Abstract
Cyclic nucleotide–gated channels are key components in the transduction of visual and olfactory signals where their role is to respond to changes in the intracellular concentration of cyclic nucleotides. Although these channels poorly select between physiologically relevant monovalent cations, the gating by cyclic nucleotide is different in the presence of Na+ or K+ ions. This property was investigated using rod cyclic nucleotide–gated channels formed by expressing the subunit 1 (or α) in HEK293 cells. In the presence of K+ as the permeant ion, the affinity for cGMP is higher than the affinity measured in the presence of Na+. At the single channel level, subsaturating concentrations of cGMP show that the main effect of the permeant K+ ions is to prolong the time channels remain open without major changes in the shut time distribution. In addition, the maximal open probability was higher when K+ was the permeant ion (0.99 for K+ vs. 0.95 for Na+) due to an increase in the apparent mean open time. Similarly, in the presence of saturating concentrations of cAMP, known to bind but unable to efficiently open the channel, permeant K+ ions also prolong the time channels visit the open state. Together, these results suggest that permeant ions alter the stability of the open conformation by influencing of the O→C transition.
Keywords: ligand-gated channel, cGMP, cAMP, tetracaine, photoreceptor
INTRODUCTION
CNG channels serve important roles in the transduction of visual and olfactory signals (Stryer, 1986, 1988; Yau and Baylor, 1989; Lagnado and Baylor, 1992; Zufall et al., 1994; Menini, 1995; Zimmerman, 1995). Their function is to sense changes in the intracellular concentration of cyclic nucleotide that occur in response to either visual or olfactory stimuli. In the visual signal cascade, light absorption by the photoreceptor results in a decrease of cGMP, which closes CNG channels present in the outer segment of the photoreceptor, leading to a hyperpolarization and a subsequent decrease in transmitter release (Stryer, 1986, 1988; Yau and Baylor, 1989; Lagnado and Baylor, 1992). In the olfactory signal cascade, odorant molecules produce an increase of cAMP, which opens CNG channels, depolarizes the olfactory neuron, and eventually triggers an action potential (Zufall et al., 1994; Menini, 1995; Zimmerman, 1995).
Although CNG channels are typically studied as homotetramers in expression systems (Gordon and Zagotta, 1995; Liu et al., 1996), in native cells they are thought to be formed by a mixture of different subunits (Chen et al., 1993; Bonigk et al., 1999; Pages et al., 2000). Each subunit contains six transmembrane segments (TMs),* including a positively charge TM4, a P region between TM5 and TM6, and a cyclic nucleotide binding domain at the intracellular COOH-terminal. The membrane topology of CNG channels is very similar to that of voltage-activated K+, Na+, and Ca2+ channels, which suggest that all these channels belong to the same superfamily (Jan and Jan, 1990). Although CNG channels are weakly voltage dependent (Karpen et al., 1988; Haynes and Yau, 1990; Taylor and Baylor, 1995; Benndorf et al., 1999), voltage is not the primary stimulus that opens them. Instead, molecules of cyclic nucleotide are required to gate these channels, which categorize them functionally as ligand-gated channels. Dose–response curves for activation of CNG channels are best fitted with Hill coefficients greater than one, which suggest that more than one molecule of cyclic nucleotide is required for channel activation (Fesenko et al., 1985; Zimmerman and Baylor, 1986; Karpen et al., 1988; Gordon and Zagotta, 1995; Liu et al., 1996; Ruiz and Karpen, 1997; Ruiz et al., 1999).
The permeation properties of CNG channels have been extensively studied, both in native and heterologous systems. The channel allows both mono and divalent cations to permeate (Fesenko et al., 1985; Haynes et al., 1986; Stern et al., 1987; Kaupp et al., 1989; Furman and Tanaka, 1990; Menini, 1990; Colamartino et al., 1991; Picones and Korenbrot, 1992; Zimmerman and Baylor, 1992; Goulding et al., 1993; Nizzari et al., 1993; Haynes, 1995a,b; Picones and Korenbrot, 1995). The permeation of monovalent cations is blocked by divalent cations (Colamartino et al., 1991; Picones and Korenbrot, 1992; Zimmerman and Baylor, 1992; Root and MacKinnon, 1993; Tanaka and Furman, 1993; Zufall and Firestein, 1993; Eismann et al., 1994; Root and MacKinnon, 1994; Nasi and del Pilar Gomez, 1999; Seifert et al., 1999) and some of the permeation properties can be best described by multiion occupancy (Furman and Tanaka, 1990; Sesti et al., 1995; Qu et al., 2001).
It has long been known that the presence of permeant ions in the pore of membrane channels can influence channel gating. For example, in acetylcholine receptors from Aplysia, the average time the channel stays open depends on the type of permeant ions present (Ascher et al., 1978). In voltage-activated potassium channels, closing becomes slower when external permeant ions are present, suggesting that somewhere in the permeation pathway a permeant ion impedes the channel from closing (Swenson and Armstrong, 1981; Matteson and Swenson, 1986; Spruce et al., 1989; Sala and Matteson, 1991). Permeant ions also influence gating in Ca-activated potassium channels (Demo and Yellen, 1992), inward rectifier potassium channels (Choe et al., 1998; Lu et al., 2001), sodium channels (Oxford and Yeh, 1985; Townsend et al., 1997; Townsend and Horn, 1997), and calcium channels (Shuba et al., 1991). The extreme case of this rather general occurrence is that the ion itself might constitute the gating particle as suggested for chloride channels (Richard and Miller, 1990; Pusch et al., 1995; Chen and Miller, 1996). In CNG channels, a linkage between selectivity for divalent cations and gating has also been described (Cervetto et al., 1988; Hackos and Korenbrot, 1999). Perhaps because CNG channels discriminate poorly among physiologically relevant monovalent cations (i.e., Na+ and K+), the interactions between the permeation of these ions and gating have been largely ignored. Recently, however, Gamel and Torre (2000) have studied a very interesting effect of K+ ions on permeation and gating of CNGA1 channels mutated at the first proline within the P region (P365T). In this mutant, K+ ions have a dual effect of blocking permeation and holding the channels open, which is indicative of some interaction between permeant ions and gating.
The present study examines the effect of monovalent cations on gating by cyclic nucleotide in wild-type CNGA1 channels. First, the apparent affinity for cGMP is increased if K+ ions, instead of Na+ ions, are permeating through the channel. Second, in the presence of subsaturating concentrations of cGMP, the main effect of permeant K+ ions is to increase the apparent time constant of the main component of the open dwell-time distribution without substantial changes in the shut time distribution, consistent with a stabilization of the open conformation. Third, in the presence of saturating concentrations of cGMP, the maximal probability of opening increased from 0.95 (for permeant Na+ ions) to 0.99 (for permeant K+ ions) as a result of an increase of the apparent dwell time for the open conformation. Fourth, when saturating concentrations of cAMP is used as the agonist, channels that visit the open state tend to stay open longer when K+ ions are permeating through the pore. These results are consistent with the idea that permeant ions influence the exit of the channel from the open conformation rather than the cGMP binding steps. Finally, blockers known to interact with the pore of CNGA1 channels respond differently to the presence of Na+ or K+ ions, suggesting that these blockers are able to sense the differences of the pore due to the presence of different ions. Collectively, these results suggest that ions within the pore of CNGA1 channels influence the response to cGMP by changing the stability of the open conformation.
