Abstract
Rectification in inward-rectifier K+ channels is caused by the binding of intracellular cations to their inner pore. The extreme sharpness of this rectification reflects strong voltage dependence (apparent valence is ∼5) of channel block by long polyamines. To understand the mechanism by which polyamines cause rectification, we examined IRK1 (Kir2.1) block by a series of bis-alkyl-amines (bis-amines) and mono-alkyl-amines (mono-amines) of varying length. The apparent affinity of channel block by both types of alkylamines increases with chain length. Mutation D172N in the second transmembrane segment reduces the channel's affinity significantly for long bis-amines, but only slightly for short ones (or for mono-amines of any length), whereas a double COOH-terminal mutation (E224G and E299S) moderately reduces the affinity for all bis-amines. The apparent valence of channel block increases from ∼2 for short amines to saturate at ∼5 for long bis-amines or at ∼4 for long mono-amines. On the basis of these and other observations, we propose that to block the channel pore one amine group in all alkylamines tested binds near the same internal locus formed by the COOH terminus, while the other amine group of bis-amines, or the alkyl tail of mono-amines, “crawls” toward residue D172 and “pushes” up to 4 or 5 K+ ions outwardly across the narrow K+ selectivity filter. The strong voltage dependence of channel block therefore reflects the movement of charges carried across the transmembrane electrical field primarily by K+ ions, not by the amine molecule itself, as K+ ions and the amine blocker displace each other during block and unblock of the pore. This simple displacement model readily accounts for the classical observation that, at a given concentration of intracellular K+, rectification is apparently related to the difference between the membrane potential and the equilibrium potential for K+ ions rather than to the membrane potential itself.
Keywords: polyamine, alkylamine, quaternaryammonium, block, voltage dependence
INTRODUCTION
Inward rectifier K+ (Kir) channels accomplish many important biological tasks, such as controlling heart rate, modulating synaptic transmission, and coupling blood glucose level to insulin secretion. Kir channels are so named because they conduct much larger inward currents at membrane voltages (V) negative to the K+ equilibrium potential (EK) than outward currents at voltages positive to EK, even when the K+ concentrations on both sides of the membrane are made equal. This property is commonly referred to as anomalous or inward rectification (Katz, 1949; Hodgkin and Horowicz, 1959; Noble, 1965; Hagiwara and Takahashi, 1974; Hagiwara et al., 1976).
The first clue to a possible mechanism came from the work of Armstrong and Binstock (1965), who showed that intracellular TEA blocks voltage-activated K+ channels of squid axon in a voltage-dependent manner, rendering them inwardly rectifying. Two decades later, Mg2+ was identified as an endogenous voltage-dependent channel blocker causing inward rectification (Matsuda et al., 1987; Vandenberg, 1987). However, its voltage dependence is too weak to explain by itself the strong inward rectification observed in many cell types and, furthermore, significant rectification remains in the nominal absence of Mg2+. Thus arose the concept of intrinsic channel gating (e.g., Kurachi, 1985; Ishihara et al., 1989; Silver and DeCoursey, 1990; Stanfield et al., 1994a), with little progress made until intracellular polyamines were found to block the channels in a strongly voltage-dependent manner (Ficker et al., 1994; Lopatin et al., 1994; Fakler et al., 1995). More recently, residual apparent intrinsic rectification of heterologously expressed wild-type IRK1 (Kir2.1) channels in the nominal absence of Mg2+ and polyamines has been traced to the presence of contaminating cationic blockers in the commonly employed recording solutions (Guo and Lu, 2000a, 2002; also see below). Therefore, inward rectification in IRK1 channels reflects nothing but the strong voltage dependence of channel block.
For high-affinity binding of cationic blockers, an acidic residue at a seemingly conserved site within the second transmembrane (M2) segment of Kir channels is crucial (Lopatin et al., 1994; Lu and MacKinnon, 1994; Stanfield et al., 1994b; Wible et al., 1994). In the case of ROMK1 (Kir1.1), an acidic residue substituted at position 171 in M2 confers a much higher affinity for blocking ions than a neutral residue does, whereas a basic residue renders the channel essentially insensitive. On the basis of these findings, it was proposed that residue 171 affects the binding of blocking ions through an electrostatic mechanism (Lu and MacKinnon, 1994). Additionally, mutations at certain acidic residues (E224 and E299) in the COOH terminus of IRK1 (Kir2.1) have been shown to affect the binding of intracellular blocking ions (Taglialatela et al., 1994, 1995; Yang et al., 1995; Kubo and Murata, 2001).
The exact source of the voltage dependence of channel block by polyamines remains unclear. Given the fact that Kir block by a tetravalent polyamine (spermine) exhibits much stronger voltage dependence than that by Mg2+ or TEA (Ficker et al., 1994; Lopatin et al., 1994, 1995; Lu and MacKinnon, 1994; Fakler et al., 1995; Spassova and Lu, 1998, 1999; Guo and Lu, 2000a, 2001), one might surmise that, as proposed by Woodhull (1973) in the case of voltage-dependent proton block of voltage-gated Na+ channels, the voltage dependence primarily reflects the movement of the charge carried by the blocker itself across (a fraction of) the electrical field. However, in the better understood case of K+ channel block by TEA (and also by Mg2+; Spassova and Lu, 1998), most if not all of the voltage dependence of block reflects the movement of K+ ions within the transmembrane electrical field as TEA and a K+ ion displace (“knock off”) one another in the pore, while TEA itself may not traverse much of the field (Armstrong and Binstock, 1965; Armstrong, 1971; Oliver et al., 1998; Spassova and Lu, 1998, 1999; for theoretical studies of channel block see, e.g., Hille and Schwarz, 1978). The one-to-one displacement model predicts the observed dissociation constant (hereafter called Kd) for TEA binding to be a linear function, and the observed valence (Zobs) to be a saturating function, of the extracellular K+ concentration ([K+ ext]). These features were indeed observed (Spassova and Lu, 1998). As shown in the quoted study, the linear dependence of Kd on [K+ ext] or, at a fixed intracellular K+ concentration ([K+ int]), the apparent exponential dependence on EK, together with the Boltzmann-like voltage dependence of Kd, leads to its apparent exponential dependence on V − EK, a classic feature of anomalous rectification (Hodgkin and Horowicz, 1959, Noble, 1965; Hagiwara and Takahashi, 1974; Hagiwara et al., 1976; Hille and Schwarz, 1978). Because of this precedent and the observation that polyamine block is also affected by the external K+ concentration (e.g., Lopatin and Nichols, 1996), the possibility should be kept in mind that the voltage dependence of channel block by polyamines results (at least in part) from the movement, across the electrical field, of multiple K+ ions residing in the long channel pore.
Pearson and Nichols (1998) examined IRK1 block by a series of bis- and mono-alkyl-amines (bis-amines and mono-amines) of varying length, a type of approach pioneered with bis-quaternary ammonium by Miller (1982) in a study of sarcoplasmic reticulum K+ channels. Finding that the apparent affinity for bis-amines increases with alkyl chain length, they concluded that hydrophobic interactions contribute to the overall binding energy. Additionally, the apparent valence of block increased with chain length from 1.5 to 4 with no sign of saturation. To explain this phenomenon, the authors hypothesized that a bis-amine is positioned in the pore in such a way that the two amine groups are equidistant from a ring of negative charges. The leading end of a long bis-amine would thus penetrate deeper into the long, narrow pore over which the electrical field drops, engendering a voltage dependence which is further enhanced by the outward displacement of K+ ions by the blocker. As for mono-amines, their affinity is much lower than that of corresponding bis-amines and, surprisingly, the apparent valence remains low (∼2) and independent of alkyl chain length. To account for this observation, the authors proposed a different binding scheme for mono-amines, in which the sole amine group binds near the ring of negative charges while the alkyl chains of varying length trail behind, pointing to the intracellular solution. In retrospect, this very intriguing study was confounded, as was another study from our group discussed below, by the following issue. With the then commonly employed recording solutions the channels exhibited definite, if modest, depolarization-induced current relaxation, resulting in inward rectification even in the absence of intracellular Mg2+ and polyamines. The presence of the seemingly “intrinsic” voltage dependence (whose artifactual nature was then unknown) resulted in underestimations of both the affinity and the valence of channel block. In addition, the use of relatively short voltage pulses may have made the estimation of steady-state parameters more difficult. In a subsequent investigation of whether polyamines cause multiple blocked states and exhibit finite permeability our group also examined bis-amine (and polyamine) block (Guo and Lu, 2000a). The apparent affinity and valence of channel block by bis-amines were found to increase with alkyl chain length. Experimental protocols ensured that all recorded currents had reached the steady state, and the blocking parameters were estimated from a steady-state equation that takes account of the finite permeability of the amines. Despite these improvements, both the affinity and the voltage dependence of channel block by bis-amines were still underestimated because of the interference of “intrinsic” voltage dependence, which can be appreciated from Eq. 13 in the quoted paper. Although these limitations did not affect the conclusions of the study regarding nonhomogenous protonation and finite permeability of polyamines (Guo and Lu, 2000a,b), they still precluded a thorough analysis of the cause underlying the voltage dependence of polyamine actions. For example, it could not be determined whether the dependence of the apparent valence on alkyl chain length had reached a true plateau at longer lengths, or was actually linear with an apparent plateau reflecting limitations imposed by adverse experimental conditions (see Fig. 12 B in Guo and Lu, 2000a).
