Abstract
Flecainide (pKa 9.3, 99% charged at pH 7.4) and lidocaine (pKa 7.6–8.0, ∼50% neutral at pH 7.4) have similar structures but markedly different effects on Na+ channel activity. Both drugs cause well-characterized use-dependent block (UDB) of Na+ channels due to stabilization of the inactivated state, but flecainide requires that channels first open before block develops, whereas lidocaine is believed to bind directly to the inactivated state. To test whether the charge on flecainide might determine its state specificity of Na+ channel blockade, we developed two flecainide analogues, NU-FL (pKa 6.4), that is 90% neutral at pH 7.4, and a quaternary flecainide analogue, QX-FL, that is fully charged at physiological pH. We examined the effects of flecainide, NU-FL, QX-FL, and lidocaine on human cardiac Na+ channels expressed in human embryonic kidney (HEK) 293 cells. At physiological pH, NU-FL, like lidocaine but not flecainide, interacts preferentially with inactivated channels without prerequisite channel opening, and causes minimal UDB. We find that UDB develops predominantly by the charged form of flecainide as evidenced by investigation of QX-FL at physiological pH and NU-FL investigated over a more acidic pH range where its charged fraction is increased. QX-FL is a potent blocker of channels when applied from inside the cell, but acts very weakly with external application. UDB by QX-FL, like flecainide, develops only after channels open. Once blocked, channels recover very slowly from QX-FL block, apparently without requisite channel opening. Our data strongly suggest that it is the difference in degree of ionization (pKa) between lidocaine and flecainide, rather than gross structural features, that determines distinction in block of cardiac Na+ channels. The data also suggest that the two drugs share a common receptor but, consistent with the modulated receptor hypothesis, reach this receptor by distinct routes dictated by the degree of ionization of the drug molecules.
Keywords: electrophysiology, local anesthetics, flecainide, arrhythmia, pharmacology
INTRODUCTION
Investigation of ion channel defects associated with the long QT syndrome (LQTS)* has provided new insights into fundamental mechanisms through which ion channel activity and its modulation by drugs control the duration of the cardiac ventricular action potential and consequently the QT interval of the electrocardiogram (EKG). In particular, the unanticipated discovery of mutations in SCN5A, the gene coding for the α subunit of the heart voltage–gated Na+ channel, associated with LQTS variant 3 (LQT-3) and the use of Na+ channel blockers to control QT prolongation in LQT-3 mutation carriers has renewed interest into the fundamental mechanism of actions of drugs that block Na+ channels (An et al., 1996; Dumaine and Kirsch, 1998; Kambouris et al., 2000; Viswanathan et al., 2001).
Flecainide, the prototypical Class 1c antiarrhythmic agent, is of particular interest because it has been shown to be more effective than lidocaine, a prototypical Class 1b agent, in the management of QT prolongation in some LQT-3 mutation carriers (Brugada et al., 1999; Benhorin et al., 2000). Additionally, flecainide is used in diagnostic tests to identify patients at risk for the Brugada syndrome, an inherited form of idiopathic ventricular fibrillation that too is associated with SCN5A mutation (Brugada et al., 1999). Clearly, understanding fundamental differences between the mechanisms of action of flecainide and lidocaine has significance, not only from the point of view of the fundamental molecular pharmacology of the cardiac Na+ channel, but also for its importance in detecting and treating these inherited arrhythmias.
Flecainide and lidocaine have similar chemical structures but markedly different effects on sodium channel activity. Both drugs promote tonic and use channel state–dependent block (block that increases with channel activity) of Na+ channels. The affinity of lidocaine is higher for inactivated compared with resting channels, and hence the stabilization of inactivated lidocaine-bound channels has been shown to underlie its state-dependent Na+ channel block (Hille, 1977a). In contrast with lidocaine, flecainide requires channels to open before it causes use-dependent block (UDB) and consequently has been associated with block of open channels (Anno and Hondeghem, 1990; Ragsdale et al., 1996; Liu et al., 2002). However, investigation of disease-associated mutant cardiac Na+ channels recently has provided evidence that although necessary, channel openings are not sufficient to explain flecainide UDB. Instead, like lidocaine, flecainide block may be determined by preferential interaction with inactivated channels (Liu et al., 2002). Further, the results of that study suggested that differential access to a common receptor might account for differences between these two drugs.
Investigation into differential access to a common receptor has been hampered by differences in the physical chemical properties of the two drugs. Lidocaine has a pKa between 7.8–8.6 and thus may be up to 50% neutral at physiological pH. In contrast, flecainide has a pKa of ∼9.3, resulting in >99% of the drug in ionized and <1% in neutral forms at pH 7.4 (Hille, 1977a; Strichartz et al., 1990). Thus, the charge on flecainide is likely to restrict access of the drug to a receptor site, confer the dependence of use-dependent block on channel openings, and account for most of the differences between it and lidocaine (Strichartz, 1973; Hille, 1977a; Chernoff and Strichartz, 1990). However, a direct test of this possibility has not been possible because of the marked differences in distribution between neutral and charged forms of each compound.
Here we employed two custom-synthesized flecainide analogues, NU-FL and QX-FL, to investigate the molecular determinants of flecainide activity. NU-FL has nearly identical hyrophobicity and very similar three-dimensional structure compared with flecainide, but has a very different pKa. As measured by titration, NU-FL has an approximate pKa value of 6.4. Consequently, it should be nearly 90% neutral at physiological pH, thus more closely resembling the ionization profile of lidocaine. QX-FL shares a very similar three-dimensional structure with the parent compound flecainide, but is fully charged at physiological pH, and thus is well suited to discriminate between hydrophilic and hydrophobic access to its receptor. We compared the effects of flecainide, NU-FL, QX-FL, and lidocaine on human heart (hH1) sodium channels expressed in human embryonic kidney (HEK) 293 cells to better understand the specific mechanism of block by flecainide. Our results indicate that like lidocaine, the tertiary flecainide analogue (NU-FL) interacts preferentially with inactivated channels without prerequisite channel openings, while flecainide (and QX-FL) is ineffective in blocking channels that inactivate without first opening. Ionized flecainide underlies UDB. Our results show marked UDB of channels by internally, but not externally, applied QX-FL, with voltage and time-dependent characteristics consistent with intracellular access to a common receptor for local anesthetic molecules. QX-FL block requires the open conformation of the channel, suggesting that channel openings unmask a preferred intracellular access route allowing QX-FL to bind to the LA receptor site in the inner mouth of the channel pore. Further, because the slow recovery of channels from QX-FL block was impeded by outer pore block by tetrodotoxin, our data also suggest that drug can diffuse away from channels via the outer pore even in the absence transitions into the open state. Our data strongly suggest that it is the difference in degree of ionization (pKa) between lidocaine and flecainide, rather than differences in their gross structural features, that determines distinction in block of cardiac Na+ channels. The data also suggest that the two drugs share a common receptor, but, as outlined in the modulated receptor hypothesis, reach this receptor by distinct routes.
MATERIALS AND METHODS
Synthesis of NU-FL (2,5-bis(2,2,2-Trifluoroethoxy)N-(2 Ethylmorpholino)Benzamide)
Crude 2,5-bis(2,2,2-trifluoroethoxy)benzoyl chloride (75 mg, 0.22 mmol), prepared by modification of previously published methods (Banitt et al., 1975), is dissolved in 10 ml of dichloromethane under inert conditions. The solution is cooled to 0°C, treated with N-aminoethylmorpholine (39 μl, 0.29 mmol), and double distilled Hünigs base (43 μl, 0.24 mmol). After reacting for 24 h at ambient temperature, the solution is concentrated, dissolved into 15 ml of 5:1 ethyl acetate:diethyl ether, and washed with equal portions of 10% sodium hydroxide, saturated sodium chloride, and distilled water. The organic layers are separated, dried over sodium sulfate, and concentrated to an oil. The oil is recrystallized from ethyl acetate:hexanes at −78°C in a CO2/acetone bath. The precipitate is collected by suction filtration, washed with cold hexanes, and dried under vacuum to yield the product (89 mg, 93%) as a white solid. TLC (EtOAc:Hex 1:5) shows one major UV active spot with Rf ∼0.75. 1H NMR (d6-DMSO, 300 MHz): δ 8.0 (br s., 1H), 7.3 (s, 1H), 7.2 (m, 2H), 4.7–4.9 (m, 2H) 3.5–3.6 (m, 4H), 3.3–3.4 (m, 2H), 2.3–2.5 (m, 6H) Mass: (APCI+) 430.6 (M+1). The material can subsequently be converted to the hydrogen chloride salt by treatment of an ether solution with 1.1 molar equivalents of concentrated HCl, filtration, and lyophilization of an aqueous solution of the salt.
