Abstract
We have investigated the interactions of a novel anionic ryanoid, 10-O-succinoylryanodol, with individual mammalian cardiac muscle ryanodine receptor channels under voltage clamp conditions. As is the case for all ryanoids so far examined, the interaction of 10-O-succinoylryanodol with an individual RyR channel produces profound alterations in both channel gating and rates of ion translocation. In the continued presence of the ryanoid the channel fluctuates between periods of normal and modified gating, indicating a reversible interaction of the ligand with its receptor. Unlike the majority of ryanoids, we observe a range of different fractional conductance states of RyR in the presence of 10-O-succinoylryanodol. We demonstrate that 10-O-succinoylryanodol is a very flexible molecule and propose that each fractional conductance state arises from the interaction of a different conformer of the ryanoid molecule with the RyR channel. The probability of channel modification by 10-O-succinoylryanodol is dependent on the transmembrane holding potential. Comparison of the voltage dependence of channel modification by this novel anionic ryanoid with previous data obtained with cationic and neutral ryanoids reveals that the major influence of transmembrane potential on the probability of RyR channel modification by ryanoids results from an alteration in receptor affinity. These investigations also demonstrate that the charge of the ryanoid has a major influence on the rate of association of the ligand with its receptor indicating that ionic interactions are likely to be involved in this reaction.
Keywords: calcium-release channel, ryanodine receptor, ryanodine
INTRODUCTION
RyRs are intracellular membrane Ca2+ release channels that possess a high-affinity binding site for the plant alkaloid ryanodine (Sutko et al., 1997). Micromolar concentrations of ryanodine, applied to single RyR channels incorporated into planar phospholipid bilayers, modify both gating and ion-handling properties, so that after the interaction of the ligand the channel enters a reduced conductance state, which has a high open probability (P o). The interaction of ryanodine with RyR is believed to bring about or stabilize a conformational change in the channel protein (Welch et al., 1997; Tanna et al., 2002). Ryanodine interaction alters the relative permeability and/or the affinity of the conduction pathway of the channel for ions (Lindsay et al., 1994). Together these changes result in altered rates of ion translocation. Covalent modification of the structure of ryanodine yields ligands (ryanoids) that compete with ryanodine for the high-affinity binding site on RyR. All ryanoids examined to date modify RyR channel gating and ion translocation; however, the rate of permeant ion translocation in the RyR–ryanoid complex is dependent on the structure of the bound ryanoid (Tinker et al., 1996). Ryanoid structure also has a profound influence on the rates of ryanoid association with and dissociation from the high affinity binding site on RyR (Tinker et al., 1996; Tanna et al., 1998).
Investigations of the interaction of structurally diverse ryanoids with individual RyR channels under voltage clamp conditions have revealed additional information concerning the mechanisms governing ryanoid binding and the resulting alteration in rates of ion translocation. A novel observation arising from these studies is that the probability of occurrence of the RyR–ryanoid complex is influenced by transmembrane holding potential (Tanna et al., 1998, 2000). We are using structurally diverse ryanoids in an attempt to understand the mechanisms underlying this voltage dependence.
In previous studies we have monitored the interactions of cationic and neutral ryanoids with individual sheep cardiac RyR channels (Tanna et al., 1998, 2000). In this report we extend these investigations by studying the influence of transmembrane holding potential on the probability of RyR channel modification by an anionic ryanoid, 10-O-succinoylryanodol. The inclusion of an anionic ryanoid in these investigations sheds new light on the mechanisms underlying the influence of transmembrane holding potential on the occurrence of the RyR–ryanoid complex and supports the proposal that the RyR channel is a sensor of transmembrane potential.
Further, the occurrence of multiple ryanoid-induced modified conductance states in the presence of 10-O-succinoylryanodol demonstrates that rates of ion translocation in the RyR–ryanoid complex can be determined by the conformation of this flexible ligand and do not require covalent modification of the structure of the ryanoid.
The novel information provided by these studies complements the findings of our previous investigations into the interaction of ryanoids with individual voltage-clamped RyR channels and contributes to a better overall understanding of the mechanisms governing the interaction of ryanoids with RyR and the mechanisms underlying alterations of ion handling in the RyR–ryanoid complex.
MATERIALS AND METHODS
Phosphatidylethanolamine was purchased from Avanti Polar Lipids, Inc. and phosphatidylcholine was from Sigma-Aldrich. [3H]-ryanodine was purchased from New England Nuclear Ltd. Standard chemicals were obtained as best available grade from BDH Ltd., Sigma-Aldrich, or Packard.
Synthesis of 10-O-Succinoylryanodol
10-O-succinoylryanodol was synthesized in two steps from ryanodol. Ryanodol was obtained from ryanodine using the method described by Wiesner (Wiesner, 1972; Sutko et al., 1997). Ryanodol was then acylated with benzyl hemisuccinate (5 equivalents) in the presence of dicyclohexylcarbodiimide (5 equivalents), a catalytic amount of triethylamine, and dimethylaminopyridine (0.03 equivalents) in a mixture of tetrahydrofuran and methylene chloride (yield 38%). Hydrogenolysis of 10-O-benzylryanodylsuccinate in tetrahydrofuran in the presence of hydrogen and 10% palladium on charcoal catalyst furnished 10-O-succinoylryanodol in 96% yield. The new compounds were characterized by proton NMR, mass spectrometry and elemental analysis.
10-O-Benzylryanodylsuccinate: 1H NMR (300 MHz, CD3OD) δ ppm: 7.33 (m, 5H, −C6H5), 5.26(d, 1 H, J = 10.6 Hz, HC10), 5.12 (s, 2 H, −CH2C6H5), 4.07 (s, 1 H, HC3), 2.67 (m, 4 H, succinate hydrogens), 2.53 (d, 1 H, J = 13.4 Hz, HAC14), 2.20–1.93 (m, 3 H, HC7, HC13, and HC9), 1.74(d, 1 H, J = 13.4 Hz, HBC14), 1.54 (m, 2 H, HC7 and HC8), 1.34 (s, 3 H, CH3C1), 1.31 (m, 1 H, HC8), 1.12 (s, 3 H, CH3C5), 1.07, 0.98, and 0.80 (3 d of 3 H each, J∼6.5 Hz, H3C18, H3C19 and H3C20); HRMS calculation for C31H38O10 (M+ − H2O): 572.2621; found: 572.2614.
10-O-Succinoylryanodol: 1H NMR (300 MHz, CD3OD) δ ppm: 5.26 (d, 1 H, J = 10.6 Hz, HC10), 4.07 (s, 1 H, HC3), 2.61 (m, 4 H, succinoyl hydrogens), 2.52 (d, 1 H, J = 13.4 Hz, HAC14), 2.15–1.98 (m, 3 H, HC7, HC13, and HC9), 1.74 (d, 1 H, J = 13.4 Hz, HBC14), 1.58 (m, 2 H, HC7 and HC8), 1.34 (s, 3 H, CH3C1), 1.31 (m, 1 H, HC8), 1.11 (s, 3 H, CH3C5), 1.07, 0.97, and 0.84 (3 d of 3 H each, J∼6.5 Hz, H3C18, H3C19, and H3C20); HRMS calculation for C24H34O10 (M+ − H2O): 482.2152; found: 482.2149.