MATERIALS AND METHODS
DNA Expression
cDNA of the subunit 1 from rod photoreceptor (Kaupp et al., 1989) was provided by Dr. William Zagotta. We subcloned the channel in the GW1-CMV vector (British Biotechnology) for expression in HEK293 cells (American Type Culture Collection). The CNGA1 channel expression plasmid (25 μg) was cotransfected with 1 μg plasmid πH3-CD8 encoding for the human CD8 lymphocyte antigen. Transfected cells were identified visually by assessing the decoration with beads coated with antibodies for CD8 (Dynal) (Jurman et al., 1994). For single channel experiments, channel DNA was lowered to 10–15 μg. Cells were transfected by electroporation (Gene Pulser II; Biorad).
Solutions and Electrophysiological Recordings
The solutions contained (in mM): 160 NaCl or KCl, 1 EGTA, 1 EDTA, 10 HEPES, pH 7.4 adjusted with NaOH or KOH, accordingly. Salts (puriss, p.a. Grades) were purchased from Fluka, divalent chelators were obtained from Sigma-Aldrich, and HEPES was acquired from American Bioanalytical. cGMP (free acid form), cAMP (free acid form), and tetracaine were purchased from Sigma-Aldrich. E12K, D13K ball peptide is a double mutation of the Shaker ball peptide: MAAVAGLYGLGKKRQHRKKQ (Zagotta et al., 1990; Murrell-Lagnado and Aldrich, 1993a,b) that was provided by Dr. Gary Yellen.
Currents from inside-out excised patches (Hamill et al., 1981) were recorded between 1–2 d after transfection, using an Axopatch 200B amplifier (Axon Instruments, Inc.). Records in the absence of cGMP were subtracted to isolate macroscopic cGMP-dependent currents. Macroscopic data analysis was performed with pClamp 8.1 (Axon Instruments, Inc.) and Origin 6.1 (Microcal Software, Inc.) software. Single channel currents were analyzed with software provided by Dr. Brad Rothberg and Karl Magleby. Macroscopic currents were sampled at 5–10 kHz and low-pass filtered between 1 and 2 kHz. Single channel recordings were sampled at 20 kHz, filtered at 5 kHz (8-pole Bessel filter; Frequency Devices) and subsequently filtered digitally at 1–2 kHz before analysis. Borosilicate glass pipettes between 1.5–2.5 MΩ were pulled for macroscopic currents and quartz pipettes between 7–15 MΩ were used for single channel recordings. Solutions were changed by manual switch.
RESULTS
Permeant Ion Dependence of Apparent Affinity for cGMP
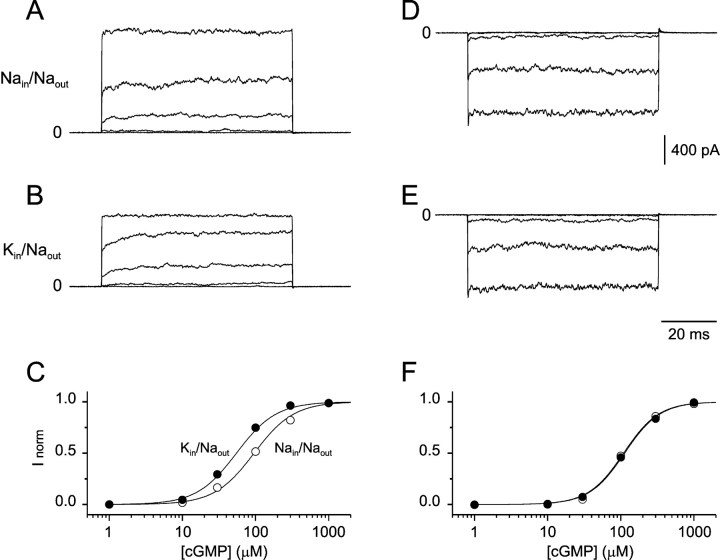
Bovine rod CNGA1 channels were studied by expressing cDNA for the subunit 1 (or α) in HEK293 cells. Inside-out patches were excised and superfused with a variety of solutions. To isolate the cGMP-dependent currents, records obtained with voltage steps to the same amplitude but without cGMP were subtracted. Fig. 1 A shows current traces in response to a voltage step from 0 to 150 mV, while the patch was superfused with Na-solution containing 1, 10, 30, 100, or 1,000 μM cGMP. A similar test was performed in the same patch, while superfusing the inner face with K-solutions, as shown in Fig. 1 B. Two differences can be clearly detected. First, the current amplitude at saturating concentrations of cGMP (Imax) is larger in Na-solution (1,350 pA) than in K-solution (950 pA). The origin of this difference will be addressed in the next section. Second, the current amplitude at 100 μM cGMP is ∼50% of Imax in Na-solution, whereas it is ∼75% in K-solutions, as if the apparent affinity (K1/2) for cGMP is higher in the presence of K+ ions. Fig. 1 C shows the normalized dose–response curves for cGMP obtained from the current traces shown in Fig. 1, A and B (open circles, Na-solutions; filled circles, K-solutions). The estimated values for the K1/2 were 94 and 52 μM cGMP for Na- and K-solutions, respectively. After patch excision, the sensitivity for cGMP can spontaneously change (Gordon et al., 1992; Molokanova et al., 1997, 1999). This could constitute a potential source of error in the experiments presented here. With this in mind, the values of K1/2 for cGMP were estimated in the same patch at negative potentials where permeant ions originate from the pipette solution, which remains unchanged during the experiment (control condition performed at the same time as the test condition shown above). Fig. 1, D and E, show current traces in response to a voltage step from 0 to −150 mV, and superfused with Na- (Fig. 1 D) or K-solution (Fig. 1 E) containing 1, 10, 30, 100, or 1,000 μM cGMP. Clearly, at negative potentials, the values of K1/2 for cGMP were similar irrespective of the solutions bathing the intracellular side of the patch (Fig. 1 F). Because CNGA1 channels poorly select between Na+ and K+ ions, the reversal potential is close to 0. Therefore, at positive potentials it is expected that most of the current will be performed by ions bathing the inside of the patch (either Na+ or K+; Fig. 1, A or B, respectively). From a set of nine patches, the average values (±SD) of K1/2 for cGMP in Na-solutions and K-solutions were 78 ± 15 μM and 47 ± 12 μM, respectively. The average ratio between these values was 1.70 ± 0.2. These observed differences at positive potentials suggest that, in CNGA1 channels, permeant ions influence gating by cGMP.
Figure 1.