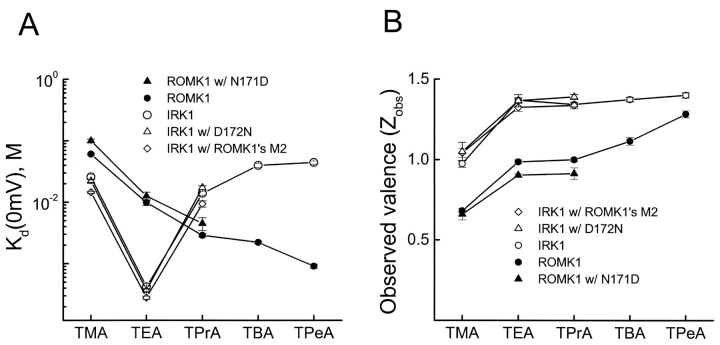
Figure 12.
Quantitative analyses of block of wild-type and mutant ROMK1 and IRK1 channels by quaternary ammoniums. (A and B) Kd(0 mV) and Zobs (mean ± SEM; n = 5–7; determined as before, see Guo and Lu, 2001) for block of wild-type and mutant IRK1 and ROMK1 channels by TMA, TEA, TPrA, tetrabutylammonium (TBA), and tetrapentylammonium (TPeA).
For the reasons discussed above, we next investigated the nature of the intrinsic voltage dependence, and found that it actually results from channel block by certain contaminants such as hydroxyethylpiperazine in HEPES and ethylenediamine in EDTA (Guo and Lu, 2000b, 2002). Besides showing that residual inward rectification results not from intrinsic gating but from voltage-dependent channel block by contaminating intracellular cations, the latter study led to the development of recording solutions practically devoid of significant blocking activity, which allowed us to carry out the present study.
MATERIALS AND METHODS
Molecular Biology and Oocyte Preparation
The cDNAs of IRK1 and KcsA-IRK1 were subcloned in pGEM-HESS plasmid, that of ROMK1 in pSPUTK plasmid (Ho et al., 1993; Kubo et al., 1993a; Lu et al., 2001). In the KcsA-IRK1 chimera, IRK1's M1 through M2 sequence is replaced by its KcsA counterpart, the NH2- and COOH-terminal junctions being formed by joining W81 of IRK1 to K14 of KcsA and G116 of KcsA to A184 of IRK1, respectively (Lu et al., 2001). All mutant cDNAs were obtained through PCR-based mutagenesis and confirmed by DNA sequencing. The cRNAs were synthesized with T7 or SP6 polymerase (Promega Corp.) using linearized cDNAs as templates. Oocytes harvested from Xenopus laevis (Xenopus One) were incubated in a solution containing NaCl, 82.5 mM; KCl, 2.5 mM; MgCl2, 1.0 mM; HEPES, pH 7.6, 5.0 mM, and collagenase, 2–4 mg/ml. The oocyte preparation was agitated at 80 rpm for 60–90 min. It was then rinsed thoroughly and stored in a solution containing NaCl, 96 mM; KCl, 2.5 mM; CaCl2, 1.8 mM; MgCl2, 1.0 mM; HEPES, pH 7.6, 5 mM, and gentamicin, 50 μg/ml. Defolliculated oocytes were selected and injected with RNA at least 2 and 16 h, respectively, after collagenase treatment. All oocytes were stored at 18°C.
Recordings and Solutions
Whole oocyte currents were recorded using a two-electrode voltage-clamp amplifier (Warner OC-725C), filtered at 1 kHz, and sampled at 5 kHz using an analogue-to-digital converter (DigiData 1200; Axon Instruments, Inc.) interfaced with a personal computer. pClamp6 software (Axon Instruments, Inc.) was used to control the amplifier and acquire the data. The resistance of electrodes filled with 3 M KCl was ∼0.3 MΩ. The bath solution contained (in mM): 100 K+ (Cl− + OH−), 0.3 CaCl2, 1 MgCl2, and 10 HEPES; pH was adjusted to 7.6 with KOH. All currents were recorded as the membrane potential was stepped from the 0 mV holding potential to test potentials between −80 and 80 mV in 10-mV increments and back to 0 mV. All records of ROMK1 and its mutants were corrected for background leak currents using the current templates obtained in the presence of synthetic tertiapin-Q at concentrations >100 × Kd (Jin and Lu, 1999). Current records of IRK-KcsA chimeric channels were corrected using the current templates obtained in the presence of 100 mM extracellular Na+ and no K+, in which these channels carry practically no currents. Currents of inside-out membrane patches of Xenopus oocytes (injected with Kir's cRNAs) were recorded with an Axopatch 200B amplifier (Axon Instruments, Inc.), filtered at 5 kHz, and sampled at 25 kHz. During current recording, the voltage across the membrane patch was first hyperpolarized from the 0 mV holding potential to −100 mV, and then stepped to various test voltages between −100 and 100 mV and back to 0 mV. Background leak current correction was performed as described previously (Lu and MacKinnon, 1994; Guo and Lu, 2000b). The intracellular solution contained (mM): 5 K2EDTA, 10 “K2HPO4 + KH2PO4” in a ratio yielding pH 8.0, and sufficient KCl to bring total K+ concentration to 100 mM, whereas in the extracellular solution 5 mM EDTA was replaced by 0.3 mM CaCl2 and 1.0 mM MgCl2 (Guo and Lu, 2002; see also Guo and Lu, 2000b). All chemicals were purchased from Fluka Chemical Corp.
RESULTS
We first tested the hypothesis that the Asp in M2 acts by an electrostatic mechanism (Lu and MacKinnon, 1994). If that residue indeed acts by local electrostatics, its electrical potential can then help determine the binding location and orientation of a given alkylamine within the pore.
Variation of Inward Rectification with the Location of the Acidic Residue in the M2 Region
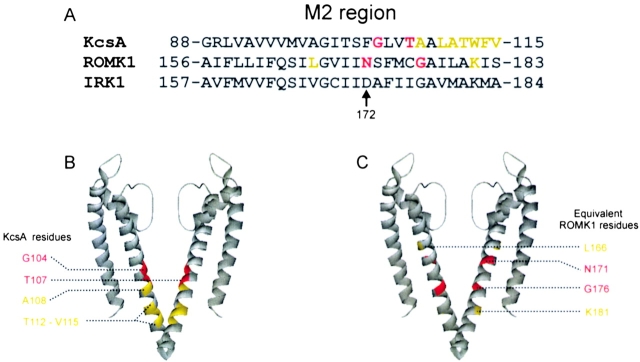
In an intact oocyte bathed in a solution containing 100 mM K+ (a nearly symmetric K+ condition), heterologously expressed IRK1 channels conduct little outward current at positive voltages compared with inward current at the corresponding negative voltages, whereas ROMK1 channels conduct robust currents. The former has acidic Asp 172 in M2, whereas the latter has neutral Asn at equivalent position 171 (Fig. 1 A; Ho et al., 1993; Kubo et al., 1993a). Replacing Asn 171 by Asp dramatically enhances ROMK1's affinity for blocking ions and confers an IRK1-like phenotype in oocytes (Lu and MacKinnon, 1994; Wible et al., 1994). If Asp acts electrostatically (Lu and MacKinnon, 1994) rather than as a specific binding site, its precise location need not be critical. To test this further, we substituted Asp for each residue (Leu166-Ser183) along the distal 2/3 of M2 that lines part of the inner pore in ROMK1; 4 of the 18 mutants express currents. Like N171D, mutation G176D confers an IRK-like phenotype, whereas the more proximal L166D or distal K181D do not (Fig. 2 , A–F). The absolute ratio of steady-state currents of the wild-type and mutant channels at 80 mV and at −80 mV is plotted against the M2 sequence in Fig. 2 G.
Figure 1.
Alignment of the M2 sequence among three Kir channels, and KcsA structural model. (A) Alignment (Ho et al., 1993; Kubo et al., 1993a; Schrempf et al., 1995; Doyle et al., 1998) of the M2 sequences of KcsA, ROMK1 and IRK1. Substituting aspartate reduces the ratio of outward to inward currents negligibly or modestly at yellow residues, but dramatically at red residues. (B) KcsA structure (Zhou et al., 2001b) with residues highlighted as in A. (C) Same structure as in B but with residues highlighted as for the ROMK1 sequence in A (position equivalence based on the sequence alignment shown in A).
Figure 2.
