Abstract
Annexin A4 (Anx4) belongs to a ubiquitous family of Ca2+-dependent membrane-binding proteins thought to be involved in membrane trafficking and membrane organization within cells. Anx4 localizes to the apical region in epithelia; however, its physiological role is unclear. We show that Anx4 exhibited binding to liposomes (phosphatidylcholine:phosphatidylserine, 1:1) in the presence of Ca2+ and binding was reversible with EDTA. Anx4 binding resulted in liposome aggregation and a reduction in membrane water permeability of 29% (P < 0.001) at 25°C. These effects were not seen in the presence of Ca2+ or Anx4 alone and were reversible with EDTA. Measurements of membrane fluidity made by monitoring fluorescence anisotropy of 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine (NBD-HPC) demonstrated that Anx4 binding rigidified the outer leaflet of the bilayer (P < 0.001), thus providing a molecular explanation for the inhibition of water flux. To determine whether Anx4 would produce similar effects on physiological membranes we constructed liposomes which recapitulated the lipid composition of the inner leaflet of the MDCK apical membrane. These membranes exhibited reductions to water permeability upon Anx4 binding (19.5% at 25°C, 31% at 37°C; P < 0.01 and P < 0.001, respectively) and to proton permeability (15% at 25°C, 19.5% at 37°C; P < 0.05). Since our in vitro experiments indicated an effect on membrane permeability, we examined localization of Anx4 in the kidney collecting duct, a region of the nephron responsible for concentrating urine through water reabsorbtion. Anx4 was shown to colocalize apically with aquaporin 2 (AQP2) in collecting duct epithelia. To test for the existence of a functional interaction between Anx4 and AQP2 we isolated AQP2-containing endosomes and exposed them to Anx4/Ca2+. Water flux rates were unchanged, indicating Anx4 does not directly regulate AQP2. We conclude that Anx4 can alter the physical properties of membranes by associating with them and regulate passive membrane permeability to water and protons. These properties represent important new functions for Anx4.
Keywords: epithelia, apical membrane, membrane fluidity, liposomes, protein binding
INTRODUCTION
Annexin A41 (Anx4)* belongs to a ubiquitous family of soluble cytoplasmic proteins that possess the unusual property of binding to and self-polymerizing on the surface of membranes in response to elevations in intracellular calcium (Kaetzel and Dedman, 1995; Gerke and Moss, 2002). Soluble monomers trimerize in the cytoplasm, bind to the membrane, and then assemble into higher order structures at the membrane interface creating a crystallization cascade in two dimensions across a large cross-sectional area of membrane (Zanotti et al., 1998). Binding to the anionic head groups of phospholipids such as phosphatidylserine (PS) occurs through a Ca2+-bridging mechanism (Swairjo et al., 1995) and is entirely reversible if Ca2+ is removed. Annexins are found in plants, fungi, nematodes, and higher vertebrates (Gerke and Moss, 2002) and yet, while much is known about their structural and biochemical properties, their cellular functions remain a challenge. Different annexins have been shown to be localized to many different cell types and to be associated with a number of different intracellular compartments. The ability to bind membranes suggest that they are well suited to play a role in membrane remodeling and organization events such as endocytosis, exocytosis, and vesicle fusion (Kaetzel et al., 1989; Gerke and Moss, 2002).
Anx4 is an epithelial isoform found at high levels in the trachea, lung, intestine, stomach and kidney and appears to be apically localized in secretory epithelia (Kaetzel et al., 1994; Kojima et al., 1994; Mayran et al., 1996; Dreier et al., 1998). Functionally it has been shown to inhibit the conductance of a Ca2+/calmodulin-dependent chloride channel in T84 cells through an interaction with the Ca2+/calmodulin-dependent protein kinase (CaM kinase) (Chan et al., 1994). The precise mechanism for this inhibition is unclear but may be due to “shielding” of the ion channel from CaM kinase. Alternatively, changing the physical state of the membrane through phospholipid binding may modify channel activity or channel accessibility. Anx4 has also been shown to play a role in kidney organogenesis in Xenopus laevis where it is localized to the luminal surface of the pronephric tubule. Ablation of the gene product results in abnormal development of the tubule (Seville et al., 2002).
A number of observations led us to investigate whether Anx4 might affect membrane permeability. These include the close association of Anx4 with the apical membrane in secretory and absorptive epithelia, alterations to the physical properties of membranes upon phospholipid binding observed with certain annexins (Megli et al., 1998; Sokolov et al., 2000), and the fact that apical membranes represent a barrier to permeation in tight epithelia. We report here that Anx4 interactions with membranes containing high concentrations of PS (50%), as well as to membranes which recreate the lipid composition of the inner surface of an epithelial apical membrane (Hill and Zeidel, 2000), did significantly reduce water permeability by reducing the fluidity of the bound leaflet. This intriguing finding led us to explore the expression pattern of Anx4 in the kidney collecting duct—a region of the nephron specialized for water reabsorption through hormone regulated insertion of water channels. Significantly, we were able to show that Anx4 colocalizes with aquaporin 2 (AQP2) subapically in the kidney medullary-collecting duct, the lumenal membrane of which is tight to passive water movement in the absence of vasopressin. Experiments designed to test whether there were direct functional interactions between Anx4 and AQP2 failed to show any effects however.
Barrier function of the plasma membrane has been explained as a function of the membrane lipid composition, of the asymmetric distribution of lipids in the bilayer, and of maintenance of that asymmetry in the apical membrane by the presence of tight junctions and the activity of phospholipid flippases (Devaux, 1992; Hill et al., 1999; Hill and Zeidel, 2000). The results presented here contribute further to this understanding and provide important new insights into how cells may regulate their membrane permeability in a rapid manner through Ca2+ signaling and the use of reversible protein–lipid interactions.
MATERIALS AND METHODS
Annexin A4 Purification
Annexin A4 was prepared and purified as described in Kaetzel et al. (1989).
Liposome Preparation
Lipids were purchased from Avanti Polar Lipids and mixed in the molar ratios specified before solubilization in chloroform:methanol (2:1). Lipid solutions containing 10 mg of total lipid were dried down under nitrogen at 37°C and were then placed in a chamber connected to a vacuum pump for 1 h to remove any residual organic solution. Dried lipids were then used immediately or stored under nitrogen at −20°C. Lipids were suspended in either 50 mM HEPES, 0.1 mM DTT, 10 mM carboxyfluorescein, pH 7.4, or in 150 mM NaCl, 20 mM HEPES, 0.1 mM DTT, 10 mM carboxyfluorescein, pH 7.4, and vortexed for 2 min. If proton permeability was to be measured, the carboxyfluorescein concentration was reduced to 1 mM. In the case of phosphatidylcholine (PC):PS liposomes, probe sonication using four bursts of 30 s followed by 3-min intervals on ice between bursts was sufficient to induce to the formation of liposomes with a radius of ∼60–80 nm. For cytoplasmic lipid liposomes this same treatment was followed by extrusion of lipids through a 200-nm polycarbonate filter 15 times, using an Avanti miniextruder. The resulting size distribution was comparable with PC:PS liposomes (Figs. 1 and 6 A). Extravesicular carboxyfluorescein was removed by gel filtration on PD-10 columns (Amersham Biosciences) and residual extravesicular carboxyfluorescein was quenched by anti-fluorescein antibody (Molecular Probes).
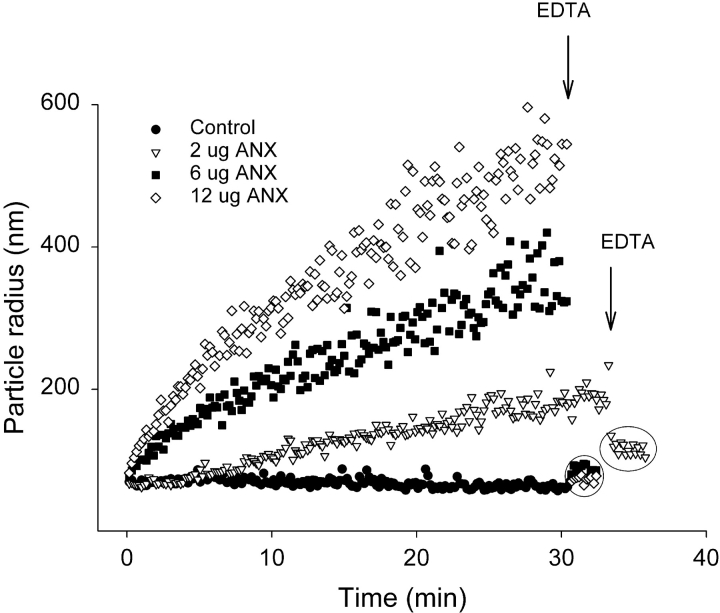
Figure 1.