Synthesis of QX-FL (N,N-Dimethyl Flecainide)
Flecainide hydrogen chloride salt (55 mg, 0.122 mmol), potassium carbonate (37 mg, 0.270 mmol), and iodomethane (0.54 mmol) are dissolved into a round bottom flask charged with 5 ml of absolute ethanol. The suspension is stirred at 25°C until TLC (20:1:1 CH2Cl2:MeOH:NH3) demonstrates only baseline material, after which time the ethanol is removed under reduced pressure, the residue is solidified by trituration with anhydrous ether, and cold distilled water (5 ml) added. The remaining solid is filtered and washed with 5 ml of cold water. The residue is collected, dissolved into 30 mL of distilled water and lyophilized to yield the product as the iodide salt (30 mg, 29%). 1H NMR (d6-DMSO, 300 MHz): δ 8.5 (t, 1H, NH), 7.1–7.3 (m, 3H), 4.7–4.8 (m, 4H), 3.9 (d, 1H), 3.2 (s, 3H), 3.0 (s, 3H), 1.2–2.1 (m, 6H). Mass (FAB+): Expected: 568 Found: 443 (M-I).
Mutagenesis and Expression of Recombinant Na+ channels
Mutations of SCN5A were engineered into WT cDNA cloned in pcDNA3.1 (Invitrogen) by overlap extension using mutation-specific primers and Quick Change site-directed mutagenesis kit (Stratagene) as described previously (Abriel et al., 2001). The presence of the mutation was confirmed by sequence analysis. WT and mutant Na+ channels were expressed in HEK293 cells as described previously (Abriel et al., 2001). Transient transfections were performed with equal amounts of Na+ channel α subunit and with hβ1, subcloned individually into the pcDNA3.1 (Invitrogen) vector (total cDNA 2.5 μg) using a previously described lipofection procedure (Abriel et al., 2001). Expression of channels was studied using patch clamp procedures 48 h after transfection.
Electrophysiology
Membrane currents were measured using whole cell patch-clamp procedures, with Axopatch 200B amplifiers (Axon Instruments, Inc.). Recordings were made at room temperature (22°C) or 32°C where indicated. The internal pipette solution contained (in mmol/L): aspartic acid 50, CsCl 60, Na2-ATP 5, EGTA 11, HEPES 10, CaCl2 1, and MgCl2 1, with pH 7.4 adjusted with CsOH (Abriel et al., 2001). External solutions consisted of (mmol/L) NaCl 130, CaCl2 2, CsCl 5, MgCl2 1.2, HEPES 10, and glucose 5, with pH 7.4 adjusted with CsOH. The external solution for cell-attached recordings was: (mM) 140 KCl, 5 HEPES, 1 MgCl2, and pH adjusted to 7.4. For cell-attached recordings, pipettes were coated with Sylgard (Dow Chemical Co.) to decrease noise and capacitance of the glass, and electrode resistance was typically 1–2 MΩ when filled with internal solution containing 110 mM NaCl, 10 mM HEPES (pH adjusted to 7.4).
In experiments designed to measure the voltage dependence of activation, external Na+ was reduced to 30 mM using n-methyl-glucamine as an Na+ substitute. Activation curves were determined by normalization of peak current versus voltage to driving force determined as the difference between test voltage and measured channel reversal potential in low external Na+. Steady-state inactivation was measured with 5-s conditioning pulses followed by a test pulse (−10 mV), with an interpulse interval of 10 s. Steady-state UDB was reached in response to trains of variable numbers of pulses (100–600×, −10 mV), at frequencies indicated in the figure legends. This was sufficiently long to induce steady-state UDB for each construct. UDB was measured as the ratio of peak current at −10 mV after and before application of a conditioning train and is reported as the percentage block of peak current.
Pclamp 8.0 (Axon Instruments, Inc.), Excel (Microsoft), and Origin (Microcal Software) were used for data acquisition and analysis. Data are presented as mean values ± SEM. P < 0.05 was considered statistically significant (using Student's t test).
RESULTS
Use-dependent Block by Flecainide and Its Tertiary Analogue
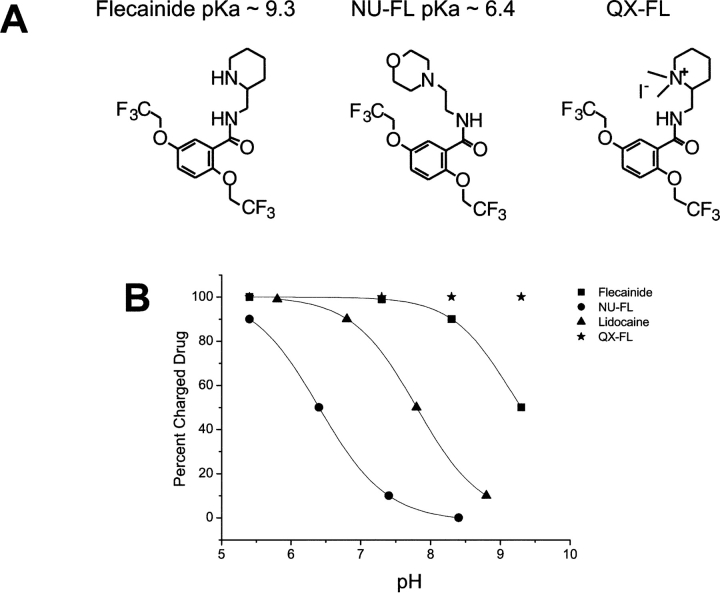
The chemical synthesis of both tertiary flecainide (NU-FL) and quaternary flecainide (QX-FL) was generally based on procedures reported in the literature for the synthesis of related flecainide analogues (Banitt et al., 1975). To the best of our knowledge these are novel flecainide analogues. Fig. 1 compares the structures of NU-FL, QX-FL, and flecainide and illustrates the relative distributions of neutral and charged drug forms as a function of solution pH. The experimentally determined pKa of NU-FL was ∼6.4. Thus, at physiologic pH (7.4), 90% of the drug is neutral. Altering the pH of drug-containing solutions alters the ionized fraction of NU-FL. At pH 8.4, the drug is 99% neutral, whereas at pH 5.4 it is 90% charged. In contrast, because the pKa of flecainide is 9.3, it remains >98% charged over this pH range, and QX-FL is fully ionized over this pH range.
Figure 1.
NU-Fl and QX-FL are novel analogues of flecainide. (A) Structural comparison of flecainide and its novel analogues NU-FL (center) and QX-FL (right). (B) Plot of estimated concentrations of charged drugs as function of pH. The pKa values of each compound are 9.3 for flecainide (▪), 6.4 for NU-FL (•), 7.8 for lidocaine (▴). At relevant physiological pH values flecainide is >99% charged, QX-FL (★) is fully ionized, lidocaine is ∼50:50, and NU-FL is >90% neutral.
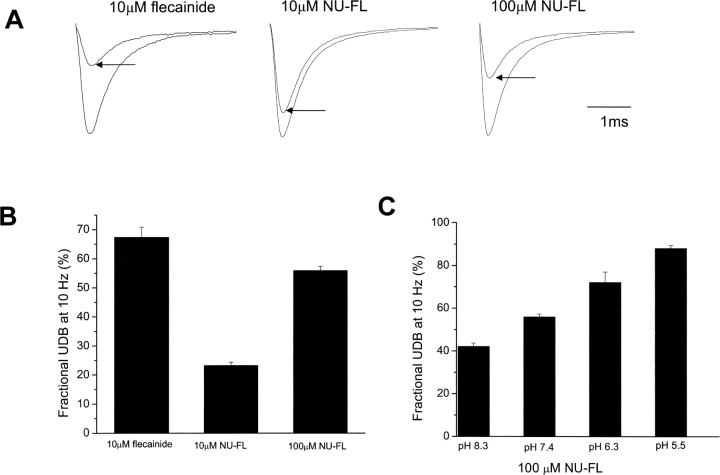
We initially focused on the actions of NU-FL to determine whether neutral or charged drug forms caused UDB. Fig. 2, A and B , shows that at similar concentrations and using similar voltage protocols, much less UDB develops in the presence of NU-FL than flecainide. Apparently at the pulse frequency of these experiments (10 Hz) the interpulse interval (75 ms) during the pulse train is sufficiently long to allow for unblock of most NU-FL bound channels. Increasing the NU-FL concentration to 100 μM such that the estimated concentration of ionized NU-FL is 10 μM results in UDB comparable to that caused by 10 μM flecainide. Because only 10% of NU-FL is charged at physiological pH, this result suggests that the block that builds with repetitive depolarization to voltages that open channels (UDB) develops predominantly by the charged forms of both flecainide and NU-FL. This might be the case if both charged and neutral drug forms gain access to a common receptor during the brief depolarizing pulses, but that, upon return to −100 mV, channels can recover quickly from neutral drug block, but not from block by ionized drugs. If this were the case, then increasing the ratio of charged to neutral drug molecules, while keeping total drug concentration constant, should increase the development of UDB with this voltage protocol. The data in Fig. 2 C indicate that this is indeed the case. As summarized in the figure, as extracellular pH is lowered and the fraction of ionized NU-FL increased, UDB also increases despite the fact that total NU-FL concentration is constant.
Figure 2.