Permeant ions influence gating by cGMP. (A) Subtracted current traces obtained from an inside-out patch in response to a voltage step from 0 to 150 mV and, bathed with Na-solutions containing 1, 10, 30, 100, or 1,000 μM cGMP. (B) Similar set of subtracted current traces obtained from the same patch but now bathed with K-solutions. (C) Normalized dose response curves for cGMP obtained from the subtracted current traces in Na-solutions (A, open circles) and in K-solutions (B, filled circles). The values of the average current from the last 20 ms at 150 mV were used to estimate the apparent affinities for cGMP. Solid lines represent Hill equation fits with a slope of 1.6 and K1/2 of 94 ± 6 μM for Na-solutions and 52 ± 2 μM for K-solutions. (D and E) Subtracted current traces obtained from the same inside-out patch in response to a voltage step from 0 to −150 mV and, under the same experimental conditions as those traces shown in A and B. (F) Normalized dose response curves for cGMP obtained from the subtracted current traces in Na-solutions (D, open circles) and in K-solutions (E, filled circles). The values of the average current from the last 20 ms at −150 mV were used to estimate the values of K1/2 for cGMP. Solid lines represent Hill equation fits with a slope of 1.8 and K1/2 of 109 ± 4 μM for Na-solutions and 113 ± 3 μM for K-solutions.
K+ Ions Stabilizes an Open Conformation of CNGA1 Channels
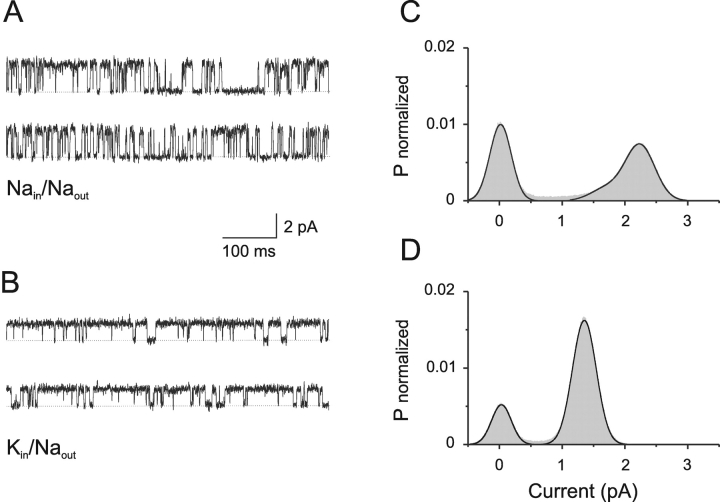
To gain a deeper understanding of the interactions between ions and gating by cGMP, a series of experiments at the single channel level were performed. Fig. 2, A and B , show the activity of a single channel in the presence of 100 μM cGMP at 70 mV in Na- and K-solutions, respectively. The permeation properties for both ions are different. Fig. 2, C and D, show the corresponding current histograms from 20 s of continuous recording in each condition. Solid lines represent fits to Gaussian functions. In Na-solution, an accurate estimation of the amplitude of the fully open state, which was 2.24 pA, required a Gaussian function with three components (Fig. 2 C). In K-solution, the current amplitude of the open state was estimated by a fit to a Gaussian function with two components. The value obtained was 1.35 pA (Fig. 2 D), which is ∼60% of the value determined in Na-solution. This difference in single channel amplitude is sufficient to account for the discrepancy of Imax observed in Na- and K-solutions using macroscopic currents (Fig. 1, A and B).
Figure 2.
Permeation of Na+ and K+ ions through CNGA1 channels. (A) Single-channel recordings at 70 mV with Na-solutions containing 100 μM cGMP. (B) Single-channel recordings from the same channel, at the same voltage, but now with K-solutions containing 100 μM cGMP. Dashed lines represent the current present when the channel is closed. (C) Amplitude histogram from 20 s of uninterrupted recording in Na-solutions. The solid line represents a three-component Gaussian function fit. The best-fit parameter value for the amplitude of the fully open state was 2.24 pA. The total area covered by the fit was 95.7%, from which 52.9% resulted by the sum of the areas for the subconductance and the open states, corresponding to a Popen of ∼0.55. (D) Amplitude histogram from 20 s of uninterrupted recording in K-solutions. The solid line represents a two-component Gaussian function fit. The best-fit parameter value for the amplitude of the open state was 1.35 pA. The total area covered by the fit was 97.5%, from which 77% is attributed to the open state, corresponding to a Popen of ∼0.79. Current records were acquired at 20 kHz, and filtered at 5 kHz. Previous to analysis, the records were digitally filtered at 2 kHz. For presentation purposes, traces shown in A and B were digitally filtered at 1 kHz.
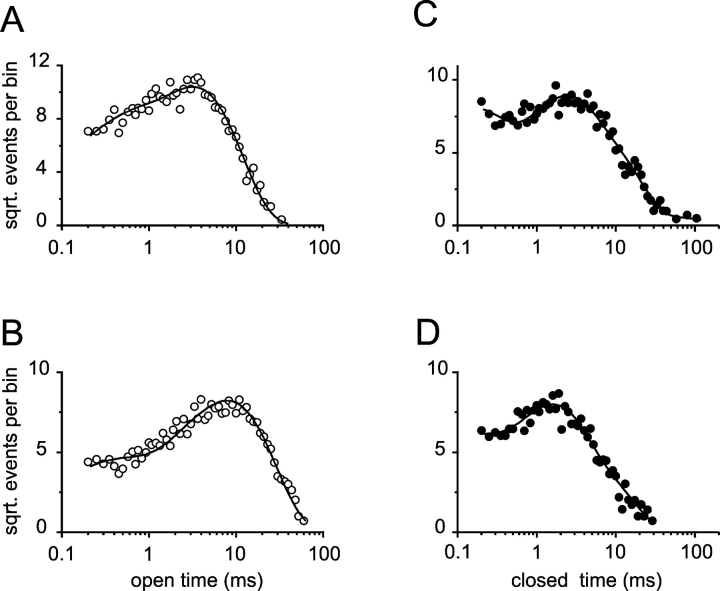
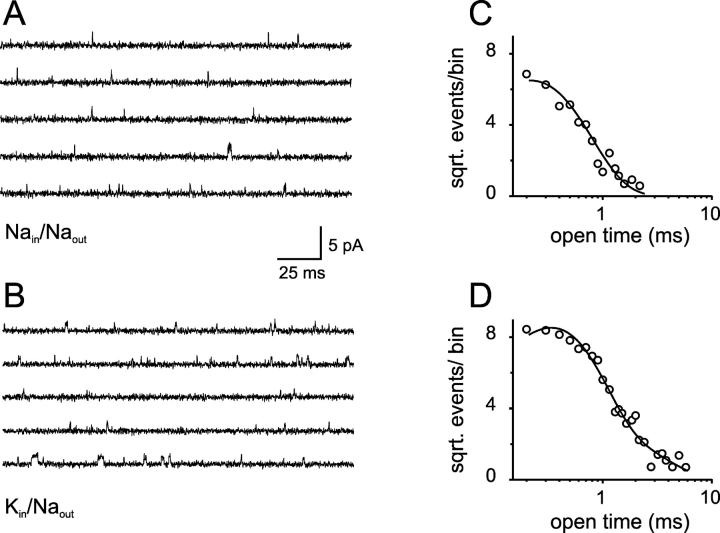
Additional changes were observed in the kinetic properties of the currents. The open time distributions in Na- and K-solutions are shown in Fig. 3, A and B , respectively. A two exponential-function fit revealed that the main effect of permeant ions in the open time distributions was to increase the apparent open time constant of the slower component from 3.2 (in Na-solutions) to 7.9 ms (in K-solutions). Fig. 3, C and D, show the closed time distributions in Na- and K-solutions, respectively. The most noticeable difference between these distributions is the presence of closed events longer than 30 ms in Na-solutions. To include these events, an additional fourth exponential component was needed. However, this component (τ∼40 ms) contributed only to 0.3% of the total area (Fig. 3 C). The parameter values for the remaining three components in Na-solutions were comparable to the parameter values obtained from a fit of a three-exponential function to the closed events observed in K-solutions (Fig. 3 D; see figure legends for details). Therefore, the main difference between the kinetics of the channel in the presence of Na- or K-solutions is that in K-solutions, the apparent mean open time of the main component of the open time distribution is 3.4 ± 0.5 (n = 3) times longer compared with Na-solutions.