Liposomes aggregate in the presence of Ca2+ and annexin A4. PC:PS liposomes (20 μg lipid) were added to 2 ml buffer containing 1 mM Ca2+. Anx4 was then added in the indicated amounts and particle size was monitored continuously by quasi-elastic light scattering at 10-s intervals. 5 mM EDTA was added where indicated. Circles indicate the data points for each experimental condition after EDTA addition.
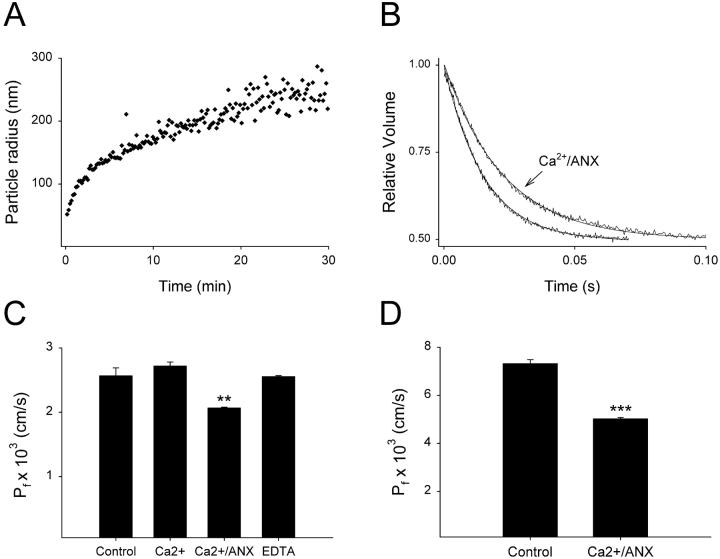
Figure 6.
AnxA4 binds to liposomes that mimic the cytoplasmic leaflet of the MDCK apical membrane and significantly reduces water permeability. (A) Quasielastic light scattering reveals that cytoplasmic leaflet liposomes aggregate in the presence of Ca2+ and Anx4. (B) Stopped-flow tracings of control and Anx-inhibited water flux in cytoplasmic lipid liposomes at 37°C. (C) Water permeability at 25°C is unaffected by Ca2+, but is significantly reduced when Anx4 is added for 20 min. EDTA reverses the inhibition (n = 3; mean ± SEM, **, P < 0.01). (D) The inhibition of water permeability is enhanced at 37°C and results in an overall reduction of 31% (P < 0.001).
Determination of Liposome Size/Liposome Aggregation Assay
Liposome sizes and size distribution were determined by quasi-elastic light scattering using a DynaPro LSR particle sizer and DYNAMICS™ data collection and analysis software. Aggregation of liposomes was monitored continuously by quasi-elastic light scattering for 30 min after addition of 1 mM CaCl2 and varying amounts of purified Anx4 protein. Measurements were made every 10 s. The optimum ratio of Anx4 protein/liposomes required to induce maximal aggregation kinetics was determined for each new liposome preparation. Typically an estimated 20-μg lipid (calculated from the known lipid mass in organic solvent initially) had 15–25 μg Anx4 added.
Water and Proton Permeability Measurements
Osmotic water permeability (Pf) was measured at 25°C or at 37°C by stopped-flow fluorometry as described previously (Lande et al., 1995; Rivers et al., 1998; Hill and Zeidel, 2000). Briefly, permeabilities were determined using a stopped-flow fluorometer (SX.18 MV; Applied Photophysics) with a measurement dead-time of <1 ms. Liposomes containing 10 mM carboxyfluorescein or carboxyfluorescein-loaded rat kidney endosomes were rapidly mixed with an equal volume of an identical buffer that had three times the osmolality due to sucrose addition. The rate of water efflux from vesicles was measured as a function of vesicle shrinkage and carboxyfluorescein self-quenching. Typically 8–10 quench curves were averaged and fit with a single exponential curve. From parameters which included the initial rate of quenching, vesicle diameter and applied osmotic gradient, Pf was calculated using MathCad software (MathSoft, Inc.). Proton permeabilities were measured by stopped-flow fluorometry as described (Rivers et al., 1998; Hill and Zeidel, 2000). This assay used pH-dependent quenching of carboxyfluorescein upon exposure of liposomes to isoosmotic buffer at a reduced pH. The pH gradient was typically 0.4–0.6 pH units which has been shown not to induce the formation of limiting electrochemical gradients (Deamer and Nichols, 1983).
Fluorescence Anisotropy
Fluorescence polarization measurements were performed on liposomes which had been equilibrated with 2 μM 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-sn-glycero-3-phosphocholine (NBD-HPC) for 30 min at room temperature in the dark. This time was shown to be sufficient for complete equilibration of the probe between aqueous and membrane phases. NBD-HPC is a lipid analogue known to label the outer leaflet of bilayers and to show virtually no flip-flop between leaflets over the time frames of the experiments (Kaiser and London, 1998). Fluorescence polarization measurements were conducted on an Aminco Bowman Series 2 luminescence spectrometer equipped with automated prism polarizers at emission/excitation wavelengths of 465/530 nm. Data collection and anisotropy analysis were performed on AB2 software supplied by Spectronic Unicam.
In vivo Loading of AQP2-containing Rat Kidney Endosomes with Carboxyfluorescein
Six female Sprague-Dawley rats (250–300 g) were anesthetized with 2.5% halothane and an incision made just right of the midline from the ventral surface at the base of the neck to expose the jugular vein. PE50 tubing was used to catheterize the jugular and 1 ml of 75 mM carboxyfluorescein in saline injected. After 15 min the kidneys were removed and the animal killed. It has been shown previously that this procedure results in the loading of AQP2-containing endosomes via endocytosis of carboxyfluorescein from the lumen of renal papillary collecting duct cells (Harris et al., 1994).
Purification of Carboxyfluorescein-loaded, AQP2-containing Endosomes from Rat Kidney
Purification of endosomes was performed according to the method of Harris et al. (1994). Endosomes were washed with EDTA-containing buffer before experiments on water permeability in order to remove any copurifying annexins.
Immunoblot Analysis of AQP2-containing Endosomes
Membrane samples were run on SDS-PAGE using 10% precast mini-gels (Gradipore) and then electrotransferred to BioRad Immun-Blot PVDF membrane. AQP2 was detected using polyclonal rabbit anti-rat AQP2 antisera (Alpha Diagnostics International) at 1:2,000 dilution and detected by ECL (Amersham Biosciences) using standard methods.
Hydration Protocol for Rats
Male Sprague-Dawley rats (Harlan Sprague-Dawley) weighing 225–250 g were housed two per cage and were maintained on a 12-h light/dark cycle. The animals were acclimated to the housing conditions for five days, during which period they had free access to tap water and standard rat chow. At the commencement of the thirsting and hydration protocol, the rats were randomly divided into three groups (n = 4 per group)—controls, thirsted, and hydrated—and subjected to the following protocol (Kishore et al., 1996) (Table I) .
TABLE I.
Hydration Protocol
| Group | 0–48 h | 48–96 h |
|---|---|---|
| Controls | Tap water ad libitum | Tap water ad libitum |
| Thirsted | 600 mM sucrose solution in tap water - ad libitum | None |
| Hydrated | 600 mM sucrose solution in tap water - ad libitum | 600 mM sucrose solution in tap water - ad libitum |
The sweetness of the sucrose solution makes the rats drink more fluid. During the last 24 h of the experimental period (72–96 h), the rats were placed in individual metabolic cages and the urine collected. The urine samples were assayed for osmolality using a freezing point depression osmometer as described previously (Kishore et al., 2000). At the end of the experimental period (96 h), the animals were killed by sodium pentobarbital overdose. Kidneys were quickly removed, rinsed in chilled phosphate-buffered saline, and the inner medullae were dissected out. The dissected tissues were flash frozen in liquid nitrogen and stored at −80°C till analyzed. The frozen inner medullary tissues were homogenized, protein concentration was determined, and solubilized for immunoblotting as described previously (Kishore et al., 2000). Kidney samples were also fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned at 5-μm thickness for immunocytochemical examination.
Immunofluorescent Localization and Immunoblotting of AQP2 and Anx4
Protein samples separated by SDS-PAGE (2 μg/lane) were transferred to nitrocellulose sheets and duplicate blots were probed with affinity-purified rabbit anti-AQP2 and sheep antiannexin IV primary antibodies 1:10,000 (Kaetzel et al., 1989) followed by ECL detection. The integrated optical density of each protein band was quantitated according to Kutcher et al. (2003) using Image Pro software (Media Cybernetics). For immunolocalization, deparaffinized sections were incubated with rabbit anti-AQP2, washed three times in PBS, labeled with fluorescein-labeled goat anti–rabbit IgG, and washed again with PBS. The sections were then incubated with sheep antiannexin IV, incubated for 45 min, washed, and then followed by Cy3-conjugated donkey anti–sheep IgG. Sections were mounted in PBS/glycerol containing 0.1% p-phenylenediamine and were viewed in a Nikon epifluorescene microscope.
