobicity of the bile acid steroid nucleus.
The Presence of Polar Groups Located on the Side of the Steroid Nucleus Opposite to Where the Hydrophobic Methyl Groups Are Located Favors Bile Acid–induced Increases in BKCa Channel Activity
Having found that several chemical substitutions in the lateral chain did not significantly affect increases in BKCa channel activity by bile acids, and that hydrophobicity of the steroid nucleus of natural bile acids favors bile acid–induced increases in channel activity, we next examined the effect of chemical modifications to groups directly attached to the steroid nucleus. Since lithocholic acid was the most effective natural bile acid tested for increasing BKCa channel activity (Fig. 5) and the hydroxyl group in the α configuration located at C3 in the bile acid molecule (a group shared by all natural bile acids) is critical for the selective interaction of these compounds with several binding proteins (for review see Stolz et al., 1989), we examined the effect of deleting this group. The resulting compound, ursocholanic acid, in spite of being substantially more hydrophobic than lithocholic acid (due to the total absence of hydroxyl groups; see Fig. 1 and Fig. 6 A for the structures), failed to increase channel activity in both cell-attached and inside-out patches in all but one patch (Fig. 6 A, middle trace, and Table I). Thus, these results prevent us from establishing a simple relationship between BKCa channel activation and hydrophobicity of the steroid nucleus: in addition to steroid nucleus hydrophobicity, the hydroxyl group in the α configuration located at C3 (or at least one hydroxyl group) favors the ability of bile acids to increase channel activity when applied to the cytosolic surface of excised inside-out patches.
To determine whether a hydroxyl group in the α configuration located at C3 is specifically required for increasing channel activity, we used “7,12 deoxycholic acid,” in which the hydroxyl group in the α configuration located at C3 is absent, but where α-hydroxyl groups are attached to the other two positions of the steroid nucleus found in natural bile acids (i.e., C7 and C12). The increase in BKCa channel activity by this agent (Fig. 6 B, top trace) demonstrates that the presence of a hydroxyl group in the α configuration does not have to be restricted to the C3 location. Moreover, the increase in channel activity produced by another compound, lithocholic acid 3-hemisuccinate (Fig. 6 B, bottom trace), in which the hydroxyl group at C3 has been substituted with a hemisuccinate (a charged group), indicates that other (even bulky) polar groups located at C3 are able to increase BKCa channel activity. Thus, a putative bile acid site to which bile acid monomers bind to increase BKCa channel activity (see discussion) should have rather lax structure activity requirements. Together, data obtained with “7,12 deoxycholic acid,” lithocholic acid 3-hemisuccinate, and ursocholanic acid indicate that polar groups (but not necessarily a hydroxyl group attached to C3) on the bile acid steroid nucleus are necessary for, or at least strongly favor, bile acid–induced increases in BKCa channel activity.
Orientation of Polar Groups:
Next, we determined whether the specific configuration (α or β) of polar groups is important for bile acid–induced increases in channel activity. Natural bile acids are relatively flat and rigid bean-shaped molecules with a concave polar and a convex hydrophobic hemisphere (i.e., they exhibit planar polarity; see shorthand representations in Figs. 6 and 7). The concave hemisphere (i.e., “below” the ring structure) is the area where the α-hydroxyl groups are found, and the convex hemisphere (i.e., “above” the ring structure) is the side of the steroid nucleus with protruding methyl groups (C18 and C19) lying above it. Concave and convex hemispheres are usually referred to as α and β planes, respectively (Carey and Small, 1972; Helenius et al., 1979; Carey, 1985). We determined whether epilithocholic acid, an isomer of lithocholic acid in which the hydroxyl group at C3 is in the β configuration, could increase BKCa channel activity. For this isomer, the hydroxyl group is axial to ring A (Figs. 1 and 6 A) but, because of the cis-junction between rings A and B, points outward and is equatorial to the overall steroid nucleus (Carey, 1985; Balducci et al., 1989). Thus, the hydroxyl group of epilithocholic acid is not located in the polar hemisphere (or α-side) of the steroid nucleus (Carey, 1985) as found in lithocholic acid. This compound (like ursocholanic acid) does not have any planar polarity, which is a feature of lithocholic acid. In addition, because of the orientation of its hydroxyl, epilithocholic acid is somewhat less hydrophobic than lithocholic acid (Carey, 1985), although it is more hydrophobic than deoxycholic acid, which is an effective enhancer of channel activity (Figs. 5 and 7 A).
Epilithocholic acid repeatedly failed to increase channel activity in both cell-attached and inside-out patches, including patches in which lithocholic acid was effective (1 cell-attached and 3 inside-out patches) (Fig. 6 A, bottom trace, and Table I).
The failure of epilithocholic acid to increase BKCa channel activity, together with increases in BKCa channel activity by naturally occurring bile acids (cholic acid methyl ester; “cholic alcohol”; “7,12 deoxycholic acid”; or lithocholic acid 3-hemisuccinate), and the failure to increase activity (in all but one patch) by ursocholanic acid seem to point to an important structural feature that favors bile acid activation of BKCa. This is the increased planar polarity of the bile acid molecule due to polar groups located in the bile acid hemisphere (α side) opposed to the hydrophobic, methyl group-containing hemisphere (β side) of the rings (Carey and Small, 1972; Carey, 1985; Balducci et al., 1989; Dopico et al., 2001).
Planar Polarity versus Hydrophobicity:
We attempted to examine the relative importance of the overall hydrophobicity of the steroid nucleus versus its degree of planar polarity on increasing BKCa channel activity by comparing the effects of two more bile acid analogues. First, we used “all β cholic acid,” which is like cholic acid but has all three of its hydroxyl groups in the β configuration, that is, in the steroid plane or above it, where the two methyl groups are located. The net effect of shifting the three hydroxyl groups is to make the β-plane much less hydrophobic and the α-plane more hydrophobic. Thus, for this compound, the difference between the polarity of the two planes is much less than it is for cholic acid. In addition, the hydroxyl groups are now located farther away from each other when compared with the location of the three hydroxyl groups when they are in the α configuration as in cholic acid, which decreases the overall hydrophobicity of the steroid nucleus (Iida and Chang, 1982; Roda et al., 1983; Carey, 1985). Moreover, the unhindered equatorial 7β-hydroxyl group has a very high potency for trapping water molecules (Carey, 1985), which also decreases the steroid ring hydrophobicity. This analogue, with a decreased steroid ring hydrophobicity (less hydrophobic than even cholic acid) and difference in the polarity between the two hemispheres, did not increase channel activity in most of the patches (poor activation occurred in one out of seven cell-attached patches and two out of seven excised, inside-out patches; Fig.7 A, top trace). This low effectiveness of “all β cholic acid” is consistent with a major role of the steroid nucleus hydrophobicity in BKCa channel activation by the bile acids, as previously shown (Fig. 5). In summary, “all β cholic acid” was a poor activator of the channel having both a decreased planar polarity and lower hydrophobicity than all naturally occurring unconjugated bile acids we tested (i.e., cholic, deoxycholic, and lithocholic acids, all effective activators of BKCa channels).
Second, we used ursodeoxycholic acid methyl ester, an analogue that has the C3 hydroxyl group in the α-plane and the C7 hydroxyl group in the β-plane. In this case, the molecule cannot be sharply divided into hydrophilic and hydrophobic hemispheres (Carey, 1985; Miyajima et al., 1988). To reduce any possible contribution of the mobile lateral charge to the polarity of either hemisphere of the ring structure, the free carboxyl in the lateral chain was replaced with a carboxy methyl ester. As demonstrated above with cholic acid methyl ester, esterification of the carboxyl in the lateral chain is not critical for the activation of these channels (Fig. 4). This molecule combines structural features with presumably opposing effects on channel activation. On one hand, there is a polar group on the polar side of the rings (i.e., the hydroxyl in the α configuration at C3), which favors activation (as shown in Fig. 6 A). In addition, being only a dihydroxy derivative, the steroid nucleus of this analogue is more hydrophobic than those of trihydroxy bile acids, such as cholic or “all β cholic” acids, and less hydrophobic than those of lithocholic or epilithocholic acids. In fact, the hydrophobicity of the steroid nucleus in ursodeoxycholic acid methyl ester (Roda et al., 1983; Miyajima et al., 1988) is intermediate between those of two “all α” compounds: cholic (with a more hydrophilic nucleus) and deoxycholic (with a slightly more hydrophobic nucleus) acids, which are both effective activators of the channel (Figs. 2 and 5 and Table I).