UDB of WT Na+ channels by flecainide and NU-FL. Currents were evoked by imposing conditioning trains of 100–600 pulses (−10 mV, 25 ms) from a holding potential of −100 mV at a frequency of 10 Hz. Pulses were applied until steady-state UDB was achieved. This pulse protocol allows for a 75-ms interpulse interval at the holding potential between conditioning pulses. (A) Examples of current traces recorded at 10 Hz before and after steady-state UDB (arrows) by flecainide (10 μM), NU-FL (10 μM), and NU-FL (100 μM). Currents are superimposed records of first and last (∼300th) pulse of a conditioning train. (B) Bar graphs summarize steady-state UDB by flecainide and NU-FL, plotted as the fraction of current blocked in response to pulse trains (n = 4–6 cells per condition). (C) Percent UDB plotted vs. pH in the presence of 100 μM NU-FL (n = 4–6 cells per condition).
As proposed above, the difference in UDB that develops for NU-FL and flecainide at pH 7.4 may be due in large part to the difference between the kinetics of the recovery from block by neutral and charged drug forms. Recovery from drug block by both NU-FL and flecainide at physiological pH is illustrated in Fig. 3 . Exponential fits to the recovery kinetic data revealed the following recovery time constants (τ1(fast) and τ2 (slow)): 55 ms and 508 ms for NU-FL, 103 ms and 2,530 ms for flecainide. Recovery is significantly faster for NU-FL than for flecainide. Because NU-FL is 90% neutral at pH 7.4, the most likely difference in the recovery kinetics is due to rapid unblock of neutral drug-bound channels and very slow unblock of charged drug-bound channels.
Figure 3.
Recovery from UDB by flecainide, QX-FL, and NU-FL. UDB was induced by trains of 100 pulses (−10 mV, 25 ms, 25 Hz) from a −100 mV holding potential. Test pulses were then imposed after variable recovery intervals at −100 mV. Currents were normalized to steady-state current levels during slow pacing (once every 30 s) and plotted against recovery interval in the absence and presence of flecainide (10 μM) and NU-FL (100 μM), and NU-FL (pH 5.5, 100 μM). Recovery from UDB by QX-FL, flecainide, and NU-FL. UDB was induced by trains of 100 pulses (−10 mV, 25 ms, 25 Hz) from a −100 mV holding potential. Test pulses were then imposed after variable recovery intervals at −100 mV. Currents were normalized to steady-state current levels during slow pacing (once every 30 s) and plotted against recovery interval in the absence and presence of test drugs. (A) Drugs studied at pH 7.4 were flecainide (10 μM), NU-FL (100 μM), and QX-FL (internal, 200 μM). (B) Similar protocols were performed in solutions buffered to pH 5.5 in the absence and presence of NU-FL (100 μM). The averaged data were fitted with a two exponential function: y(t) = y0 + A1 × exp-t/τ1 + A2 × exp-t/τ2), where t is the recovery time, τ1 and τ2 are the recovery time constant, A1 and A2 are fractional amplitudes of each component, and y0 is the estimated steady-state fraction of recovered current. n = 3–5 cells per condition.
Recovery from NU-FL block in solutions buffered to pH 5.5, when the ratio of neutral to charged forms of NU-FL are reversed, is greatly slowed, consistent with this hypothesis. Finally, we also illustrate recovery from channel block by the permanently charged analogue QX-FL in panel A, which is remarkably similar to recovery from flecainide block. At pH 7.4 there are both neutral and ionized forms of flecainide and NU-FL, and thus the time course of recovery from block for these drugs are complex. As proposed by Hille and colleagues in the analysis of the pH dependence of use-dependent block of Na+ channels in muscle and nerve (Hille, 1977a,b; Schwarz et al., 1977), during interpulse intervals bound charged drug is trapped within the channel until the drug molecule is deprotonated. Neutral drug, which is less restricted (Hille, 1977a), can dissociate from the channel at these voltages between conditioning pulses. At pH 5.5 (Fig. 3 B), when ∼90% of NU-FL molecules are ionized, the recovery from inactivation closely resembles that of flecainide at pH 7.4 (Fig. 3 A), suggesting similar kinetics when both drugs are ionized. At physiological pH, the fact that the recovery from block is faster for NU-FL than for flecainide may simply be due to the greater contribution (90%) of drug block by the neutral NU-FL component compared, while flecainide remains >99% charged. On the other hand, according the scheme described above, it is possible that deprotonation of NU-FL, which can occur when channels are closed and at rest (Schwarz et al., 1977), may occur faster than deprotonation of flecainde, and that the differences in recovery kinetics occur not only because of the greater fraction of neutral NU-FL molecules at this pH, but also because the ionized bound drug deprotonates faster than ionized bound flecainde leave the vicinity of the receptor via a hydrophobic pathway. Our data thus indicate that UDB develops predominantly as a function of differences between the recovery kinetics of ionized and neutral drug molecules. This profile closely resembles that of lidocaine, raising the question as to whether or not the molecular pharmacology of lidocaine and NU-FL, including channel state dependence of block, share similar profiles.
Channel Openings Are Required for Block by Charged, but Not Neutral, Flecainide
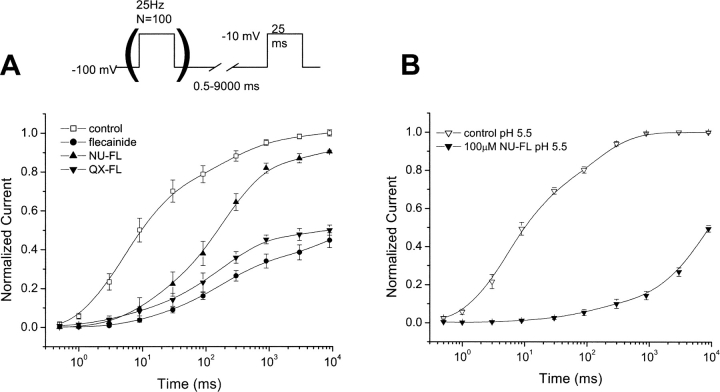
UDB by flecainide requires that channels first open before block develops (Ragsdale et al., 1996; Liu et al., 2002). If this dependence on channel openings is due to restricted receptor access caused by the predominantly ionized form of the drug, this requirement should be reduced or eliminated for NU-FL, a greater fraction of which is neutral at pH 7.4. Fig. 4 shows that this is in fact the case. Shown in the figure are currents measured at a fixed voltage, but after trains of conditioning pulses have been applied to a series of voltages. Reduction in current measured during the test pulse is a reflection of block that develops in a use-dependent manner during the conditioning pulse trains (Ragsdale et al., 1994, 1996). As has been shown previously by us (Liu et al., 2002), flecainide block develops during the conditioning train, but only after pulses are applied to voltages more positive than −45 mV, a voltage near the activation threshold for Na+ channels. A similar profile emerged for the voltage dependence of channel block by QX-FL (filled circles), indicating that channel openings are a common prerequisite for QX-FL and flecainide Na+ channel block. This suggests that the open state conformation is necessary for the ionized form of the drug to reach its receptor. In contrast with the experiments illustrated in Fig. 2, the pulse frequency (25 Hz) in these experiments, which allows only a 15-ms interpulse interval between conditioning pulses, was sufficiently fast to promote channel block by NU-FL during the conditioning trains. UDB by NU-FL is apparent after conditioning voltages as negative as −80 mV, voltages consistent with a decrease in channel availability that is not dependent on channel openings.
Figure 4.
Voltage dependence of UDB of Na+ channels by flecainide, QX-FL, and NU-FL. Currents were recorded before (control) and after (test) application of conditioning pulses (100 pulses, 25 Hz) of varying amplitude in the presence of flecainide (10 μM), QX-FL (100 μM, internal), and NU-FL (100 μM). The pulse protocol allows for a 15-ms interpulse interval at the holding potential during the conditioning trains. Test pulses were preceded by a 100 ms pulse-free interval at the holding potential (−100 mV) to allow drug-free channels to recover from inactivation. (A) Current traces from experiments with flecainide (right), QX-FL (middle), and NU-FL (left) elicited by a control depolarization before and by test depolarization after conditioning trains to −80 mV∼0 mV. (B) Normalized block is plotted vs. conditioning pulse amplitude for NU-FL (▪), QX-FL (•), and flecainide (▵). Normalized block was determined as the fraction of test pulse current (normalized to control current) reduced by the conditioning train (n = 3–4 cells per measurement).