Statistics
Testing for significant differences was performed by Student's t test.
RESULTS
Since Anx4 is known to aggregate membranes upon binding (Kaetzel et al., 2001) we developed an assay based upon this phenomenon in order to optimize the ratio of protein to lipid in our experiments. Using quasielastic light scattering we measured the change in average particle size over time in a solution of liposomes (PC:PS, 1:1 molar ratio) containing varying amounts of purified Anx4. Fig. 1 shows that addition of Anx4 to liposomes in the presence of 1 mM Ca2+ resulted in a time-dependent increase in particle size, which is consistent with the progressive aggregation of liposomes into larger and larger clusters. Addition of EDTA to chelate Ca2+ and reverse Anx4 lipid binding caused an extremely rapid dissociation of aggregates back to free liposomes. In Fig. 1 varying amounts of Anx4 were added to 20 μg of liposomes. Addition of 18 μg Anx4 resulted in identical aggregation kinetics as was seen for 12 μg (unpublished data). It is clear that the rate of aggregation is dependent on the ratio of Anx4/lipid and that it is a saturable process. If aggregation was allowed to continue beyond 30 min a second distribution of particle sizes with a much larger radius (thousands of nm) became detectable and more prominent with time. This was probably due to the aggregation of aggregates into very large assemblies. This assay represented a simple means by which we could determine the optimum ratio of protein to lipid in order to maximize membrane-binding effects for different liposome preparations and for different membrane compositions.
The water permeability of PC:PS liposomes was tested with stopped-flow fluorometry, which measures the rate of self-quench of entrapped carboxyfluorescein when liposomes shrink in response to a rapidly imposed osmotic gradient. The rate of shrinkage is directly proportional to the rate of water efflux and hence the membrane permeability. Values for osmotic water permeability calculated using this technique have been shown to correlate very well with values derived from near membrane measurements of Na+ gradients in the vicinity of planar bilayers (Pohl et al., 1997; Krylov et al., 2001). Ca2+ (1 mM) or Anx4 protein added alone did not alter the permeability of the membrane after 20 min (Fig. 2 A). EDTA (5 mM) resulted in a slight increase in permeability which was statistically significant (P < 0.05). However, when Ca2+ and Anx4 were added simultaneously to induce protein–membrane interactions, there was a highly significant (P < 0.001) 29% drop in the membrane water permeability from 5.83 × 10−3 cm/s to 4.14 × 10−3 cm/s. Stopped-flow tracings and fitted single exponential curves showing liposome shrinkage under control and annexin-bound conditions are shown in Fig. 2 B. Addition of EDTA to Anx-bound liposomes restored permeability to control levels (Fig. 2 A), demonstrating that the effect was completely reversible under conditions, which would be expected to remove calcium and therefore protein from the membrane surface (see Fig. 1). These data clearly indicate that Anx4 membrane binding alters the biophysical properties of the membrane in such a way as to limit passive water diffusion. Since the protein can reliably be assumed to bind only to the outer leaflet and each leaflet in a bilayer has been shown to offer independent resistances to water permeation (Negrete et al., 1996; Krylov et al., 2001), it is possible to calculate that the effect on Anx-binding on the outer leaflet is to reduce its permeability by 45% compared with a protein-free leaflet.
Figure 2.
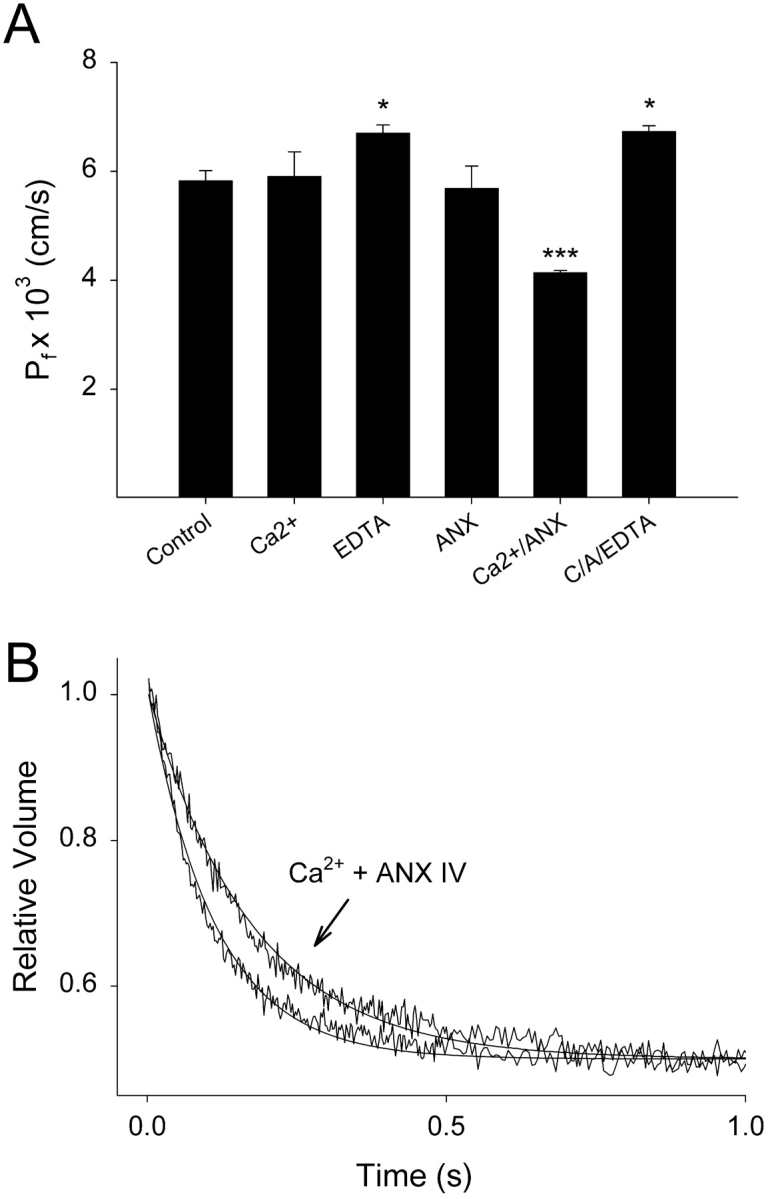
Water permeability of PC:PS liposomes is reduced upon Ca2+-induced binding of annexin A4 to membranes. Water permeability of liposomes was measured by stopped-flow fluorometry 20 min after additions were made. (A) Addition of Ca2+ or Anx4 alone did not affect the osmotic water permeability coefficient (Pf) of the liposomal membrane. Addition of EDTA alone slightly increased water permeability (P < 0.05). Addition of Ca2+ and Anx4 together for 20 min resulted in a highly significant reduction in membrane water permeability (P < 0.001), which was reversible within 2 min by the subsequent addition of 5 mM EDTA (C/A/EDTA; n = 3–6 for each condition, mean ± SEM). (B) Stopped-flow tracings showing relative rates of vesicle shrinkage following exposure of liposomes to a sudden osmotic gradient. Liposomes treated with Ca2+ and Anx4 shrink more slowly than controls. Single exponential curves have been fitted to the fluorescence data.
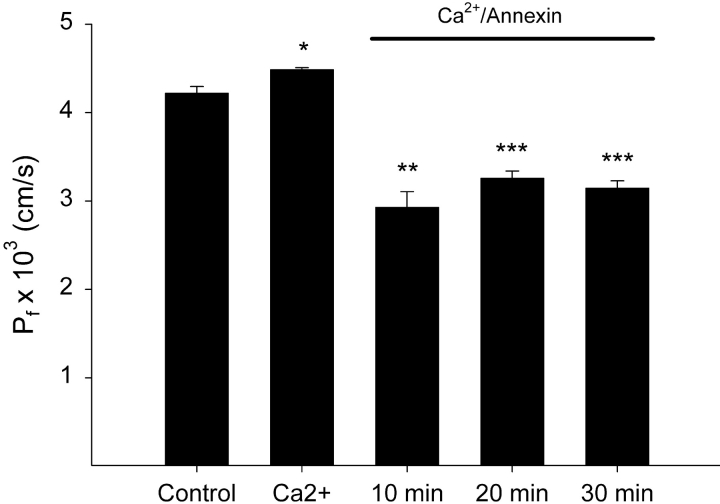
The kinetics of this process were explored (Fig. 3) . After 10 min of Anx4 binding, the water permeability of the liposomal membrane was maximally reduced. This reduction was stably maintained for up to 30 min. Ca2+ alone induced a small but significant increase in water permeability; however, this effect was not seen consistently. These data demonstrated that Anx4 binding is rapid, the effect on membrane permeability is sustained and implied that liposomal aggregation (as shown in Fig. 1) did not affect our ability to measure permeability. It also implied that aggregation per se was not causing changes to membrane order with resulting alterations to permeability.
Figure 3.