On the other hand, ursodeoxycholic acid methyl ester has a decreased hydrophobicity of the β side of the steroid nucleus (where the methyl groups are located) due to the presence of an equatorial, water-trapping, 7β-hydroxyl group (see above), which reduces the planar polarity of the molecule. Ursodeoxycholic acid methyl ester increased channel activity (Fig. 7 A, bottom trace, and Table I), but appears not to be as effective as deoxycholic or cholic acids. A diminished effectiveness of ursodeoxycholic acid methyl ester versus deoxycholic acid would be consistent with a reduced planar polarity and/or hydrophobicity of the steroid nucleus of this synthetic analogue when compared with that of the natural dihydroxy derivative. On the other hand, a diminished effectiveness of ursodeoxycholic acid methyl ester versus cholic acid, which has smaller hydrophobicity of the nucleus but increased planar polarity, would be consistent with a major role for planar polarity as a structural feature favoring channel activation by bile acids.
In summary, the results with “all β cholic acid” and ursodeoxycholic acid methyl ester, taken together with the near lack of effect on channel activity obtained with epilithocholic acid or ursocholanic acid, indicate that the location of polar groups in a plane (α-plane) opposite to the hydrophobic hemisphere of the steroid rings, which emphasizes the planar polarity of these steroids, favors BKCa channel activation. This structural feature is largely reduced in all four of these compounds when compared with all naturally occurring bile acids.
All of the experiments described so far to examine important structural features for bile acid activation of BKCa channels were performed with a concentration of 100 μM of the bile acid analogue in the puffer pipette. However, we needed to establish whether the failure of compounds with diminished planar polarity, such as epilithocholic acid, could be overcome by higher concentrations of the analogue, and/or the differential effects of a pair of analogues (e.g., lithocholic versus epilithocholic acid) could still be maintained over a wider concentration range. Because of the minor structural change preventing epilitholic acid from being an effective channel activator, for these experiments we compared the effects of this agent and lithocholic acid at a known concentration using the larger gravity fed pipette system (materials and methods) to avoid possible dilution of the agonist that can occur with puffer pipettes. When we tried 1-mM concentrations of these analogues in a few patches, we routinely lost the gigaseals. Furthermore, membrane disruption and loss of gigaseals were observed in 4 out of 13 patches using a concentration of 333 μM. These experimental problems are consistent with what might happen when aqueous concentrations of these monohydroxylated analogues approach their CMC, which is in the very low mM range (Carey and Small, 1972; Roda et al., 1983, 1990; Heuman and Bajaj, 1994). Bile acids at their CMC start to solubilize membrane components and disrupt membrane permeability (Coleman et al., 1980; Oelberg et al., 1990). Moreover, the transition from monomers to micelles for all bile acids, as opposed to typical anionic detergents, is not abrupt but occurs gradually over a relatively broad concentration range (Roda et al., 1983; Heuman and Bajaj, 1994).
Nevertheless, we were able to get recordings from applications of 333-μM concentrations of either lithocholic or epilithocholic acid to the same inside-out patch and evaluate channel activity. There was a large variation in the magnitude of the responses. There were four patches to which both analogues were applied for complete experiments and where lithocholic caused a marked increase in channel activity. In two of these patches, epilithocholic acid caused no effect or even a possible decrease in activity. In the other two patches, epilithocholic acid caused an increase in activity. However, these responses (353 and 193% of control activity) were markedly smaller than those evoked by 333 μM lithocholic acid on the same patches (646 and 300% of control activity, respectively). The average response induced by epilithocholic acid, therefore, was much smaller than that produced by lithocholic acid: 174 ± 76 vs. 1,557 ± 1,371% of control (n = 4). There was one patch where both agents caused no marked change in channel activity.
Both epilithocholic and lithocholic acid, even at these concentrations close to their CMCs, failed to modify the channel unitary current amplitude: 98.5 ± 2 and 99.9 ± 0.7% of control values, for epilithocholic and lithocholic acid. This finding, i.e., changes in channel activity without modification of unitary current amplitude, is similar to that obtained with bile acids and analogues at lower concentrations (see above), and contrasts with results obtained with other anionic detergents, cholesterol, and/or lipid curvature–inducing amphiphiles on a variety of ion channels (Sawyer et al., 1989; Sawyer and Andersen, 1989; Lundbæk and Andersen, 1994; Chang et al., 1995a,b; Bezrukov, 2000).
Finally, given the difficulties outlined above to test the effect of very hydrophobic, monohydroxy derivatives over a wide concentration range (i.e., concentrations of epilithocholic acid below 100 μM were ineffective, and concentrations of this analogue and of lithocholic acid near 1 mM disrupted many patches), we next obtained concentration-response curves examining the increase in BKCa channel activity by dihydroxy derivatives in inside-out patches. As pointed out above, an increase in the number of hydroxyl groups attached to the steroid nucleus decreases the hydrophobicity of the steroid nucleus and the molecule. The decreased hydrophobicity of dihydroxy derivatives allowed us to obtain a concentration-response curve over a wide and pathophysiologically relevant concentration range.
Deoxycholic and “epideoxycholic” acids (Fig. 1) possess rather similar hydrophobicity (Roda et al., 1983; Carey, 1985; Miyajima et al., 1988) but markedly different planar polarity. In deoxycholic acid, both axial α hydroxyl groups (at C3 and C12) are located in the side of the rings opposite to the side where the methyl groups are attached, which divides the molecule into two clear-cut hemispheres: hydrophobic (convex, where methyl groups are attached) and polar (concave, where hydroxyl groups are attached). On the other hand, epideoxycholic is characterized by its axial α hydroxyl (at C12) facing the concave hemisphere and its equatorial β hydroxyl (at C3) in the plane of the steroid rings, which reduces the planar polarity of the molecule (Carey, 1985; Miyajima et al., 1988). Fig. 7 B shows that within a wide concentration range (3 μM to 1 mM), the concentration-response curve to “epideoxycholic” is shifted to the right when compared with that of deoxycholic acid. Since CMCs for dihydroxy derivatives are well above 1 mM under our recording conditions (Carey and Small, 1972; Helenius et al., 1979; Roda et al., 1983; Heuman and Bajaj, 1994), the figure indicates that the compound with increased planar polarity, at any given monomeric concentration tested, produces increases in channel activity higher than those produced by the compound with reduced planar polarity. Given the presence of an unknown number of BKCa channels present in the patches, it was impossible to obtain a “ceiling effect” for these analogues, so the shift to the right may be due to “epideoxycholic acid” having a decreased efficacy, relative affinity, and/or access to its receptor site(s) (see discussion), when compared with those of deoxycholic acid.
In summary, the poor effectiveness of “all β cholic acid,” ursodeoxycholic acid methyl ester, “epideoxycholic,” epilithocholic, and ursocholanic acids, when compared with that of naturally occurring bile acids, indicate that the location of polar groups in a plane (α-plane) opposite to the hydrophobic hemisphere of the steroid rings, favors BKCa channel activation. This structural feature, which emphasizes the planar polarity of bile acids, is largely reduced in all five of these analogues when compared with that of all naturally occurring bile acids.
Responses of Outside-out Patches to Bile Acids and Bile Acid Analogues
Cell membrane composition is characterized by leaflet asymmetry (Deveaux, 1991). To test for a possible modulatory role of membrane asymmetry in bile acid–induced increases in BKCa channel activity, we determined whether possible differential effects would occur when a given compound was applied to the extracellular versus the intracellular membrane leaflet. Thus, bile acid action on BKCa channel activity was explored in outside-out patches, where analogues were applied to the extracellular side of the membrane patch, and possible differential effects were addressed by comparing a given analogue action in outside-out patches to its effect in inside-out patches. As shown in Table I, cholic, deoxycholic, and lithocholic acids when applied to outside-out patches increased BKCa channel activity (10 out of 10 patches), as found with cell-attached or inside-out patches. In addition, ursocholanic acid, which was almost always ineffective when applied to both inside-out and cell-attached patches, produced activation in four out of seven outside-out patches. In three of the patches in which ursocholanic acid increased channel activity, lithocholic acid was applied to the same patch, and was consistently more effective: NPo values reached 162 vs. 442, 210 vs. 2,600, and 653 vs. 11,157% of controls, in the presence of ursocholanic versus lithocholic acid, respectively. In a fourth paired outside-out patch experiment, lithocholic acid increased channel activity to 9,138% of control while ursocholanic acid failed to do so (98.9% of control). These increases in channel activity by lithocholic acid are similar to those reported from inside-out patches (Fig. 5). In addition, the differential effect of lithocholic versus ursocholanic acid in outside-out patches seems to indicate that the presence of polar groups attached to the steroidal nucleus favors channel activation by bile acids, as concluded from inside-out patch experiments.