Figure 3.
Permeant K+ ions stabilize the open conformation of CNGA1 channels: subsaturating cGMP concentration. (A and B) Open dwell-time distributions with Na- and K-solutions, respectively. The solid lines represent two-exponential fits. For Na-solution the best-fit parameter values were: τ1 = 0.4 ms, A1 = 0.23, τ 2 = 3.2 ms, A2 = 0.77. For K-solution the best-fit parameter values were: τ1 = 0.3 ms, A1 = 0.16, τ 2 = 7.9 ms, A2 = 0.84. (C and D) Closed time distributions with Na- and K-solutions, respectively. For Na-solution, the solid line represents a four-exponential fit. The best-fit parameter values were: τ1 = 0.13 ms, A1 = 0.36, τ2 = 1.92 ms, A2 = 0.40, τ3 = 5.78 ms, A3 = 0.23, τ4 = 40 ms, A4 = 0.003. For K-solution, the solid line represents a three-exponential fit. The best-fit parameter values were: τ1 = 0.11 ms, A1 = 0.24, τ2 = 1.40 ms, A2 = 0.59, τ3 = 4.36 ms, A3 = 0.17.
The single channel gating kinetics of CNG channels are rather complex, with multiple open and closed states, and fast transitions between them. However, in order to establish the origin of the observed differences, a simplified kinetic model for cGMP activation was used:
In this model, the observed effect of K+ ions on the open time distribution is achieved by slowing the rate constant of the O→C transition, i.e., K+ ions stabilize the open conformation of the channel. To further support this conclusion, saturating concentrations of cGMP were used so that channels will tend to dwell either in the fully bound closed or open states. Because the O→C2cGMP transition is independent of cGMP, the discrepancy in the open time distributions should persist even at saturating concentrations of cGMP. Fig. 4, A and B , show single channel recordings in the presence of 2 mM cGMP at 70 mV in Na- and K-solutions, respectively. The corresponding open time distributions are shown in Fig. 4, C and D. At 2 mM cGMP, these distributions can be fitted with a single-exponential function. As expected, the apparent mean open time when the channel was permeating K+ ions (54 ms) is longer that the apparent mean open time when the channel was permeating Na+ ions (10 ms). In K-solutions, the apparent mean open time of the open time distribution is 3.9 ± 1.6 (n = 2) times longer compared with Na-solutions. Inspection of the shut time distribution reveals that the effect of ions on the gating by cGMP is not exclusively at the O→C transition (Fig. 4, F and G). Even at saturating concentrations of cGMP, there is an additional component in the shut time distribution with a time constant of ∼6 ms when the channel was permeating Na+ ions. However, the contribution of this component to the total area is relatively small, ∼1%.
Figure 4.
Permeant K+ ions stabilize the open conformation of CNGA1 channels: saturating cGMP concentration. (A) Single-channel recordings at 70 mV with Na-solutions containing 2 mM cGMP. (B) Single channel recordings from the same channel, voltage, and cGMP concentration, but now with K-solutions. Dashed lines represent the current present when the channel is closed. (C and D) Open dwell-time distributions with Na- and K-solutions, respectively. The solid lines represent single-exponential fits. The best-fit parameter values for the time constants were 9.7 ms for Na-solution and 54.3 ms for K-solution. (E and F) Closed time distributions with Na- and K-solutions, respectively. For Na-solution, the solid line represents a two-exponential fit. The best-fit parameter values were: τ1 = 0.47 ms, A1 = 0.99, τ2 = 5.7 ms, A2 = 0.01. For K-solution, the solid line represents a single-exponential fit with a time constant of 0.45 ms. Current records were acquired at 20 kHz, and filtered at 5 kHz. Previous to analysis, the records were digitally filtered at 1 kHz.
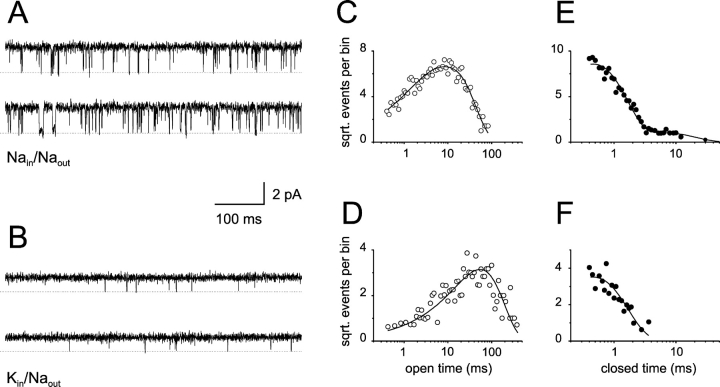
If the observed differences in open time distribution are independent of agonist binding then they should persist with agonists that have very different efficacies. As shown in Fig. 5 , K+ ions produced qualitatively similar results when cAMP was used as ligand. In a patch containing ∼7–10 channels (determined by activation with saturated cGMP concentration), activation by 5 mM cAMP never produced open events longer than 3 ms when the currents were performed by Na+ ions (Fig. 5, A and C). However, when K+ ions were permeating, open events longer than 3 ms were frequently observed (Fig. 5, B and D; same patch as Fig. 5, A and C).
Figure 5.
Permeant K+ ions stabilize the open conformation of CNGA1 channels: saturating cAMP concentration. (A) Single-channel recordings at 70 mV in a patch with ∼7–10 channels and bathed with Na-solution containing 5 mM cAMP. (B) Single channel recordings from the same patch, voltage, and cAMP concentration, but now bathed with K-solution. (C and D) Open dwell-time distributions with Na- and K-solutions, respectively. The solid lines represent a single-exponential fit for Na-solution and a two-exponential fit for K-solution. For Na-solution, the best-fit parameter value was 0.2 ms. For K-solution, the best-fit parameter values were: τ1 = 0.33 ms, A1 = 0.93, τ2 = 1.04 ms, A2 = 0.07. Current records were acquired at 20 kHz and filtered at 5 kHz. Previous to analysis, the records were digitally filtered at 2 kHz. For presentation purposes, traces shown in A and B were digitally filtered at 1 kHz. Similar results were observed in three patches.
Together, these results suggest that most of the effects of permeant ions on gating by cGMP can be explained by changes in the stability of the open conformation.