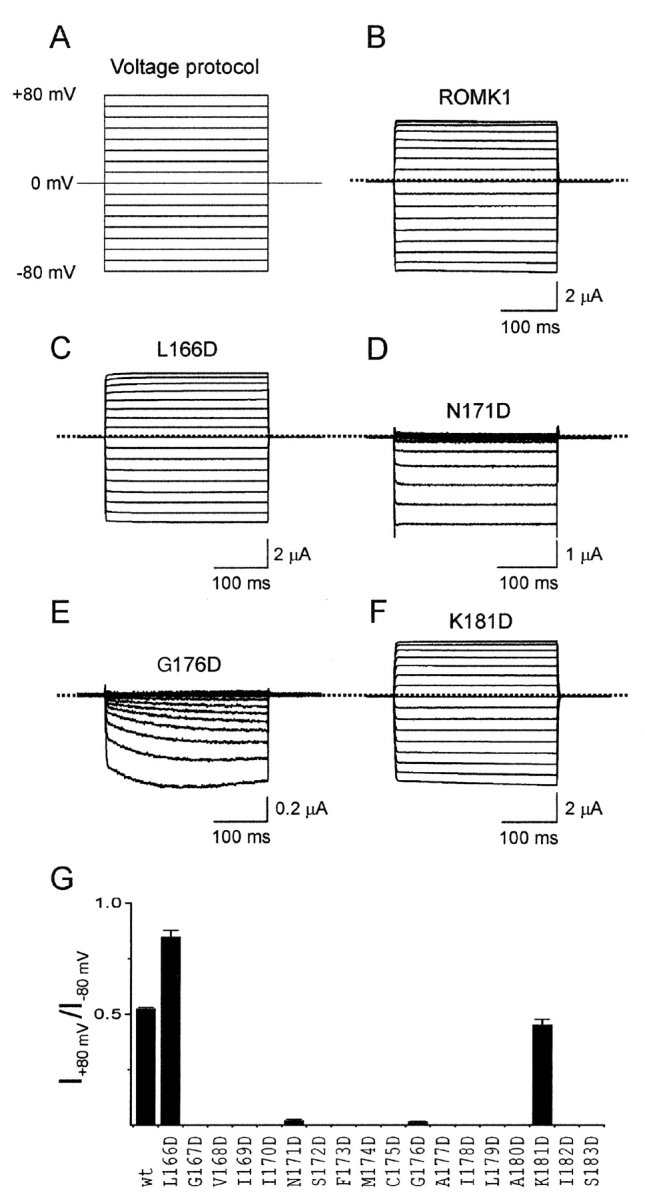
Effects of substituting aspartate in M2 of ROMK1 on inward rectification. (A) Voltage pulse protocol. (B-F) Currents of ROMK1 and four mutants containing Asp at various positions in M2. (G) Absolute ratio of steady-state currents (mean ± SEM; n = 5–12) at 80 and at −80 mV for wild-type and Asp-substituted channels, plotted against the M2 sequence.
To gain structural insight, we performed similar studies with an IRK1 construct whose pore (M1-M2) sequence is replaced by KcsA's (Fig. 3 A; Lu et al., 2001; materials and methods); 7 of the 17 mutants (G99D–V115D) express current. Dramatic reductions in outward current (compared with inward current) occur when Asp replaces KcsA's G104 or T107 (Fig. 3, B and C; for the T107D channel see also Lu et al., 2001). In the chimeric channels containing G104D and T107D (or ROMK1 containing G176D), the slow relaxation of inward currents upon hyperpolarization is reminiscent of that in Kir3.1/3.4 (GIRK1/CIR) channels (Dascal et al., 1993; Kubo et al., 1993b; Krapivinsky et al., 1995). However, substituting Asp at the more internal residues A108 or T112 - V115 produces only modest or negligible effects (T112D is shown in Fig. 3 D). The absolute ratio of steady-state currents at 80 mV and at −80 mV is plotted against the M2 sequence in Fig. 3 E. For structural reference, we highlight, on the KcsA structure (Zhou et al., 2001b), those KcsA and ROMK1 residues whose mutation to Asp preserves a functional channel (Fig. 1, B and C). Red residues correspond to those whose replacement dramatically reduces the ratio of outward to inward currents, yellow residues to those where modest or negligible effects are seen. Thus, the effect of Asp is not absolutely site-specific, although it does vanish as the mutation is moved out of the cavity. These findings strongly support the notion that Asp acts mainly by a local electrostatic mechanism (Lu and MacKinnon, 1994).
Figure 3.
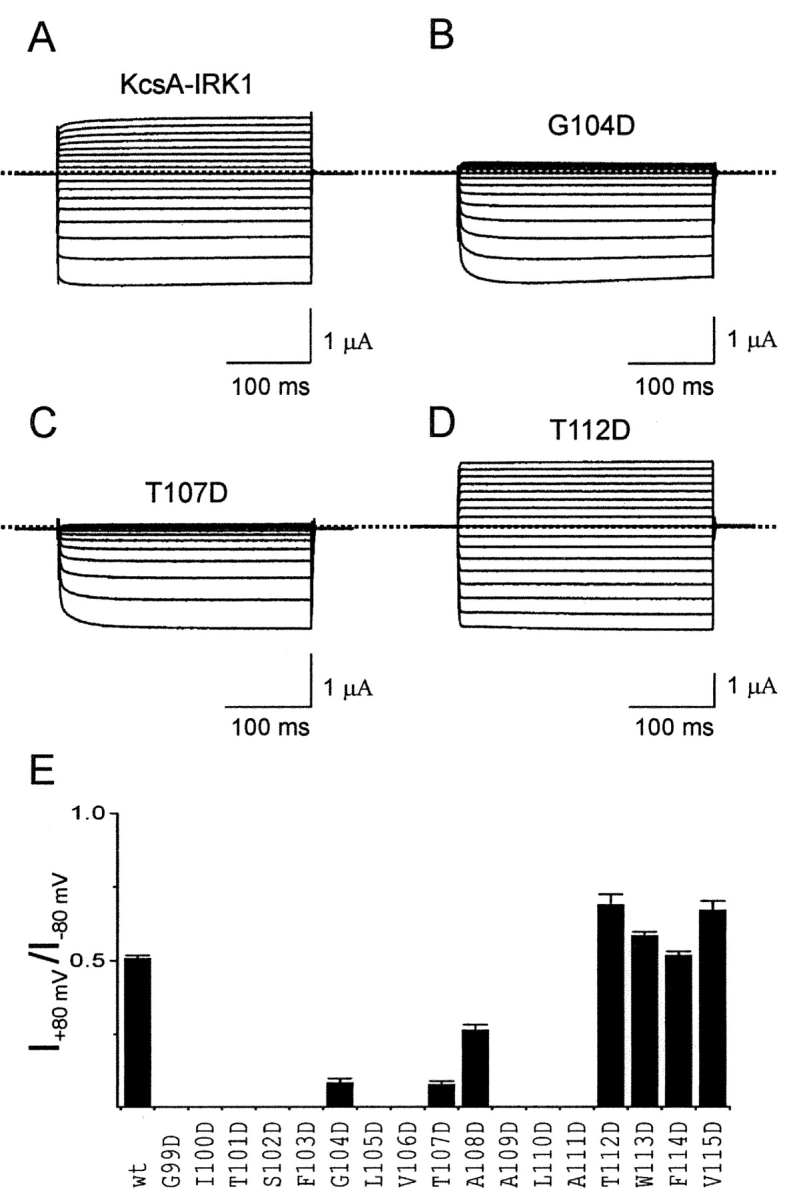
Effects of substituting aspartate in M2 of KcsA-IRK1 on inward rectification. (A–D) Currents of original KcsA-IRK1 and mutants containing Asp at three representative positions. (E) Absolute ratio of steady-state currents (mean ± SEM; n = 5–10) at 80 and at −80 mV for the original and the Asp-substituted KcsA-IRK1 constructs.
Channel Block by Alkylamines
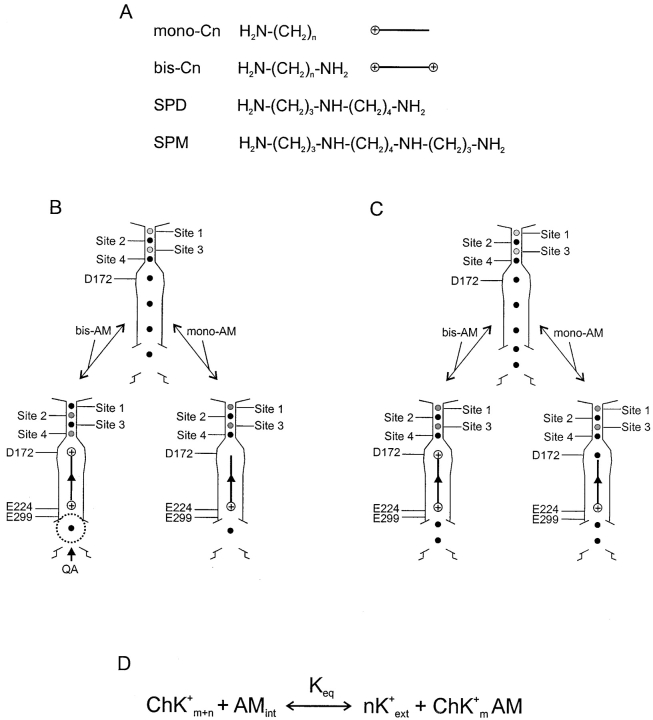
In the steady state, the apparent valence of IRK1 block by the polyamines spermidine (SPD) or spermine (SPM) is approximately five (Guo and Lu, 2000a,b; for chemical structures see Fig. 4 A). Since the voltage dependence does not reflect intrinsic gating (Guo and Lu, 2000b, 2002), this high valence must reflect the movement within the electrical field of charges on the polyamine and/or K+ ions. To determine the source of the voltage dependence, we systematically and quantitatively characterized how specific mutations affect IRK1 block by two series of alkylamines of varying length (Fig. 4 A): alkyl-mono-amines (mono-Cn) or alkyl-bis-amines (bis-Cn) with an amine at only one or both ends, respectively (n designates the number of methylene groups in the alkyl chain).