We next evaluated the response in BKCa channel activity to polar compounds, for which flip-flopping across the membrane is very slow. First, “all β cholic acid” (a compound with a highly polar steroid nucleus and minimal planar polarity), which failed to readily increase channel activity in inside-out patches (Table I), produced channel activation in two out of two cases when applied to outside-out patches. Second, we explored the effect of taurolithocholic acid, which is a polar compound (due to a largely ionized group in the lateral chain) with a relatively hydrophobic nucleus. This natural analogue readily increased channel activity in inside-out patches, but failed to do so in cell-attached patches (Fig. 4). Taurolithocholic acid readily increased channel activity in three out of three outside-out patches, its effectiveness being similar to that reported in inside-out patches. Since taurolithocholic acid can only flip across the bilayer very slowly (Cabral et al., 1987; Kamp and Hamilton, 1993; Donovan and Jackson, 1997), a fast and reversible increase in channel activity when this natural compound is applied to either inside-out or outside-out patches, seems to indicate that bile acids can increase BKCa channel activity by working from either side of the membrane. These data also suggest that membrane leaflet symmetry is not necessary for bile acid action on BKCa channel activity.
An explanation for the effectiveness of negatively charged steroids when applied to outside-out patches is that they preferentially intercalate into the outer leaflet of the lipid bilayer causing the outer membrane surface charge to be more negative. This would have a similar effect on the channel as making the membrane potential more positive (Hille, 1992), increasing BKCa channel activity. However, the application of either taurolithocholic acid or ursocholanic acid to the extracellular surface of an outside-out patch in a high ionic strength solution (310 mM NaCl) to shield surface charge (MacKinnon et al., 1989; MacKinnon and Miller, 1989) produced an increase in channel activity similar to that produced by either of these agents when applied to the same patch in the usual solutions (Table I, legend).
Bile Acid–induced Increases in BKCa Channel Activity May Be a Widespread Phenomenon in Different Smooth Muscle Cell Types
We determined whether the enhancement of BKCa channel activity by naturally occurring bile acids occurs in single cells isolated from other vascular, and even nonvascular, smooth muscle. Both lithocholic acid and its tauroconjugate increased BKCa channel activity in single smooth muscle cells freshly isolated from the rabbit main pulmonary artery which, unlike the mesenteric artery, is a nonresistive vessel (Table II). Lithocholic acid (100 μM in the application pipette) was effective when applied to patches both in the inside-out and the cell-attached configurations. The characteristics of this activation paralleled those already described above for bile acid–induced channel activation in the mesenteric artery (i.e., immediate onset upon application of lithocholic acid and full reversibility after removal and washout of the agent). Furthermore, taurolithocholic acid was as effective as lithocholic acid in enhancing channel activity when applied to the cytosolic surface of inside-out patches, but was ineffective when applied to the extrapatch surface of cell-attached patches (Table II). These findings are similar to those previously demonstrated in the mesenteric artery (Fig. 4 and Table I).
TABLE II.
Increase in BKCa Channel Activity by Different Natural Bile Acids and Synthetic Analogues in Smooth Muscle Cells Freshly Isolated from Rabbit Main Pulmonary Artery and Gallbladder, Recorded in Either Cell-attached (C-A) or Inside-out (I-O) Patches
| C-A | I-O | |
|---|---|---|
| Main pulmonary artery | ||
| Lithocholic acid | 6/6 | 4/4 |
| Taurolithocholic acid | 0/3 | 2/2 |
| Gallbladder | ||
| Deoxycholic acid | 2/2 | 2/2 |
Results are expressed as the ratio of the number of positive results to the number of patches tested. Lithocholic acid and its tauroconjugate were applied at 100-μM concentrations in the puffer pipette to pulmonary artery cells, and deoxycholic acid was applied at a concentration of 3 mM in the puffer pipette to gallbladder cells. Compounds were prepared from stock solutions and diluted in bath solution to the final concentration (materials and methods). Solvents used to prepare the stock solutions were equally diluted and applied onto the patches (controls). The DMSO-ethanol dilution (<0.03%/<0.3% vol/vol) did not noticeably modify channel activity in pulmonary artery cells (C-A, 0/3; I-O, 0/3). Likewise, the DMSO-ethanol dilution (<0.9%/<9% vol/vol) did not noticeably modify channel activity in gallbladder cells (C-A, 0/2; I-O, 0/2).
We also examined the effect of deoxycholic acid on the activity of BKCa channels in single smooth muscle cells freshly isolated from the rabbit gallbladder. 3 mM deoxycholic acid dramatically increased channel activity in both cell-attached (n = 2) and inside-out patches (n = 2), with control solutions having no effect (n = 4).
Taken together, these results, obtained in vascular smooth muscle cells from either the pulmonary or the systemic circulation and in gallbladder, suggest the possibility that an increase in BKCa channel activity by bile acids may be common to many smooth muscle cell types.
DISCUSSION
Bile Acids Directly Activate BKCa Channels
Using both whole-cell and single-channel recording patch-clamp techniques we have demonstrated for the first time the activation of BKCa channels by naturally occurring bile acids and synthetic analogues. This effect of bile acids on BKCa channels occurs without a change in the unitary conductance (Fig. 3 A), indicating that the bile acid–induced increase in whole-cell BKCa currents can be attributed solely to its effect on channel gating, which results in an overall increase in steady-state NPo. We found that the bile acid–induced increase in activity occurred with a parallel shift to the left in the activity-voltage relationship (i.e., no change in the “slope factor”; Fig. 3 B), suggesting that the voltage sensing component of the channel is not affected by these negatively charged steroids. Our results indicate that bile acids increase channel activity by interacting with the channel protein complex itself or some closely associated membrane component.
Putative Targets in the Membrane for Bile Acid Action on BKCa Channels
Our results from inside-out patches show that the increase in BKCa channel activity by natural bile acids is higher the more hydrophobic the bile acid (Fig. 5). This relationship may reflect a direct interaction between bile acids and: (1) a hydrophobic site on a membrane protein (including the channel complex itself); (2) hydrophobic, lipidic domains throughout the lipid bilayer; or (3) a hydrophobic domain in the lipid bilayer at the lipid–protein interface.
Protein Site(s): An ion channel protein–bile acid direct interaction is quite possible since there is wide precedence for the interaction of polypeptides or proteins and bile acid monomers (Roda et al., 1990), which, as discussed below, are most likely the molecular form responsible for increasing BKCa channel activity. The dissociation constants (K ds) for bile acid binding to selective affinity sites on circulating plasma proteins range from 2–5 μM (lithocholic acid binding to albumin) to 2,500 μM (cholic acid binding to delipidated human lipoprotein) (Roda et al., 1982; Ceryak et al., 1993). A similar range of K ds is found for the binding of bile acids to a variety of cytosolic binding proteins: from 0.5 μM (lithocholic acid binding to the Ya class of glutathione S-transferases) to 1,000 μM (cholic acid and derivatives binding to fatty acid binding protein) (reviewed in Stolz et al., 1989). The ileal Na+/bile acid polypeptidic cotransporter, following cloning and expression in Xenopus oocytes, displays similar competitive substrate inhibition by taurodeoxycholic acid and apparent affinity (Km = 48 μM) as the native brush border cotransporter studied in native membranes (Mullins et al., 1992). All of these affinity constants are within the range of concentrations reported here for bile acids to effectively enhance BKCa channel activity (3–1,000 μM; Table I, legend). In addition, the high affinity, specific binding of bile acids to albumin correlates with the hydrophobicity of bile acid monomers, with the K d for lithocholic acid (5 μM) being smaller than that for deoxycholic acid (25 μM) and smaller still than that for cholic acid (333 μM; Roda et al., 1982; Picó et al., 1989; Ceryak et al., 1993). A similar order and range of affinities is found for the binding of these bile acids to the nuclear farnesoid X receptor (Parks et al., 1999). This order is identical to that reported in our study for the effectiveness of bile acids activating BKCa channels (Fig. 5). Likewise, an increase in the number of hydroxyls and, thus, polarity decreases the affinity of bile acids for a shared site on human delipidated lipoproteins (Ceryak et al., 1993).