Ions in the Pore Might Be Responsible for Changing the Rate of the O→C Transition
Are these effects on gating the result of ions bound outside the pore or are they the consequence of ions interacting within the permeation pathway? To help clarify the nature of the interaction between ions and gating by cGMP, two different molecules known to interact with the pore of CNGA1 channels were used as probes for ion-pore occupancy. These molecules are the so-called ball peptide and the local anesthetic tetracaine; both have been shown to act from the intracellular side of the channel (Kramer et al., 1994; Fodor et al., 1997b). If ions in the pore alter the gating by cGMP, then the blockers, which interact with the pore of CNGA1 channels, might be able to detect these differences.
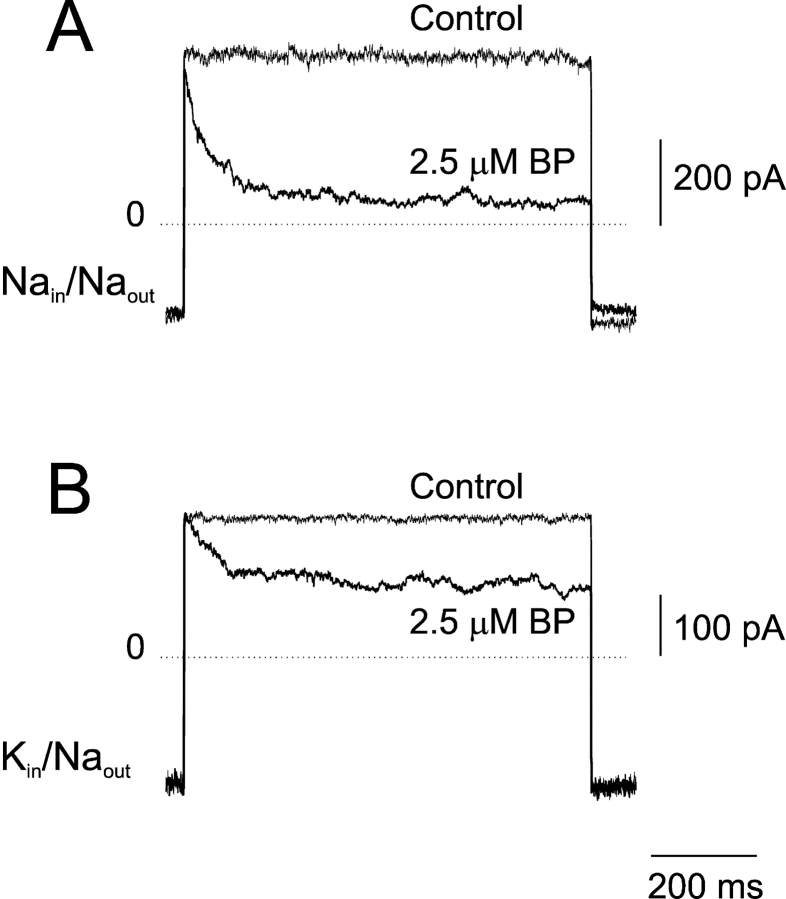
When applied internally, the ball peptide preferentially blocks CNGA1 channels in the open state (Kramer et al., 1994). Because K+ ions stabilize the open conformation of the channel, the immediate expectation is that the ball peptide should have a higher apparent affinity in K-solutions. Fig. 6 A shows subtracted current traces, acquired with Na-solutions, in response to a voltage step from −90 to 110 mV in control conditions (2 mM cGMP) and with 2.5 μM E12K, D13K synthetic ball peptide added. The equivalent experiment is shown in Fig. 6 B in the presence of K-solutions. In Na-solutions, the kinetics of blockade are faster and the extent of block is larger; this suggests that the apparent affinity for ball peptide is higher in Na-solutions. This result is opposite to the prediction based on the assumption that the only interactions between ball peptide and permeant ions are a consequence of open state stabilization. Therefore, this result can be interpreted as a sign that the ball peptide is able to sense the differences of the pore when either Na+ or K+ ions are present.
Figure 6.
Permeant ions interact with intracellular ball peptide: a pore blocker molecule. (A) Superimposed subtracted current traces in response to a voltage step from −90 to 110 mV with bath Na-solutions containing 2 mM cGMP in control conditions and with 2.5 μM E12K, D13K synthetic ball peptide added. In these experimental conditions, the apparent affinity for ball peptide (KDBP) was 0.3 μM. The value of KDBP is defined as the concentration of ball peptide needed to block 50% of the current assuming a simple model of a single blocker molecule binding to a single site (Michaelis-Menten model). KDBP = {If [BP]/(1 − If)} where If is the fractional current remained after blockade and [BP] is the concentration of ball peptide used. (B) Superimposed subtracted current traces in response to the same voltage protocol but now with bath K-solutions. In these experimental conditions, the KD for ball peptide was 3.4 μM. Similar results were observed in three patches.
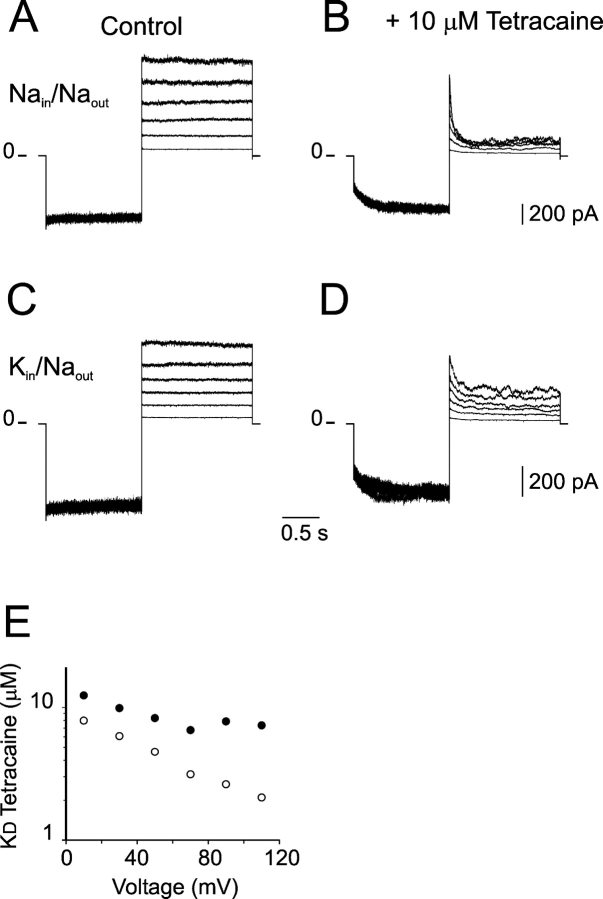
Tetracaine is a local anesthetic that blocks CNGA1 channels with two important properties: the blockade is voltage dependent and the blocker binds almost exclusively to the closed state (Fodor et al., 1997b). Fig. 7 A shows subtracted current traces obtained in Na-solutions with 2 mM cGMP. Equivalent set of current traces, but with 10 μM tetracaine added, are shown in Fig. 7 B. The voltage protocol consisted of a prepulse to −110 mV to promote unbinding of tetracaine (see Fig. 7 B) followed by voltage steps to positive potential to assess blockade by tetracaine. The apparent affinity for tetracaine (KDTet) was estimated from the fraction of current blocked and assuming that one molecule of tetracaine blocks the channel (Fodor et al., 1997b). The values of KDTet estimated from the current traces shown in Fig. 7, A and B, are plotted in Fig. 7 E (open circles), which are consistent with previous observations of tetracaine blockade of CNGA1 channels expressed in Xenopus oocytes using similar Na-solutions (Fodor et al., 1997b). The same experiment was repeated, in the same patch, but now with K-solutions (Fig. 7, C–E, filled circles). As predicted from the properties of tetracaine blockade, the apparent affinity for tetracaine is lower in K-solutions. However, if the shift in the C↔O distribution with different ionic species is the sole determinant for blockade, the KDTet versus voltage relationship should be linear and parallel for Na- and K-solutions. As shown in Fig. 7 E, that is not the case. The values of KDTet obtained in K-solutions showed a deviation from linearity, which opens the possibility that tetracaine is not only blocking but may also be permeating at positive potentials >60 mV. Again, this result can be interpreted as a sign that tetracaine is able to sense the differences of the pore by the presence of Na+ or K+ ions, and somehow the presence of K+ ions favors the permeation of tetracaine.