Figure 4.
Blocker structures, channel models, and blocking reaction scheme. (A) Chemical structures of mono-Cn, bis-Cn, SPD, and SPM. (B and C) Models for voltage dependence of channel block by alkylamines. Model B has four K+ ions in the inner pore, and the binding of a blocking amine “pushes” the K+ ions to sites 1 and 3 from sites 2 and 4 in the narrow outer pore (gray versus black dots). The dotted circle at the internal end of the pore represents a blocking QA. Model C has five K+ ions in the inner pore, and the binding of an amine does not affect the binding of K+ ions in the outer pore. The arrowheads midway through the vertical lines that represent the alkyl chains symbolize the fact that these lines represent a series of alkylamines of increasing chain length. (D) One-step steady-state model for channel block by an amine molecule. The total number of K+ ions in a conducting channel (Ch) is m + n. Binding of an intracellular amine (AMint) to the channel pore displaces n K+ ions to the extracellular solution (nK+ ext), and vice versa. The voltage-dependent equilibrium constant (Keq) is defined in the text.
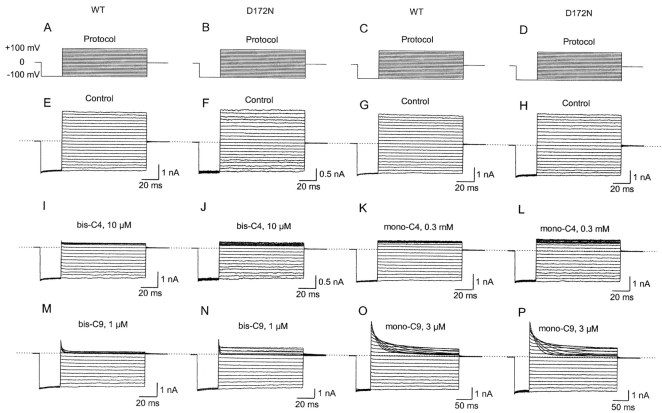
Fig. 5 shows sample currents recorded from IRK1 and its D172N mutant with and without two representative alkyl-bis-amines: bis-C4 and bis-C9, or the corresponding alkyl-mono-amines: mono-C4 and mono-C9. In the absence of alkylamines (Fig. 5, E–H), the currents underwent no relaxation and displayed linear steady-state I-V relationships, confirming that no significant contaminating blocking agent is present in the intracellular solution. In both wild-type and mutant IRK1, short amines (Fig. 5, I–L) act faster and with lower affinity than long ones (Fig. 5, M–P). Also noteworthy is that bis-C9 blocks with much faster kinetics and higher affinity than mono-C9 does, in both wild-type (Fig. 5, M versus O) and mutant channels (N versus P).
Figure 5.
Block of IRK1 and its D172N mutant by alkylamines. Data in each column were collected from a separate oocyte. (A–D) Voltage protocols. (E–P) Wild-type and mutant currents without (E–H) and with bis-C4 (I and J), mono-C4 (K and L), bis-C9 (M and N), or mono-C9 (O and P).
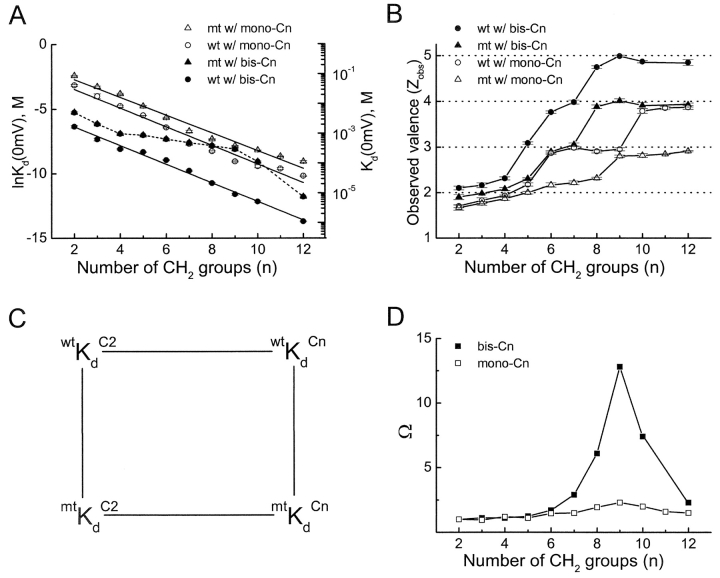
Fig. 6 summarizes, in terms of both Kd (at 0 mV) and Zobs of channel block, our quantitative analysis of steady-state block of wild-type and D172N mutant IRK1 channels by 10 alkyl-bis-amines and 11 alkyl-mono-amines of varying length. We will review the blockers in that order. The averaged data, plotted in Fig. 6, A and B, were obtained from records of the kind shown in Fig. 5. Fig. 6 A shows that mutation D172N reduces IRK1's affinity up to 40-fold for long bis-amines (e.g., bis-C9) but only two- to threefold for short ones (bis-C2 to bis-C4; compare filled circles and triangles). To illustrate the phenomena more clearly, we computed the Asp 172–blocker coupling coefficient Ω (Hidalgo and MacKinnon, 1995) from thermodynamic cycles (Fig. 6 C), using bis-C2 as a reference (Fig. 6 D, filled squares). The value of Ω remains near unity for chain lengths up to C6. Further lengthening yields a sharp peak at bis-C9 followed by a decrease, as if the leading amine of bis-C9 approaches Asp 172 most closely, while that of bis-C8 or bis-C10, respectively, falls slightly short of or overshoots Asp 172. (Additionally or alternatively, the drop-off in the value of Ω may reflect buckling of the alkyl chain.) We thus surmise that with increasing chain length, the leading amine of bis-amines reaches further in the outward direction and encounters an increasing electrostatic potential from Asp 172, whereas the trailing amine remains near the same internal site and encounters little potential (Fig. 4 B). This model predicts the following two phenomena.
Figure 6.
Quantitative analyses of steady-state alkylamine block of wild-type and D172N mutant IRK1 channels. (A) Natural logarithm of Kd(0 mV) (determined as before [Guo and Lu, 2000a]; mean ± SEM; n = 6–10; all error bars within the symbols) for mono- and bis-alkyl-amines in wild-type and mutant channels, plotted against the number of methylene groups in the alkyl chain. The three lines are linear fits to the data, yielding (top to bottom) slopes of 0.68, 0.71, and 0.71, and Y-intercepts at “zero alkyl chain length” of −1.54, −1.96, and −4.93. The fourth (dotted) curve was drawn by hand. (B) Zobs (determined as in Guo and Lu, 2000a) of wild-type and mutant channel block by alkyl-mono- and bis-amines plotted against chain length; mean ± SEM; n = 6–10; connected by straight lines. (C) An illustration of thermodynamic cycles (Hidalgo and MacKinnon, 1995). The four corners are Kds (0 mV) of wild-type and mutant channels for mono- or bis-C2 and for a given mono- or bis-Cn. Ω = (wtKd C2 × mtKd Cn)/(mtKd C2 × wtKd Cn), where Kds (all at 0 mV) are taken from A. (D) Ω values, computed as in C, plotted against chain length. Bis-C11 is not available commercially.
First, the apparent valence of voltage-dependent block should increase with chain length because, as the leading amine reaches deeper into the pore, it displaces more K+ ions out of the inner pore and through the selectivity filter across which most of the transmembrane voltage drops steeply. Indeed, Zobs increases with chain length in apparent steps of approximately one, reaching a plateau of approximately five at about that of bis-C8 (filled circles in Fig. 6 B; compare Pearson and Nichols, 1998; Guo and Lu, 2000a). (The lower plateau valence for block of the D172N mutant channel (filled triangles) probably reflects reduced K+ occupancy.) Since the leading amine of bis-C8 is near Asp 172 and therefore near the inner end of the selectivity filter, it is not surprising that bis-C8 displaces the greatest number of K+ ions, yielding essentially the strongest voltage dependence. Bis-C8 (Fig. 6 B) and spermidine (Guo and Lu, 2000a,b), about equally long, cause block with comparable voltage dependence (Z = ∼5). Longer bis-amines (Fig. 6 B) or spermine (Guo and Lu, 2000a, b), however, do not exhibit much stronger voltage dependence, which must mean that the leading amine rarely enters the narrow selectivity filter itself, in accord with the observation that alkylamines (including polyamines) have only a very low probability of traversing the entire pore (Guo and Lu, 2000a).