Finally, the stereospecificity of bile acid–induced increases in BKCa channel activity, demonstrated with the differential effects of mono-, dihydroxy-, or trihydroxyderivatives (e.g., lithocholic versus epilithocholic acid, deoxycholic versus “epideoxycholic” acid, or cholic versus “all β cholic” acid), is also consistent with an action of bile acid monomers on a protein site. It is interesting to note that for another steroid, estradiol, whereas the naturally occurring 17β isomer is highly effective for increasing BKCa channel activity in arterial smooth muscle, its synthetic 17α isomer is not (Kitazawa et al., 1997). This effect of estradiol appears to be due to binding to the β subunit of the BKCa channel (Valverde et al., 1999). However, stereospecificity itself is not enough to claim a protein site of action, since it also has been used to argue for a lipid site of action for anesthetic steroids. Data obtained with two pairs of anesthetic steroids, differing only in the configuration of the hydroxyl group in C3, showed that the ability to disorder the hydrocarbon phase of liposome bilayers correlates very well with anesthetic potency; the active isomer (α) produces a marked increase in disorder, whereas the inactive isomer (β) does not (Lawrence and Gill, 1975).
The fact that bile acid derivatives having dissimilar polar groups (e.g., hemisuccinate or hydroxyls), or having hydroxyl groups located in various locations in the steroid nucleus (C3 versus C7 and C12), are all effective enhancers of BKCa channel activity does not rule out a protein–bile acid interaction (Hagenbuch et al., 1990).
Lipid Target:
Instead of resulting from a direct binding of bile acid to a protein site, potentiation of BKCa channel activity could result from bile acid incorporation into the membrane lipid phase, which may eventually change the lipid environment around the channel protein and thereby modify protein function (Schwenk et al., 1977; Lundbæk et al., 1996; Cantor, 1999). Some bilayer physical properties, such as monolayer equilibrium curvature (Lundbæk and Andersen, 1994; Chang et al., 1995a,b; Lundbæk et al., 1996), lipid packing stress or lateral pressure profile (Cantor, 1997; Bezrukov, 2000), and/or stiffness (Lundbæk et al., 1996) might be extremely sensitive to changes in bilayer composition that would result from bile acid insertion. According to this theory, small changes in bile acid concentrations in the aqueous/lipid interphase with consequent incorporation of bile acids into the bilayer modify one or more bilayer properties, which, in turn, results in changes in the conformation of the protein and its function (Cantor, 1997).
A variety of lipidic detergents and lysophospholipids, all promoting positive (convex) curvature of the lipid monolayer and/or decrease in bilayer stiffness, have been reported to rapidly and reversibly modify the activity of several ion channels, including gramicidin, alamethicin, and voltage-gated Ca2+ channels. On the other hand, compounds that promote negative curvature and/or increase bilayer stiffness have been reported to modify ion channel activity in the opposite direction (Sawyer et al., 1989; Lundbæk and Andersen, 1994; Lundbæk et al., 1996; Bezrukov et al., 1998). The changes in channel activity have been attributed to the modification of monolayer equilibrium curvature and/or bilayer stiffness caused by these agents (Lundbæk and Andersen, 1994; Lundbæk et al., 1996). In particular, the activity of BKCa channels reconstituted into lipid bilayers has been reported to be increased by increasing the phospholipid cross sectional area, which promotes a positive curvature monolayer, and decreased by cholesterol, which favors negative curvature (Chang et al., 1995a,b). Similar changes in BKCa channel function resulting from modification of the bilayer cholesterol content have been reported with BKCa channel-forming hslo subunits reconstituted into lipid bilayers (Crowley et al., 2002) and native BKCa channels in vascular smooth muscle cells (Bolotina et al., 1989).
Bile acids, as well as several other chemically unrelated detergents (Sawyer et al., 1989), are positive curvature-forming lipids. At very low concentrations (<2 wt%), sodium cholate has been shown to promote positive curvature in a phosphatidylethanolamine monolayer/water system (Thurmond et al., 1991). A problem that arises in extrapolating the effect of these low bile acid concentrations on monolayer curvature as a possible mechanism underlying BKCa channel activation, is that the mole fraction of bile acid in the smooth muscle membrane during our experiments is unknown. If bile acids interact with bilayer lipids as they do with each other in simple micelles, we may obtain a mole fraction from the aqueous concentration and the CMC, as fully described for other amphiphiles in Sawyer et al. (1989). Then, we may speculate that in our experiments, the molar fraction should have ranged between 0.1 and 0.3 for lithocholic acid and between 0.009 and 0.027 for cholic acid, which are values similar to those from Thurmond et al. (1991). However, in this study, the samples were thermally equilibrated for 1 h before [31P]NMR spectra were obtained. Thus, equilibrium conditions were very different from ours, in which bile acids in solution were applied to a patch of membrane for several seconds with the resulting channel activation being fast and fully reversible (Figs. 2 [B and C], 4, 6, and 7), which makes it unclear whether this mechanism can apply to our experiments. In addition, was BKCa channel activation by bile acids solely due to their positive curvature-inducing property when applied to inside-out patches, it is not clear from this analysis, why lithocholic acid is much more effective than epilithocholic (with similar CMC, and, thus, similar molar fraction) or deoxycholic acid is more effective than “epideoxycholic acid” (with similar CMC and similar molar fraction) or, more importantly, lithocholic acid is much more effective than ursocholanic acid (which has lower CMC and, thus, higher molar fraction). However, it should be noted that this analysis, which includes bile acid aqueous concentration, CMC, molar fraction, and monolayer equilibrium curvature modification capability, does not take into account differential affinity of structural analogues for membrane lipids, nor does it consider the particular spatial arrangement of a given analogue in the lipid bilayer. The equatorial hydroxyl of epilithocholic or “epideoxycholic” acids might well allow these analogues to be inserted in a very different way from the way lithocholic or deoxycholic acids with their axial hydroxyls insert, which eventually results in differential effects on lipid curvature.
Taurolithocholic acid, with a largely ionized lateral chain can only flip across natural membranes very slowly (Cabral et al., 1987; Kamp and Hamilton, 1993; Donovan and Jackson, 1997). The increase in channel activity induced by taurolithocholic acid in both inside-out and outside-out patches is fast enough to make flip-flopping to a particular leaflet unlikely. Thus, should an alteration of the monolayer curvature equilibrium be a mechanism partially or totally responsible for bile acid–induced increase in BKCa channel activity, this alteration would be evoked by insertion of this polar bile acid from either leaflet of the membrane.
In summary, it remains to be established whether bile acid–induced increase in BKCa channel activity, indeed, results as a consequence of direct binding to the channel protein, is secondary to penetration and incorporation into the membrane lipid phase, or due to a combination of these possibilities (Carey, 1985; Heuman and Bajaj, 1994).
Bile Acid Monomers Are Likely the Molecular Form Responsible for Causing Increases in BKCa Channel Activity in Inside-out Patches
Micelle Formation:
One of the most important parameters in the evaluation of the biological actions of bile acids is their CMC (Balducci et al., 1989). Above the CMC, bile acid micelles in solution induce nonspecific membrane damage, partial membrane micellization, and/or removal of membrane proteins and lipids (Helenius et al., 1979; Coleman et al., 1980; Roda et al., 1983). Bile acids were able to effectively increase arterial smooth muscle BKCa channel activity at concentrations well below (3 μM in the application pipette) their CMCs, which would be above 1 mM under the conditions used (Carey and Small, 1972; Helenius et al., 1979; Roda et al., 1983; Heuman and Bajaj, 1994). In particular, highly polar compounds, such as cholic acid and taurolithocholic acid, which are characterized by very high CMCs and concentrations needed to achieve membrane solubilization (Helenius et al., 1979), repeatedly increased channel activity when applied in the low micromolar range to excised patches (Figs. 5, 4 A, and Table I.). Thus, it is very unlikely that bile acid–induced increases in BKCa channel activity result from nonspecific membrane damage produced by bile acid micelles in solution.
