Abstract
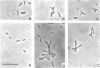
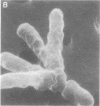
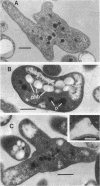
Cowpea-type Rhizobium sp. strain 32H1 and Rhizobium japonicum USDA 26 and 110 grown on a glutamate-mannitol-gluconate agar medium showed increases in the number of pleomorphic cells coincident with their acetylene-reducing activity. Pleomorphs appeared to be inhibited in growth nonuniformly, because acetylene-reducing cultures were mixtures of rod, branched (V, Y, and T), and other irregularly shaped cells. In contrast, strain USDA 10 consistently failed to reduce acetylene, even though it also could grow and yield pleomorphic cells under various conditions. With minimal inhibitory supplements (5 micrograms per ml of medium) of nalidixic acid and novobiocin as cell division inhibitors, an increase in pleomorphic cells was observed, but the inhibited cultures displayed lower acetylene-reducing activity. A study of pleomorphic cells derived in different ways indicated that not all pleomorphs reduce acetylene.
Full text
PDF

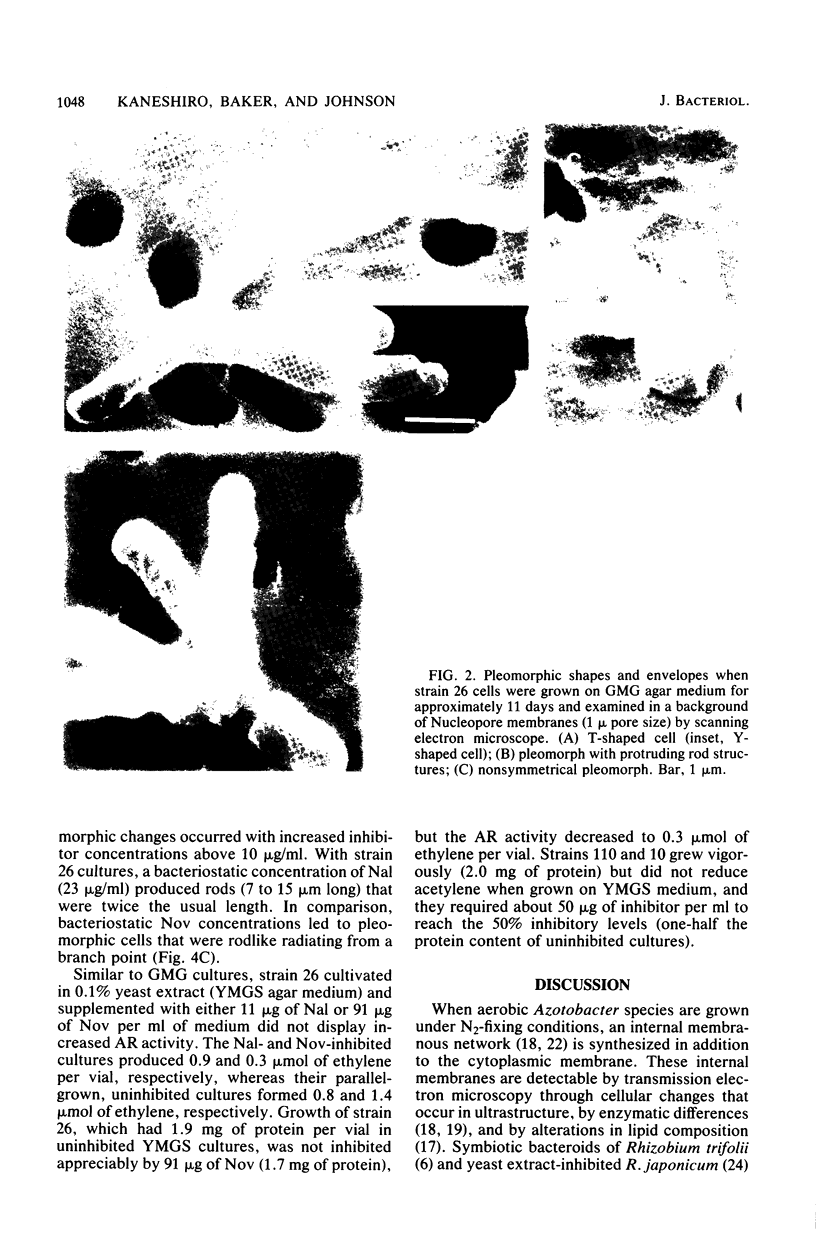


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal A. K., Shantharam S., Verma D. P. Changes in the outer cell wall of Rhizobium during development of root nodule symbiosis in soybean. Can J Microbiol. 1980 Sep;26(9):1096–1103. doi: 10.1139/m80-182. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Hedtke S. Isolation of bacteria, transforming bacteria, and bacteroids from soybean nodules. Plant Physiol. 1977 Nov;60(5):771–774. doi: 10.1104/pp.60.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Orr E., Holland I. B. Inhibition of deoxyribonucleic acid gyrase: effects on nucleic acid synthesis and cell division in Escherichia coli K-12. J Bacteriol. 1980 Apr;142(1):153–161. doi: 10.1128/jb.142.1.153-161.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörcher R., Wilcockson J., Werner D. Screening for mutants of Rhizobium japonicum with defects in nitrogen fixing ability. Z Naturforsch C. 1980 Sep-Oct;35(9-10):729–732. doi: 10.1515/znc-1980-9-1013. [DOI] [PubMed] [Google Scholar]
- Jordan D. C., Coulter W. H. On the cytology and synthetic capacities of natural and artificially produced bacteroids of Rhizobium leguminosarum. Can J Microbiol. 1965 Aug;11(4):709–720. doi: 10.1139/m65-094. [DOI] [PubMed] [Google Scholar]
- Marcus L., Kaneshiro T. Lipid composition of Azotobactervinelandii in which the internal membrane network is induced or repressed. Biochim Biophys Acta. 1972 Nov 2;288(2):296–303. doi: 10.1016/0005-2736(72)90250-7. [DOI] [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangborn J., Marr A. G., Robrish S. A. LOCALIZATION OF RESPIRATORY ENZYMES IN INTRACYTOPLASMIC MEMBRANES OF AZOTOBACTER AGILIS. J Bacteriol. 1962 Oct;84(4):669–678. doi: 10.1128/jb.84.4.669-678.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBRISH S. A., MARR A. G. Location of enzymes in Azotobacteragilis. J Bacteriol. 1962 Jan;83:158–168. doi: 10.1128/jb.83.1.158-168.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantharam S., Gow J. A., Bal A. K. Fractionation and characterization of two morphologically distinct types of cells in Rhizobium japonicum broth culture. Can J Microbiol. 1980 Feb;26(2):107–114. doi: 10.1139/m80-016. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. E. Nondividing, Bacteroid-Like Rhizobium trifolii: In Vitro Induction Via Nutrient Enrichment. Appl Environ Microbiol. 1979 Dec;38(6):1173–1178. doi: 10.1128/aem.38.6.1173-1178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- van Brussel A. A., Costerton J. W., Child J. J. Nitrogen fixation by Rhizobium sp. 32H1. A morphological and ultrastructural comparison of asymbiotic and symbiotic nitrogen-fixing forms. Can J Microbiol. 1979 Mar;25(3):352–361. doi: 10.1139/m79-055. [DOI] [PubMed] [Google Scholar]