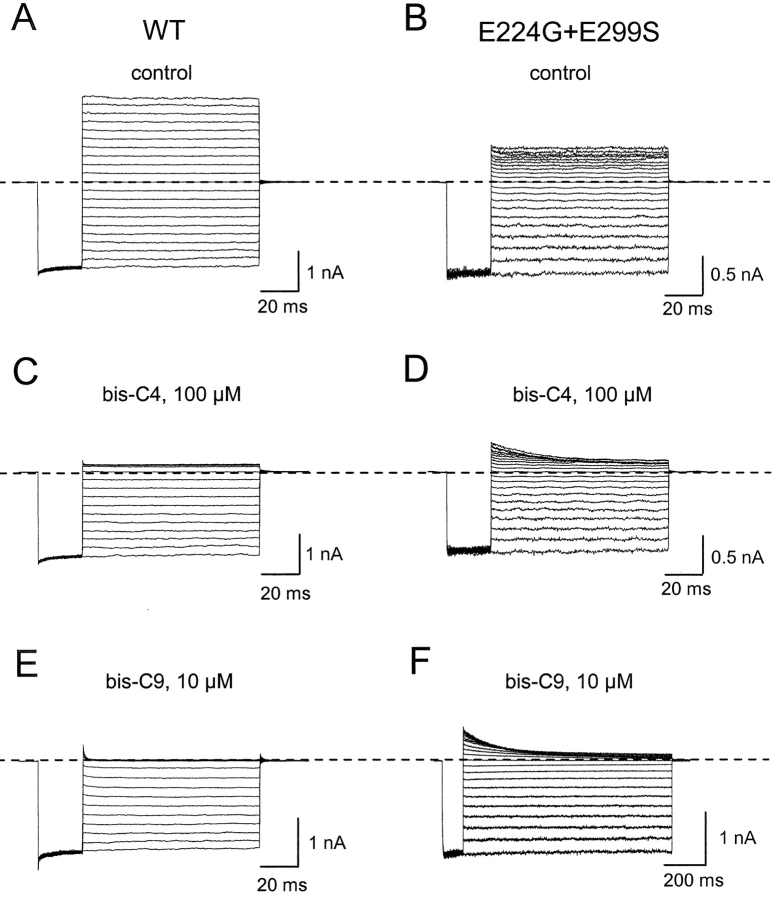
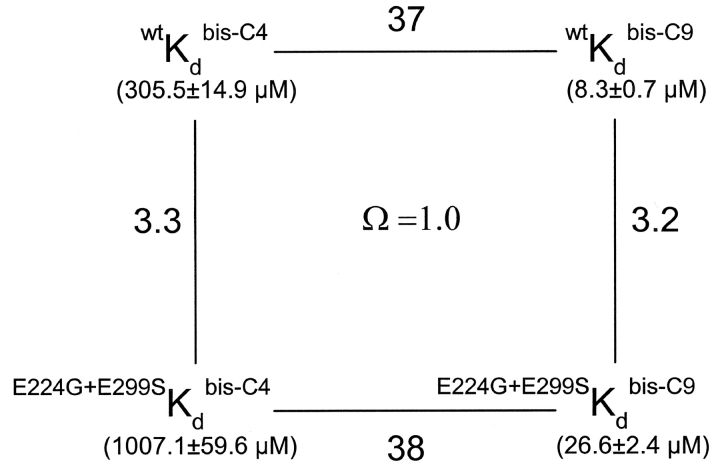
A second prediction is as follows. COOH-terminal mutations E224G and E299S have been shown to affect the binding of blocking ions (Taglialatela et al., 1994, 1995; Yang et al., 1995; Kubo and Murata, 2001). Fig. 7 shows current records of wild-type IRK1 and the mutant containing both E224G and E299S. The double mutation affects the kinetics profoundly and the affinity of channel block by both bis-C4 and bis-C9 modestly, and renders the I-V relation nonlinear. Additionally, it reduces the apparent valence of channel block; for example, Zobs for block by bis-C9 is 2.2 ± 0.1 (mean ± SEM, n = 5) in mutant channels compared with 5.0 ± 0.1 (n = 8) in wild-type IRK1. The reductions in both the outward current and the valence of block are consistent with a reduced presence of K+ ions in the inner pore. If the model we propose is correct and the trailing amine of bis-amines, whether short or long, stays near the same internal site and interacts with E224 and E299, then the double mutation should lower the channel affinity for bis-C4 and for bis-C9 by the same factor. With the coupling energy thus independent of bis-amine length, Ω computed with Kds of bis-C4 and bis-C9 in the wild-type and the double mutant channels should therefore be near unity, as we indeed observe (Fig. 8) .
Figure 7.
Block of wild-type and double mutant (E224G + E299S) IRK1 channels by a short and a long alkyl-bis-amine. Currents of wild-type (A, C, and E) and mutant (B, D, and F) channels recorded in the absence (A and B) and presence of bis-C4 (C and D) or bis-C9 (E and F).
Figure 8.
Thermodynamic analysis of block of wild-type and double mutant (E224G + E299S) IRK1 channels by a short and a long alkyl-bis-amine. The four corners of the thermodynamic cycle are Kds (0 mV) (mean ± SEM; n = 5–8) of wild-type and mutant channels for bis-C4 and bis-C9. The numbers around the cycle are the ratios of adjacent Kds. Ω = (wtKd bis-C4 × E224G+E299SKd bis-C9)/(E224G+E299SKd bis-C4 × wtKd bis-C9).
In an intuitively plausible model of pore block by mono-amines (Pearson and Nichols, 1998), the sole amine always binds at the same site in the pore while the alkyl tails of varying length point to the intracellular solution. If this were the case, the rate constant for channel block by a given mono-amine should, from statistical considerations, be twofold smaller than that by the corresponding bis-amine, and the voltage dependence of channel block should be unaffected by chain length. Neither prediction was verified, as discussed below. The reverse (tail first) orientation should therefore be considered (Fig. 4 B), even though it would appear energetically less favorable [indeed, Kd(0 mV) for any given mono-amine is ∼20-fold higher than for the corresponding bis-amine (open versus filled circles in Fig. 6 A)]. If the trailing amine group of all mono-amines binds near the same internal site, the coupling energy between the mono-amines and Asp 172 should not vary with their length, which was verified experimentally (Fig. 6 D, open squares). With ever longer alkyl tails approaching the selectivity filter, the voltage dependence of channel block should increase as more K+ ions are displaced outwardly across the selectivity filter (Fig. 6 B, open circles). This is indeed what we observed (compare Pearson and Nichols, 1998). The difference in maximal valence of channel block between the mono- and bis-amines (four versus five; open versus filled circles in Fig. 6 B) is consistent with the view that the leading amine in bis-amines either shifts the K+-binding configuration in the selectivity filter (Fig. 4 B, sites 2 and 4 versus 1 and 3; Morais-Cabral et al., 2001; Zhou et al., 2001b), or repels an extra K+ ion from the inner pore (Fig. 4 C). Although in an open pore the transmembrane voltage drops mainly across the outer, narrow K+ selectivity filter, once the pore is blocked the transmembrane electrical field is expected to redistribute toward the internal side. The leading amine of long bis-amines may therefore sense a fraction of the electrical field, and thus contribute somewhat to the extra apparent valence associated with channel block by long bis-amines.
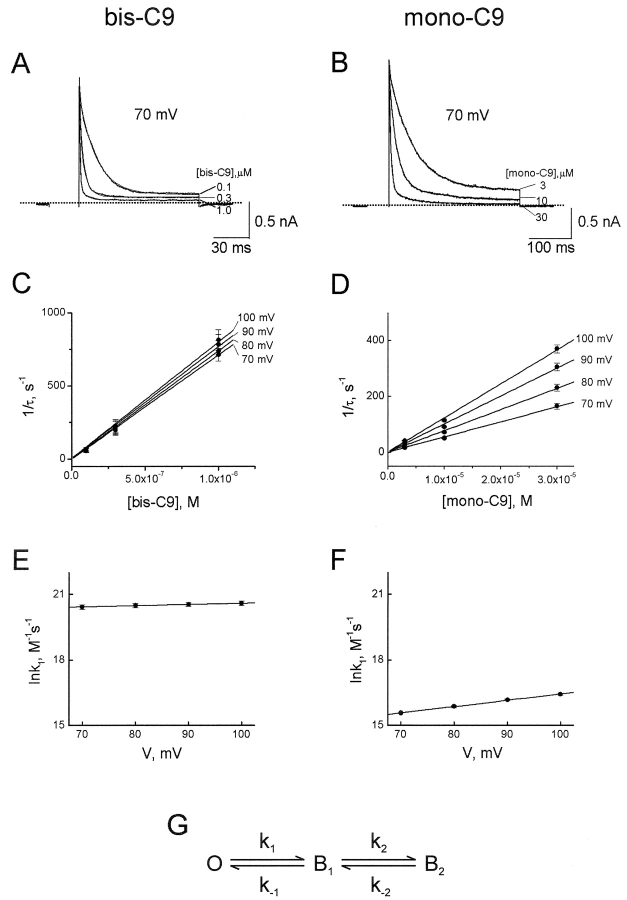
A channel probably becomes nonconducting as soon as the leading end of an alkylamine enters the pore, and before the blocker reaches its final position. That is, there may be two (or more) blocked states (Fig. 9 G). To gain insight into the kinetics of channel block by alkyl-mono-amines and bis-amines, we examined how the rate of current relaxation after a voltage jump varies with blocker concentration and membrane voltage. Fig. 9 illustrates two examples (bis-C9 and mono-C9); Fig. 10 summarizes the results from five alkyl-bis-amines and six alkyl-mono-amines whose blocking kinetics, over practical ranges of voltage and concentration, were sufficiently slow to permit quantitative analysis. Fig. 9, A and B, shows single-exponential fits to current transients elicited by stepping the voltage from 0 to 70 mV in the presence of three concentrations of bis-C9 and mono-C9, respectively. Reciprocals of the fitted time constants (1/τ) are plotted against blocker concentration in Fig. 9, C and D. Regardless of the number of subsequent closed states, the slopes of plots in Fig. 9, C and D, reflect the rate constant of formation of the first blocked state (k1 in Fig. 9 G). To determine the voltage dependence of k1, we plotted its natural logarithm against membrane voltage and fitted the plot with a straight line (Fig. 9, E and F; collected results for all alkylamines tested are shown in Fig. 10). If more than one blocked state exists, the interpretation of the Y-intercept in Fig. 9, C and D is not straightforward since it does not simply reflect the unblocking rate constant k−1 (Fig. 9 G). Extensive kinetic studies to determine k−1 and any additional rate constants for transitions between blocked states (k2, k−2, etc.), and their voltage dependence, are beyond the scope of the present study.