10-O-succinoylryanodol was stored as a stock solution in 50% aqueous ethanol at −20°C.
Isolation of RyR and Single Channel Data Acquisition and Analysis
Heavy sarcoplasmic reticulum membrane vesicles were prepared from sheep cardiac muscle (Sitsapesan and Williams, 1990) and RyR channels isolated and reconstituted into unilamellar liposomes (Lindsay and Williams, 1991) as described previously.
Planar phospholipid bilayers were formed from a suspension of phosphatidylethanolamine in n-decane and individual RyR channels incorporated into the bilayer following methods described previously (Lindsay and Williams, 1991). Single channel current fluctuations were monitored with K+ as the charge carrying species (Tanna et al., 1998). Data were stored on digital audio tape. For analysis, data were replayed, low-pass filtered with an 8-pole Bessel filter, and digitized using Satori (Intracel, Cambridge, UK). Single channel current amplitudes were measured from digitized data by monitoring relative current amplitudes between cursors placed at the center of the noise associated with open, closed and ryanoid-modified conductance states. The amplitudes of ryanoid-induced conductance states are quoted as fractional conductance (FC); that is the chord conductance of the modified conductance state expressed as a proportion of the full conductance.
The interaction of 10-O-succinoylryanodol with an individual RyR channel under voltage clamp conditions results in the occurrence of reduced conductance states with high open probability. In the continued presence of this ryanoid an individual channel alternates between periods of normal gating (fluctuations between open and closed conductance levels) and modified gating (fluctuations between the ryanoid-modified conductance level and the closed conductance level). Durations of individual dwell times in the normal and ryanoid-modified states were determined by direct measurement of digitised data. To obtain sufficient events for analysis, these parameters were obtained from steady-state recordings lasting at least 6 min.
As is the case for other ryanoids for which we have monitored the kinetics of interaction with single RyR channels (Tanna et al., 1998, 2000); apparent rate constants for the association (k on) and dissociation (k off) of 10-O-succinoylryanodol were determined from the mean dwell times in the unmodified and ryanoid-modified conductance states (Eqs. 1 and 2) (Tanna et al., 1998):
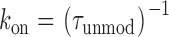 |
(1) |
and
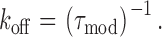 |
(2) |
If the rates of association and dissociation of the ryanoid with its receptor are influenced by transmembrane holding potential, the rate constants at a given voltage will be described by the following relationships:
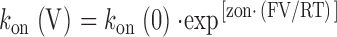 |
(3) |
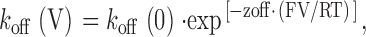 |
(4) |
where k(V) and k(0) are the rate constants at a particular voltage and at 0 mV, respectively, and z is the valence of the appropriate reaction. Plots of the natural logarithm of k on and k off against holding potential should be linear with slopes zonF/RT and −zoffF/RT and intercepts ln[k on(0)] and ln[k off(0)] respectively. The total voltage dependence (ztotal) of the reaction is then zon + zoff.
The high affinity ryanoid binding site on RyR is only accessible when the channel is in an open conformation (Serysheva and Hamilton, 1998); as a consequence rates of association of ryanoids with RyR are dependent on channel open probability (Tanna et al., 1998). In the studies reported in this communication variations in Po were minimized by the inclusion of up to 200 μM EMD 41000 (McGarry and Williams, 1994) in the solution at the cytosolic face of the channel and quoted values of kon are normalized to a Po of 1.0 (Tanna et al., 2000). Channel open probability in the presence of the ryanoid was determined by monitoring this parameter in the sections of the recorded data during which the channel was gating normally (ryanoid present but not bound). P o in these periods was determined by 50% threshold analysis (Sitsapesan and Williams, 1994).
Molecular Modeling of the Conformational Freedom of 10-O-succinoylryanodol
To estimate the conformational space of 10-O-succinoylryanodol we employed molecular dynamics simulations using SYBYL (Tripos, Inc.). The solvent was simulated using two separate methods. In the simplest case an aqueous solution was considered to be a continuum using a distance-dependent dielectric to approximate the shielding by the water dipoles. In the second case, water was explicitly included in the calculations. A box of water molecules was built surrounding the ryanoid and molecular dynamics was performed on the ensemble using periodic boundary conditions, holding temperature (either 300 or 310°K), and volume constant. Simulation times varied between 10 and 1,000 ps. The conformers are the result of exhaustive minimization (up to 100,000 iterations) of all conformational snapshots obtained in the simulations. These conformers are sorted according to the root mean square (rms) difference between conformers. A requirement for a large rms difference reduces the number of conformers whereas a low rms threshold will include a larger number of conformers. In all cases the conformers used are local minima in the energy surface of 10-O-succinoylryanodol. The large number of conformational minima is consistent with a simple calculation considering only the succinoyl pendent group. If the torsional angles of the five rotatable bonds (connecting the terpene and the carboxyl group) have three rotational minima, 243 possible distinct minima can be obtained. The succinoyl group is floppy and family analysis of either single rotatable bonds or all five failed to produce distinct groupings of side chain trajectories. Therefore, conformers of the succinoylryanodol were grouped by the similarity of the three torsional angles most proximal to the fused (terpene) ring system of ryanodol. For example, it is possible to plot the three torsional angles on an X, Y, and Z-axis of a three-dimensional graph and look for clusters of points indicating conformers with similar values of all three torsional angles. The dividing lines between groups were vague and the determination of boundaries between groups was somewhat arbitrary. To provide a consistent method of grouping the conformers a hierarchical cluster analysis (as implemented in SYBYL) of all five bonds was used to group the conformations of the succinoyl side chain. The level of the analysis was arbitrarily set to divide the ensemble of conformers into 30 groups. The conformers covered a wide range of orientations that ranged from straight chains to curled arrangements reminiscent of six-membered rings. The conformers were more-or-less randomly divided among the groups indicating little preference for any conformation (see results). The viscous drag of the explicit solvent slowed the motion of the succinoyl but did not change the diversity of conformers. The explicit presence of water caused a small redistribution of conformers from that observed in implicit solvent. The number of extended conformers increased relative to the number of curled conformers (unpublished data). In all of the cases examined, no conformer group accounted for >22% of the total number of observed conformers.
[3H]-ryanodine Binding to Populations of Sarcoplasmic Reticulum Membrane Vesicles
The dissociation constants quoted in Table I were determined as described previously (Welch et al., 1994, 1996).
TABLE I.