Figure 7.
Permeant ions interact with intracellular tetracaine: a pore blocker molecule. (A and B) Superimposed subtracted current traces in response to a voltage protocol that includes a prepulse to −110 mV followed by voltage jumps to potentials between 10 and 110 mV. In A the current traces in bath Na-solution with 2 mM cGMP are shown, and in B similar traces but with 10 μM tetracaine added are shown. (C and D) Superimposed subtracted current traces from the same patch and in response to the same voltage protocol as before, but now with bath K-solutions. In C the current traces with 2 mM cGMP are shown, and in D similar traces but with 10 μM tetracaine added are shown. (E) Voltage dependence of the KD for tetracaine (KDTet) in bath Na-solutions (open circles) and in bath K-solutions, (filled circles). The value of KDTet is defined as the concentration of tetracaine needed to block 50% of the current assuming a simple model of a single blocker molecule binding to a single site. KDTet = {If [T]/(1 − If)}, were If is the fractional current remained after blockade and [T] is the concentration of tetracaine used. Similar results were observed in three patches.
Together, these results suggest that the permeation properties for Na+ and K+ ions in CNGA1 channels are different. These differences in permeation might be related to the observed differences in gating by cGMP, which will be consistent with the idea that ions in the pore of CNGA1 channels are linked to the conformational changes responsible for opening and closing of the pore.
DISCUSSION
Linkage of Permeant Ions and Gating in CNGA1 Channels
The properties of permeation or gating have been extensively studied in CNG channels (see introduction for references). However, little attention has been paid to the relationship between these properties. Using heterologous expression of CNGA1 channels from rod photoreceptor, this study shows that activation by cGMP is influenced by the nature of the permeant ion. Using Na+ or K+ as permeant ions, it was shown that the apparent affinity for cGMP is higher when CNGA1 channels are permeating K+ ions, which suggests that permeant ions influence gating by cGMP.
Kinetic Origin of the Changes in Apparent Affinity for cGMP by Permeant Ions
Because electrical currents are being used to estimate channel activity, changes in sensitivity to cGMP can originate from changes in the actual binding affinity for cyclic nucleotide (binding steps) or by changes in the stability of the open conformation of the channel. Three lines of evidence support the second possibility. First, experiments done at subsaturating concentrations of cGMP showed that permeant ions mostly affect the open dwell time distribution of single channel currents. When K+ ions were carrying the current, the apparent time constant of the main open state is significantly longer than the one measured when Na+ ions were permeating. Second, if ions influenced the actual binding affinity for cGMP, it is expected that the use of saturating concentrations of cyclic nucleotide would attenuate the differential effect of K- versus Na-containing intracellular solutions. As shown in Fig. 4, using saturating concentrations of cGMP, the observed differences in the gating properties of CNGA1 channels when Na+ or K+ ions were permeating were similar to those observed at subsaturating concentrations of cGMP (Fig. 3). At all concentrations of cGMP tested the main effect of K+ ions was to increase the apparent time constant of the main component of the open dwell time distribution. Third, permeant K+ ions were also able to produce open events that were consistently longer than those observed with permeant Na+ ions when saturating concentrations of cAMP were used to gate the channels, implying that the differences on gating induced by permeant ions were not affected by the nature of the agonist. Together, these results strongly suggest that permeant K+ ions influence gating by stabilizing the open conformation of the channel, i.e., by reducing the rate constant of exiting the open state.
Ions in the Pore Influence the Stability of the Open Conformation of CNA1G Channels
Because the gate that open and closes the channel is likely to be located within the permeation pathway (Fodor et al., 1997a; Becchetti and Roncaglia, 2000; Liu and Siegelbaum, 2000; Flynn and Zagotta, 2001), it is conceivable that ions, while permeating, could interfere with the process of gating. As a result, the observed differences of the gating properties of CNGA1 channels permeating Na+ or K+ ions would be a consequence of the different interactions between each ionic species and the pore of the channel. The use of pore blockers indicates that indeed Na+ and K+ ions might interact differently. For example, it was found that the intracellular ball peptide, known to preferentially block the channel in the open state (Kramer et al., 1994), has a higher affinity in the presence of Na+ as permeant ions. This is the opposite of what one would predict from the fact that K+ ions stabilize the open state of CNGA1 channels, as if the presence of Na+ ions in the pore might create a more favorable environment for blockade of the ball peptide. Similarly, tetracaine seems to permeate at positive voltages in the presence of K+ ions but not in the presence of Na+ ions. In addition, the difference of single channel amplitudes when CNGA1 channels are permeating either Na+ or K+ ions also suggests that each ionic species interacts somehow differently with the pore of the channel.
All the results presented are consistent with the idea that monovalent cation permeation and gating are related processes in CNGA1 channels. Progress in understanding the molecular mechanisms of ion permeation in KcsA potassium channels (Doyle et al., 1998; Zhou et al., 2001; Zhou and MacKinnon, 2002) provides a basis for interpreting the results described here. In those studies, the atomic nature of the interactions between permeant ions and the pore of KcsA channels, and how these interactions vary with both the concentration and species of permeant ion, offer physical insights into how permeant ions might affect gating in KcsA channels. For example, high-resolution structures of the selectivity filter in high K+ (200 mM) and low K+ (3 mM) revealed significant conformational differences. In low K+, the carbonyl oxygen of Val 76 is pointing away from the pore and the α-carbon of Gly 77 is twisted inward, resulting in a nonconductive selectivity filter where K+ ions can only occupy two of the four possible sites (Zhou et al., 2001). In addition, the selectivity filter can make small adjustments to the size of the permeant ion. If the ion is bigger than K+, like Cs+, the carbonyl oxygen slightly rotates to accommodate the bigger ion (Zhou and MacKinnon, 2002). In light of the fact that KcsA architecture might well represent a prototype for ionic membrane channels (Doyle et al., 1998), it is not hard to imagine that permeant ions will also directly interact with the pore of CNGA1 channels and these interactions somehow might be coupled to gating by cGMP.
Figure .