Similarly, it is unlikely that the increase in BKCa channel activity by bile acids (≤100 μM) is secondary to a nonspecific interaction of bile acids with lipids within the bilayer with the consequent formation of mixed micelles (i.e., partial membrane micellization). First, channel activation by bile acids and analogues was rapidly and effectively “washed out” with bath solution after application ceased. Second, there was no evidence of a large nonspecific ionic leak even after repeated applications of active compounds to the same patch membrane and/or 5 min of continuous application. Third, lithocholic acid 3-hemisuccinate was as effective as lithocholic acid in enhancing channel activity in spite of the fact that the addition of a negative charge (hemisuccinate) to the steroid nucleus is known to strongly disfavor self-association of bile acids into micelles (Roda et al., 1983; Carey, 1985). Fourth, bile acid analogues like ursocholanic and epilithocholic acid, which would have the same capability of forming mixed micelles as natural bile acids, were for the most part ineffective in enhancing channel activity.
Together, these findings do not support the idea of a “detergent” action on the cellular membrane produced by bile acid micelles, formed either in solution or after the interaction with membrane lipids, underlying bile acid–induced increases in BKCa channel activity.
Dimer or Oligomer Formation:
Even at concentrations below the CMC, bile acids may form dimers or slightly larger aggregates (Balducci et al., 1989), a critical number of hydroxyl substituents being essential for aggregation (Roda et al., 1983). In fact, the mode of insertion of both trihydroxy and, in particular, dihydroxy bile acids into a lipid bilayer or natural membrane may be in a dimeric, even oligomeric, form (Helenius et al., 1979; Shiao et al., 1993). These small aggregates consist of two to four molecules of bile acids with their hydrophilic sides facing inwards, bound by hydrogen bonds between the hydroxyl groups (Carey, 1985). This model, which describes the formation of bile acid dimers or oligomers intercalating in a lipid environment, implies a direct relationship between the number of hydroxyl groups and the capacity of a bile acid to form aggregates (Carey, 1985; Schubert et al., 1986; Schubert and Schmidt, 1988). However, the fact that trihydroxy bile acids, such as cholic acid or “all β cholic acid” were less effective activators of BKCa channels than deoxycholic acid and, furthermore, that this dihydroxy bile acid induced a less marked enhancement of channel activity than the monohydroxylated lithocholic acid (for which dimer formation is strongly disfavored; Fig. 5), suggests that these bile acid dimers or oligomers are not the predominant form of bile acid responsible for increasing BKCa channel activity.
However, it is still possible for small bile acid aggregates to be formed at low concentrations, based on a hydrophobic interaction between the β-planes. In this form of aggregation, the higher the total hydrophobic index of the bile acid molecule, the higher its capability for forming aggregates (Carey and Small, 1972; Miyajima et al., 1988). The fact that two very hydrophobic derivatives, such as epilithocholic and ursocholanic acids (for which this form of aggregation would be strongly favored), were almost always ineffective activators of BKCa channels (Fig. 6 A), indicates that these aggregates are unlikely the primary form of bile acid responsible for increasing channel activity.
Thus, by a process of elimination, we conclude that the bile acid–induced increase in BKCa channel activity is primarily due to a direct action of bile acid monomers in the perfusion solution. Consistent with this conclusion, is our result that “7,12 deoxycholic acid,” with hydroxyl groups located at C7 and C12 in the “polar hemisphere” of the molecule was as effective as deoxycholic acid, with hydroxyl groups at C3 and C12 in the polar hemisphere. Furthermore, ursodeoxycholic acid methyl ester and “epideoxycholic acid,” both having one hydroxyl in the polar hemisphere and the other in the hydrophobic hemisphere, were less effective. It is known that the intrinsic monomeric lipophilicity of a bile acid is independent of the distribution of hydroxyls but related to the ratio of the polarity of the hydrophobic and hydrophilic hemispheres (Roda et al., 1990).
Structural Features of the Bile Acid Molecule and Bile Acid–induced BKCa Channel Activation in Inside-out Patches
Our results from inside-out patches demonstrate that several major substitutions in the lateral chain, including the nature of the ionic group (unconjugated or taurine-conjugated), the elimination of negative charge, and the introduction of groups with different degrees of polarity (methyl ester or primary alcohol) and length (4 or 5-C atoms) do not appear to modify channel activation by bile acids. In contrast, results with both naturally occurring bile acids and synthetic analogues indicate that hydrophobicity of the bile acid steroid nucleus favors bile acid–induced channel activation. In addition to steroid nucleus hydrophobicity, our data suggest that the presence of at least one polar group attached in the α configuration to the steroid rings in the bile acid molecule favors activation of BKCa channels by bile acids. This structural feature agrees with previous findings, which indicate that polar groups, in particular hydroxyl groups, are critical for the modulation of ion channel function by different steroids including bile acids. For example, deoxycholic acid has been shown to suppress the peak Na+ conductance in squid axons. For these experiments, it was felt that the hydrophilic groups of the bile acid are probably the most active moieties in “binding” to the Na+ channel (Wu et al., 1980). In addition, steroids lacking hydroxyl groups, such as methylprednisolone and 2-methylfluorohydrocortisone, have been found to be inactive on Na+ and K+ conductances in squid giant axons, whereas analogues with these hydroxyl groups are active (Henkin et al., 1973).
We interpreted that the existence of one or more polar groups in the α-plane of the bile acid molecule favors bile acid activation of BKCa channels because of the increase in the planar polarity of the bile acid molecule that results from that particular orientation of the polar groups. First, our data show that all active compounds, whether naturally occurring bile acids or synthetic analogues, share the structural feature of planar polarity. Second, compounds without planar polarity, disregarding different degrees of steroid nucleus hydrophobicity (such as ursocholanic and epilithocholic acids), were mostly inactive. Third, compounds with decreased or minimal planar polarity (ursodeoxycholic and “all β cholic” acid, respectively) were mild or ineffective activators of the BKCa channel. Finally, compounds with similar hydrophobicity but markedly different planar polarity, such as deoxycholic and “epideoxycholic” acids, showed a differential effectiveness for increasing channel activity, the analogue with the greater planar polarity being more effective.
Although a minimal planar polarity favors an increase in channel activity, our data demonstrate that, additionally, the overall hydrophobicity of the steroid nucleus does seem to be important. For example, lithocholic acid has greater hydrophobicity but less planar polarity than cholic acid, yet the former causes greater activation of BKCa channels. How these two properties (planar polarity and hydrophobicity) quantitatively contribute to the ability of the bile acid molecule to interact with the putative binding site(s) remains to be determined. However, it should be clear from our results with inside-out patches that, for natural bile acids, the requisite planar polarity for channel activation is obtained with the hydroxyl group(s) located in the α-plane (concave hemisphere) of these flattened rigid amphiphiles.
Possible Role of Bile Acid Activation of BKCa Channels in Bile Acid Relaxation of Smooth Muscle
The vasodilating action of bile acids in the micromolar to low millimolar range has been widely characterized both in vitro and in vivo (for review see Bomzon and Ljubuncic, 1995). In addition, bile acids (30–300 μM) have been shown to relax gallbladder smooth muscle (Sunagane et al., 1990; Xu et al., 1997), intestine (Sunagane et al., 1986), and uterus (Uruno et al., 1975). However, the cellular and molecular mechanisms underlying this widespread, nonspecific relaxation of smooth muscle by bile acids are not well understood.
An increase in K+ efflux secondary to the opening of K+ channels by bile acids would repolarize the smooth muscle membrane which, in turn, would limit Ca2+ influx through voltage-gated Ca2+ channels and, therefore, would lead to relaxation. The activation of BKCa channels has been reported to control membrane potential and the degree of contraction and/or contractility in both vascular (Brayden and Nelson, 1992; Asano et al., 1993; Jaggar et al., 2000) and nonvascular (Suarez-Kurtz et al., 1991; Anwer et al., 1993) smooth muscle. Previous studies that showed bile acid–induced increases in K+ currents were performed in nonsmooth muscle preparations (Binah et al., 1987; Kotake et al., 1989; Devor et al., 1993). In particular, an increase in a Ca2+-sensitive conductance in colonic secretory cells by taurodeoxycholic acid led Dharmsathaphorn et al. (1989) to postulate an involvement of Ca2+-dependent K+ channels in taurodeoxycholic acid effects on secretion in these cells. These previous studies did not address the particular channel type targeted by bile acids.