Parameters Derived from the Variations in Rates of Ryanoid Association with, and Dissociation from, Individual RyR2 Channels and Binding to Populations of Channels in Intact SR Vesicles
| 10-O-succinoylryanodol (formal charge −1) |
Ryanodol (formal charge 0) |
21-amino-9a-hydroxyryanodine (formal charge +1) |
|
|---|---|---|---|
| Slope (zonF/RT) | 0.021 ± 0.003 (r = 0.75) | 0.041 ± 0.003 (r = 1) | 0.051 ± 0.002 (r = 1) |
| zon | 0.54 | 1.03 | 1.29 |
| Slope (−zoffF/RT) | −0.028 ± 0.005 (r = 0.75) | −0.019 ± 0.002 (r = 1) | −0.034 ± 0.002 (r = 1) |
| zoff | 0.70 | 0.48 | 0.87 |
| ztotal | 1.24 | 1.51 | 2.16 |
| kon at 0 mV (μM−1s−1) | 0.002 | 0.035 | 0.365 |
| koff at 0 mV (s−1) | 0.082 | 0.095 | 0.990 |
| Kd at 0 mV (μM) | 35.620 | 2.810 | 2.790 |
| Kd (binding, μM) | 19.5 ± 4.0a | 7.0 ± 2.0,a 5.8 ± 0.9b | 3.7 ± 0.6b |
Sheep cardiac SR.
Rabbit skeletal SR.
Comparative Molecular Field Analysis (CoMFA)
The conformational minima of ryanoid molecules were determined as described in previous publications (Welch et al., 1994, 1997).
RESULTS
Multiple Fractional Conductance States Induced by 10-O-Succinoylryanodol
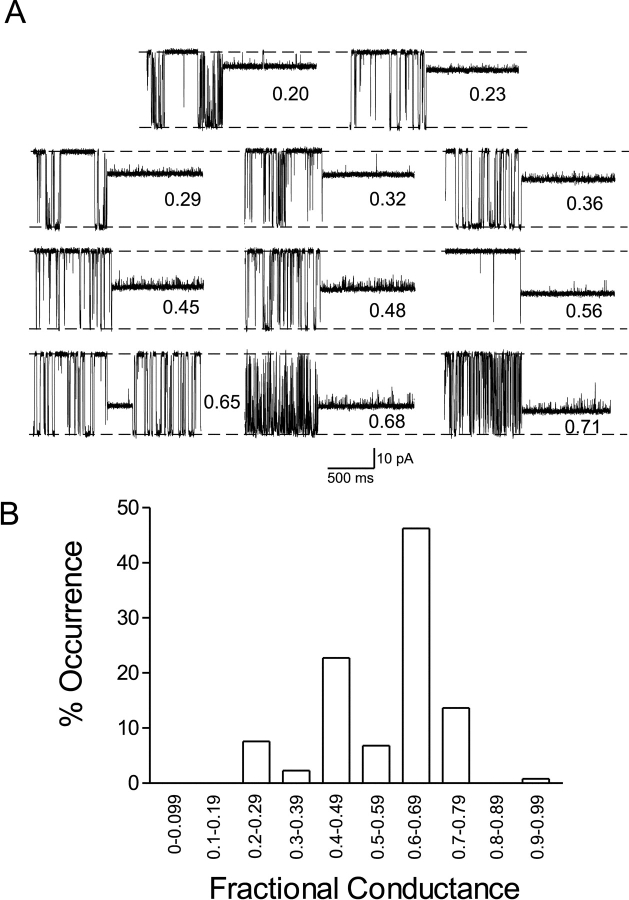
The addition of micromolar concentrations of 10-O-succinoylryanodol to the solution bathing the cytosolic face of a RyR channel results in the modification of channel function. The interaction of 10-O-succinoylryanodol with RyR produces a dramatic increase in channel open probability and a reduction in rates of ion translocation; on dissociation of the ryanoid from the channel RyR function returns to normal. Fig. 1 shows current fluctuations recorded from individual sheep cardiac RyR channels at a holding potential of <60 mV with 10 μM 10-O-succinoylryanodol in the solution at the cytosolic face of the channel. A striking and uncommon feature of the modification of RyR channel function after the interaction of 10-O-succinoylryanodol is the occurrence of modified conductance states of differing fractional conductance. 11 representative modification events are shown in Fig. 1 A. In each example, the interaction of the ryanoid with the RyR channel results in a marked elevation of channel open probability; however, the rate of ion translocation in the ryanoid-modified channel varies significantly with values of fractional conductance ranging from 0.20 to 0.71. Using the method of measurement described in materials and methods we have observed a total of 33 different modified conductance states induced by the interaction of 10-O-succinoylryanodol with individual RyR channels with values of fractional conductance ranging from 0.20 to 0.93. Given the relative noise of the modified conductance states induced by 10-O-succinoylryanodol, and the resolution attainable in our experiments, it is clearly not possible to define each of these states unequivocally as a separate ryanoid-modified conductance state of RyR. For this reason we have grouped the individual modified conductance states in bins each representing 10% of unitary conductance of the RyR channel and have displayed the probability of occurrence of the various states in Fig. 1 B. It would appear that unlike the vast majority of ryanoids, such as ryanodine, that invariably induce a single modified conductance state in RyR, the interaction of 10-O-succinoylryanodol with the open RyR channel can result in the occurrence of a very wide range of modified conductance states.
Figure 1.
(A) Representative current fluctuations of an RyR2 channel monitored in symmetrical 610 mM K+ at a holding potential of 60 mV in the presence of 10 μM 10-O-succinoylryanodol in the solution at the cytosolic face of the channel. Each panel shows the modification of gating and conductance resulting from the association of 10-O-succinoylryanodol with the channel. In all cases channel opening is depicted as a downward deflection in the trace. Association of 10-O-succinoylryanodol with RyR2 results in the occurrence of a range of modified conductance states. The fractional conductance of each event (see materials and methods) is indicated. (B) Histogram showing the probability of occurrence of fractional conductance states observed in the presence of 10-O-succinoylryanodol. Measured states were allocated to bins each representing 10% of channel full conductance.