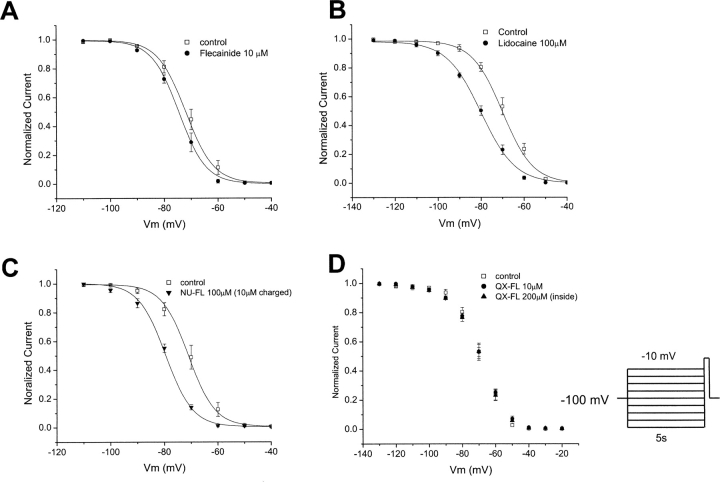
The data of Fig. 4 suggest a preferential interaction of neutral flecainide (NU-FL) with inactivated channels that does not require channel openings to develop, a suggestion that predicts drug-dependent alteration of the voltage dependence of channel availability. Thus, we compared the effects of NU-FL with lidocaine and flecainide on the voltage dependence of channel availability in the absence of trains of conditioning pulses to first open channels. Under these conditions, as previously shown (Liu et al., 2002), flecainide has little effect on channel availability (Fig. 5 A). In contrast, lidocaine (Fig. 5 B) causes a well-documented negative shift in channel availability under the same voltage conditions. The tertiary flecainide analogue NU-FL (100 μM, ∼10 μM charged) also shifts channel availability without conditioning pulses, similar to lidocaine but in contrast to flecainide (Fig. 5 C). Thus, although nearly identical to flecainide in structure, NU-FL interacts with the inactivated state without mandatory channel openings similar to lidocaine, a drug with a significant neutral component at physiological pH. When similar experiments are carried at pH 8.0, solutions for which a greater fraction of NU-FL is neutral, a lower total NU-FL concentration causes a similar shift in channel availability, providing additional evidence that it is the neutral form of NU-FL that interacts with the inactivated channel in these experiments (unpublished data).
Figure 5.
Effects of flecainide, NU-FL, and lidocaine on steady-state inactivation of WT Na+ channels. Steady-state inactivation was measured with 5-s conditioning pulses followed by a test pulse (−10 mV) with an interpulse of 10 s. Graphs show normalized current plotted against conditioning pulse voltage. Smooth lines are according to 1/{1 + exp[(Vc − V1/2)/k]}, where Vc is conditioning potential, V1/2 is voltage for which half the channel are not available, and k is a slope factor. The drug-induced shift in channel availability (ΔV1/2) was determined from V1/2,drug − V1/2,control using V1/2 values obtained from fits of the data. ΔV1/2 is −2.3 ± 0.29 mV with 10 μM flecainide, −9.17 ± 1.04 mV with 100 μM NU-FL, −10.28 ± 0.61 mV with 100 μM lidocaine (n = 4–6 cells per condition).
NU-FL: High-affinity Block of Inactivated Channels
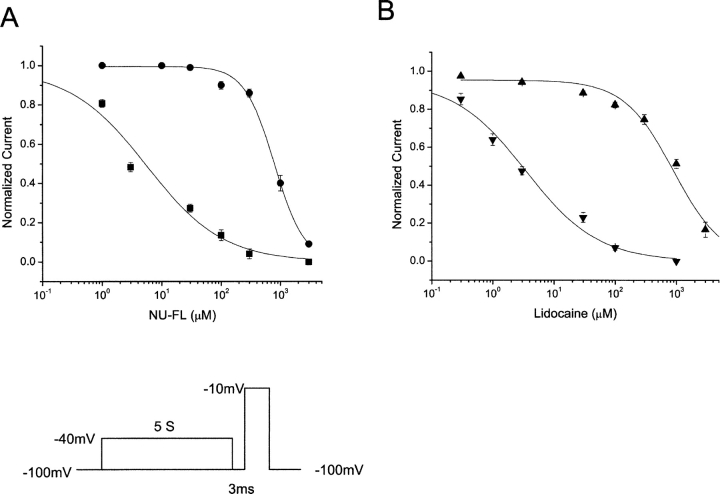
We next determined whether NU-FL, like lidocaine, has a higher affinity for the inactivated rather than the resting state and illustrate the results of these experiments in Fig. 6 . A voltage protocol was used to inactivate (5-s pulse to −40 mV), but not open, most channels in the absence and presence of drug. A test pulse to −10 mV, preceded by a 3-ms return to the negative (−100 mV) holding potential to allow drug-free, but not drug-bound, channels to recover from inactivation was applied to assay available channels. The concentration dependence of the block of channels during this test pulse with and without conditioning pulses provides an estimate of rested state (no conditioning pulse) and inactivated state (with conditioning pulse) channel block (Weiser et al., 1999). Concentration-response curves obtained using this protocol showed that the neutral analogue inhibited Na+ channels after conditioning pulses (inactivated state block) with an EC50 value of 5.32 ± 0.51 μM, which is comparable to that obtained for lidocaine (3.46 ± 0.33 μM) using the same voltage protocol. Rested state block (no prepulse) revealed an EC50 values of 794 ± 39 μM (NU-FL) and 895 ± 55 μM (lidocaine). Thus, NU-FL and lidocaine displayed 150- and 250-fold higher affinities for inactivated compared with resting Na+ channels. The data suggest that a simple change in the hydrophobicity (charge) of flecainide from predominantly charged to neutral results in a dramatic change flecainide action: block of inactivated channels without prerequisite channel openings.
Figure 6.
Affinity of resting and inactivated states of WT Na+ channels for NU-FL and lidocaine. The block of resting Na+ channels was investigated by depolarizing to −10 mV from a holding potential of −100 mV. The block of inactivated Na+ channels was measured using 5-s depolarizing prepulse to −40 mV to inactivated most channels and the membrane potential then was returned to the holding potential for 3 ms to allow drug-free channels to recover from inactivation. A test pulse to −10 mV then was applied. The block of peak test pulse current is plotted as a function of drug concentration. The smooth curves are the best fits of the Hill equation 1/((1 + ([drug]/EC50)n) to the data, these gave estimates of EC50 for drug binding to resting and inactivated channels. For the resting channels, EC50 are 794 ± 39.3 μM for NU-FL (•) and 895 ± 55.4 μM for lidocaine (▴). For the inactivated channels, EC50 are 5.32 ± 0.51 μM for NU-FL (▪) and 3.46 ± 0.33 μM for lidocaine (▾) (n = 3–5 cells per condition).
Flecainide Blocks Na+ Channels via an Intracellular Pathway
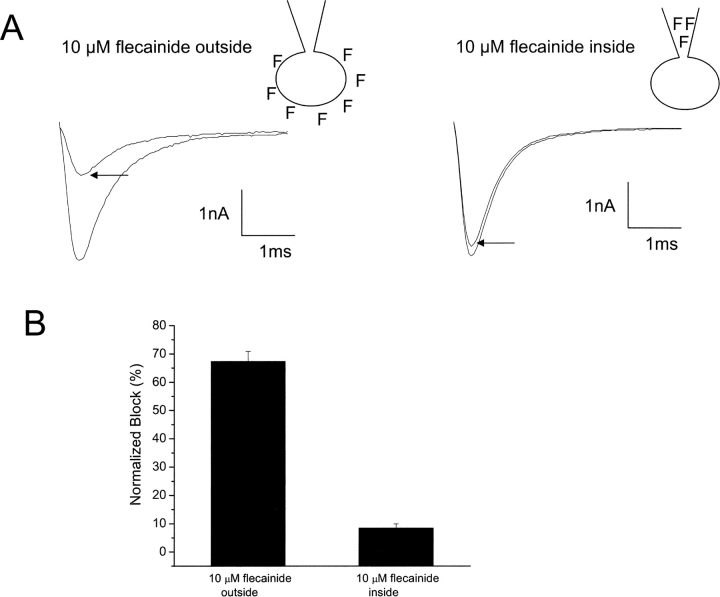
Delineating drug access to its receptor is critical in understanding the structural basis of drug action. Fig. 7 shows that, in the whole-cell recording mode, external, but not internal, application of flecainide promotes UDB of heart Na+ channels. The figure shows records and summary data of experiments in which trains of repetitive depolarizing pulses (10 Hz, −10 mV, 25 ms) were applied to cells expressing cardiac Na+ channels in the presence of flecainide (10 μM). As shown in the figure, including flecainide in the recording pipette (cells were dialyzed for 3 min before applying UDB protocols) has almost no effect on Na+ channel current, whereas bath application of the same flecainide concentration causes marked UDB of the channels (67.3 ± 3.5% vs. 8.4 ± 1.5% for internal application, P < 0.01, n = 4–5 cells). Increasing the flecainide (10 μM) dialysis time from 3 to 10 min increased the amount of UDB measured to 15 ± 1.0%, still less than four times the amount of UDB induced by external flecainide (10 μM) application. These data are consistent with extracellular, but not intracellular, access to a receptor for flecainide. However, although predominantly charged, a small fraction of flecainide molecules (<1%) is neutral at physiological pH, and thus another interpretation of these results is possible. It is possible that neutral flecainide diffuses across the cell membrane, reaches equilibrium within the cell and again partitions into charged (>99%) and neutral (<1%) forms such that drug block occurs via intracellular flecainide. Pipette application of the drug would be expected to have little effect as drug applied essentially as a point source in the cell, diffuses out of the cell via its neutral component and would be quickly diluted and washed away in the extracellular bath.
Figure 7.
Test of sidedness of flecainide block using whole-cell recordings. (A) UDB of WT Na+ channels by external and internal application of flecainide in whole-cell configurations. Currents were evoked by imposing conditioning trains of 100–600 pulses (−10 mV, 25 ms) from a holding potential of −100 mV at a frequency of 10 Hz. Pulses were applied until steady-state UDB was achieved, as indicated by the arrows. (B) Bar graphs summarize steady-state UDB by external and internal application of flecainide (10 μM) plotted as the fraction of current blocked in response to pulse trains. n = 4–6 cells per condition.