Reductions in water permeability effected by Anx4-binding occur rapidly and are stable. Water permeability of PC:PS liposomes was measured after addition of Ca2+ and then at defined times after addition of Anx4 (n = 3, data shown is mean ± SEM; **, P < 0.01, ***, P < 0.001).
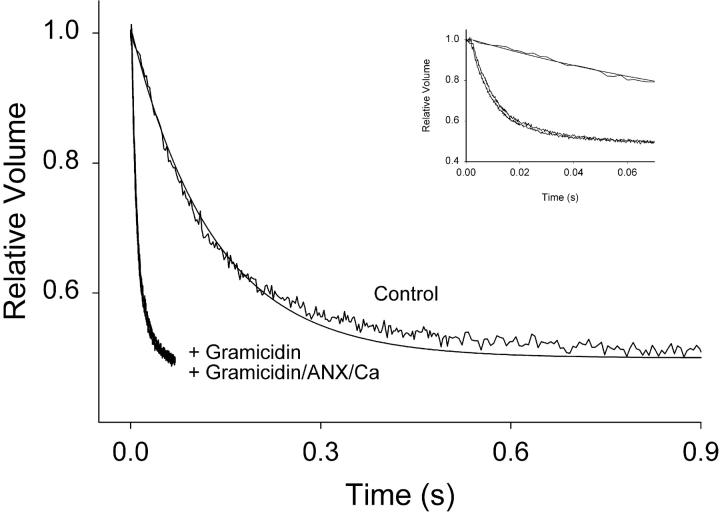
To establish that the aggregation of liposomes was not producing an unstirred layer, which could induce an apparent reduction in membrane permeability, we performed an experiment with gramicidin, a pore-forming peptide that dramatically increases membrane permeability to water. We reasoned that if the reductions in water permeability we had seen with Anx binding were due to the formation of unstirred layers, then a similar low Pf value should be obtained even for gramicidin-treated membranes. However, if the reductions were due to biophysical changes to the properties of the bilayer, then gramicidin-treated and gramicidin-treated plus Ca2+ and Anx should show no difference in permeability due to the fact that the membrane has essentially been removed as a barrier to water flow. As can be seen in Fig. 4 , the addition of 1 μM gramicidin dramatically increased the rate of water flux across the membrane 15-fold compared with control membranes. However, there was no change in the rate of water flux when Anx4 and Ca2+ were added to the gramicidin-treated liposomes for 10 min. Detail of the initial 70 ms of the same three curves is shown in the inset and demonstrates that the gramicidin-treated membranes with or without Ca/Anx are superimposable. Clearly, in the presence of gramicidin there was no change to the water flux rate as a consequence of Anx binding. Therefore unstirred layers do not contribute to the permeability reductions noted earlier.
Figure 4.
Reductions in membrane water permeability are not due to the formation of unstirred layers on liposomal aggregation. Water permeability was measured in PC:PS liposomes and in liposomes treated with 1 μM gramicidin for 10 min, which resulted in dramatically increased osmotic water permeability. When gramicidin-treated liposomes were incubated in the presence of 1 mM Ca2+ and Anx4 for 30 min the rate of water permeability did not change (inset shows an expanded scale over the first 70 ms and demonstrates superposition of the two curves). If unstirred layers were providing extra resistance to water flux, then it would dramatically manifest itself under these conditions where the membrane has been effectively removed as a barrier to water permeation.
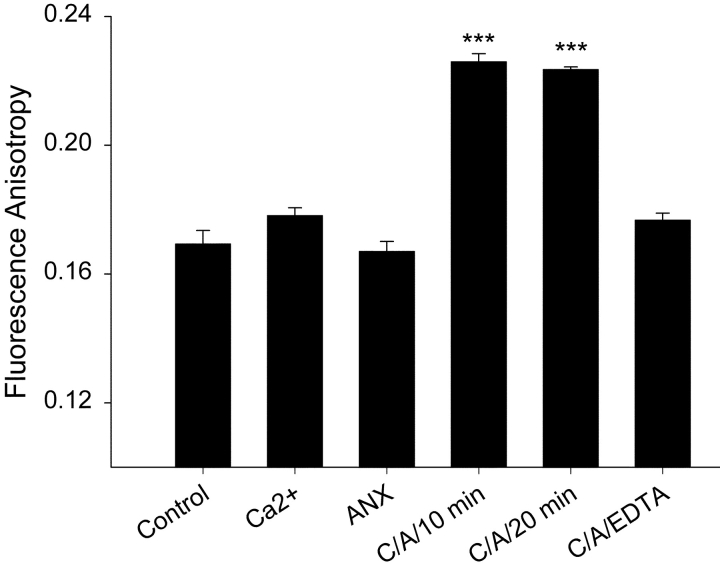
To explore further the nature of the membrane interaction we measured membrane fluidity using fluorescence anisotropy. We used a phospholipid analogue (NBD-HPC) as an anisotropy probe that localizes to the outer leaflet of the bilayer. When tested under a range of conditions it could be shown that Anx4 membrane binding induced a rigidification of the outer leaflet, as indicated by a significant increase (P < 0.001) in the anisotropy of the probe (Fig. 5) . Anx4 or Ca2+ alone had no effect on membrane fluidity and addition of EDTA to Anx-bound liposomes restored leaflet fluidity to control levels. These data demonstrated a significant alteration in phospholipid order in the outer leaflet of the membrane after Anx4 polymerization on the surface. Since NBD-HPC reports on the microenvironment of the region close to the headgroup (Kaiser and London, 1998) rather than deeper in the hydrophobic core of the bilayer, it is likely that the increased rigidity encountered by the probe reflects limitations to head group or near head group acyl chain motion.
Figure 5.
Water-permeability reductions induced by annexin A4 binding are due to rigidification of the outer leaflet of the liposomal membrane. Polarized fluorescence anisotropy was performed on PC:PS liposomes incubated for 30 min with 2 μM NBD-HPC. Polarized excitation and emission fluorescence was monitored at 465 and 530 nm, respectively. Addition of 1 mM Ca2+ or 15 μg Anx4 alone for 10 min did not alter fluorescence anisotropy. Addition of Ca2+ and Anx4 (C/A) simultaneously resulted in a significant rigidification (as measured by increased anisotropy) of the outer leaflet of the liposomal membrane. Subsequent addition of EDTA resulted in an increase in fluidity to values exhibited by the control. (n = 3, data shown is mean ± SEM; ***, P < 0.001).
While the data shown thus far indicate a reproducible and reversible effect on the membrane permeability of PC:PS liposomes, we wished to explore whether more physiologically relevant membranes would also be affected. To this end we constructed liposomes that resemble the inner leaflet of an epithelial apical membrane. Since Anx4 has been localized to subapical regions of various epithelia, including the kidney collecting duct (Fig. 8), and has been shown to regulate the activity of apical membrane proteins such as chloride channels (Kaetzel et al., 1994), it is reasonable to assume that annexin A4 may bind to the inner surface of the plasma membrane. We have previously modeled the inner and outer leaflet of the MDCK apical membrane and explored the permeability properties of liposomes which recapitulated those leaflets (Hill and Zeidel, 2000; Krylov et al., 2001). Therefore, it seemed reasonable to use the known composition of this membrane to test whether annexin A4 could reduce its water permeability. Liposomes comprised of (by molar percentage) phospatidylethanolamine (PE), 40.1%; PS, 20.1%; phosphatidylinositol (PI), 2.5%; and cholesterol, 37.3%, were first tested for their ability to aggregate in the presence of Ca2+ and Anx4. As seen in Fig. 6 A, aggregation kinetics were similar to that observed with PC:PS liposomes. Anx4 in the presence of Ca2+ was indeed able to significantly reduce the water permeability of these membranes (Fig. 6, B and C), although the effect at 25°C was modest (19.5% reduction). When the water permeability was assayed at 37°C a substantial reduction of 31% upon Anx4 binding was noted (Fig. 6 D). This data clearly indicates the potential for Anx4 to reduce water permeability across the apical membrane of epithelial cells.
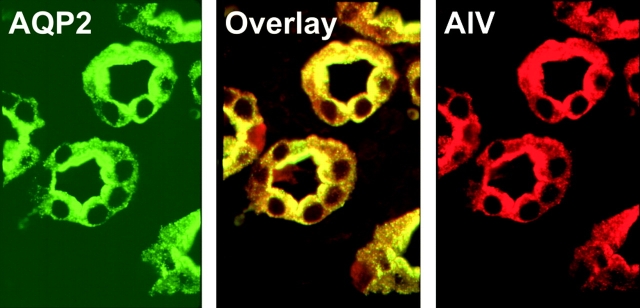
Figure 8.
Immunolocalization of AQP2 (green, left) and Anx4 (red, right), and colocalization by double exposure (yellow, center) in normal rat kidney inner medullary–collecting ducts.