The activation of the BKCa channel type of Ca2+-activated K+ channels by micromolar concentrations of natural bile acids shown here in both systemic and pulmonary vascular smooth muscle types provides one mechanism that may underlie, or at least contribute to, the relaxant effects of bile acids on smooth muscle. The BKCa channel activity increase produced by bile acids in arterial smooth muscle cells was shown here to be immediate in onset and fully reversible. These characteristics are expected from physiological regulators and would eventually support the contention that bile acids are physiological regulators of ileal blood flow (Kvietys et al., 1981; Thomas et al., 1991).
Under physiological conditions, systemically circulating levels of bile acids in fasting humans are usually 2–10 μM, which is sixfold lower than those in the portal circulation (Scharschmidt, 1982; Ceryak et al., 1993; Güldütuna et al., 1993). These circulating bile acids are >95% bound to albumin (Burke et al., 1971; Roda et al., 1982). Thus, one could speculate that circulating low concentrations of free bile acids would not have a major role in increasing BKCa channel activity and relaxing systemic arterial smooth muscle under normal physiological conditions. In contrast, a significant spillover of bile acids from the portal to the systemic circulation occurs in a wide variety of human pathological conditions (Lewis et al., 1969; Tarantino et al., 1989; Greco and Mingrone, 1993; Hamdan and Stacey, 1993). This results in a 10–100-fold (even reaching 1 mM) higher systemic bile acid concentration (Ceryak et al., 1993; Greco and Mingrone, 1993). These levels are within and even above the concentrations of bile acids shown here to enhance BKCa channel activity and possibly contribute to systemic vasorelaxation. In fact, it has been suggested that increased levels of bile acids in plasma are associated with the hypotension of obstructive jaundice (Bomzon et al., 1984). There is a strong correlation between the magnitude of portosystemic shunting and elevations in plasma bile acid concentrations in portal hypertensive men (Ohkubo et al., 1984). Moreover, the depletion of bile acids in portal hypertensive rats reduces portal blood flow (Thomas et al., 1991).
In hepatobiliary disease, there may not only be an overall increase in systemic bile acid levels, but also a change in composition. Lithocholic acid (both free and conjugated) levels, which normally constitute 5–10% of total bile acids, can dramatically increase in liver disease (Greco and Mingrone, 1993). Interestingly, we found that both lithocholic acid and its tauroconjugate produced a robust increase in BKCa channel activity in systemic arterial muscle, which is more marked than that evoked by deoxycholic or cholic acid, the latter two being more abundant than lithocholic acid in humans under normal physiological conditions.
Finally, a possible contribution of the bile acid–induced increase in BKCa channel activity to vasorelaxation and hypotension in human disease would result not only when bile acid levels increase, but also when circulating albumin, which binds bile acids, decreases. This situation may be observed in analbuminemia and advanced stages of liver cirrhosis (Scharschmidt, 1982; Rothschild et al., 1988).
Acknowledgments
We thank Drs. M.L. Lewbart, J.A. Hamilton, and R.S. Cantor for helpful discussions, Drs. David Kupfer and Ann Rittenhouse for critical reading of earlier versions of the manuscript, and P. Tilander, K. O'Connor, B. Packard, and D. Idrizovic for their excellent technical assistance.
This work was supported by National Institutes of Health grants DK31620 and AA11560.
Footnotes
Abbreviations used in this paper: BKCa, large conductance, Ca2+-activated K+; CMC, critical micellar concentration; Po, open probability.
References
- Anwer, K., C. Oberti, G.J. Perez, N. Perez-Reyes, J.K. McDougall, M. Monga, B.M. Sanborn, E. Stefani, and L. Toro. 1993. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am. J. Physiol. 265:C976–C985. [DOI] [PubMed] [Google Scholar]
- Asano, M., K. Masuzawa-Ito, and T. Matsuda. 1993. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 108:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balducci, R., A. Roda, and R.S. Pearlman. 1989. A theoretical model for the critical micelle concentration of bile salts. J. Sol. Chem. 18:355–368. [Google Scholar]
- Beuers, U., M.H. Nathanson, C.M. Isales, and J.L. Boyer. 1993. Tauroursodeoxycholic acid stimulates hepatocellular exocytosis and mobilizes extracellular Ca2+ mechanisms defective in cholestasis. J. Clin. Invest. 92:2984–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezrukov, S.M. 2000. Functional consequences of lipid packing stress. Curr. Opin. Colloid Interface Sci. 5:237–243. [Google Scholar]
- Bezrukov, S.M., R.P. Rand, I. Vodyanoy, and V.A. Parsegian. 1998. Lipid packing stress and polypeptide aggregation: alamethicin channel probed by proton titration of lipid charge. Faraday Discuss. 111:173–183. [DOI] [PubMed] [Google Scholar]
- Binah, O., I. Rubinstein, A. Bomzon, and O.S. Better. 1987. Effects of bile acids on ventricular muscle contraction and electrophysiological properties: studies in rat papillary muscle and isolated ventricular myocites. Naunyn Schmiedebergs Arch. Pharmacol. 335:160–165. [DOI] [PubMed] [Google Scholar]
- Bolotina, V., V. Omelyanenko, B. Heyes, U. Ryan, and P. Bregestovski. 1989. Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflügers Arch. 415:262–268. [DOI] [PubMed] [Google Scholar]
- Bomzon, A., and P. Ljubuncic. 1995. Bile acids as endogenous vasodilators? Biochem. Pharmacol. 49:581–589. [DOI] [PubMed] [Google Scholar]
- Bomzon, A., J.P. Finberg, D. Tovbin, S.G. Naidu, and O.S. Better. 1984. Bile salts, hypotension and obstructive jaundice. Clin. Sci. 67:177–183. [DOI] [PubMed] [Google Scholar]
- Brayden, J.E., and M.T. Nelson. 1992. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 256:532–535. [DOI] [PubMed] [Google Scholar]
- Bregestovski, P.D., V.M. Bolotina, and V.N. Serebryakov. 1989. Fatty acid modifies Ca2+-dependent potassium channel activity in smooth muscle cells from the human aorta. Proc. R. Soc. Lond. Biol. Sci. 237:259–266. [DOI] [PubMed] [Google Scholar]
- Burke, C.W., B. Lewis, D. Panveliwalla, and S. Tabaqchali. 1971. The binding of cholic acid and its taurine conjugate to serum proteins. Chim. Clin. Acta. 32:207–214. [DOI] [PubMed] [Google Scholar]
- Cabral, D.J., D.M. Small, H.S. Lilly, and J.A. Hamilton. 1987. Trans-bilayer movement of bile acids in model membranes. Biochemistry. 26:1801–1804. [DOI] [PubMed] [Google Scholar]
- Cantor, R.S. 1997. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Physiol. Chem. 101:1723–1725. [Google Scholar]
- Cantor, R.S. 1999. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 76:2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, M.C. 1985. Physical-chemical properties of bile acids and their salts. Sterols and Bile Acids. H. Danielsson and J. Sjoval, editors. Elsevier Science Publishers, Amsterdam. 345–403.