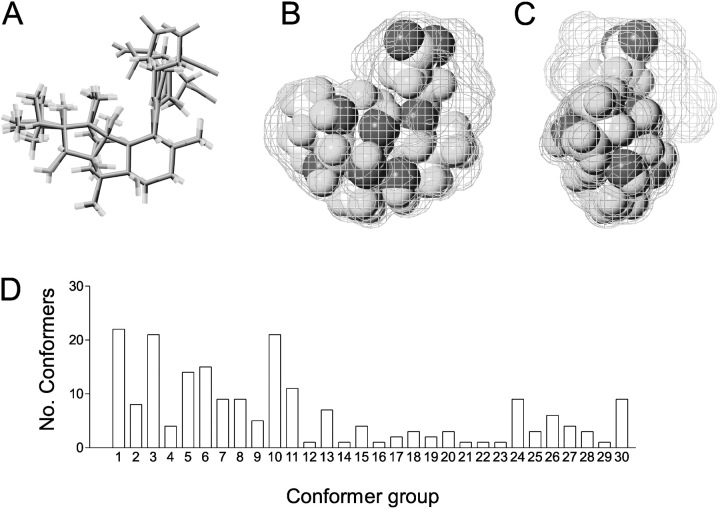
Information on a potential mechanism underlying this unusual property of 10-O-succinoylryanodol can be obtained from an investigation of the flexibility of the ryanoid molecule. Unlike ryanodine, which has a fixed and rigid structure, 10-O-succinoylryanodol is a very flexible molecule that can exist in many conformers. This flexibility arises from rotation around the innermost bonds of the pendant succinoyl group and the resulting variations in structure are depicted in Fig. 2 . Fig. 2 A shows superimposed structures representing six local conformational minima of 10-O-succinoylryanodol in molecular dynamics simulations of the motion of the ryanoid. 200 conformers were filtered to remove conformational minima that did not differ from each other by at least 1 Å root mean square. Fig. 2, B and C, are orthogonal views of the ryanoid in which the wire frames are swept volumes of 200 conformers found in an extended molecular dynamics run. The space-fill structure in these figures is the average conformation of the most highly populated group of conformers identified using hierarchical cluster analysis of the 200 conformers. Considerable variation in structure arises from movement of the succinoyl group relative to the rest of the molecule and this results in the existence of multiple conformers of 10-O-succinoylryanodol. In the light of this information it is tempting to propose that the various modified conductance states of RyR observed after the interaction of 10-O-succinoylryanodol with the channel result from the interaction of different conformers of the ryanoid with the open channel.
Figure 2.
(A) Superimposed figures representing six local conformational minima of 10-O-succinoylryanodol found in molecular dynamics simulations of the motions of the ryanoid. 200 conformers were filtered to remove conformational minima which did not differ by at least 1 Å rms from each other. B and C are orthogonal views of the ryanoid. The wire frames are the swept volumes of 200 conformers found in an extended molecular dynamics run. The space-fill structure is the average conformation of the most highly populated group of conformers identified using hierarchical analysis of the 200 conformers. D is a histogram displaying the distribution of 10-O-succinoylryanodol conformers found in molecular dynamics simulations of the motions of the ryanoid. Using hierarchical analysis, the 200 conformers were collected into 30 groups according to the conformational similarity of the succinoyl pendant group.
The data in Fig. 2 D shows the result of an investigation into the distribution of the 10-O-succinoylryanodol conformers found in molecular dynamics simulations of the motion of the ryanoid. Using hierarchical analysis, the 200 conformers were collected into 30 groups according to conformational similarity of the pendant succinoyl group. This analysis demonstrates a broad spread in the distribution of the 200 conformers with only 3 of the 30 groups containing >20% of the available conformers.
A comparison of the distribution of 10-O-succinoylryanodol conformers (Fig. 2 D) with the probability of occurrence of the various fractional conductance states of RyR induced by this ryanoid (Fig. 1 B) lends support to the proposal that different fractional conductance states arise as the result of the interaction of different conformers of the ryanoid with the channel.
The Influence of Transmembrane Holding Potential on the Probability of RyR Channel Modification by 10-O-succinoylryanodol
Previous investigations have shown that the probability of RyR channel modification of function by nonionic and cationic (+1) ryanoids is influenced by transmembrane holding potential. Although quantitative differences between the ryanoids exist, in both cases rates of ryanoid association increase and rates of ryanoid dissociation decrease as holding potential is shifted from negative to positive values. We have extended the investigation of the influence of ryanoid charge on the kinetics of ryanoid interaction with RyR by monitoring rates of association and dissociation of 10-O-succinoylryanodol; an anionic ryanoid, with individual sheep cardiac muscle RyR channels under voltage clamp conditions.
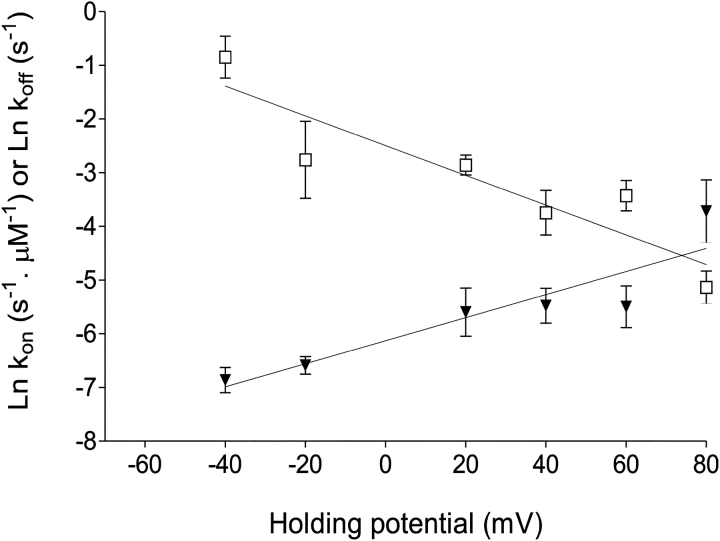
The influence of holding potential on the interaction of 10-O-succinoylryanodol with a representative RyR channel is shown in Fig. 3 . In the presence of a fixed concentration of 10-O-succinoylryanodol the probability of the channel being in a ryanoid-modified state increases progressively as holding potential is changed from −40 to 80 mV. As highlighted earlier, several different 10-O-succinoylryanodol–induced fractional conductance states occurred in the course of the experiment. Variations in mean rates of 10-O-succinoylryanodol association with RyR and dissociation from RyR with changing holding potential are shown in Fig. 4 . For the purposes of this analysis, we have ignored the variation in amplitude of the 10-O-succinoylryanodol–induced fractional conductance states and measured the rates of association and dissociation as if all modified states were identical. This analysis yields average rate constants and allows us to compare the kinetics and analysis with conventional ligand binding isotherms measured using ensembles of receptors. Since all states are not equally populated, the rates of association and dissociation will be different for each of the modified states. However, as at the present time it is not possible to assign each modified conductance state to a particular conformer of the ryanoid, such a detailed analysis is not warranted in this communication.
Figure 3.