Water permeation across phospholipid bilayers is thought to occur via a solubility-diffusion mechanism in which water molecules must first “dissolve” into the proximal hydrophobic environment of the membrane close to the head groups and then to diffuse across the bilayer (Finkelstein, 1987). Most small, uncharged solutes are believed to use this mechanism also. Protons, however, have anomolously high permeabilities when compared with other cationic species and it is thought they cross bilayers by a completely different mechanism that may utilize “water wires” (Nagle and Morowitz, 1978; Pomes and Roux, 1996; Brewer et al., 2001) or, alternatively, proton carriers such as free fatty acids to transport protons from one side of the bilayer to the other (Gutknecht, 1987b; Brown and Brand, 1991). We wished to see whether Anx4 binding could alter proton permeability. In Fig. 7 it can be seen that proton permeation was inhibited by Anx4 binding but was unchanged by the presence of Ca2+ alone. Data on the left shows the permeability coefficients at 25°C, whereas the right hand bars indicate the effect at 37°C with proton permeability reductions of 15% (P < 0.05) and 19.5% (P < 0.05), respectively.
Figure 7.
Anx4 binding to cytoplasmic lipid liposomes significantly reduces proton permeability. Proton permeability was measured by stopped-flow fluorometry under conditions in which liposomes were exposed to a pH gradient. pH-induced changes to fluorescence were monitored and proton permeability coefficients were calculated. Addition of 1 mM Ca2+ for 20 min had no effect on proton permeability at 25°C however addition of Ca2+ and annexin A4 together for 20 min significantly reduced proton permeability by 15%. At 37°C (right hand bars) proton permeability under control conditions was enhanced and the inhibitory effect of Anx4-binding resulted in a 20% inhibition (n = 3; mean ± SEM; P < 0.05).
Because we had shown that the membrane-organizing effects of Anx4 could regulate the water permeability of epithelial membranes we investigated whether Anx4 was present in the kidney-collecting duct due to its key role in the urine concentration mechanism and volume homeostasis. We were also interested to know whether there might exist a functional relationship between Anx4 and AQP2 that is normally found either in subapical vesicles or inserted into the apical membrane. Groups of rats were either deprived of water, allowed to drink ad libitum, or were hydrated (see materials and methods). There was a twofold increase in the urine osmolality in thirsted rats as compared with the control group (4,252 ± 897 mosmol/kg H2O vs. 2,004 ± 89 mosmol/kg H2O; mean ± SE). On the other hand, the hydrated rats showed a fivefold decrease in the urine osmolality as compared with the controls (394 ± 43 mosmol/kg H2O).
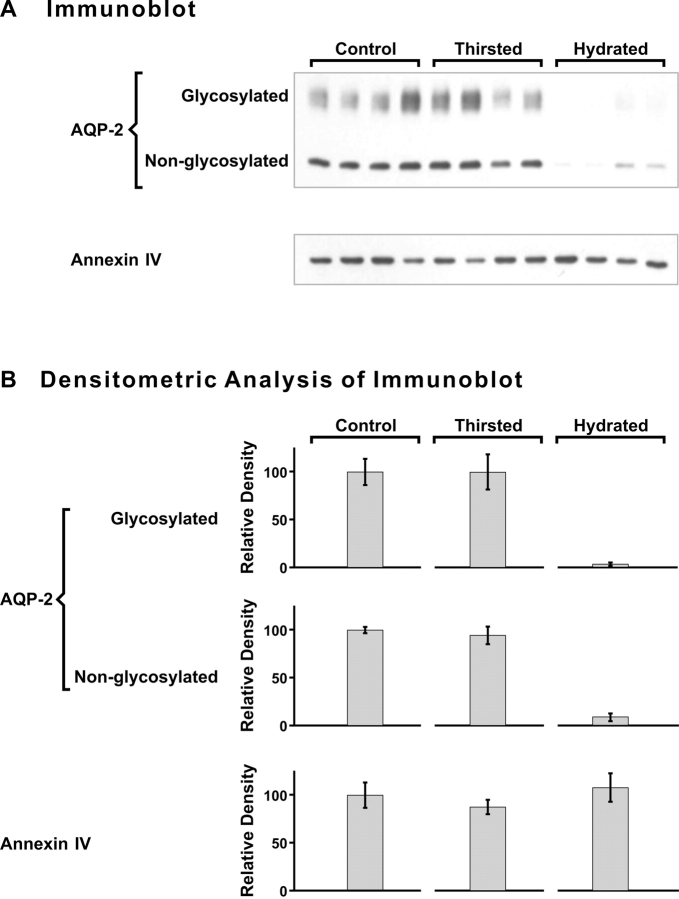
Kidneys were removed and immunoblot analysis performed to determine relative levels of Anx4 and AQP2 protein. In Fig. 8 it can be seen by immunofluorescence that both Anx4 and AQP2 are abundantly expressed in the collecting duct epithelial cells. Both proteins show a strong colocalization in the apical region (Fig. 8, overlay). In Fig. 9 A it can be seen by immunoblotting that Anx4 was present at a consistent level regardless of the hydration status of the animals, whereas AQP2 was dramatically down-regulated in hydrated animals. Quantitation by densitometry shown in Fig. 9 B illustrate that there were no significant changes in Anx4 levels with different hydration states and no significant difference between AQP2 under control and thirsted states. Given that Anx4 and AQP2 were present in close physical proximity we wished to know whether Anx4 could directly regulate AQP2 water channels and therefore the membrane permeability of the collecting duct. To answer this question we used an in vivo dye loading technique in which anesthetized rats were i.v. injected with carboxyfluorescein, thereby permitting rapid uptake of the dye into AQP2-containing endosomes in the kidney (see materials and methods). After loading, the kidneys were removed and carboxyfluorescein-loaded endosomes purified and tested for functional water channels. Fig. 10 A shows a fivefold enrichment for AQP2 in the purified endosome fraction compared with homogenate and Fig. 10 B demonstrates rapid water flux in these vesicles that is completely inhibited by mercuric chloride. When Anx4 was added to endosomes at 1:1 and 2:1 ratios of Anx4 to endosome protein concentration (concentrations sufficient to fully saturate potential binding sites) there was no effect on water flux. Fig. 10 C shows that the kinetics of water permeation were unchanged upon addition of a 2:1 ratio of Anx4/endosomal protein. Thus, our results demonstrated no direct regulation of AQP2 function by Anx4.
Figure 9.
Immunoblot analysis of Anx4 and AQP2 in rat kidney inner medulla. (A) Samples of kidney inner medulla from control, thirsted, and hydrated rats (each lane represents an individual animal) were probed with anti-AQP2 and anti-Anx4. AQP2 is present in nonglycosylated and glycosylated forms. (B) Densitometric quantitation of protein bands in A (bars show mean ± SEM). Although the levels of AQP2 decrease dramatically with hydration, Anx4 levels remain constant during thirsting and hydration.
Figure 10.
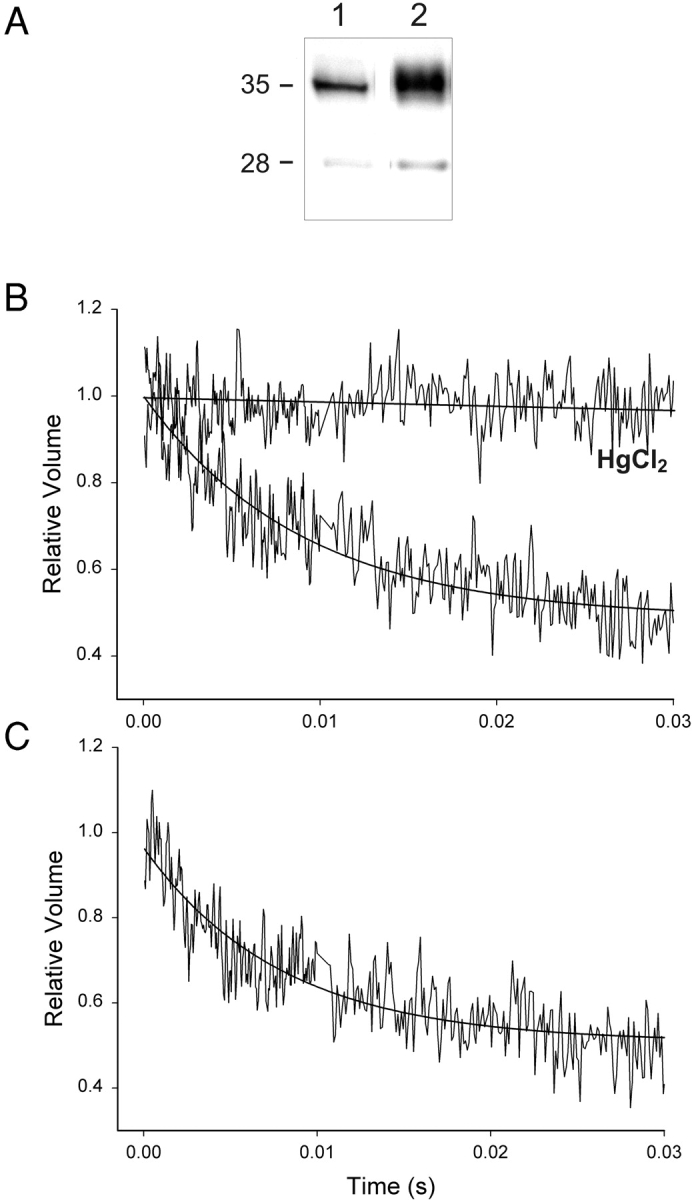
Aquaporin 2–containing endosomes from control rat kidney show Hg2+-inhibitable water flux that is unchanged by addition of Anx4/Ca2+. (A) Endosomes enriched in AQP2 were isolated from rat kidney after in vivo loading with carboxyfluorescein as described in materials and methods. Western blotting of equivalent protein loaded lanes (10 μg) revealed that the purified fraction was fivefold enriched for AQP2 (lane 2) when compared with homogenate (lane 1). (B) AQP2-containing endosomes exhibited rapid water transport which was completely inhibited by treatment of membranes with 1 mM HgCl2 for 30 min (C) AQP2-containing endosomes treated with Ca2+ and Anx4 for 30 min exhibited water transport kinetics that were identical to untreated membranes. Anx4 was added to endosomes at 1:1 and 2:1 protein ratios (2:1 shown). Results shown are representative of three separate experiments.