- Carey, M.C., and D.M. Small. 1972. Micelle formation by bile salts. Arch. Int. Med. 130:506–527. [PubMed] [Google Scholar]
- Ceryak, S., B. Bouscaren, and H. Fromm. 1993. Comparative binding of bile acids to serum lipoproteins and albumin. J. Lipid Res. 34:1661–1674. [PubMed] [Google Scholar]
- Chang, H.M., R. Reitstetter, and R. Gruener. 1995. a. Lipid-ion interactions: increasing phospholipid headgroup size but not ordering acyl chains alters reconstituted channel behavior. J. Membr. Biol. 145:13–19. [DOI] [PubMed] [Google Scholar]
- Chang, H.M., R. Reitstetter, R.P. Mason, and R. Gruener. 1995. b. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J. Membr. Biol. 143:51–63. [DOI] [PubMed] [Google Scholar]
- Clarke, A.L., S. Petrou, J.V. Walsh, Jr., and J.J. Singer. 1995. Modulation of large conductance Ca2+-activated K+ channel activity by charged lipids: structural requirements. Soc. Neurosci. Abstr. 21:1326. [Google Scholar]
- Coleman, R., P.J. Lowe, and D. Billington. 1980. Membrane lipid composition and susceptibility to bile salt damage. Biochim. Biophys. Acta. 599:294–300. [DOI] [PubMed] [Google Scholar]
- Combettes, L., B. Berthon, E. Doucet, S. Erlinger, and M. Claret. 1989. Characteristics of bile acid-mediated Ca2+ release from permeabilized liver cells and liver microsomes. J. Biol. Chem. 264:157–167. [PubMed] [Google Scholar]
- Crowley, J.J., S.N. Treistman, and A.M. Dopico. 2002. Cholesterol antagonizes ethanol potentiation of human BKCa channel-forming α subunits in binary phospholipid bilayers. Biophys. J. 82:586a. (Abstr.) [DOI] [PubMed] [Google Scholar]
- Deveaux, P.F. 1991. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 30:1163–1173. [DOI] [PubMed] [Google Scholar]
- Devor, D.C., M.C. Sekar, R.A. Frizzell, and M.E. Duffey. 1993. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84). J. Clin. Invest. 92:2173–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn, K., P.A. Huott, P. Vongkovit, G. Beuertein, S.J. Pandol, and H.V. Ammon. 1989. Cl− secretion induced by bile salts. A study of the mechanism of action based on a cultured colonic epithelial cell line. J. Clin. Invest. 84:45–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, J.M., and A.A. Jackson. 1997. Transbilayer movement of fully ionized taurine-conjugated bile salts depends upon bile acid concentration, hydrophobicity, and membrane cholesterol content. Biochemistry. 36:11444–11451. [DOI] [PubMed] [Google Scholar]
- Dopico, A.M., M.T. Kirber, J.J. Singer, and J.V. Walsh, Jr. 1994. a. Membrane stretch directly activates large conductance Ca2+ -activated K+ channels in mesenteric artery smooth muscle cells. Am. J. Hypertens. 7:82–89. [DOI] [PubMed] [Google Scholar]
- Dopico, A.M., J.V. Walsh, Jr., and J.J. Singer. 1994. b. Bile acids directly activate large conductance Ca2+-activated K+ (CAK) channels in vascular smooth muscle cells. Biophys. J. 66:A437. (Abstr.) [DOI] [PubMed] [Google Scholar]
- Dopico, A.M., J.V. Walsh, Jr., and J.J. Singer. 2001. Structural features of the steroidal nucleus that favor activation of BKCa channels by bile acids. Biophys. J. 80:203a. (Abstr.) [Google Scholar]
- Dopico, A.M., V. Anantharam, and S.N. Treistman. 1998. Ethanol increases the activity of Ca2+-dependent K+ (mslo) channels: functional interaction with cytosolic Ca2+. J. Pharmacol. Exp. Ther. 284:258–268. [PubMed] [Google Scholar]
- Greco, A., and G. Mingrone. 1993. Serum bile acid concentrations in mild liver cirrhosis. Clin. Chim. Acta. 221:183–189. [DOI] [PubMed] [Google Scholar]
- Güldütuna, S., T. You, W. Kurts, and U. Leuschner. 1993. High performance liquid chromatographic determination of free and conjugated bile acids in serum, liver biopsies, bile, gastric juice and feces by fluorescence labeling. Clin. Chim. Acta. 214:195–207. [DOI] [PubMed] [Google Scholar]
- Hagenbuch, B., H. Lübbert, B. Stieger, and P.J. Meier. 1990. Expression of the hepatocyte Na+/bile acid cotransporter in Xenopus laevis oocytes. J. Biol. Chem. 265:5357–5360. [PubMed] [Google Scholar]
- Hamdan, H., and N.H. Stacey. 1993. Mechanism of trichloroethylene-induced elevation of individual serum bile acids. I. Correlation of trichloroethylene concentrations to bile acids in rat serum. Toxicol. Appl. Pharmacol. 121:291–295. [DOI] [PubMed] [Google Scholar]
- Helenius, A., D.R. McCaslin, E. Fries, and C. Tanford. 1979. Properties of detergents. Methods Enzymol. 56:734–749. [DOI] [PubMed] [Google Scholar]
- Henkin, R.I., D.L. Gilbert, I. Stillman, and R. DiPolo. 1973. Ineffectiveness of adrenocorticosteroids and adrenocorticotropin in altering Na-K currents in squid giant axon. Experientia. 29:555–556. [DOI] [PubMed] [Google Scholar]
- Heuman, D.M., and R. Bajaj. 1994. Ursodeoxycholate conjugates protect against disruption of cholesterol-rich membranes by bile salts. Gastroenterology. 106:1333–1341. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels in Excitable Membranes. 2nd ed. Sinauer Associates, Inc., Sunderland, MA. 607 pp.
- Igimi, H., and M.C. Carey. 1980. pH-Solubility relations of chenodeoxycholic and ursodeoxycholic acids: physical-chemical basis for dissimilar solution and membrane phenomena. J. Lipid Res. 21:72–90. [PubMed] [Google Scholar]
- Iida, T., and F.C. Chang. 1982. Potential bile acid metabolites. 7.1 3,7,12-trihydroxy-5β-cholanic acids and related compounds. J. Org. Chem. 47:2972–2978. [Google Scholar]
- Jaggar, J.H., V.A. Porter, W.J. Lederer, and M.T. Nelson. 2000. Calcium sparks in smooth muscle. Am. J. Physiol. 278:C235–C256. [DOI] [PubMed] [Google Scholar]
- Kamp, F., and J.A. Hamilton. 1993. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 32:11074–11085. [DOI] [PubMed] [Google Scholar]
- Kirber, M.T., R.W. Ordway, L.H. Clapp, J.V. Walsh, Jr., and J.J. Singer. 1992. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 297:24–28. [DOI] [PubMed] [Google Scholar]
- Kitazawa, T., E. Hamada, K. Kitazawa, and A.K.M. Gaznabi. 1997. Non-genomic mechanism of 17β-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J Physiol. 499:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake, H., T. Itoh, M. Watanabe, I. Hisatome, J. Hasegawa, and H. Mashiba. 1989. Effect of bile acid on electrophysiological properties of rabbit sino-atrial node in vitro. Br. J. Pharmacol. 98:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume, H., K. Takagi, T. Satake, H. Tokuno, and T. Tomita. 1990. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J. Physiol. 424:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvietys, P.R., J.M. McLendon, and D.N. Granger. 1981. Postprandial intestinal hyperemia: role of bile salts in the ileum. Am. J. Physiol. 241:G469–G477. [DOI] [PubMed] [Google Scholar]
- Lawrence, D.K., and E.W. Gill. 1975. Structurally specific effects of some steroid anesthetics on. spin-labeled liposomes. Mol. Pharmacol. 11:280–286. [PubMed] [Google Scholar]
- Lewis, B., S. Tabaqchali, D. Panveliwalla, and I.D.P. Wootton. 1969. Serum-bile-acids in the stagnant-loop syndrome. Lancet. Feb. 1:219–220. [DOI] [PubMed] [Google Scholar]
- Lundbæk, J.A., and O.S. Andersen. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk, J.A., P. Birn, J. Girshman, A.J. Hansen, and O.S. Andersen. 1996. Membrane stiffness and channel function. Biochemistry. 35:3825–3830. [DOI] [PubMed] [Google Scholar]
- MacKinnon, R., and C. Miller. 1989. Functional modification of a Ca2+-activated K+ channel by trimethyloxonium. Biochemistry. 28:8087–8092. [DOI] [PubMed] [Google Scholar]
