Abstract
We have previously demonstrated the presence of a cyclic GMP (cGMP)-dependent calcium-activated inward current in vascular smooth-muscle cells, and suggested this to be of importance in synchronizing smooth-muscle contraction. Here we demonstrate the characteristics of this current. Using conventional patch-clamp technique, whole-cell currents were evoked in freshly isolated smooth-muscle cells from rat mesenteric resistance arteries by elevation of intracellular calcium with either 10 mM caffeine, 1 μM BAY K8644, 0.4 μM ionomycin, or by high calcium concentration (900 nM) in the pipette solution. The current was found to be a calcium-activated chloride current with an absolute requirement for cyclic GMP (EC50 6.4 μM). The current could be activated by the constitutively active subunit of PKG. Current activation was blocked by the protein kinase G antagonist Rp-8-Br-PET-cGMP or with a peptide inhibitor of PKG, or with the nonhydrolysable ATP analogue AMP-PNP. Under biionic conditions, the anion permeability sequence of the channel was SCN− > Br− > I− > Cl− > acetate > F− >> aspartate, but the conductance sequence was I− > Br− > Cl− > acetate > F− > aspartate = SCN−. The current had no voltage or time dependence. It was inhibited by nickel and zinc ions in the micromolar range, but was unaffected by cobalt and had a low sensitivity to inhibition by the chloride channel blockers niflumic acid, DIDS, and IAA-94. The properties of this current in mesenteric artery smooth-muscle cells differed from those of the calcium-activated chloride current in pulmonary myocytes, which was cGMP-independent, exhibited a high sensitivity to inhibition by niflumic acid, was unaffected by zinc ions, and showed outward current rectification as has previously been reported for this current. Under conditions of high calcium in the patch-pipette solution, a current similar to the latter could be identified also in the mesenteric artery smooth-muscle cells. We conclude that smooth-muscle cells from rat mesenteric resistance arteries have a novel cGMP-dependent calcium-activated chloride current, which is activated by intracellular calcium release and which has characteristics distinct from other calcium-activated chloride currents.
Keywords: chloride channels, calcium signaling, calcium oscillations, cyclic nucleotides
INTRODUCTION
In previous work on small arteries (Gustafsson et al., 1993; Gustafsson and Nilsson, 1994), it was suggested that the regular or irregular oscillation in diameter or tone of vessels—vasomotion—originated from interplay between a cytosolic oscillator (calcium release from intracellular stores) (Berridge and Galione, 1988) and membrane potential variations. Vasomotion in the small arteries was also characteristically dependent on smooth-muscle levels of cGMP and therefore influenced by the endothelium. We have suggested that membrane potential variations serve to synchronize intracellular oscillators in the smooth-muscle cells, with membrane potential variations being driven by the cytosolic oscillator (Peng et al., 2001). We were able to define a membrane current that was critically dependent on cyclic GMP and activated by intracellular calcium release. This cGMP-dependent Ca2+-activated current seemed to have all properties required to participate in the reciprocal interaction of membrane potential and intracellular calcium release.
In the present work we have examined the characteristics of this Ca2+-activated current. Although this current was shown to be a Ca2+-activated Cl- current (ICl(Ca)), it has several characteristics that are distinct from previously described ICl(Ca) (Large and Wang, 1996; Frings et al., 2000). The previously characterized current has properties such as time and voltage dependence, sensitivity to pharmacological blockers such as niflumic acid (Large and Wang, 1996), a characteristic permeability sequence for halides (Frings et al., 2000), and specific interactions with multivalent cations (Tokimasa and North, 1996). However, the properties of the cGMP-dependent chloride current in smooth-muscle cells from mesenteric small arteries described here differs in many of these aspects. For comparison we have therefore also characterized ICl(Ca) from pulmonary arterial myocytes, where it has been characterized in detail previously (Yuan, 1997; Greenwood and Large, 1999; Greenwood et al., 2001). The new channel seems to combine properties characteristic for different families of Cl− channels. Brief preliminary reports of part of the present results have been given elsewhere (Matchkov et al., 2001, 2003).
MATERIALS AND METHODS
Cell Isolation
All experiments were conducted in accordance with the Danish national guidelines for animal research. Male Wistar rats (12–18-wk-old) were killed with CO2. The mesentery and in some cases the pulmonary artery was removed from the rats and placed into an ice-cold physiological salt solution. Branches of small mesenteric arteries were dissected out and cleaned from connective tissue. All arteries were opened longitudinally, and endothelium and remaining blood were removed by gently rubbing the luminal side of the arteries.