DISCUSSION
Annexins possess a common large core region consisting of four conserved motifs of ∼70 amino acids. Each mediates the phospholipid and Ca2+-binding functions that all family members share in common. Isoforms differ from each other primarily in the NH2 terminus, a region which is postulated to confer upon each annexin its unique location and cellular function. Annexin A4, like annexin A1 and A2 has been shown to aggregate artificial vesicles and specific subcellular compartments like secretory chromaffin granules (Zaks and Creutz, 1990; Kaetzel et al., 2001). The ability of Anx4 to aggregate a set quantity of liposomes was shown to be a saturable function of the amount of protein added to the cuvette. At concentrations of Anx4 sufficient to maximally stimulate liposome aggregation it can be assumed that most if not all membrane surfaces were fully coated with protein.
Junker and Creutz (1994) showed that Anx4 exhibited the following lipid binding preferences: cardiolipin > PS = PA > PG > PI > PIP2 > PE >> PC and that protein binding has complex kinetics that depend on head group charge, head group size, Ca2+ availability, protein concentration, lipid concentration, and interestingly, the precise combinations of lipids in the membrane. For example, PE in combination with PC was a fairly poor substrate for binding; however, PE in combination with PS proved to be much better than PS alone. This synergism is intriguing because the inner leaflet of the plasma membrane has precisely this combination of lipids augmented by the presence of PI, another lipid Anx4 has high affinity for (Junker and Creutz, 1994). Our experiments confirm the affinity of Anx4 for membranes of this composition (Fig. 6) and the subsequent effects on membrane ordering.
The physiological role of Anx4 continues to evolve. It has been shown to regulate chloride efflux from an immortalized epithelial cell line; however, this occurs by an indirect mechanism involving CaM kinase. Anx4 is neither an inhibitor of, nor a substrate for the kinase. Therefore it is unclear how Anx4 exerts its effects on ion conductance. One possibility must include an alteration to the membrane state in which the channel resides. Annexin A5 has been shown to decrease membrane fluidity in small unilamellar vesicles (Megli et al., 1998) and in mitochondria (Megli et al., 2000) and to increase the conductance properties of planar lipid bilayers (Sokolov et al., 2000). Indeed, Sokolov et al. (2000) showed that both Anx A5 and Anx A12 binding to planar bilayers had profound effects on the functional properties of the membrane. They observed a 60% decrease in nonactin-induced conductance and alterations to the characteristic properties of alamethicin-induced conductance. The authors rule out changes to surface charge or dipole potential as mechanisms by which annexins affect membrane conductance and speculate that altered viscosity, phase separation, or changes in curvature could be responsible. These observations suggested a potential global or local organizing effect as a means to transduce calcium signaling via annexin-binding. We demonstrate in these series of experiments that Anx4 has substantial effects on membrane fluidity and that this rigidification of the membrane is sufficient to reduce the overall water permeability in not only highly enriched PS-containing membranes but also in membranes with physiological concentrations of PS, PE, and PI. Thus, major effects on membrane conductance properties may be a functional characteristic shared by many annexins.
The degree to which water is inhibited from permeating across the contact leaflet can be calculated, since individual leaflets in a bilayer offer independent resistances to water permeation (Hill et al., 1999; Krylov et al., 2001). This calculation reveals that the permeability of the inner leaflet of an epithelial plasma membrane is reduced 48% by Anx4 binding. As this protein is found apically in a range of epithelia, it is ideally located to exert rapid and reversible effects on membrane permeability (Kaetzel et al., 1994; Dreier et al., 1998). The question arises as to whether this level of inhibition has physiological importance. Most epithelia, with several notable exceptions (these include urothelium, cortical collecting duct, thick ascending limb of Henle, and stomach) are not faced with the task of maintaining steep osmotic gradients between lumen and interstitium and therefore do not need to be impermeable. Indeed the opposite seems to be more likely with permeability enhancement due to the aquaporin family of water channels being found in an expanding list of tissues and cell types. Virtually all tissues faced with a need to secrete or absorb fluid appear to use aquaporins to mediate near isoosmotic water transport (Ma and Verkman, 1999; Verkman et al., 2000; Verkman, 2002) and yet, paradoxically, the consequences of ablating these proteins is often mild unless the animal is placed under some degree of hydration-related stress. Conceivably, fluid secreting epithelia which express Anx4 may be faced with microosmotic or transient gradients and thus the possibility exists for inappropriate volume expansion or contraction. Anx4-binding may prevent cells from becoming hyper- or hypovolemic. For different reasons, the apical membrane of the inner medullary–collecting duct (IMCD) which is somewhat water-tight in the absence of vasopressin and which we have shown coexpresses Anx4 (Fig. 8), may be made even tighter through Ca2+-dependent annexin binding. For example in isolated perfused tubule studies, cortical collecting duct had an osmotic water permeability of 1.5 × 10−3 cm/s while IMCD was 6 × 10−3 cm/s (Masilamani et al., 2000). Anx4 could contribute to barrier properties in specific segments such as the IMCD by reducing the fluidity of the inner leaflet. Control of membrane permeability has been thought to be a static property of cell membranes, dictated primarily by lipid composition. These results introduce a novel paradigm for cellular regulation of permeability. It is also worth noting that while the discussion has focused on water as the permeating species, a reduction in membrane fluidity will also restrict the diffusion of a number of other important small nonionic solutes e.g., urea and ammonia (Hill et al., 1999; Hill and Zeidel, 2000).
Of interest was the observation that proton permeability was also restricted by Anx4. It is unclear whether this function is of physiological significance. Given the presence of acidified compartments within cells, Anx4 could play a role in restricting proton leakage from endosomes and/or lysosomes; however, such a function is speculative and further studies will be required to establish such a role. The mechanism by which Anx4 reduces proton flux is likely to be different from that of water since it has been shown in a number of studies that proton permeability does not correlate well with membrane fluidity (Gutknecht, 1987b; Wilkes et al., 1989; Lande et al., 1995) (see Rossignol et al., 1982; Deamer and Nichols, 1989; Krishnamoorthy and Krishnamoorthy, 2001; Wan et al., 2002 for alternative viewpoints). Currently, four models have been proposed to explain anomolously high proton permeation rates (compared with other cations) across phospholipid bilayers. The first invokes the concept of “water wires” that allow protons to move through membranes by hopping along a chain of hydrogen-bonded water molecules (Nagle and Morowitz, 1978; Deamer and Nichols, 1989; Pomes and Roux, 1996; Brewer et al., 2001). Conceivably, Anx4 binding could sterically reduce access to water within the bilayer and thereby inhibit proton flux. The second model suggests that free fatty acids within membranes can become protonated and then flip across to the other monolayer before releasing the proton on the other side (Gutknecht, 1987a). Although this is a less likely possibility in our system due to the use of purified lipids to create membranes, it is feasible that the reduction in fluidity which accompanies Anx4 binding could reduce highly active contaminant fatty acid translocation rates. The third model envisages the presence of transient pores or hydrated defects that would allow faster proton flux by avoiding the Born energy barrier associated with passage through the low dielectric hydrophobic interior of the bilayer. Paula et al. (1996) examined the effect of bilayer thickness on proton permeation and concluded that transient pores could explain proton permeation behavior for lipid bilayers having up to 18 carbon acyl chains but that solubility-diffusion better predicted longer chain phospholipids. The fourth postulates the existence of protonated and deprotonated water clusters within the bilayer (Haines, 2001). It is certainly conceivable that Anx4 binding could inhibit the formation of defects at the membrane surface by ordering the lipid head groups.