Figure 9.
Kinetics of voltage jump-induced IRK1 current relaxations in the presence of bis- and mono-amines. (A and B) Current traces at three concentrations of bis-C9 (A) or mono-C9 (B) elicited by stepping membrane voltage from 0 to 70 mV. The curves superimposed on the current transients are single exponential fits. (C and D) Reciprocals of the time constants (1/τ; mean ± SEM, n = 5), obtained from the fits shown in A and B, are plotted against bis-C9 (C) and mono-C9 (D) concentrations and fitted with straight lines. (E and F) Natural logarithms of the slopes (k1 in G) of the linear fits shown in C and D are plotted against membrane voltage and fitted with straight lines: ln k1 = ln k1(0 mV) + z1VF/RT. (G) A kinetic model with one open (O) and two blocked states (B1 and B2).
Figure 10.
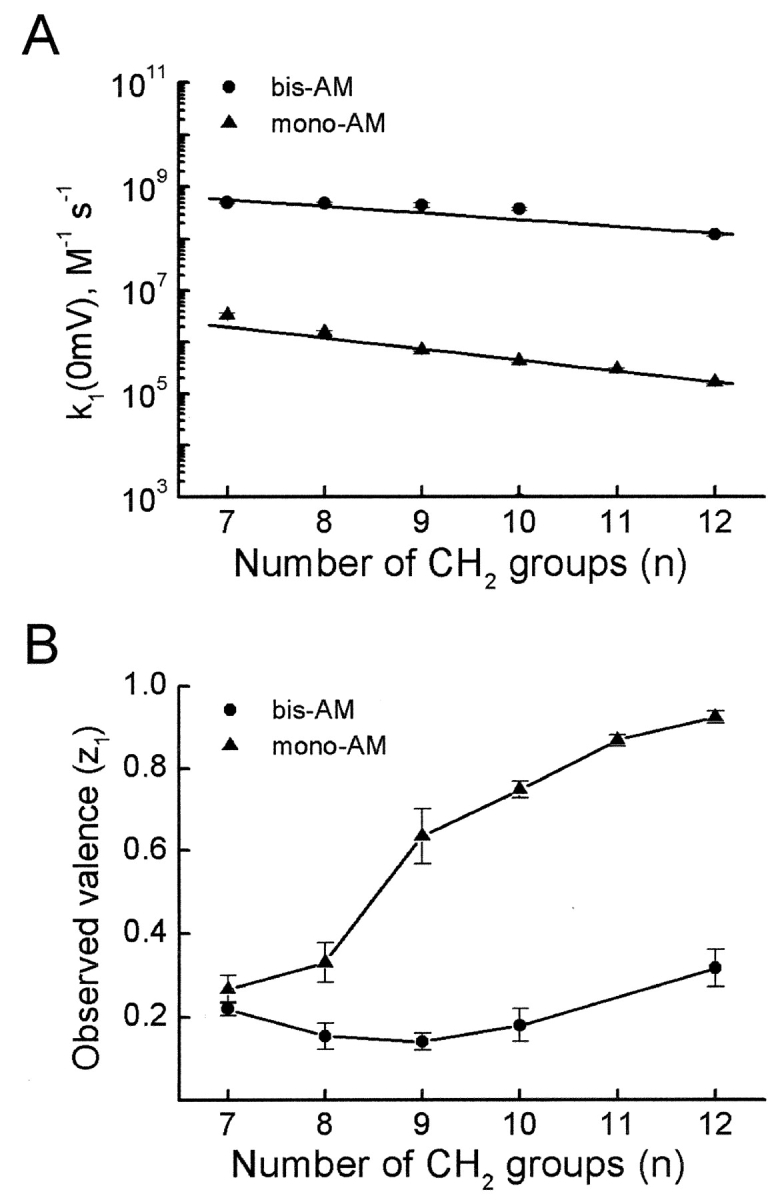
Characteristics of rate constants (k1 in Fig. 9 G) for block of IRK1 by alkyl-bis- and mono-amines of varying chain length. The values (mean ± SEM; n = 5) were obtained from linear fits as illustrated for alkyl-bis- and mono-C9 in Fig. 9, E and F. (A) Rate constants extrapolated to 0 mV, k1(0 mV), for IRK1 block by bis-amines and mono-amines are plotted against alkyl chain length. (B) Observed valence of rate constants (z1) for IRK1 block by bis-amines and mono-amines, plotted against alkyl chain length.
Fig. 10 summarizes, from plots such as those illustrated in Fig. 9, E and F, the values of blocking rate constant k1(0 mV) and the associated observed valence (z1) for several bis-amines and mono-amines whose blocking kinetics are sufficiently slow to allow analysis. The behavior of k1 is informative in two ways. First, k1(0 mV) for a given mono-amine is generally ∼600-fold lower than that for the corresponding bis-amine, as expected if, without electrostatic focusing, an uncharged tail has a low probability of entering the pore and if, with positive charges at both ends, a bis-amine is more likely to adopt an extended conformation which facilitates entry. Second, blocking rate constant k1 for the alkylamines tested exhibits only weak or no voltage dependence (z1 ≤ 1), which suggests that these blockers render the channel nonconducting as soon as, or even before, they displace the first K+ ion. Most of the steady-state voltage dependence of channel block, reflected in Kd, must therefore arise in the subsequent closed transition(s), when most of the K+ ions are displaced and “pushed” across the transmembrane electrical field, and/or conversely, when external K+ ions move across the electrical field to dislodge the amine from the inner pore.
Channel Block by Quaternary Ammonium Ions
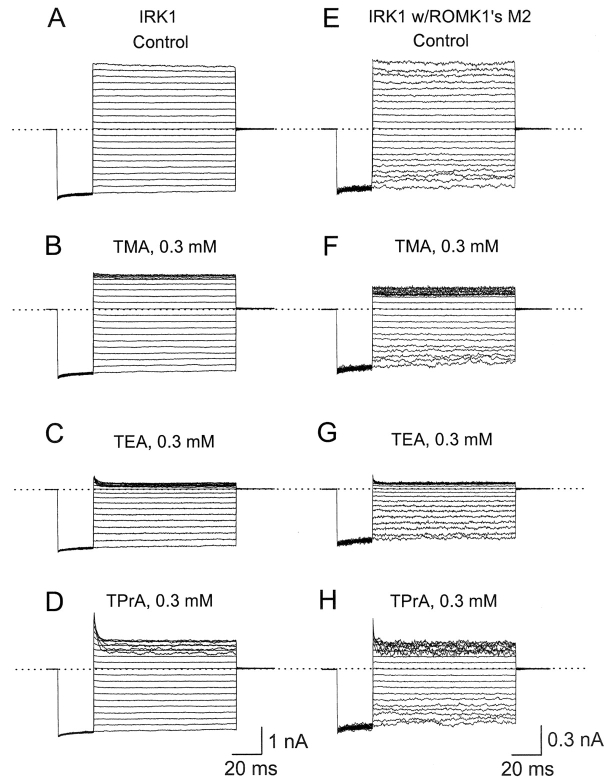
A crystallographic study shows that tetrabutylantimony ion (a quaternary ammonium (QA) analogue) binds in the cavity of KcsA (Zhou et al., 2001a). Were this true also for IRK1, according to our model, a QA on the way to its binding site in the cavity would sweep all K+ ions present in the inner pore across the selectivity filter, resulting in a high apparent valence. Instead, the observed valence is only ∼1 (Guo and Lu, 2001), which suggests that IRK1 has a more intracellular (less deep) site. To explore this possibility, we reviewed and further studied the blocking behaviors of QAs in two rectifiers. ROMK1 binds larger QAs with increasing affinity, whereas IRK1 has an exquisitely high affinity and selectivity for TEA (Guo and Lu, 2001). As shown before (Guo and Lu, 2001) and replotted in Fig. 12 A for later comparison, these differences persist regardless of whether residue 171/172 in ROMK1/IRK1 is Asn or Asp. Furthermore, we found here that even when its entire M2 sequence is replaced by that of ROMK1, IRK1's high affinity and exquisite selectivity for TEA remain practically unchanged (Fig. 11 , and Fig. 12 A, open diamonds). Together, these results and our finding (Fig. 12 B) that the valence of IRK1 block by QAs is ∼1 are consistent with IRK1 having a specific internal QA site where binding of a QA displaces only the one (or two) innermost K+ ion(s) (Fig. 4, B and C). Nevertheless, a QA that binds in the cavity may still exhibit low valence if it is small enough to bypass K+ ions in the inner pore.