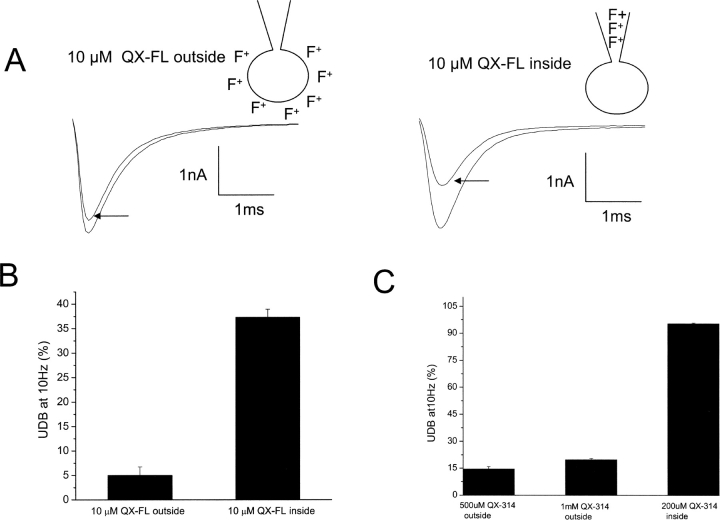
To determine whether or not this may be the case, we repeated the experiments of Fig. 7, but replaced flecainide by QX-FL (10 μM) to determine whether removal of the small fraction of neutral flecainide affects the apparent sidedness of drug action on cardiac Na+ channels. UDB was measured under conditions of both external and internal drug application and the sidedness of drug block was opposite to that for flecainide. As shown in Fig. 8 , UDB by externally applied QX-FL was very weak; however, internal application of QX-FL (3 min dialysis time before assaying for UDB) resulted in marked UDB. Because QX-FL cannot diffuse across the cell membrane, these data clearly indicate that an intracellular pathway dominates for ionized flecainide block of heart Na+ channels. These results, very similar to those obtained in similar experiments with the lidocaine analogue QX-314 (Fig. 8 C), show that the small fraction of neutral flecainide molecules complicates the analysis of the sidedness of flecainide action.
Figure 8.
Internally applied QX-FL blocks whole-cell Na+ channel activity. (A) UDB of WT Na+ channels by external and internal application of QX-FL in whole-cell configurations. Currents were evoked by imposing conditioning trains of 100–600 pulses (−10 mV, 25 ms) from a holding potential of −100 mV at a frequency of 10 Hz. Pulses were applied until steady-state UDB was achieved. (B) Bar graphs summarize steady-state UDB by external and internal application of QX-FL (10 μM) plotted as the fraction of current blocked in response to pulse trains. n = 4–6 cells per condition. (C) Bar graphs summarize steady-state UDB by external and internal application of QX-314 plotted as the fraction of current blocked in response to pulse trains. n = 4–6 cells per condition.
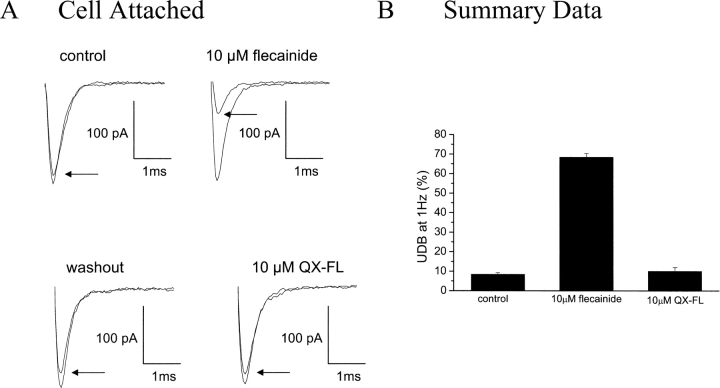
Together, it is likely that external flecainide diffuses into cells through rapid equilibrium via its neutral component, and, once inside, equilibrium is again established with >99% of intracellular drug ionized. To further test this hypothesis, using cell-attached recordings of Na+ channel activity, we applied flecainide to the cell outside of the patched area. Under these conditions, external drug application should only block channels if access to the drug-binding site occurs via a pathway that allows the drug to enter the cytoplasm by passing through the cell membrane. As shown in Fig. 9 , external application of flecainide (10 μM) indeed causes UDB of channels recorded within the cell-attached patch, and this block is completely, and rapidly, reversible upon washout. In contrast, channels within the patch are insensitive to external QX-FL (10 μM), as expected for a drug lacking a neutral component.
Figure 9.
Cell-attached recordings reveal flecainide access to LA-receptor through the cell membrane. (A) UDB of WT Na+ channels by external application of flecainide and QX-FL in cell-attached configurations. Currents were evoked by imposing conditioning trains of 100–200 pulses (−20 mV, 25 ms) from a holding potential of −120 mV at a frequency of 1 Hz. Pulses were applied until steady-state UDB was achieved. (B) Bar graphs summarize steady-state UDB by external application of flecainide and QX-FL plotted as the fraction of current blocked in response to pulse trains. n = 2–3 cells per condition.
Common Molecular Determinants of Lidocaine, NU-FL, and QX-FL Channel Block
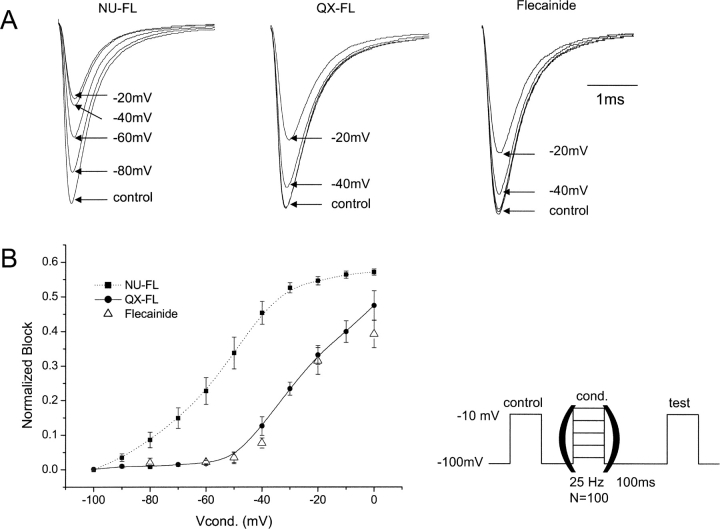
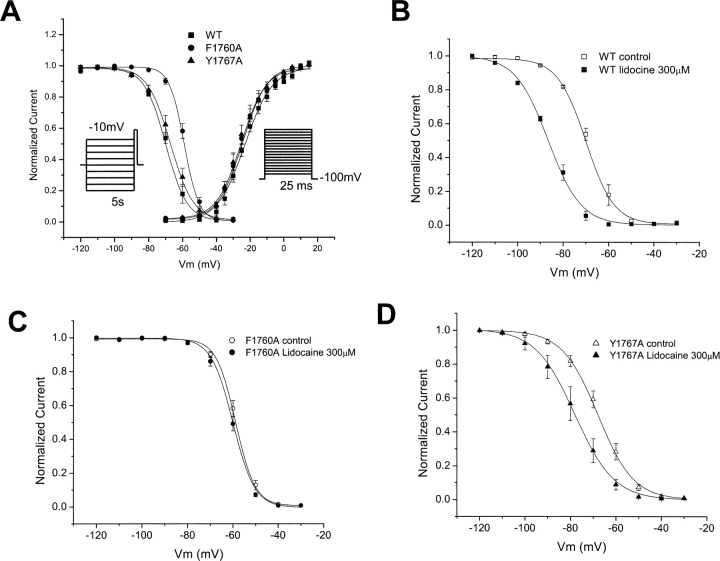
Local anesthetics have been shown to interact with specific sites in domains I, III, and IV of voltage-gated Na channels (Ragsdale et al., 1994, 1996; Wang et al., 1998; Catterall, 2002; Yarov-Yarovoy et al., 2002). Because of the differences in physical chemical properties of lidocaine and flecainide, the effects of site-specific mutations on drug block of channels has required different voltage protocols (Ragsdale et al., 1994, 1996), suggesting that the different drug structures may define unique interaction sites (Li et al., 1999). In particular, the mutations F1764A and Y1771A in transmembrane segment IVS6 of the brain type IIA Na+ channel α subunit dramatically reduced block of inactivated channels by lidocaine (Ragsdale et al., 1996). We prepared the homologous mutants F1760A and Y1767A in the human cardiac sodium channel α subunit and tested the effects of these mutations on block by NU-FL and QX-FL. As was the case for the brain channel, we find that these mutations have little effect on drug-free channel activity although the F1760A mutation causes a positive shift of channel availability (Fig. 10 A). We first determined if the homologous mutations in the human cardiac channel would disrupt the lidocaine-induced hyperpolarization of channel availability. Our findings, consistent with those reported in the literature for mutations of the rat cardiac Na+ channel, indicate that both mutations reduce this shift, but the effect is most pronounced for the F1760A mutation (Fig. 10, C and D).