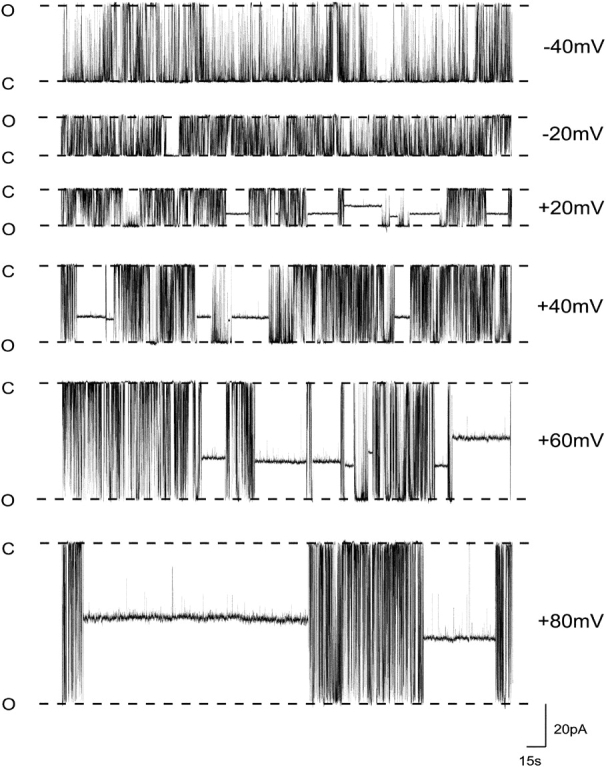
The influence of transmembrane holding potential on the probability of modification of RyR2 channel function by 10-O-succinoylryanodol. Recordings of current fluctuations of an individual representative RyR2 channel in symmetrical 610 mM K+ with 10 μM 10-O-succinoylryanodol in the solution at the cytosolic face of the channel at holding potentials ranging from −40 to 80 mV. The probability of occurrence of a ryanoid-induced modified conductance state increases as holding potential is made more positive. O, open; C, closed.
Figure 4.
The influence of transmembrane holding potential on the rate of association of 10-O-succinoylryanodol with (▾) and dissociation from (□) individual RyR2 channels. Rates were determined from dwell times in the unmodified and modified gating states of channels in the presence of 10 μM 10-O-succinoylryanodol in symmetrical 610 mM K+. Each point is the mean ± SEM of 4–7 experiments. The solid lines were obtained by linear regression. Parameters calculated from these plots are quoted in Table I.
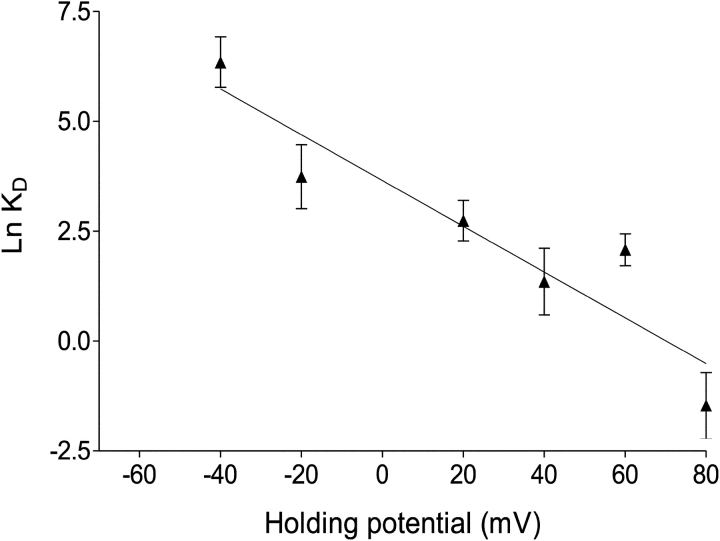
The data in Fig. 4 demonstrate that variations in the probability of channel modification by 10-O-succinoylryanodol with changing holding potential arise from voltage-dependent changes in both kon and koff. As is the case with a nonionic ryanoid (ryanodol) (Tanna et al., 2000) and a cationic (+1) ryanoid (21-amino-9α-hydroxyryanodine) (Tanna et al., 1998), rates of association of 10-O-succinoylryanodol (charge = −1) increase and rates of dissociation decrease as holding potential shifts from negative to positive values. The relationship between the dissociation constant (KD) of 10-O-succinoylryanodol and transmembrane holding potential is shown in Fig. 5 . The KD at 0 mV derived from this plot is 35.62 μM and is comparable with the value obtained for binding isotherms with populations of sheep cardiac sarcoplasmic reticulum membrane vesicles (19.5 ± 4 μM, Table I).
Figure 5.
The variation in the kinetic dissociation constant (Kd) of 10-O-succinoylryanodol with holding potential. Each point was calculated as Kd = koff (s−1)/kon (μM−1 s−1) using the data shown in Figure 4. The solid line was obtained by linear regression. Parameters calculated from the plot are quoted in Table I.
A comparison of the parameters for the interaction of 10-O-succinoylryanodol with individual RyR channels under voltage clamp conditions described in this communication with equivalent values determined for ryanoids with a formal charge of 0 or 1 indicates that, irrespective of charge, the influence of holding potential on the probability of ryanoid interaction is qualitatively similar. The probability of interaction increases as holding potential is made more positive and in all cases this reflects qualitatively similar alterations in rates of association and dissociation of the ryanoid. However the charge of the ryanoid does have a very marked influence on the absolute rates of association and dissociation.
Table I shows values of kon, koff, and KD at 0 mV for 10-O-succinoylryanodol (charge = −1), ryanodol (charge = 0) and 21-amino-9α-hydroxyryanodine (charge = +1). It is clear that, at least for these three ryanoids, the association rate constant of the ryanoid with RyR is strongly dependent on the charge of the ligand. In comparison with the neutral ryanoid, ryanodol, the association rate constant of the negatively charged ryanoid, 10-O-succinoylryanodol is decreased by an order of magnitude, while that of the positively charged ryanoid is increased by an order of magnitude.
As reported above, ligand charge causes an order of magnitude change in the association rate constant, whereas the values of zF/RT vary only slightly with ligand charge (Table I). Whereas ionic interactions between ligand and receptor are important in the transition state for ryanoid binding and release, ligand charge has little role in the voltage-induced modification of the receptor.
Dissociation rate constants at 0 mV of ryanodol and 10-O-succinoylryanodol are comparable, whereas that of 21-amino-9α-hydroxyryanodine is an order of magnitude greater. Variations in association and dissociation rate constants underlie the markedly different dissociation constants of these ryanoids at 0 mV (Table I). The excellent agreement between the thermodynamic dissociation rate constants measured for these ryanoids in SR vesicles (Table I) and the dissociation constants inferred from the rate constants is important confirmation that the kinetic phenomena reported here are relevant to the RyR in the intact SR membrane.
DISCUSSION
The Consequences of the Interaction of 10-O-succinoylryanodol on the Function of the RyR2 Channel
In earlier investigations into the functional consequence of ryanoid interaction with individual RyR2 channels under voltage clamp conditions we have demonstrated that the rate of monovalent cation translocation in the ryanoid-modified channel is dependent on structural features of the bound ryanoid molecule (Tinker et al., 1996) and have identified specific loci on the ryanoid molecule that are involved (Welch et al., 1997). Although both electrostatic and steric features of the ryanoid influence the fractional conductance induced after the interaction of the ligand with the RyR channel, electrostatic effects are more important. The 10-position of the ryanoid has the greatest influence on rates of ion translocation in the ryanoid-modified channel. Increased positive potential, and increased steric bulk, at this position correlate with low fractional conductance. Despite being the major locus for altered ion translocation, the 10-position has relatively little influence on the probability of interaction of ryanoids with the high-affinity binding site on the RyR channel.