Although we have emphasized the possible global significance of permeability reductions, another possibility is worth considering. Namely, that Anx4–membrane interactions occur on a much more localized scale in response to calcium signals. Spatially restricted calcium signaling could result in microdomain formation involving protein on the inner surface of the plasma membrane. In this event, alterations to membrane permeability are likely to be secondary manifestations rather than primary events. The role of Anx4 in creating microdomains could involve effects on membrane-embedded proteins mediated through alterations to membrane fluidity. While speculative, it is certainly true that some membrane proteins are able to have their activities modulated by the physical state of the membrane. Examples include the EGF receptor that binds more ligand, has increased autophosphorylation and is inhibited from being internalized in response to the removal of cholesterol from the membrane in which it resides (Pike and Casey, 2002). Other examples include Na/K-ATPase, which increases its activity depending on the lipids into which it is reconstituted (Cornelius, 2001), and the epithelial sodium channel that is activated by localization to cholesterol and sphingolipid enriched microdomains within the apical membrane of epithelial cells (Hill et al., 2002; Shlyonsky et al., 2003). In the case of Anx4, membrane ordering effects may alter the activity of membrane-resident proteins, alter protein conformation in ways which facilitate, or inhibit interactions with other proteins or even affect the trafficking of vesicles.
Our in vitro studies indicated that Anx4 can control membrane water permeability and others have shown that the protein localized to the apical pole in various epithelia. We therefore sought to examine the intracellular distribution of Anx4 in principal cells of the kidney-collecting duct. These cells control urine osmolality by increasing or decreasing the permeability of their apical membrane through hormonally regulated insertion or retrieval of AQP2 water channels. Our experiments demonstrated a striking colocalization of the two proteins, leading us to wonder whether Anx4 might exert a direct regulatory effect on the water channel. Since it has been shown recently that Ca2+ signaling appears to be important in AQP2 membrane insertion (Chou et al., 2000), there exists a common regulatory pathway which is known to affect the localization of both proteins. To investigate whether a direct interaction exists we exposed isolated AQP2-containing endosomes to calcium and Anx4. Under these conditions there was no alteration to AQP2-mediated water flux, therefore indicating that Anx4 is unlikely to affect AQP2 activity directly. As Anx4 is able to alter membrane fluidity it is conceivable that any effects on principal cell membrane permeability could be mediated through alterations to AQP2 trafficking rather than function.
The kidney appears to express a limited repertoire of annexins, some of which exhibit specificity for particular regions of the nephron. Annexin A5 has been found in distal tubules but not proximal (Matsuda et al., 2001), whereas annexin A1 is present in epithelia of the Bowman's capsule, the macula densa and the collecting duct, but shows little immunostaining in the medullary-thick ascending limb (McKanna et al., 1992). Little information is available, however, about the subcellular distribution of annexins in kidney, so it is difficult to speculate on whether there may be other annexin isoforms present coincident with Anx4 in principal cells. Annexin 13b is exclusively localized to intestine and kidney and has been shown to participate in apical membrane trafficking in MDCK cells, but precise immunohistochemical localization in kidney was not performed (Fiedler et al., 1995). It will be interesting to determine whether this isoform is present in collecting duct and whether it participates in the trafficking of AQP2-containing vesicles.
We conclude that Anx4 is localized apically in IMCD and can, in theory, regulate membrane permeability and alter membrane fluidity. Questions that remain to be answered are whether its actions occur on a global scale or are restricted to microdomain formation on membrane surfaces with direct or indirect effects on resident apical proteins. These experiments indicate a potential new paradigm for the control of membrane permeability that utilizes rapid and reversible protein–lipid interactions rather than modifications to membrane lipid composition.
Acknowledgments
We wish to thank Susan Meyers for performing the intravenous injections.
This work was supported by National Institutes of Health Grants DK43955 (to M. Zeidel) and DK46433 (to J. Dedman and M. Kaetzel) and by the Smith Kline Beecham Young Investigator Grant of the National Kidney Foundation (to W. Hill).
David C. Gadsby served as editor.
Bellamkonda K. Kishore's present address is Division of Nephrology and Hypertension, University of Utah Health Sciences Center and VA Salt Lake City Health Care System, Salt Lake City, UT 84132.
Footnotes
Previously known as annexin IV. New nomenclature adopted at the 50th Harden Conference on Annexins, Wye College, UK; September, 1999.
Abbreviations used in this paper: Anx4, annexin A4; AQP2, aquaporin 2; IMCD, inner medullary–collecting duct; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine.
References
- Brewer, M.L., U.W. Schmitt, and G.A. Voth. 2001. The formation and dynamics of proton wires in channel environments. Biophys. J. 80:1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G.C., and M.D. Brand. 1991. On the nature of the mitochondrial proton leak. Biochim. Biophys. Acta. 1059:55–62. [DOI] [PubMed] [Google Scholar]
- Chan, H.C., M.A. Kaetzel, A.L. Gotter, J.R. Dedman, and D.J. Nelson. 1994. Annexin IV inhibits calmodulin-dependent protein kinase II-activated chloride conductance. A novel mechanism for ion channel regulation. J. Biol. Chem. 269:32464–32468. [PubMed] [Google Scholar]
- Chou, C.L., K.P. Yip, L. Michea, K. Kador, J.D. Ferraris, J.B. Wade, and M.A. Knepper. 2000. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J. Biol. Chem. 275:36839–36846. [DOI] [PubMed] [Google Scholar]
- Cornelius, F. 2001. Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry. 40:8842–8851. [DOI] [PubMed] [Google Scholar]
- Deamer, D.W., and J.W. Nichols. 1983. Proton-hydroxide permeability of liposomes. Proc. Natl. Acad. Sci. USA. 80:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer, D.W., and J.W. Nichols. 1989. Proton flux mechanisms in model and biological membranes. J. Membr. Biol. 107:91–103. [DOI] [PubMed] [Google Scholar]
- Devaux, P.F. 1992. Protein involvement in transmembrane lipid asymmetry. Annu. Rev. Biophys. Biomol. Struct. 21:417–439. [DOI] [PubMed] [Google Scholar]
- Dreier, R., K.W. Schmid, V. Gerke, and K. Riehemann. 1998. Differential expression of annexins I, II, and IV in human tissues: an immunohistochemical study. Histochem. Cell Biol. 110:137–148. [DOI] [PubMed] [Google Scholar]
- Fiedler, K., F. Lafont, R.G. Parton, and K. Simons. 1995. Annexin XIIIb: a novel epithelial-specific annexin is implicated in vesicular traffic to the apical plasma membrane. J. Cell Biol. 128:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, A. 1987. Water Movement through Lipid Bilayers, Pores, and Plasma Membranes: Theory and Reality. Wiley-Interscience, New York.