Figure 10.
Effects of mutations of key residues of LA-receptor on Na+ channel gating and lidocaine block. (A) Averaged inactivation and activation curves for WT, F1760A, and Y1767A Na+ channels. The voltage-dependence for the inactivation was measured as described in Fig. 5. The voltage dependence of activation was measured by normalizing currents measured during pulses (25 ms) from −80 mV to 50 mV (5-mV increments) to driving force. Experimental data were fitted with Boltzmann relationships (Fig. 5) to obtain the parameters that follow. For inactivation: V1/2 (mV) = −69.2 ± 0.79 (WT); −60.6 ± 0.51 (F1760A); and −67.5 ± 0.63 (Y1767A). The slope factor, VK is 6.46 ± 0.36 (WT); 5.41 ± 0.18 (F1760A); 6.74 ± 0.33 (Y1767A). For activation: V1/2 (mV) is −25.3 ± 1.2 (WT); −23.3 ± 1.4 (F1760A); and −23.8 ± 1.3 (Y1767A). The slope factor, VK, is 7.1 ± 0.9 (WT); 7.2 ± 0.7 (F1760A); and 7.4 ± 0.6 (Y1767A). n = 4–6 cells per condition. (B–D) Mutations hhF1760A and hhY1767A reduce block of inactivated Na+ channel for lidocaine. Steady-state inactivation was measured as described in Fig. 5. Experimental data were fitted with Boltzmann relationships (Fig. 5) and the drug-induced shift in channel availability (ΔV1/2) was determined from V1/2,drug − V1/2,control using V1/2 values obtained from fits of the data. For WT, F1760A, and Y1767A, ΔV1/2 (mV) is −17.65 ± 1.17, −1.1 ± 0.30, and −9.67 ± 0.65 with 300 μM lidocaine, respectively (n = 4–6 cells per condition).
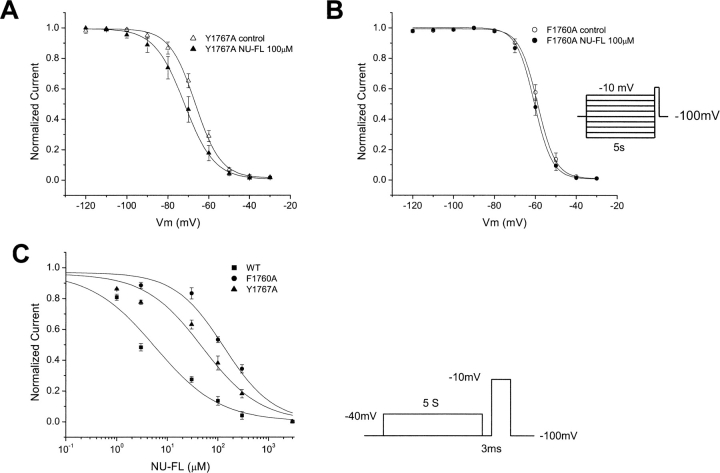
The F1760A and Y1767A mutations in human heart Na+ channels have a very similar effect on the interactions of NU-FL with channel availability. As is the case for lidocaine, the F1760A mutation virtually ablates the NU-FL–induced hyperpolarization of the availability curve, whereas the Y1767A mutation reduces, but does not eliminate, this shift (Fig. 11, A and B) . In light of the fact that the mutations disrupt both the shift of the inactivation curve induced by the neutral analogue we reasoned that the affinity of the neutral analogue for the inactivated state of the mutant channels should likewise be attenuated. Consequently, and similar to the previously reported effects on lidocaine (Ragsdale et al., 1996), both mutations alter the affinity of NU-FL for block of inactivated channels (Fig. 11 C).
Figure 11.
Effects of mutations of LA-receptor on NU-FL block. (A and B) Mutations F1760A and Y1767A reduce block of inactivated Na+ channel for NU-FL. Steady-state inactivation was measured as described in Fig. 5. Experimental data were fitted with Boltzmann relationships (Fig. 5). The drug-induced shift in channel availability (ΔV1/2) was determined from V1/2,drug − V1/2,control using V1/2 values obtained from fits of the data. For WT, F1760A, and Y1767A, ΔV1/2 (mV) is −9.17 ± 1.04, −1.57 ± 0.15, and −4.65 ± 0.57 with 100 μM NU-FL, respectively. n = 3–5 cells per condition. (C) Mutation F1760A and Y1767A reduce the affinity of inactivated channel for NU-FL. The block of inactivated Na+ channels was measured as described in Fig. 6. Experimental data were fitted with the Hill equation; these gave estimates of EC50 for NU-FL binding to the inactivated channels. For WT, F1760A, and Y1767A, EC50 for NU-FL are 5.32 ± 0.51 μM, 134.9 ± 9.96 μM, and 53.7 ± 5.55 μM, respectively (n = 3–5 cells per condition).
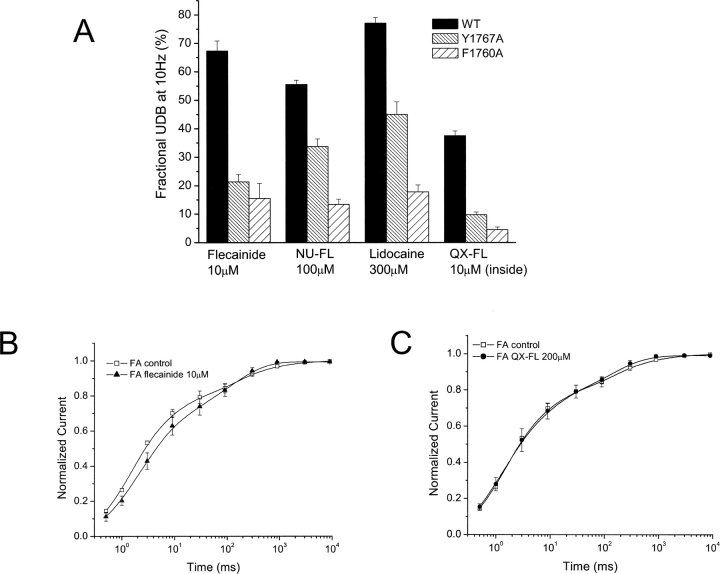
Our findings support the notion of a common binding site in the channel pore for both NU-FL and lidocaine. In addition, these data clearly suggest a relationship that the charge carried by flecainide most likely acts to restrict its access to this common site of action. This suggestion is consistent with the data summarized in Fig. 12 A that indicate that these two IVS6 mutations disrupt Na+ channel UDB by NU-FL in the same rank order as they disrupt UDB by flecainide and lidocaine despite the fact that, as shown above, NU-FL UDB is dominated by charged drug interactions with the channel. Furthermore, as shown in Fig. 12 A, we find that the F1760A mutation has pronounced effects on UDB by QX-FL. The F1760A mutation causes an eightfold reduction (37.3 ± 1.7% for WT vs. 4.0 ± 0.9% for F1760A, n = 3–5 cells) in UDB block by QX-FL (applied internally), an effect similar to that on UDB by externally applied flecainide. Further, both flecainide and QX-FL have no effects on the recovery rate of F1760A mutant channels (Fig. 12, B and C). These data support the notion of a common binding site in the pore for lidocaine, flecainide, and both neutral and quaternary flecainide analogues.
Figure 12.
UDB of WT and mutant Na+ channels by flecainide, NU-FL, QX-FL, and lidocaine. (A) Currents were evoked by imposing conditioning trains of 100–600 pulses (−10 mV, 25 ms) from a holding potential of −100 mV at a frequency of 10 Hz. Pulses were applied until steady-state UDB was achieved. Bar graphs summarize steady-state UDB by flecainide (10 μM), NU-FL (100 μM), QX-FL (100 μM, internal), and lidocaine (300 μM) plotted as the fraction of current blocked in response to pulse trains. (B) Recovery of F1760A channels from drug block, measured as described in Fig. 3 legend, for flecainide (10 μM, left) and QX-FL (100 μM, internal, right).