Figure 11.
Block of the wild-type and a mutant IRK1 by quaternary ammoniums. Currents of the wild-type and an IRK1 construct containing ROMK1's M2 sequence recorded in the absence (A, E) and presence of tetramethylammonium (TMA; B and F), tetraethylammonium (TEA; C and G), or tetrapropylammonium (TPrA; D and H).
DISCUSSION
To produce effective rectification, a blocker evidently needs to carry a charge at both ends of a hydrophobic chain of a certain length, such as bis-C9. Our analysis suggests the following model: One end of the blocker binds near an intracellular site in its interaction with the pore, whereas the other extends toward the selectivity filter, displacing a number of K+ ions (Fig. 4 B). As to kinetics, the leading amine electrostatically guides its entry, significantly increasing the blocking rate compared with a mono-amine of comparable length (Fig. 10 A). As to energetics, the electrostatic interaction between the leading amine and Asp 172 contributes up to 1.5 kcal/mol to the interaction energy, as computed from Ω in Fig. 6 D. Additionally, from the slope of a linear fit of ln Kd(0 mV) against alkyl chain length (Fig. 6 A), we estimate the hydrophobic interactions between the alkyl chain and the pore to increase by 0.4 kcal/mol per methylene group (Fig. 6 A). Finally, extrapolating the linear fit for block of D172N channels by mono-amines to “zero alkyl chain length” (Fig. 6 A, open triangles), we estimate the interaction energy between the trailing amine and the relevant channel residues at 0.9 kcal/mole. Most of that energy can be accounted for by interactions involving COOH-terminal E224 and E299, since neutralizing them together reduces the binding energy by 0.7 kcal/mol (vertical comparisons; Fig. 8).
Strikingly, the observed valences of channel block by long mono-amines and bis-amines (open and closed circles in Fig. 6 B), and by the polyamines spermidine and spermine (Guo and Lu, 2000a,b) are uniformly approximately four or five, even though these blockers carry very different charges (from one to four). Therefore, perhaps counterintuitively, the voltage dependence of apparent blocker affinity must primarily reflect movement of K+ ions across the electrical field in the pore, and not of the blocking amine itself, during block and/or unblock when the amine and K+ ions displace each other (Fig. 4 D; see also Armstrong and Binstock, 1965; Armstrong, 1971; Spassova and Lu, 1998, 1999). At 0 mV, the blocking reaction depicted in Fig. 4 D, which assumes obligatory coupling between the movements of the blocking amine and K+ ions, is governed by an equilibrium constant,
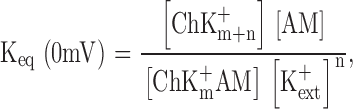 |
(1) |
and the fraction of channels not blocked, or normalized macroscopic conductance, is given by:
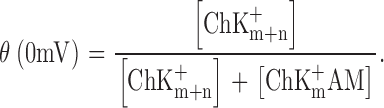 |
(2) |
Combining Eqs. 1 and 2, and assuming that the electrical potential energy in the reaction is a simple exponential (Boltzmann) function, with valence Zobs, of the transmembrane potential V, we have,
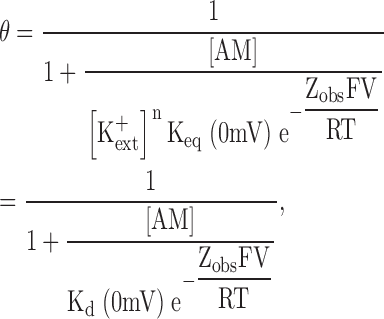 |
(3) |
where F, R, and T have their usual meaning, and the relation with the Kd(0 mV) plotted in Fig. 6 A is made explicit. Substituting the Nernst equation for [K+ ext] in Eq. 3 yields,
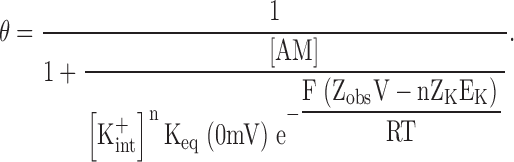 |
(4) |
In the limit where the observed voltage dependence results solely from the movement of K+ ions across the transmembrane electrical field, the numerical value of Zobs equals the sum of charges in all K+ ions (nZK) moving across the electrical field, and Eq. 4 reduces to:
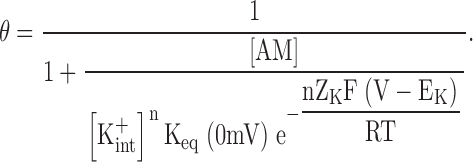 |
(5) |
That is, at a given [K+ int], the normalized macroscopic conductance is a function of the electrochemical potential for K+ ions (V − EK) rather than of the membrane potential itself. To summarize, mutual displacement between K+ ions and the blocker causes a linear dependence of Kd on [K+ ext]n (Spassova and Lu, 1998, 1999), or an exponential dependence on EK at fixed [K+ int]. If, furthermore, the entire observed voltage dependence results solely from charges carried by K+ ions, Kd becomes an exponential function of V − EK (compare Eqs. 3 and 5; see also Spassova and Lu, 1998, 1999).
Eq. 5 is exactly equivalent to the empirical equation of Hagiwara and Takahashi (1974) (shown below) that describes quantitatively the signature property of inward rectifiers, i.e., the dependence of their relative macroscopic conductance on both extracellular K+ and membrane voltage (Hodgkin and Horowicz, 1959; Hagiwara and Takahashi, 1974; Hagiwara et al., 1976; Hille and Schwarz, 1978; Hagiwara and Yoshii, 1979; Leech and Stanfield, 1981),
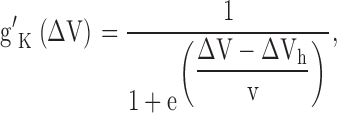 |
where g′K = θ and ΔV = V − EK.
Comparison with our Eq. 5 reveals the theoretical underpinnings of their empirical terms:
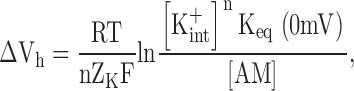 |
and
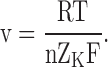 |
The latter identity yields n = ∼4 for anomalous rectifiers in starfish eggs (Fig. 4 B in Hagiwara and Takahashi, 1974).
To produce an apparent valence as high as approximately five for IRK1 block, the long inner pore lined by residues in both M2 and the COOH terminus in the channel protein must contain as many as four or five K+ ions. Regardless of the precise number of K+ ions, they must be effectively displaced by the intracellular blocker, out of the inner pore and across the transmembrane electrical field, for blocker binding to acquire the steep voltage dependence (apparent valence) we observe. Interestingly, valences for block of both wild-type and mutant channels by either mono- or bis-amines appear to increase with chain length in steps of approximately one (Fig. 6 B), as if K+ ion displacement occurs in discrete steps as the leading end of alkylamines of increasing length approaches the K+ selectivity filter. Discrete displacement may occur as long as K+ ions cannot exchange position with (or bypass) the blocker, even if they can do so among themselves (i.e., mix) in the inner pore. If indeed K+ ions present in (part of) the inner pore are not truly in a single file and therefore can mix, the value of the maximal valence of channel block need not be the same as the value of the Ussing flux ratio exponent for these channels (n′ = 2.2; Stampe et al., 1998), since the exponent is determined by the number of K+ ions that move along the pore in true single-file fashion, i.e., unable to exchange position with one another (Hodgkin and Keynes, 1955; Begenisich and De Weer, 1980). The ability of small metal ions in the inner pore to mix also helps explain why Mg2+ blocks with an apparent valence of only about one, even though it binds at a site just internal to the narrow ion selectivity filter in IRK1 (e.g., Fujiwara and Kubo, 2002; for Mg2+ block of ROMK1 see, e.g., Lu and MacKinnon, 1994).
Together, our results suggest that the following properties make IRK1 an effective rectifier. First, IRK1 has many negatively charged residues in its pore to interact with positively charged amines in the blocker; this results in a fast blocking rate and a substantial amount of interaction energy. Second, its inner pore must also be lined with many hydrophobic residues whose interactions with the methylene groups in a polyamine make up most of the binding energy. Finally, the COOH termini of IRK1 must extend the pore internally (Taglialatela et al., 1994, 1995; Yang et al., 1995; Kubo and Murata, 2001) beyond what is conserved among various types of K+ channels (Lu et al., 2001), and harbor additional K+ ions whose residence there is favored by the presence of some acidic residues. Coupling of the movement of a large number of K+ ions across the transmembrane electrical field to the binding and unbinding of a long polyamine leads to strong voltage dependence of channel block and, consequently, sharp rectification. Our conclusions based on functional studies are entirely compatible with the crystal structure of Kir3.1 cytoplasmic termini that has just been solved by Nishida and MacKinnon (2002).