Altered rates of ion translocation in RyR after the interaction of ryanodine result from changes in both the relative permeability of ions within the channel and the affinity of the channel for permeant cations (Lindsay et al., 1994). Investigations of ion handling and block in ryanoid-modified channels (Lindsay et al., 1994; Tanna et al., 2001) and correlations between structural features of a range of ryanoids and RyR fractional conductance (Welch et al., 1997) indicate that changes in the rates of cation translocation are likely to be brought about through an allosteric mechanism in which the interaction of a particular ryanoid with the high affinity binding site on the channel induces or stabilizes conformational alterations in the conduction pathway of the channel that result in a characteristic alteration in the rate of ion translocation and hence fractional conductance.
On first inspection, the observation of multiple fractional conductance states of RyR in the presence of 10-O-succinoylryanodol would appear to be at odds with the mechanism set out above; this ryanoid does not induce a single characteristic fractional conductance state. However, the mechanisms underlying these observations are revealed by molecular dynamics simulations of the motion of this ryanoid molecule. As a consequence of movement of the pendant succinoyl group 10-O-succinoylryanodol is an extremely flexible molecule that can exist in a very large number of roughly isoenergetic conformers. Our observation of many fractional conductance states in the presence of this ryanoid is consistent with the proposal that each fractional conductance state results from the interaction of an individual conformer of 10-O-succinoylryanodol with an open RyR channel. These findings support and extend conclusions drawn from experiments in which we observed three fractional conductance states induced in RyR by 21-p-nitrobenzoylamino-9α-hydroxyryanodine (Tanna et al., 2001). Molecular dynamics simulations of the motions of this ryanoid demonstrated the occurrence of three major conformers resulting from different locations of the bulky p-nitrobenzoyl tail. Unlike the conformers of 10-O-succinoylryanodol described in this communication the three major conformers of 21-p-nitrobenzoylamino-9α-hydroxyryanodine are not isoenergetic and exist in a ratio of 73:24:1%. Experiments in which the interaction of this ryanoid with individual RyR2 channels were monitored revealed a striking correlation between the occurrence of the various fractional conductance states and the probability of occurrence of the three major conformers, with 73% at 0.27, 20% at 0.17, and 7% at 0.59.
As outlined above, we have monitored the amplitude of fractional conductance states induced by the interaction of many ryanoids, of differing structure, with individual RyR channels (Tinker et al., 1996; unpublished data). Using a subset of these ryanoids we have constructed a comparative molecular field analysis (CoMFA)* of fractional conductance and ryanoid structure and have used this to predict the fractional conductance of identified conformers of 10-O-succinoylryanodol. The ryanoid molecules used in the construction of the CoMFA (3-O-(pyridyl-3-carbonyl)ryanodol, 9,12-didehydroryanodine, 9β-21-epoxyryanodine, 8β-hydroxy-10-O-methyl-10-epiryanodine, 10-O-guanidinopropionylryanodine, ryanodine, ryanodol, 10-β-alanylryanodine, 3-O-(tetrahydropyridyl-3-carbonyl)ryanodol, 8β-amino-9α-hydroxyryanodine) were chosen for the lack of ambiguity in both conformation and induced fractional conductance. Using this CoMFA the fractional conductance of 36 widely differing conformers of 10-O-succinoylryanodol were predicted. The predicted fractional conductances varied from 0.50 to 0.90 of the unmodified channel. These values are within the range of experimentally determined values of fractional conductance induced by this ryanoid (0.20–0.93); however, it is not immediately clear why the full range of observed values is not predicted. Possible explanations could include (a) that the ryanoids used in the CoMFA basis set are insufficient to detect conformers that induce small fractional conductance states, (b) that the use of other physicochemical fields may be better suited to estimate the fractional conductance states induced by different conformers of 10-O-succinoylryanodol, and (c) 10-O-succinoylryanodol may bind to RyR in more than one orientation (Welch et al., 1996). It is clear, however, that different unique conformers of 10-O-succinoylryanodol can induce different, unique, fractional conductance states.
The wide range of fractional conductance states seen in the presence of 10-O-succinoylryanodol (0.20–0.93) indicates that the movement of the pendant succinoyl group will yield conformers with very different electrostatic and steric features at and around the 10-position and emphasizes the importance of this position in the determination of ryanoid-induced alterations in ion translocation in RyR. The observation of multiple conductance states in the presence of both 21-p-nitrobenzoylamino-9α-hydroxyryanodine and 10-O-succinoylryanodol demonstrates that the RyR binding site does not act as a template and select a single conformation of the ligand. The RyR site will accept multiple conformers and alter rates of ion translocation in response to the formation of isomeric ligand–receptor complexes.
Although molecular dynamics simulations of 10-O-succinoylryanodol demonstrate enormous flexibility of this molecule in solution, observations of modified conductance states of RyR induced by the interaction of this ryanoid suggest that once bound the structure of the ryanoid changes little, if at all. There is no evidence for isomerization within the ligand–RyR complex with either 21-p-nitrobenzoylamino-9α-hydroxyryanodine or 10-O-succinoylryanodol derivatives. Significant changes in structure of the bound ryanoid might be expected to result in shifts between different fractional conductance states. We have no evidence for significant alterations in fractional conductance during a 10-O-succinoylryanodol or 21-p-nitrobenzoylamino-9α-hydroxyryanodine–induced modified-conductance event. A dissociation and rebinding event appears to be the mechanism of formation of multiple conductance states.
Alterations in the Probability of RyR Channel Modification by 10-O-succinoylryanodol with Changing Transmembrane Holding Potential
A comparison of the influence of transmembrane holding potential on the probability of RyR channel modification by ryanoids with charges of 1, 0, and −1 indicates that irrespective of charge the probability of occurrence of altered function is low at negative and high at positive holding potentials. In all cases an alteration of holding potential from negative to positive values results in an increase in the rate of ryanoid association and a decrease in the rate of ryanoid dissociation.
This observation demonstrates that the predominant mechanism underpinning the influence of transmembrane holding potential on ryanoid interaction with RyR, and hence altered channel function, is a potential-driven change in receptor affinity. Our previous observation of similarities in voltage dependence of the interaction of cationic and neutral ryanoids with RyR (Tanna et al., 2000) was consistent with this proposal but was not in itself sufficient to establish that transmembrane potential has a direct effect on the receptor. By monitoring the influence of transmembrane potential on the interaction of an anionic ryanoid with RyR we have demonstrated that the effect of applied potential varies little among three ryanoids that vary drastically in electronic distribution. Qualitatively, if ligand-charge were a significant determinant of the voltage-induced change in the affinity of the receptor for ryanoid, reversal of the formal charge on the ryanoid would reverse the voltage dependence of interaction. This is not the case: the effect of the applied field is the same regardless of the ionic nature of the ryanoid. In all cases that we have investigated, as applied potential becomes more positive kon increases, koff decreases, and KD decreases. While differences in the electronic field of the bound ryanoid do influence the conductance of the RyR channel (Welch et al., 1997) and the association rate constant (Table I), the electronic field of the ryanoid has little role in voltage-induced changes in ryanoid binding.