QX-FL Unblocks Na+ Channels without Channel Openings
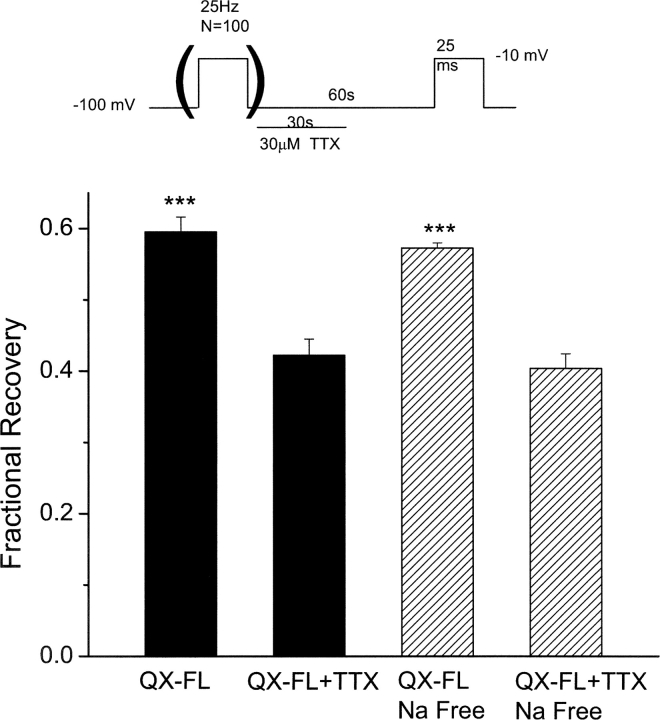
Interestingly, channels recover from QX-FL block (very slowly) at negative potentials where channel openings are rare or nonexistent (Fig. 3), despite the requirement of channel openings for the development of QX-FL block. Using single channel measurements, we tested for, but did not find evidence of, infrequent channel openings at the −100mV holding potential, and thus conclude that a pathway must exist via which the ionized drug may exit from closed channels. Tetrodotoxin (TTX) is thought to block the extracellular mouth of Na+ channel (Hille, 1975; Terlau et al., 1991) and has been used to examine whether permanently charged local anesthetics can exit through the extracellular end of the pore (Qu et al., 1995). To test whether QX-FL exits from closed channels through the extracellular mouth of the pore, the rates of recovery from QX-FL UDB were measured in the present of 30 μM TTX, which blocks ∼95% of resting cardiac Na channels in a rapidly reversible manner. If QX-FL exits through the outer mouth of the pore, TTX should reduce the rate of recovery of QX-FL block. Consistent with this prediction, as illustrated in Fig. 13 , exposure of 30 μM TTX for 30 s during a total 60-s recovery period significantly reduces the rate of recovery after UDB (P < 0.01, n = 4–6 cells). To determine whether or not this TTX-induced reduction in recovery from block might be due, in part, to inhibition of Na+ entry through open channels during the recovery period, we tested the effects of Na+-removal on recovery from block. As shown in Fig. 13, we found no difference in the effects of TTX on recovery whether or not Na+ was present in the external solution. Thus, the extracellular mouth of the pore participates in the exit route for QX-FL apparently whether or not channels are in the open conformation.
Figure 13.
Outer pore block by TTX impedes recovery from QX-FL block. WT channels were blocked by QX-FL (100 μM, internal application). UDB was induced by trains of 100 pulses (−10 mV, 25 ms, 25 Hz) from a −100 mV holding potential. Test pulses were then imposed after 60-s intervals at −100 mV to assay recovery from block. The protocol was performed in the absence and then in the presence of TTX (30 μM) during the first 30 s of the 60-s recovery period (see schematic). The bars graphs summarize the fraction of recovery from QX-FL block in the absence and presence of TTX in external solutions containing 130 mM NaCl. The hatched bars summarize similar experiments recorded in Na-free conditions (Na+ was replaced by n-methyl-glucamine). n = 4–6 cells per condition. ***, P < 0.05 compared with TTX-free conditions in both Na+ -containing and Na+-free solutions.
DISCUSSION
The results of this study show for the first time that the degree of ionization of flecainide molecules at physiological pH defines the mechanism of action of the drug and confers upon the drug molecules the prerequisite, during repetitive activity, that channels must first open before channel block can accumulate (UDB). Reduction of the pKa of the drug molecule from 9.3 to 6.4 yielded a flecainide analogue (NU-FL) for which 90% of the drug molecules are neutral at pH 7.4, compared with <1% neutral flecainide molecules at the same pH. With this change in drug structure, the molecular pharmacology of the custom synthesized drug was remarkably similar to the well-characterized Na+ channel blocker lidocaine.
The Charged Form of Flecainide Is the Active Form for UDB
UDB by LA drugs is the hallmark of their antiarrhythmic activity, it enables these drugs to be more effective when the frequency of action potentials is high, such as in ventricular tachycardia (Rosen et al., 1975; Rosen and Wit, 1983; Wit and Rosen, 1983). During UDB, channel block accumulates because of incomplete recovery of drug-bound channels during diastole (interstimulus intervals). Slowed recovery of drug-bound channels can be explained by a combination of the modulated-receptor hypothesis and the guarded-receptor model (Hille, 1977a; Hondeghem and Katzung, 1977; Starmer et al., 1984). The modulated receptor hypothesis proposes that higher affinity for LA drugs during activated and inactivated gating states slows unbinding of drug and, consequently, recovery of the drug-bound channels (Hille, 1977a; Hondeghem and Katzung, 1977). The guarded-receptor model emphasized that a state-dependent availability of the drug access path to and from the binding site influence apparent binding and unbinding kinetics (Starmer et al., 1984). Both of these hypotheses suggest that there is common binding site for tertiary amine LAs. In this study, our data clearly showed that UDB develops predominantly by the charged forms of both flecainide and NU-FL. What are the explanations for the weakness of UDB of Na+ channels by the neutral form of flecainide? First, the neutral form may occupy a binding site that is distinct from the binding site for charged form of flecainide. However, our data didn't support the existence of a separate neutral LA binding site apart from the charged form binding site. Second, the recovery time course of the neutral form of flecainide is fast (too fast to accumulate the UDB) and is comparable to recovery time course of fast Na inactivation (Hille, 1977a; Yeh and Tanguy, 1985). As a result, upon dissociation neutral flecainide simply diffuses away through a hydrophobic pathway. This possibility is likely as the recovery from NU-FL block in solutions buffered to pH 5.5, when the ratio of neutral to charged forms of NU-FL are reversed, is greatly slowed, consistent with the higher UDB level at the same condition. Thus, our results clearly indicate that the charged form of flecainide is the active form that underlies UDB, which is the reported mechanism of action of lidocaine (Strichartz, 1973; Wang et al., 1995).
Flecainide Access to an Intracellular Pathway: A Role of Neutral Drug
If flecainide is 99% ionized at physiological pH, how does external flecainide application result in internal access of the charged form of the drug to the inner mouth of the channel pore? The results presented in Fig. 9 provide a clue to this question. Because flecainide, applied outside the area under the patch electrode, blocked channels recorded in cell-attached patches, the drug had to diffuse across the cell membrane in order to reach the patched channels. This must occur via the neutral flecainide component, which crosses the membrane and then equilibrates in the intracellular solution again in neutral (1%) and charged (99%) forms. A similar sequence must also occur across the membrane of the patch, but in this case both the difference in membrane area (patch to whole cell) and the volume of the absorbing solution (pipette volume compared with volume of the cell), would limit the drug concentration of the pipette solution. That this is likely the case is evidenced by the rapid reversibility of drug block, which occurs when the extra-patch flecainide is removed in these experiments (Fig. 9).
An indirect role of the neutral flecainide component in the development of use-dependent block of externally applied drug is also supported by the experiments of Figs. 8 and 9, which show that QX-FL is not effective when applied externally outside of the patched area (Fig. 9), but when applied intracellularly, as effectively as extracellularly applied flecainide (Fig. 7). The simplest interpretation of these results is that neutral flecainide diffuses across the lipid bilayer of the cell membrane, equilibrates in the intracellular compartment where, as in the extracellular solution, 99% of the flecainide molecules in equilibrium are ionized. Use-dependent block that occurs, most likely develops as a consequence of the intracellular charged flecainide molecules.
Receptor Access Distinguishes Lidocaine and Flecainide Activity and Underlies Distinct Therapeutic Profiles
As has previously been the case for the quaternary lidocaine analogue QX-314, QX-FL provides unique insight into the access of a charged flecainide analogue to a common local anesthetic receptor-binding site on the Na+ channel. Studies using alanine-scanning mutagenesis have provided a detailed picture of components of a local anesthetic receptor site for which residues on the S6 segments of domains I, III, and IV contribute (Ragsdale et al., 1994, 1996; Wang et al., 1998; Catterall, 2002; Yarov-Yarovoy et al., 2002). Alanine-scanning mutagenesis indicates that residues in at least domains III and IV that contribute to the receptor site are likely to face the inner pore region of the channel (Catterall, 2002; Yarov-Yarovoy et al., 2002), and that rotational movement of S6 segments during activation and inactivation are likely to alter access to this site from the intracellular region of the channel (Perozo et al., 1999; del Camino et al., 2000).
We have shown previously that flecainide UDB requires channels to open but that block is very sensitive to alterations in channel inactivation (Liu et al., 2002). We now find that ionized flecainide, which accounts for the distinct UDB of the drug, gains access to its receptor via an intracellular pathway after channels open. Mutation of the key Phe residue in the local anesthetic receptor disrupts channel block by lidocaine, flecainide, neutral flecainide (NU-FL), and ionized flecainide (QX-FL). Together, these results are very consistent with a common receptor shared by lidocaine and flecainide, access to which is restricted by differences in ionization of the two compounds as predicted by the modulated receptor hypothesis. Our data strongly suggest that transitions into the open state change intracellular access of ionized flecainide to the receptor site defined by residues on the S6 segments lining the inner pore, and like lidocaine, block by flecainide is stabilized by further transitions into the inactivated state (Liu et al., 2002).