Acknowledgments
We thank P. De Weer for critical review and discussion of our manuscript, R. MacKinnon for helpful comments on our manuscript, K. Ho and S. Hebert for ROMK1 cDNA, L.Y. Jan for IRK1 cDNA, J. Yang for IRK1 cDNA subcloned in the pGEM-HESS vector, and R. MacKinnon and E. Perozo for KcsA cDNA.
This study was supported by National Institutes of Health grant GM55560. Z. Lu is the recipient of an Independent Scientist Award from the National Institutes of Health (HL03814).
David C. Gadsby served as editor.
References
- Armstrong, C.M. 1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C.M., and L. Binstock. 1965. Anomalous rectification in the squid giant axon injected with tetraethylammonium chloride. J. Gen. Physiol. 48:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich, T., and P. De Weer. 1980. Potassium flux ratio in voltage-clamped squid giant axons. J. Gen. Physiol. 76:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal, N., N.F. Lim, W. Schreibmayer, W. Wang, N. Davidson, and H.A. Lester. 1993. Expression of an atrial G-protein-activated potassium channel in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 90:6596–6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D.A., C.J. Morais, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Fakler, B., U. Brandle, E. Glowatzki, S. Weidemann, H.P. Zenner, and J.P. Ruppersberg. 1995. Strong voltage-dependent inward rectification of inward rectifier K + channels is caused by intracellular spermine. Cell. 80:149–154. [DOI] [PubMed] [Google Scholar]
- Ficker, E., M. Taglialatela, B.A. Wible, C.M. Henley, and A.M. Brown. 1994. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 266:1068–1072. [DOI] [PubMed] [Google Scholar]
- Fujiwara, Y., and Y. Kubo. 2002. Ser165 in the second transmembrane region of the Kir2.1 channel determines its susceptibility to blockade by intracellular Mg2+. J. Gen. Physiol. 120:677–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2000. a. Mechanism of IRK1 channel block by intracellular polyamines. J. Gen. Physiol. 115:799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2000. b. Pore block versus intrinsic gating in the mechanism of inward rectification in the strongly-rectifying IRK1 channel. J. Gen. Physiol. 116:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2001. Kinetics of inward-rectifier K+ channel block by quaternary alkylammonium ions: Dimension and properties of the inner pore. J. Gen. Physiol. 117:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2002. IRK1 inward rectifier K+ channels exhibit no intrinsic rectification. J. Gen. Physiol. 120:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, S., S. Miyazaki, and N.P. Rosenthal. 1976. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J. Gen. Physiol. 67:621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara, S., and K. Takahashi. 1974. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J. Membr. Biol. 18:61–80. [DOI] [PubMed] [Google Scholar]
- Hagiwara, S., and M. Yoshii. 1979. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J. Physiol. 292:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo, P., and R. MacKinnon. 1995. Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science. 268:307–310. [DOI] [PubMed] [Google Scholar]
- Hille, B., and W. Schwarz. 1978. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72:409–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, K., C.G. Nichols, W.J. Lederer, J. Lytton, P.M. Vassilev, M.V. Kanazirska, and S.C. Hebert. 1993. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 362:31–38. [DOI] [PubMed] [Google Scholar]
- Hodgkin, A.L., and R.D. Keynes. 1955. The potassium permeability of a giant nerve fibre. J. Physiol. 128:61–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, A.L., and P. Horowicz. 1959. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J. Physiol. 148:127–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara, K., A. Mitsuiye, A. Noma, and M. Takano. 1989. The Mg2+ block and intrinsic gating underlying inward rectification of the K+ current in guinea-pig cardiac myocytes. J. Physiol. 419:297–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, W., and Z. Lu. 1999. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 38:14286–14293. [DOI] [PubMed] [Google Scholar]
- Katz, B. 1949. Les constantes électriques de la membrane du muscle. Arch. Sci. Physiol. 2:285–299. [Google Scholar]
- Krapivinsky, G., E.A. Gordon, K. Wickman, B. Velimirovic, L. Krapivinsky, and D.E. Clapham. 1995. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+ channel proteins. Nature. 374:135–141. [DOI] [PubMed] [Google Scholar]
- Kubo, Y., and Y. Murata. 2001. Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K+ channel. J. Physiol. 531:645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, Y., T.J. Baldwin, Y.N. Jan, and L.Y. Jan. 1993. a. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 362:127–133. [DOI] [PubMed] [Google Scholar]
- Kubo, Y., E. Reuveny, P.A. Slesinger, Y.N. Jan, and L.Y. Jan. 1993. b. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 364:802–806. [DOI] [PubMed] [Google Scholar]
- Kurachi, Y. 1985. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J. Physiol. 366:365–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech, C.A., and P.R. Stanfield. 1981. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J. Physiol. 319:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin, A.N., E.N. Makhina, and C.G. Nichols. 1994. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 372:366–369. [DOI] [PubMed] [Google Scholar]
- Lopatin, A.N., E.N. Makhina, and C.G. Nichols. 1995. The mechanism of inward rectification of potassium channels: “long-pore plugging” by cytoplasmic polyamines. J. Gen. Physiol. 106:923–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin, A.N., and C.G. Nichols. 1996. [K+] dependence of polyamine-induced rectification in inward rectifier potassium channels (IRK1, Kir2.1). J. Gen. Physiol. 108:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., A.M. Klem, and Y. Ramu. 2001. Ion conduction pore is conserved among potassium channels. Nature. 413:809–813. [DOI] [PubMed] [Google Scholar]
- Lu, Z., and R. MacKinnon. 1994. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature. 371:243–246. [DOI] [PubMed] [Google Scholar]
- Matsuda, H., A. Saigusa, and H. Irisawa. 1987. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 325:156–159. [DOI] [PubMed] [Google Scholar]
- Miller, C. 1982. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J. Gen. Physiol. 79:869–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Nishida, M., and R. MacKinnon. 2002. Structural basis of inward rectification: Cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell. 111:957–965. [DOI] [PubMed] [Google Scholar]
- Noble, D. 1965. Electrical properties of cardiac muscle attributable to inward going (anomalous) rectification. J. Cell. Comp. Physiol. 66:127–136. [Google Scholar]
- Oliver, D., H. Hahn, C. Antz, J.P. Ruppersberg, and B. Fakler. 1998. Interaction of permeant and blocking ions in cloned inward-rectifier K+ channels. Biophys. J. 74:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, W.L., and C.G. Nichols. 1998. Block of the Kir2.1 channel pore by alkylamine analogues of endogenous polyamines. J. Gen. Physiol. 112:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf, H., O. Schmidt, R. Kummerlen, S. Hinnah, D. Muller, M. Betzler, T. Steinkamp, and R. Wagner. 1995. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 14:5170–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, M.R., and T.E. DeCoursey. 1990. Intrinsic gating of inward rectifier in bovine pulmonary artery endothelial cells in the presence and absence of internal Mg2+. J. Gen. Physiol. 96:109–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova, M., and Z. Lu. 1998. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J. Gen. Physiol. 112:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova, M., and Z. Lu. 1999. Tuning the voltage dependence of tetraethylammonium block with permeant ions in an inward-rectifier K+ channel. J. Gen. Physiol. 114:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampe, P., J. Arreola, P. Perez-Cornejo, and T. Begenisich. 1998. Nonindependent K+ movement through the pore in IRK1 potassium channels. J. Gen. Physiol. 112:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield, P.R., N.W. Davies, P.A. Shelton, I.A. Khan, W.J. Brammar, N.B. Standen, and E.C. Conley. 1994. a. The intrinsic gating of inward rectifier K+ channels expressed from the murine IRK1 gene depends on voltage, K+ and Mg2+. J. Physiol. 475:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield, P.R., N.W. Davies, P.A. Shelton, M.J. Sutcliffe, I.A. Khan, W.J. Brammar, and E.C. Conley. 1994. b. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J. Physiol. 478:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela, M., E. Ficker, B.A. Wible, and A.M. Brown. 1995. C-terminus determinants for Mg2+ and polyamine block of the inward rectifier K+ channel IRK1. EMBO J. 14:5532–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela, M., B.A. Wible, R. Caporaso, and A.M. Brown. 1994. Specification of pore properties by the carboxyl terminus of inwardly rectifying K+ channels. Science. 264:844–847. [DOI] [PubMed] [Google Scholar]
- Vandenberg, C.A. 1987. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc. Natl. Acad. Sci. USA. 84:2560–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wible, B.A., M. Taglialatela, E. Ficker, and A.M. Brown. 1994. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature. 371:246–249. [DOI] [PubMed] [Google Scholar]
- Woodhull, A.M. 1973. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61:687–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Y.N. Jan, and L.Y. Jan. 1995. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 14:1047–1054. [DOI] [PubMed] [Google Scholar]
- Zhou, M., J.H. Morais-Cabral, S. Mann, and R. MacKinnon. 2001. a. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, and R. MacKinnon. 2001. b. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]