- MacKinnon, R., R. Latorre, and C. Miller. 1989. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 28:8092–8099. [DOI] [PubMed] [Google Scholar]
- McManus, O.B. 1991. Calcium-activated potassium channels: regulation by calcium. J. Bioenerg. Biomembr. 23:537–560. [DOI] [PubMed] [Google Scholar]
- Mistry, D.K., and C.J. Garland. 1998. Characteristics of single, large-conductance calcium-dependent potassium channels (BKCa) from smooth muscle cells isolated from rabbit mesenteric artery. J. Membr. Biol. 164:125–138. [DOI] [PubMed] [Google Scholar]
- Miyajima, K., K. Machida, T. Taga, H. Komatsu, and M. Nakagaki. 1988. Correlation between the hydrophobic nature of monosaccharides and cholates, and their hydrophobic indices. J. Chem. Soc. Faraday Trans. 84:2537–2544. [Google Scholar]
- Mullins, J.G.L., R.B. Beechey, G.W. Gould, F.C. Campbell, and S.P. Shirazi-Beechey. 1992. Characterization of the ileal Na+/bile salt co-transporter in brush border membrane vesicles and functional expression in Xenopus laevis oocytes. Biochem. J. 285:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelberg, D.G., S.A. Downey, and M.M. Flynn. 1990. Bile salt-induced intracellular Ca++ accumulation in type II pneumocytes. Lung. 168:297–308. [DOI] [PubMed] [Google Scholar]
- Ohkubo, H., K. Okuda, S. Iida, K. Ohnishi, S. Ikawa, and I. Makimo. 1984. Role of portal and splenic vein shunts and impaired hepatic extraction in the elevated serum bile acids in liver cirrhosis. Gastroenterology. 86:514–520. [PubMed] [Google Scholar]
- Pak, J.-M., and S.S. Lee. 1993. Vasoactive effects of bile salts in cirrhotic rats: in vivo and in vitro studies. Hepatology. 18:1175–1181. [PubMed] [Google Scholar]
- Parks, D.J., S.G. Blanchard, R.K. Bledsoe, G. Chandra, T.G. Consler, S.A. Kliewer, J.B. Stimmel, T.M. Willson, A.M. Zavacki, D.D. Moore, and J.M. Lehmann. 1999. Bile acids: natural ligands for an orphan nuclear receptor. Science. 284:1365–1368. [DOI] [PubMed] [Google Scholar]
- Petrou, S., R.W. Ordway, J.A. Hamilton, J.V. Walsh, Jr., and J.J. Singer. 1994. Structural requirements for charged lipid molecules to directly increase or suppress K+ channel activity in smooth muscle cells. J. Gen. Physiol. 103:471–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó, G., M. Favazza, and C. Gatti. 1989. Effect of bile salts on proton binding to human serum albumin. J. Gen. Physiol. Biophys. 8:515–519. [PubMed] [Google Scholar]
- Roda, A., G. Cappelleri, R. Aldini, E. Roda, and L. Barbara. 1982. Quantitative aspects of the interaction of bile acids with human serum albumin. J. Lipid Res. 23:490–495. [PubMed] [Google Scholar]
- Roda, A., A.F. Hofmann, and K.J. Mysels. 1983. The influence of bile salt structure on self-association in aqueous solutions. J. Biol. Chem. 258:6362–6370. [PubMed] [Google Scholar]
- Roda, A., A. Minutello, M.A. Angellotti, and A. Fini. 1990. Bile acid structure-activity relationship: evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J. Lipid Res. 31:1433–1443. [PubMed] [Google Scholar]
- Rothschild, M.A., M. Oratz, and S.S. Schreiber. 1988. Serum albumin. Hepatology. 8:385–401. [DOI] [PubMed] [Google Scholar]
- Sawyer, D.B., and O.S. Andersen. 1989. Platelet-activating factor is a general membrane perturbant. Biochim. Biophys. Acta. 987:129–132. [DOI] [PubMed] [Google Scholar]
- Sawyer, D.B., R.E. Koeppe, II, and O.S. Andersen. 1989. Induction of conductance heterogeneity in gramicidin channels. Biochemistry. 28:6571–6583. [DOI] [PubMed] [Google Scholar]
- Scharschmidt, B.F. 1982. Bile formation and cholestasis, metabolism and enterohepatic circulation of bile acids, and gallstone formation. Hepatology: A Textbook of Liver Disease. D. Zakim and T.D. Boyer, editors. W.B. Saunders Co., Philadelphia. 297–351.
- Schubert, R., and K.-H. Schmidt. 1988. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry. 27:8787–8794. [DOI] [PubMed] [Google Scholar]
- Schubert, R., K. Beyer, H. Wolburg, and K.-H. Schmidt. 1986. Structural changes in membranes of large unilamellar vesicles after binding of sodium cholate. Biochemistry. 25:5263–5269. [DOI] [PubMed] [Google Scholar]
- Schwenk, M., L.R. Schwarz, and H. Greim. 1977. Taurolithocholate inhibits taurocholate uptake by isolated hepatocytes at low concentrations. Naunyn Schmiedebergs Arch. Pharmacol. 298:175–179. [DOI] [PubMed] [Google Scholar]
- Shiao, Y.-J., J.-C. Chen, C.-N. Wang, and C.-T. Wang. 1993. The mode of action of primary bile salts on human platelets. Biochim. Biophys. Acta. 1146:282–293. [DOI] [PubMed] [Google Scholar]
- Singer, J.J., and J.V. Walsh, Jr. 1987. Characterization of calcium-activated potassium channels in single smooth muscle cells using the patch-clamp technique. Pflügers Arch. 408:98–111. [DOI] [PubMed] [Google Scholar]
- Stolz, A., H. Takikawa, M. Ookhtens, and N. Kaplowitz. 1989. The role of cytoplasmic proteins in hepatic bile acid transport. Annu. Rev. Physiol. 51:161–176. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz, G., M.L. Garcia, and G.J. Kaczorowski. 1991. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J. Pharmacol. Exp. Ther. 259:439–443. [PubMed] [Google Scholar]
- Sunagane, N., T. Kobori, T. Uruno, and K. Kubota. 1986. Mechanisms of spasmolytic action of bile salts in depolarized guinea-pig taenia colli. J. Pharmacobiodyn. 9:473–478. [DOI] [PubMed] [Google Scholar]
- Sunagane, N., T. Kobori, T. Uruno, and K. Kubota. 1990. Possible mechanisms of spasmolytic action of bile salts on the isolated guinea-pig gallbladder. Jap. J. Smooth Muscle Res. 26:143–150. [DOI] [PubMed] [Google Scholar]
- Sung, J.Y., E.A. Shaffer, and J.W. Costerton. 1993. Antibacterial activity of bile salts against common biliary pathogens. Dig. Dis. Sci. 38:2104–2112. [DOI] [PubMed] [Google Scholar]
- Tarantino, G., S. Cambri, A. Ferrara, M. Marzano, A. Liberti, G. Vellone, and A.F.L. Ciccarelli. 1989. Concentrazione sierica degli acidi biliari e ipertensione portale di pazienti cirrotici. Possibile correlazione. Eur. Rev. Med. Pharmacol. Sci. 11:195–205. [PubMed] [Google Scholar]
- Thomas, S.H., T. Joh, and J.N. Benoit. 1991. Role of bile acids in splanchnic hemodynamic response to chronic portal hypertension. Dig. Dis. Sci. 36:1243–1248. [DOI] [PubMed] [Google Scholar]
- Thurmond, R.L., G. Lindblom, and M. Brown. 1991. Effect of bile salts on monolayer curvature of a phosphatidylethanolamine/water model membrane system. Biophys. J. 60:728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno, T., I. Takayanagi, K. Kubota, and K. Takagi. 1975. Actions of bile salts and of papaverine and intracellular cyclic AMP in isolated rat uterus. Eur. J. Pharmacol. 32:116–119. [DOI] [PubMed] [Google Scholar]
- Valverde, M.A., P. Rojas, J. Amigo, D. Cosmelli, P. Orio, M.I. Bahamonde, G.E. Mann, C. Vergara, and R. Latorre. 1999. Acute activation of maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 285:1929–1931. [DOI] [PubMed] [Google Scholar]
- Wu, C.H., P.J. Sides, and T. Narahashi. 1980. Interaction of deoxycholate with the sodium channel of squid axon membranes. J. Gen. Physiol. 76:355–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q.-W., S.M. Freedman, and E.A. Shaffer. 1997. Inhibitory effect of bile salts on gallbladder smooth muscle contractility in the guinea pig in vitro. Gastroenterology. 112:1699–1706. [DOI] [PubMed] [Google Scholar]
- Zimniak, P., J.M. Little, A. Radominska, D.G. Oelberg, M.S. Anwer, and R. Lester. 1991. Taurine-conjugated bile acids act as Ca2+ ionophores. Biochemistry. 30:8598–8604. [DOI] [PubMed] [Google Scholar]