- Gerke, V., and S.E. Moss. 2002. Annexins: from structure to function. Physiol. Rev. 82:331–371. [DOI] [PubMed] [Google Scholar]
- Gutknecht, J. 1987. a. Proton conductance through phospholipid bilayers: water wires or weak acids? J. Bioenerg. Biomembr. 19:427–442. [DOI] [PubMed] [Google Scholar]
- Gutknecht, J. 1987. b. Proton/hydroxide conductance and permeability through phospholipid bilayer membranes. Proc. Natl. Acad. Sci. USA. 84:6443–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines, T.H. 2001. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 40:299–324. [DOI] [PubMed] [Google Scholar]
- Harris, H.W., M.L. Zeidel, I. Jo, and T.G. Hammond. 1994. Characterization of purified endosomes containing the antidiuretic hormone-sensitive water channel from rat renal papilla. J. Biol. Chem. 269:11993–12000. [PubMed] [Google Scholar]
- Hill, W.G., B. An, and J.P. Johnson. 2002. Endogenously expressed epithelial sodium channel is present in lipid rafts in A6 cells. J. Biol. Chem. 277:33541–33544. [DOI] [PubMed] [Google Scholar]
- Hill, W.G., R.L. Rivers, and M.L. Zeidel. 1999. Role of leaflet asymmetry in the permeability of model biological membranes to protons, solutes, and gases. J. Gen. Physiol. 114:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, W.G., and M.L. Zeidel. 2000. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J. Biol. Chem. 275:30176–30185. [DOI] [PubMed] [Google Scholar]
- Junker, M., and C.E. Creutz. 1994. Ca(2+)-dependent binding of endonexin (annexin IV) to membranes: analysis of the effects of membrane lipid composition and development of a predictive model for the binding interaction. Biochemistry. 33:8930–8940. [DOI] [PubMed] [Google Scholar]
- Kaetzel, M.A., H.C. Chan, W.P. Dubinsky, J.R. Dedman, and D.J. Nelson. 1994. A role for annexin IV in epithelial cell function. Inhibition of calcium-activated chloride conductance. J. Biol. Chem. 269:5297–5302. [PubMed] [Google Scholar]
- Kaetzel, M.A., and J.R. Dedman. 1995. Annexins: novel Ca2+-dependent regulators of membrane function. News Physiol. Sci. 10:171–176. [Google Scholar]
- Kaetzel, M.A., P. Hazarika, and J.R. Dedman. 1989. Differential tissue expression of three 35-kDa annexin calcium-dependent phospholipid-binding proteins. J. Biol. Chem. 264:14463–14470. [PubMed] [Google Scholar]
- Kaetzel, M.A., Y.D. Mo, T.R. Mealy, B. Campos, W. Bergsma-Schutter, A. Brisson, J.R. Dedman, and B.A. Seaton. 2001. Phosphorylation mutants elucidate the mechanism of annexin IV-mediated membrane aggregation. Biochemistry. 40:4192–4199. [DOI] [PubMed] [Google Scholar]
- Kaiser, R.D., and E. London. 1998. Location of diphenylhexatriene (DPH) and its derivatives within membranes: comparison of different fluorescence quenching analyses of membrane depth. Biochemistry. 37:8180–8190. [DOI] [PubMed] [Google Scholar]
- Kishore, B.K., C.M. Krane, D. Di Iulio, A.G. Menon, and W. Cacini. 2000. Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney Int. 58:701–711. [DOI] [PubMed] [Google Scholar]
- Kishore, B.K., B. Mandon, N.B. Oza, S.R. DiGiovanni, R.A. Coleman, N.L. Ostrowski, J.B. Wade, and M.A. Knepper. 1996. Rat renal arcade segment expresses vasopressin-regulated water channel and vasopressin V2 receptor. J. Clin. Invest. 97:2763–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, K., H. Utsumi, H. Ogawa, and I. Matsumoto. 1994. Highly polarized expression of carbohydrate-binding protein p33/41 (annexin IV) on the apical plasma membrane of epithelial cells in renal proximal tubules. FEBS Lett. 342:313–318. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy, I., and G. Krishnamoorthy. 2001. Probing the link between proton transport and water content in lipid membranes. J. Physical. Chem. B. 105:1484–1488. [Google Scholar]
- Krylov, A.V., P. Pohl, M.L. Zeidel, and W.G. Hill. 2001. Water permeability of asymmetric planar lipid bilayers: leaflets of different composition offer independent and additive resistances to permeation. J. Gen. Physiol. 118:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher, L.W., S.R. Beauman, E.I. Gruenstein, M.A. Kaetzel, and J.R. Dedman. 2003. Nuclear CaMKII inhibits neuronal differentiation of PC12 cells without affecting MAPK or CREB activation. Am. J. Physiol. Cell Physiol. 101152/ajpcell 005102002. [DOI] [PubMed]
- Lande, M.B., J.M. Donovan, and M.L. Zeidel. 1995. The relationship between membrane fluidity and permeabilities to water, solutes, ammonia, and protons. J. Gen. Physiol. 106:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T., and A.S. Verkman. 1999. Aquaporin water channels in gastrointestinal physiology. J. Physiol. 517:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamani, S., M.A. Knepper, and M.B. Burg. 2000. Urine concentration and dilution. Brenner and Rector's The Kidney. B.M. Brenner, editor. Saunders, Philadelphia. 595–635.
- Matsuda, R., N. Kaneko, Y. Horikawa, F. Chiwaki, M. Shinozaki, T. Ieiri, T. Suzuki, and N. Ogawa. 2001. Localization of annexin V in rat normal kidney and experimental glomerulonephritis. Res. Exp. Med. (Berl.). 200:77–92. [DOI] [PubMed] [Google Scholar]
- Mayran, N., V. Traverso, S. Maroux, and D. Massey-Harroche. 1996. Cellular and subcellular localizations of annexins I, IV, and VI in lung epithelia. Am. J. Physiol. 270:L863–L871. [DOI] [PubMed] [Google Scholar]
- McKanna, J.A., A. Chuncharunee, K.A. Munger, J.A. Breyer, S. Cohen, and R.C. Harris. 1992. Localization of p35 (annexin I, lipocortin I) in normal adult rat kidney and during recovery from ischemia. J. Cell. Physiol. 153:467–476. [DOI] [PubMed] [Google Scholar]
- Megli, F.M., M. Mattiazzi, T. Di Tullio, and E. Quagliariello. 2000. Annexin V binding perturbs the cardiolipin fluidity gradient in isolated mitochondria. Can it affect mitochondrial function? Biochemistry. 39:5534–5542. [DOI] [PubMed] [Google Scholar]
- Megli, F.M., M. Selvaggi, S. Liemann, E. Quagliariello, and R. Huber. 1998. The calcium-dependent binding of annexin V to phospholipid vesicles influences the bilayer inner fluidity gradient. Biochemistry. 37:10540–10546. [DOI] [PubMed] [Google Scholar]
- Nagle, J.F., and H.J. Morowitz. 1978. Molecular mechanisms for proton transport in membranes. Proc. Natl. Acad. Sci. USA. 75:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete, H.O., R.L. Rivers, A.H. Goughs, M. Colombini, and M.L. Zeidel. 1996. Individual leaflets of a membrane bilayer can independently regulate permeability. J. Biol. Chem. 271:11627–11630. [DOI] [PubMed] [Google Scholar]
- Paula, S., A.G. Volkov, A.N. Van Hoek, T.H. Haines, and D.W. DeAm. 1996. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 70:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, L.J., and L. Casey. 2002. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry. 41:10315–10322. [DOI] [PubMed] [Google Scholar]
- Pohl, P., S.M. Saparov, and Y.N. Antonenko. 1997. The effect of a transmembrane osmotic flux on the ion concentration distribution in the immediate membrane vicinity measured by microelectrodes. Biophys. J. 72:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomes, R., and B. Roux. 1996. Structure and dynamics of a proton wire: a theoretical study of H+ translocation along the single-file water chain in the gramicidin A channel. Biophys. J. 71:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers, R., A. Blanchard, D. Eladari, F. Leviel, M. Paillard, R.A. Podevin, and M.L. Zeidel. 1998. Water and solute permeabilities of medullary thick ascending limb apical and basolateral membranes. Am. J. Physiol. 274:F453–F462. [DOI] [PubMed] [Google Scholar]
- Rossignol, M., P. Thomas, and C. Grignon. 1982. Proton permeability of liposomes from natural phospholipid mixtures. Biochim. Biophys. Acta. 684:195–199. [DOI] [PubMed] [Google Scholar]
- Seville, R.A., S. Nijjar, M.W. Barnett, K. Masse, and E.A. Jones. 2002. Annexin IV (Xanx-4) has a functional role in the formation of pronephric tubules. Development. 129:1693–1704. [DOI] [PubMed] [Google Scholar]
- Shlyonsky, V.G., F. Mies, and S. Sariban-Sohraby. 2003. Epithelial sodium channel in detergent-resistant membrane microdomains. Am. J. Physiol. Renal Physiol. 284:F182–F188. [DOI] [PubMed] [Google Scholar]
- Sokolov, Y., W.S. Mailliard, N. Tranngo, M. Isas, H. Luecke, H.T. Haigler, and J.E. Hall. 2000. Annexins V and XII alter the properties of planar lipid bilayers seen by conductance probes. J. Gen. Physiol. 115:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swairjo, M.A., N.O. Concha, M.A. Kaetzel, J.R. Dedman, and B.A. Seaton. 1995. Ca(2+)-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 2:968–974. [DOI] [PubMed] [Google Scholar]
- Verkman, A.S. 2002. Aquaporin water channels and endothelial cell function. J. Anat. 200:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman, A.S., M.A. Matthay, and Y. Song. 2000. Aquaporin water channels and lung physiology. Am. J. Physiol. Lung Cell Mol. Physiol. 278:L867–L879. [DOI] [PubMed] [Google Scholar]
- Wan, F.Y., Y.N. Wang, and G.J. Zhang. 2002. Influence of the physical states of membrane surface area and center area on lysosomal proton permeability. Arch. Biochem. Biophys. 404:285–292. [DOI] [PubMed] [Google Scholar]
- Wilkes, J.M., H.J. Ballard, D.T. Dryden, and B.H. Hirst. 1989. Proton permeability and lipid dynamics of gastric and duodenal apical membrane vesicles. Am. J. Physiol. 256:G553–G562. [DOI] [PubMed] [Google Scholar]
- Zaks, W.J., and C.E. Creutz. 1990. Annexin-chromaffin granule membrane interactions: a comparative study of synexin, p32 and p67. Biochim. Biophys. Acta. 1029:149–160. [DOI] [PubMed] [Google Scholar]
- Zanotti, G., G. Malpeli, F. Gliubich, C. Folli, M. Stoppini, L. Olivi, A. Savoia, and R. Berni. 1998. Structure of the trigonal crystal form of bovine annexin IV. Biochem. J. 329:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]