Interestingly, despite the apparent restricted access to inner pore receptor from closed, rested states, we find that channels blocked by charged flecainide (QX-FL) recover from channel block, albeit very slowly, at negative potentials even in the absence of channel openings (Fig. 3). Thus there must be a pathway available for drug exit at rest. The facts that outer pore block by TTX slows the recovery of channels from QX-Fl block, even at voltages in which channels do not open, and that removal of extracellular Na+ has no effect on the recovery from channel block provide strong evidence that the drug can dissociate from channels at rest and diffuse slowly through the outer pore in a pathway to permit very slow recovery from block. Thus, in many ways the fundamental mechanisms of action of QX-FL and the charged lidocaine analogue QX-314 are very similar, indicating that it is the differences in distribution of charged and neutral forms of flecainide and lidocaine and not differences in chemical structures that accounts for the distinct voltage-dependent profiles of the two drugs. As predicted by the modulated receptor hypothesis (Hille, 1977a; Hondeghem and Katzung, 1977), it thus appears that hydrophobic and hydrophilic access pathways to a common LA receptor in fact can explain the differences in activities of these two important drugs and must be addressed in considering novel analogues that may be useful in the treatment of cardiac electrical defects that are caused by inherited mutations of SCN5A, the gene coding for the α subunit of the cardiac Na+ channel.
That flecainde has proven to be particularly useful in the treatment of variant 3 of the long QT syndrome and as a diagnostic tool in identifying potential Brugada syndrome patients has raised the interest in understanding the mechanistic basis of mutation-altered interactions of the drug with cardiac Na+ channels. Since the neutral components of lidocaine and flecainide, as evidenced by the data presented in this study, appear to interact with a common receptor and cause nearly identical changes in channel activity, it is most likely mutation-induced alteration in the access of the charged form of flecainide to a common LA receptor site that accounts for the unique therapeutic efficacy of this drug. Analysis of the effects of flecainide on disease-associated mutations that independently alter channel mean open time and/or the voltage dependence of channel availability has provided evidence in support of this view (Liu et al., 2002).
Acknowledgments
We thank Drs. Colleen Clancy and Michihiro Tateyama for helpful discussion concerning this work.
This work was supported by National Institutes of Health grants 1R01 HL-56810-05 (R.S. Kass) and 1P01-HL-67849-01 (R.S. Kass).
David Gadsby served as editor.
Footnotes
Abbreviations used in this paper: QTS, long QT syndrome; UDB, use-dependent block.
References
- Abriel, H., C. Cabo, X.H. Wehrens, I. Rivolta, H.K. Motoike, M. Memmi, C. Napolitano, S.G. Priori, and R.S. Kass. 2001. Novel arrhythmogenic mechanism revealed by a long-qt syndrome mutation in the cardiac na(+) channel. Circ. Res. 88:740–745. [DOI] [PubMed] [Google Scholar]
- An, R.H., R. Bangalore, S.Z. Rosero, and R.S. Kass. 1996. Lidocaine block of LQT-3 mutant human Na+ channels. Circ. Res. 79:103–108. [DOI] [PubMed] [Google Scholar]
- Anno, T., and L.M. Hondeghem. 1990. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ. Res. 66:789–803. [DOI] [PubMed] [Google Scholar]
- Banitt, E.H., W.E. Coyne, J.R. Schmid, and A. Mendel. 1975. Antiarrhythmics. N-(aminoalkylene)trifluoroethoxybenzamides and N-(aminoalkylene)trifluoroethoxynaphthamides. J. Med. Chem. 18:1130–1134. [DOI] [PubMed] [Google Scholar]
- Benhorin, J., R. Taub, M. Goldmit, B. Kerem, R.S. Kass, I. Windman, and A. Medina. 2000. Effects of flecainide in patients with new SCN5A mutation: mutation- specific therapy for long-QT syndrome? Circulation. 101:1698–1706. [DOI] [PubMed] [Google Scholar]
- Brugada, J., R. Brugada, and P. Brugada. 1999. Brugada syndrome. Arch. Mal. Coeur Vaiss. 92:847–850 [PubMed] [Google Scholar]
- Catterall, W.A. 2002. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found. Symp. 241:206–218. [PubMed] [Google Scholar]
- Chernoff, D.M., and G.R. Strichartz. 1990. Kinetics of local anesthetic inhibition of neuronal sodium currents. pH and hydrophobicity dependence. Biophys. J. 58:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino, D., M. Holmgren, Y. Liu, and G. Yellen. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature (Lond.). 403:321–325. [DOI] [PubMed] [Google Scholar]
- Dumaine, R., and G.E. Kirsch. 1998. Mechanism of lidocaine block of late current in long Q-T mutant Na+ channels. Am. J. Physiol. 274:H477–H487. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1975. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys. J. 15:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 1977. a. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 69:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 1977. b. The pH-dependent rate of action of local anesthetics on the Node of Ranvier. J. Gen. Physiol. 69:475–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondeghem, L.M., and B.G. Katzung. 1977. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim. Biophys. Acta. 472:373–398. [DOI] [PubMed] [Google Scholar]
- Kambouris, N.G., H.B. Nuss, D.C. Johns, E. Marban, G.F. Tomaselli, and J.R. Balser. 2000. A revised view of cardiac sodium channel “blockade” in the long-QT syndrome. J. Clin. Invest. 105:1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H.L., A. Galue, L. Meadows, and D.S. Ragsdale. 1999. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Mol. Pharmacol. 55:134–141. [DOI] [PubMed] [Google Scholar]
- Liu, H., M. Tateyama, C.E. Clancy, H. Abriel, and R.S. Kass. 2002. Channel openings are necessary but not sufficient for use-dependent block of cardiac Na(+) channels by flecainide: Evidence from the analysis of disease-linked mutations. J. Gen. Physiol. 120:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., D.M. Cortes, and L.G. Cuello. 1999. Structural rearrangements underlying K+-channel activation gating. Science. 285:73–78. [DOI] [PubMed] [Google Scholar]
- Qu, Y., J. Rogers, T. Tanada, T. Scheuer, and W.A. Catterall. 1995. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel. Proc. Natl. Acad. Sci. USA. 92:11839–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale, D.S., J.C. McPhee, T. Scheuer, and W.A. Catterall. 1994. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 265:1724–1728. [DOI] [PubMed] [Google Scholar]
- Ragsdale, D.S., J.C. McPhee, T. Scheuer, and W.A. Catterall. 1996. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA. 93:9270–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, M.R., B.F. Hoffman, and A.L. Wit. 1975. Electrophysiology and pharmacology of cardiac arrhythmias. V. Cardiac antiarrhythmic effects of lidocaine. Am. Heart J. 89:526–536. [DOI] [PubMed] [Google Scholar]
- Rosen, M.R., and A.L. Wit. 1983. Electropharmacology of antiarrhythmic drugs. Am. Heart J. 106:829–839. [DOI] [PubMed] [Google Scholar]
- Schwarz, W., P.T. Palade, and B. Hille. 1977. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys. J. 20:343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer, C.F., A.O. Grant, and H.C. Strauss. 1984. Mechanisms of use-dependent block of sodium channels in excitable membranes by local anesthetics. Biophys. J. 46:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz, G.R. 1973. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J. Gen. Physiol. 62:37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz, G.R., V. Sanchez, G.R. Arthur, R. Chafetz, and D. Martin. 1990. Fundamental properties of local anesthetics. II. Measured octanol:buffer partition coefficients and pKa values of clinically used drugs. Anesth. Analg. 71:158–170. [DOI] [PubMed] [Google Scholar]
- Terlau, H., S.H. Heinemann, W. Stuhmer, M. Pusch, F. Conti, K. Imoto, and S. Numa. 1991. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 293:93–96. [DOI] [PubMed] [Google Scholar]
- Viswanathan, P.C., C.R. Bezzina, A.L. George, Jr., D.M. Roden, A.A. Wilde, and J.R. Balser. 2001. Gating-dependent mechanisms for flecainide action in SCN5A-linked arrhythmia syndromes. Circulation. 104:1200–1205. [DOI] [PubMed] [Google Scholar]
- Wang, G.K., C. Quan, and S. Wang. 1998. A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated mu1 Na+ channels. Pflugers Arch. 435:293–302. [DOI] [PubMed] [Google Scholar]
- Wang, Z., B. Fermini, and S. Nattel. 1995. Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J. Pharmacol. Exp. Ther. 272:184–196. [PubMed] [Google Scholar]
- Weiser, T., Y. Qu, W.A. Catterall, and T. Scheuer. 1999. Differential interaction of R-mexiletine with the local anesthetic receptor site on brain and heart sodium channel alpha-subunits. Mol. Pharmacol. 56:1238–1244. [DOI] [PubMed] [Google Scholar]
- Wit, A.L., and M.R. Rosen. 1983. Pathophysiologic mechanisms of cardiac arrhythmias. Am. Heart J. 106:798–811. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy, V., J.C. McPhee, D. Idsvoog, C. Pate, T. Scheuer, and W.A. Catterall. 2002. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel alpha subunit in voltage-dependent gating and drug block. J. Biol. Chem. 277:35393–35401. [DOI] [PubMed] [Google Scholar]
- Yeh, J.Z., and J. Tanguy. 1985. Na channel activation gate modulates slow recovery from use-dependent block by local anesthetics in squid giant axons. Biophys. J. 47:685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]