Acknowledgments
I would like to thank Kenton Swartz, Enrico Nasi, and Joe Mindell for helpful discussions, Emily Stern for subcloning cG7 into the mammalian GW vector, and Tara Ogren and Deepa Srikumar for a steady supply of transfected cells.
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper: BP, ball peptide; If, fractional current remained after blockade; Imax, current amplitude at saturating concentrations of cGMP; K1/2, apparent affinity for cGMP; KDTet, apparent affinity for tetracaine; KDBP, apparent affinity for ball peptide;TM, transmembrane segment.
References
- Ascher, P., A. Marty, and T.O. Neild. 1978. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J. Physiol. 278:177–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti, A., and P. Roncaglia. 2000. Cyclic nucleotide-gated channels: intra- and extracellular accessibility to Cd2+ of substituted cysteine residues within the P-loop. Pflugers Arch. 440:556–565. [DOI] [PubMed] [Google Scholar]
- Benndorf, K., R. Koopmann, E. Eismann, and U.B. Kaupp. 1999. Gating by cyclic GMP and voltage in the alpha subunit of the cyclic GMP- gated channel from rod photoreceptors. J. Gen. Physiol. 114:477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonigk, W., J. Bradley, F. Muller, F. Sesti, I. Boekhoff, G.V. Ronnett, U.B. Kaupp, and S. Frings. 1999. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 19:5332–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto, L., A. Menini, G. Rispoli, and V. Torre. 1988. The modulation of the ionic selectivity of the light-sensitive current in isolated rods of the tiger salamander. J. Physiol. 406:181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T.Y., and C. Miller. 1996. Nonequilibrium gating and voltage dependence of the ClC-0 Cl− channel. J. Gen. Physiol. 108:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T.Y., Y.W. Peng, R.S. Dhallan, B. Ahamed, R.R. Reed, and K.W. Yau. 1993. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 362:764–767. [DOI] [PubMed] [Google Scholar]
- Choe, H., H. Sackin, and L.G. Palmer. 1998. Permeation and gating of an inwardly rectifying potassium channel. Evidence for a variable energy well. J. Gen. Physiol. 112:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamartino, G., A. Menini, and V. Torre. 1991. Blockage and permeation of divalent cations through the cyclic GMP-activated channel from tiger salamander retinal rods. J. Physiol. 440:189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo, S.D., and G. Yellen. 1992. Ion effects on gating of the Ca2+-activated K+ channel correlate with occupancy of the pore. Biophys. J. 61:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D.A., J. Morais Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69-77. [DOI] [PubMed] [Google Scholar]
- Eismann, E., F. Muller, S.H. Heinemann, and U.B. Kaupp. 1994. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc. Natl. Acad. Sci. USA. 91:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko, E.E., S.S. Kolesnikov, and A.L. Lyubarsky. 1985. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 313:310–313. [DOI] [PubMed] [Google Scholar]
- Flynn, G.E., and W.N. Zagotta. 2001. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 30:689–698. [DOI] [PubMed] [Google Scholar]
- Fodor, A.A., K.D. Black, and W.N. Zagotta. 1997. a. Tetracaine reports a conformational change in the pore of cyclic nucleotide-gated channels. J. Gen. Physiol. 110:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor, A.A., S.E. Gordon, and W.N. Zagotta. 1997. b. Mechanism of tetracaine block of cyclic nucleotide-gated channels. J. Gen. Physiol. 109:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman, R.E., and J.C. Tanaka. 1990. Monovalent selectivity of the cyclic guanosine monophosphate-activated ion channel. J. Gen. Physiol. 96:57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamel, K., and V. Torre. 2000. The interaction of Na+ and K+ in the pore of cyclic nucleotide- gated channels. Biophys. J. 79:2475–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, S.E., D.L. Brautigan, and A.L. Zimmerman. 1992. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 9:739–748. [DOI] [PubMed] [Google Scholar]
- Gordon, S.E., and W.N. Zagotta. 1995. Subunit interactions in coordination of Ni2+ in cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 92:10222–10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding, E.H., G.R. Tibbs, D. Liu, and S.A. Siegelbaum. 1993. Role of H5 domain in determining pore diameter and ion permeation through cyclic nucleotide-gated channels. Nature. 364:61–64. [DOI] [PubMed] [Google Scholar]
- Hackos, D.H., and J.I. Korenbrot. 1999. Divalent cation selectivity is a function of gating in native and recombinant cyclic nucleotide-gated ion channels from retinal photoreceptors. J. Gen. Physiol. 113:799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, O.P., A. Marty, E. Neher, B. Sakmann, and F.J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Haynes, L.W. 1995. a. Permeation and block by internal and external divalent cations of the catfish cone photoreceptor cGMP-gated channel. J. Gen. Physiol. 106:507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, L.W. 1995. b. Permeation of internal and external monovalent cations through the catfish cone photoreceptor cGMP-gated channel. J. Gen. Physiol. 106:485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, L.W., A.R. Kay, and K.W. Yau. 1986. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 321:66–70. [DOI] [PubMed] [Google Scholar]
- Haynes, L.W., and K.W. Yau. 1990. Single-channel measurement from the cyclic GMP-activated conductance of catfish retinal cones. J. Physiol. 429:451–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan, L.Y., and Y.N. Jan. 1990. A superfamily of ion channels. Nature. 345:672. [DOI] [PubMed] [Google Scholar]
- Jurman, M.E., L.M. Boland, Y. Liu, and G. Yellen. 1994. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 17:876–881. [PubMed] [Google Scholar]
- Karpen, J.W., A.L. Zimmerman, L. Stryer, and D.A. Baylor. 1988. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc. Natl. Acad. Sci. USA. 85:1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp, U.B., T. Niidome, T. Tanabe, S. Terada, W. Bonigk, W. Stuhmer, N.J. Cook, K. Kangawa, H. Matsuo, T. Hirose, et al. 1989. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 342:762–766. [DOI] [PubMed] [Google Scholar]
- Kramer, R.H., E. Goulding, and S.A. Siegelbaum. 1994. Potassium channel inactivation peptide blocks cyclic nucleotide-gated channels by binding to the conserved pore domain. Neuron. 12:655–662. [DOI] [PubMed] [Google Scholar]
- Lagnado, L., and D. Baylor. 1992. Signal flow in visual transduction. Neuron. 8:995–1002. [DOI] [PubMed] [Google Scholar]
- Liu, D.T., G.R. Tibbs, and S.A. Siegelbaum. 1996. Subunit stoichiometry of cyclic nucleotide-gated channels and effects of subunit order on channel function. Neuron. 16:983–990. [DOI] [PubMed] [Google Scholar]
- Liu, J., and S.A. Siegelbaum. 2000. Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels. Neuron. 28:899–909. [DOI] [PubMed] [Google Scholar]
- Lu, T., L. Wu, J. Xiao, and J. Yang. 2001. Permeant ion-dependent changes in gating of Kir2.1 inward rectifier potassium channels. J. Gen. Physiol. 118:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson, D.R., and R.P. Swenson, Jr. 1986. External monovalent cations that impede the closing of K channels. J. Gen. Physiol. 87:795–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini, A. 1990. Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J. Physiol. 424:167–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini, A. 1995. Cyclic nucleotide-gated channels in visual and olfactory transduction. Biophys. Chem. 55:185–196. [DOI] [PubMed] [Google Scholar]