While the mechanisms underlying voltage-dependent alterations in ryanoid binding are not yet known, our experiments indicate that the primary event is a direct modulation of the receptor by the applied field. The change in the receptor is detected by ryanoid binding. Apparently, conformational change is an intrinsic property of the receptor and not the result of a peculiar electronic configuration of the ligand–receptor complex. As noted in the previous paragraph, the effect of the applied field is the same regardless of the ionic nature of the ryanoid. The small differences in the influence of voltage on the dissociation constants of different ryanoids can be explained in terms of the scheme shown in Fig. 6 A (see also Tanna et al., 2000). There is no a priori reason that the relative binding of ryanoids should be the same in the voltage-modified states. For example, a cationic and an anionic ryanoid may bind equally well to the RyR2 conformer that dominates at negative holding potentials but the cationic ryanoid may bind more avidly to the conformer that dominates at positive holding potentials (Fig. 6 A) (Tanna et al., 2000). Difference in binding determinants account for the small differences in the voltage sensitivity of the dissociation constants of the three different ryanoids.
Figure 6.
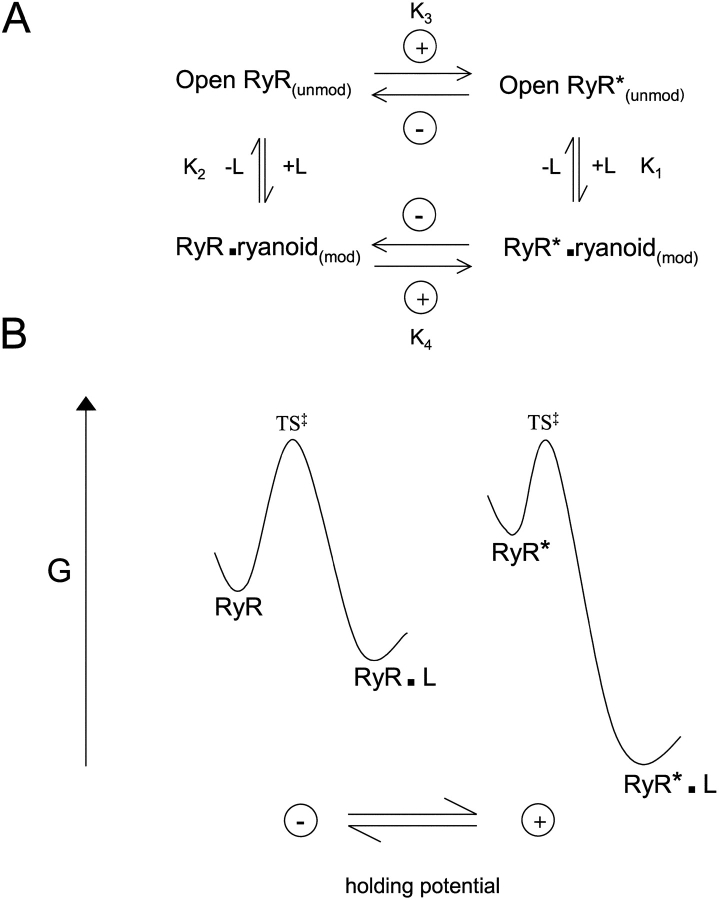
(A) Scheme summarizing the influence of transmembrane potential on the interaction of ryanoids with the high-affinity binding site of RyR (Tanna et al., 2000). L represents the ligand (any ryanoid) and + and − indicate the application of positive and negative holding potentials to the cytosolic side of the channel. A minimum of two forms of the vacant receptor exist in this model (open RyR which is low affinity and open RyR* which is high affinity). (B) Rate theory representation of scheme A. A shift from a negative to a positive holding potential increases the free energy of the free RyR (RyR to RyR*) and decreases the free energy of the RyR–ryanoid complex (RyR.L to RyR*.L). See text for details.
One can illustrate the effect of voltage on rate constants by use of Eyring rate theory (Eyring, 1935) (Fig. 6 B). It is presumed that a transition state exists between the free receptor and the ligand–receptor complex. Rates are determined by the magnitude of the free energy of the transition state (G±) relative to the free receptor and receptor–ligand complex. The energies of the transition state and the ryanoid–receptor complex will differ with each receptor–ryanoid pair (and account for the differences in the absolute rate and dissociation constants). The effects of applied potential modulate the free energies of reactants. Because increasing positive potential increases kon and decreases koff and KD, it is clear that applied potential does more that alter the transition state free energy. For simplicity, let us assume that transition state free energy depends on the nature of the ryanoid but not on applied field. The data are consistent with a mechanism in which increasing positive potential increases the free energy of the free RyR2, whereas increasing positive potential decreases the free energy of all three RyR2–ryanoid complexes. This is not the behavior expected if ligand charge were a major factor in the voltage-induced changes.
A similar phenomenon to that reported here has been noted previously in batrachotoxin-activated Na+ channels. The modulation of conductance by guanidinium toxins in these channels is sensitive to trans-membrane holding potential. As is the case with the interaction of ryanoids with RyR, the voltage-induced modulation is independent of the ionic state of the ligand (Moczydlowski et al., 1984; Green et al., 1987). Moczydlowski et al. (1984) suggested that these observations might reflect a mechanism essentially identical to that described in Fig. 6 A, involving a voltage-driven isomerization of both empty and ligated forms of the receptor. In contrast, Green et al. (1987) proposed that altered conductance was associated with voltage-sensitive conformational changes that occurred after toxin binding. The resolution of the detailed mechanism governing the voltage-dependent modulation of ryanoid binding to RyR will be the subject of future investigations.
We have demonstrated previously that, at least in the case of 21-amino-9α-hydroxyryanodine, the high-affinity binding site on RyR is only accessible from the cytosolic side of the channel protein (Tanna et al., 1998). Therefore, ryanoids reach the high-affinity binding site on RyR from the bulk solution at the cytosolic face of the channel. The variation in rates of association of ryanoids with different charge reported here might indicate that access to the high-affinity site can be influenced by a region of the channel that has a fixed net negative charge. Independent evidence for the existence of significant negative charge at the cytosolic entrance to the conduction pathway of the RyR channel has been provided by experiments in which rates of association of K+ channel N-type inactivation peptide, added to the solution at the cytosolic face of the RyR channel, were increased dramatically by an increase in net charge of the peptide from three to seven (Mead et al., 1998). This, together with the demonstration that mutations of residues within the putative conduction pathway of the RyR channel can influence ryanodine binding (Chen et al., 2002), suggests that the ryanoid binding site is likely to be located within the conduction pathway of the RyR channel. However, the very limited direct influence of transmembrane holding potential on the interaction of charged ryanoids with RyR and the earlier observation that bound ryanoids do not interact directly with translocated ions (Welch et al., 1997) indicates that the high-affinity binding site is not located a significant distance into the voltage drop across the channel.