- Molokanova, E., F. Maddox, C.W. Luetje, and R.H. Kram. 1999. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. J Neurosci. 19:4786-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova, E., B. Trivedi, A. Savchenko, and R.H. Kram. 1997. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci. 17:9068–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado, R.D., and R.W. Aldrich. 1993. a. Energetics of Shaker K channels block by inactivation peptides. J. Gen. Physiol. 102:977–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado, R.D., and R.W. Aldrich. 1993. b. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J. Gen. Physiol. 102:949–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi, E., and M. del Pilar Gomez. 1999. Divalent cation interactions with light-dependent K channels. Kinetics of voltage-dependent block and requirement for an open pore. J. Gen. Physiol. 114:653–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzari, M., F. Sesti, M.T. Giraudo, C. Virginio, A. Cattaneo, and V. Torre. 1993. Single-channel properties of cloned cGMP-activated channels from retinal rods. Proc. R. Soc. Lond. B Biol. Sci. 254:69–74. [DOI] [PubMed] [Google Scholar]
- Oxford, G.S., and J.Z. Yeh. 1985. Interactions of monovalent cations with sodium channels in squid axon. I. Modification of physiological inactivation gating. J. Gen. Physiol. 85:583–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages, F., M. Ildefonse, M. Ragno, S. Crouzy, and N. Bennett. 2000. Coexpression of alpha and beta subunits of the rod cyclic GMP-gated channel restores native sensitivity to cyclic AMP: role of D604/N1201. Biophys. J. 78:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones, A., and J.I. Korenbrot. 1992. Permeation and interaction of monovalent cations with the cGMP-gated channel of cone photoreceptors. J. Gen. Physiol. 100:647–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picones, A., and J.I. Korenbrot. 1995. Permeability and interaction of Ca2+ with cGMP-gated ion channels differ in retinal rod and cone photoreceptors. Biophys. J. 69:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch, M., U. Ludewig, A. Rehfeldt, and T.J. Jentsch. 1995. Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature. 373:527–531. [DOI] [PubMed] [Google Scholar]
- Qu, W., A.J. Moorhouse, A.M. Cunningham, and P.H. Barry. 2001. Anomalous mole-fraction effects in recombinant and native cyclic nucleotide-gated channels in rat olfactory receptor neurons. Proc. R. Soc. Lond. B Biol. Sci. 268:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, E.A., and C. Miller. 1990. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 247:1208–1210. [DOI] [PubMed] [Google Scholar]
- Root, M.J., and R. MacKinnon. 1993. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 11:459–466. [DOI] [PubMed] [Google Scholar]
- Root, M.J., and R. MacKinnon. 1994. Two identical noninteracting sites in an ion channel revealed by proton transfer. Science. 265:1852–1856. [DOI] [PubMed] [Google Scholar]
- Ruiz, M., R.L. Brown, Y. He, T.L. Haley, and J.W. Karpen. 1999. The single-channel dose–response relation is consistently steep for rod cyclic nucleotide-gated channels: implications for the interpretation of macroscopic dose–response relations. Biochemistry. 38:10642–10648. [DOI] [PubMed] [Google Scholar]
- Ruiz, M.L., and J.W. Karpen. 1997. Single cyclic nucleotide-gated channels locked in different ligand- bound states. Nature. 389:389–392. [DOI] [PubMed] [Google Scholar]
- Sala, S., and D.R. Matteson. 1991. Voltage-dependent slowing of K channel closing kinetics by Rb+. J. Gen. Physiol. 98:535–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert, R., E. Eismann, J. Ludwig, A. Baumann, and U.B. Kaupp. 1999. Molecular determinants of a Ca2+-binding site in the pore of cyclic nucleotide-gated channels: S5/S6 segments control affinity of intrapore glutamates. EMBO J. 18:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti, F., E. Eismann, U.B. Kaupp, M. Nizzari, and V. Torre. 1995. The multi-ion nature of the cGMP-gated channel from vertebrate rods. J. Physiol. 487:17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba, Y.M., V.I. Teslenko, A.N. Savchenko, and N.H. Pogorelaya. 1991. The effect of permeant ions on single calcium channel activation in mouse neuroblastoma cells: ion-channel interaction. J. Physiol. 443:25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce, A.E., N.B. Standen, and P.R. Stanfield. 1989. Rubidium ions and the gating of delayed rectifier potassium channels of frog skeletal muscle. J. Physiol. 411:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, J.H., H. Knutsson, and P.R. MacLeish. 1987. Divalent cations directly affect the conductance of excised patches of rod photoreceptor membrane. Science. 236:1674–1678. [DOI] [PubMed] [Google Scholar]
- Stryer, L. 1986. Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 9:87–119. [DOI] [PubMed] [Google Scholar]
- Stryer, L. 1988. Molecular basis of visual excitation. Cold Spring Harb. Symp. Quant. Biol. 53:283–294. [DOI] [PubMed] [Google Scholar]
- Swenson, R.P., Jr., and C.M. Armstrong. 1981. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 291:427–429. [DOI] [PubMed] [Google Scholar]
- Tanaka, J.C., and R.E. Furman. 1993. Divalent effects on cGMP-activated currents in excised patches from amphibian photoreceptors. J. Membr. Biol. 131:245–256. [DOI] [PubMed] [Google Scholar]
- Taylor, W.R., and D.A. Baylor. 1995. Conductance and kinetics of single cGMP-activated channels in salamander rod outer segments. J. Physiol. 483:567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, C., H.A. Hartmann, and R. Horn. 1997. Anomalous effect of permeant ion concentration on peak open probability of cardiac Na+ channels. J. Gen. Physiol. 110:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, C., and R. Horn. 1997. Effect of alkali metal cations on slow inactivation of cardiac Na+ channels. J. Gen. Physiol. 110:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau, K.W., and D.A. Baylor. 1989. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 12:289–327. [DOI] [PubMed] [Google Scholar]
- Zagotta, W.N., T. Hoshi, and R.W. Aldrich. 1990. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 250:568–571. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., and R. MacKinnon. 2002. High resolution chrystallographic analysis of ion permeation in KcsA. Biophys. J. 82:1702A. [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel- Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]
- Zimmerman, A.L. 1995. Cyclic nucleotide gated channels. Curr. Opin. Neurobiol. 5:296–303. [DOI] [PubMed] [Google Scholar]
- Zimmerman, A.L., and D.A. Baylor. 1986. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 321:70–72. [DOI] [PubMed] [Google Scholar]
- Zimmerman, A.L., and D.A. Baylor. 1992. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J. Physiol. 449:759–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall, F., and S. Firestein. 1993. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J. Neurophysiol. 69:1758–1768. [DOI] [PubMed] [Google Scholar]
- Zufall, F., S. Firestein, and G.M. Shepherd. 1994. Cyclic nucleotide-gated ion channels and sensory transduction in olfactory receptor neurons. Annu. Rev. Biophys. Biomol. Struct. 23:577–607. [DOI] [PubMed] [Google Scholar]