In summary, the experiments presented in this communication describe for the first time the interaction of an anionic ryanoid with individual RyR channels under voltage clamp conditions. Our observation of numerous fractional conductance states in RyR channels exposed to 10-O-succinoylryanodol, and the demonstration that this ryanoid can exist in a range of approximately isoenergetic conformers, emphasize the importance of the 10 position in the ryanoid molecule in determining rates of ion translocation in the RyR–ryanoid complex. These observations support the contention that ion translocation in the RyR–ryanoid complex can be determined solely by the conformation of the ryanoid molecule and is not dependent on other interactions between the ligand and receptor that could result from alterations in the covalent structure of the ryanoid.
The observed dependence of the probability of 10-O-succinoylryanodol interaction with RyR on transmembrane holding potential is consistent with the proposal that the major influence of potential on the association of ryanoids with and dissociation of ryanoids from RyR is exerted via a voltage-driven alteration in receptor affinity. The dependence of absolute rates of ryanoid association with RyR on ryanoid charge indicates that fixed negative charge at the cytosolic entrance to the conduction pathway is likely to influence the likelihood of ryanoid interaction with the RyR high-affinity binding site.
Acknowledgments
We wish to thank Mr. M. Dodier, Department de Chimie, Universite de Sherbrooke and Noah Duffy and Tracy Kipke, Department of Biochemistry, University of Nevada, Reno, for technical assistance in the synthesis of 10-O-succinoylryanodol and [3H]-ryanodine binding assays respectively.
This work was supported by funds from the British Heart Foundation, the Wellcome Trust, the National Science Foundation (MCB 9817605), the Molecular Modeling/Graphics Core Facility, University of Nevada, Reno, The Universite de Sherbrooke Fonds de Recherche a Allocation Interne (FRAI) and the National Institutes of Health (HL 53677 and AR 45112).
Olaf S. Andersen served as editor.
Footnotes
Abbreviation used in this paper: CoMFA, comparative molecular field analysis.
References
- Chen, S.R.W., P. Li, M.C. Zhao, X.L. Li, and L. Zhang. 2002. Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys. J. 82:2436–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyring, H. 1935. The activated complex in chemical reactions. J. Chem. Phys. 3:107–115. [Google Scholar]
- Green, W.N., L.B. Weiss, and O.S. Andersen. 1987. Batrachotoxin-modified sodium channels in planar lipid bilayers. Characterization of saxitoxin- and tetrodotoxin-induced channel closures. J. Gen. Physiol. 89:873–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, A.R.G., A. Tinker, and A.J. Williams. 1994. How does ryanodine modify ion-handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel? J. Gen. Physiol. 104:425–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, A.R.G., and A.J. Williams. 1991. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1064:89–102. [DOI] [PubMed] [Google Scholar]
- McGarry, S.J., and A.J. Williams. 1994. Activation of the sheep cardiac sarcoplasmic reticulum Ca2+-release channel by analogues of sulmazole. Br. J. Pharmacol. 111:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead, F.C., D. Sullivan, and A.J. Williams. 1998. Evidence for negative charge in the conduction pathway of the cardiac ryanodine receptor channel provided by the interaction of K+ channel N-type inactivation peptides. J. Membr. Biol. 163:225–234. [DOI] [PubMed] [Google Scholar]
- Moczydlowski, E., S. Hall, S.S. Garber, G.R. Strichartz, and C. Miller. 1984. Voltage-dependent blockade of muscle Na+ channels by guanidinium toxins. J. Gen. Physiol. 84:687–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serysheva, I.I., and S.L. Hamilton. 1998. Ryanodine binding sites on the sarcoplasmic reticulum Ca2+ release channel. The Structure and Function of Ryanodine Receptors. R. Sitsapesan and A.J. Williams, editors. Imperial College Press, London. 95–109.
- Sitsapesan, R., and A.J. Williams. 1990. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 423:425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan, R., and A.J. Williams. 1994. Gating of the native and purified cardiac SR Ca2+-release channel with monovalent cations as permeant species. Biophys. J. 67:1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko, J.L., J.A. Airey, W. Welch, and L. Ruest. 1997. The pharmacology of ryanodine and related compounds. Pharmacol. Rev. 49:53–98. [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J.L. Sutko, and A.J. Williams. 1998. Interactions of a reversible ryanoid (21-amino-9α-hydroxy-ryanodine) with single cardiac ryanodine receptor channels. J. Gen. Physiol. 112:55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J.L. Sutko, and A.J. Williams. 2000. The interaction of a neutral ryanoid with the ryanodine receptor channel provides insights into the mechanisms by which ryanoid binding is modulated by voltage. J. Gen. Physiol. 116:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J.L. Sutko, and A.J. Williams. 2001. Ryanoid modification of the cardiac muscle ryanodine receptor channel results in relocation of the tetraethylammonium binding site. J. Gen. Physiol. 117:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna, B., W. Welch, L. Ruest, J.L. Sutko, and A.J. Williams. 2002. Excess noise in modified conductance states following the interaction of ryanoids with cardiac ryanodine receptor channels. FEBS Lett. 516:35–39. [DOI] [PubMed] [Google Scholar]
- Tinker, A., J.L. Sutko, L. Ruest, P. Deslongchamps, W. Welch, J.A. Airey, K. Gerzon, K.R. Bidasee, H.R. Besch, Jr., and A.J. Williams. 1996. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys. J. 70:2110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, W., S. Ahmad, J.A. Airey, K. Gerzon, R.A. Humerickhouse, H.R. Besch, Jr., L. Ruest, P. Deslongchamps, and J.L. Sutko. 1994. Structural determinants of high-affinity binding of ryanoids to the vertebrate skeletal muscle ryanodine receptor: A comparative molecular field analysis. Biochemistry. 33:6074–6085. [DOI] [PubMed] [Google Scholar]
- Welch, W., J.L. Sutko, K.E. Mitchell, J.A. Airey, and L. Ruest. 1996. The pyrrole locus is the major orienting factor in ryanodine binding. Biochemistry. 35:7165–7173. [DOI] [PubMed] [Google Scholar]
- Welch, W., A.J. Williams, A. Tinker, K.E. Mitchell, P. Deslongchamps, J. Lamothe, K. Gerzon, K.R. Bidasee, H.R. Besch, Jr., J.A. Airey, et al. 1997. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry. 36:2939–2950. [DOI] [PubMed] [Google Scholar]
- Wiesner, K. 1972. The structure of ryanodine. Adv. Org. Chem. 8:295–316. [Google Scholar]