Subsequently, in most experiments papain digestion (Schubert et al., 2001) was used to isolate smooth-muscle cells. The arteries were stored overnight at 4°C in a solution for enzymatic digestion of the arteries (for composition see below). Before experiments, the microtube with the vessels was incubated for 5–10 min at 37°C, after which the vessels were transferred to a low-calcium solution. Single cells were released by trituration with a polyethylene pipette into the experimental solution. This method consistently produces a high yield of relaxed smooth-muscle cells, which respond reversibly to vasoconstrictors and remain viable for at least 3 h (Clapp and Gurney, 1991).
In experiments where BAY K8644 was used to activate calcium channels, cells were instead isolated by the collagenase-elastase method of Petkov (Petkov et al., 2001). This method produces smooth-muscle cells that express a high density of voltage-gated calcium currents.
Patch-clamp Recording
All experiments were made at room temperature (22–24°C). Patch pipettes were prepared from borosilicate glass (PG15OT-7.5; Harvard Apparatus), pulled on a P-97 puller (Sutter Instrument Co.) and fire polished to achieve tip resistances in the range of 2–5 MΩ.
Recordings were made with an Axopatch 200B amplifier (Axon Instruments, Inc.) in whole-cell configuration. Data were sampled at a rate of 2 kHz and filtered at 1 kHz. Data acquisition and analysis were done with the software package Clampex 7 for Windows (Axon Instruments, Inc.). Only cells with essentially no leak current (seal resistance >2 GΩ) and a low access resistance (5–10 MΩ) were used, and the stability of these parameters was tested regularly during the course of the experiment. Series resistance and capacitive current were routinely compensated. Junction potentials were estimated using the junction potential calculator of pClamp 7 (Axon Instruments, Inc.) and data were corrected for these.
Currents were recorded at a holding potential of −60 mV. Current-voltage characteristics were obtained either from ramp protocols or from a voltage-step protocol. Voltage ramps were performed from −60 to +60 mV with a duration of 100 ms, unless specified otherwise. In the voltage-step protocol currents were evoked by pipette solutions containing calcium buffered at 900 nM (Fixed-Ca solution, see below). This technique has been used to activate ICl(Ca) in studies on smooth muscle (Greenwood et al., 2001; Britton et al., 2002) and directly activates the chloride current at a clamped concentration of calcium. The current-voltage relationship of the activated current (after subtraction of baseline current) was determined by stepping the potentials (20-mV steps) between −90 and +90 mV, maintaining the voltage at each step for 2 s. Current amplitudes were averaged over the last 0.1 s before the end of the test pulse.
To obtain the relative permeabilities for anions under biionic conditions the current was activated by application of ionomycin in symmetrical chloride conditions. Current-voltage relationships were obtained by 2-s voltage steps covering the range −70 to +90 mV in 20-mV increments from a holding potential of −90 mV. The protocol was performed initially in symmetric, chloride-containing solution, and then repeated during extracellular superfusion with the substitute anion. Finally, chloride-containing solution was reapplied and a control measurement was made. Current values were taken from the last 0.1 s of the 2-s voltage step. Ionomycin was present throughout. Results were discarded from analysis if the initial and final measurements in the presence of chloride differed in characteristics.
Drug Application
Drugs were either applied to the bath or superfused locally over the patched cell (except experiments in biionic conditions, where drugs were applied in the solutions for flow exchange with the rate 5 ml/min; see above). For local superfusion, a specially designed electric manipulator (Danish Myo Technology) was used to position the application pipettes immediately before application and to move them out of the solution immediately afterwards. In this way seepage of drugs onto the cells before or after application was eliminated.
The standard protocol consisted of breaking the membrane and waiting 5 min for equilibration. Then a control recording was made, followed by a 10 min wash out. Finally, the drug was superfused for 1 min followed by another current recording in the continued presence of the drug.
Solutions
The solution used for enzymatic digestion of arteries contained (in mM): NaCl 110, KCl 5, MgCl2 2, KH2PO4 0.5, NaH2PO4 0.5, NaHCO3 10, CaCl2 0.16, EDTA 0.49, Na-HEPES 10, glucose 10, taurine 10 at pH 7.0, as well as 1.5 mg ml−1 papain, 1.6 mg ml−1 albumin, and 0.4 mg ml−1 dl-dithiothreitol. The collagenase/elastase enzyme solution contained (in mM): NaCl 110, KCl 5.6, MgCl2 1.2, Na-HEPES 10, glucose 20, taurine 20, Na-pyruvate 5 at pH 7.4, as well as 1 mg ml−1 collagenase type 1 (Worthington Biochemical Corporation), 4.85 μg ml−1, elastase type I (Sigma-Aldrich), 1 mg ml−1 soybean trypsin inhibitor, 1 mg ml−1 albumin.
The active subunit of protein kinase G (PKG) was obtained by trypsinization of PKG as described by White et al. (1993). In brief, 10 μg PKG Iα was dissolved in 300 μl water. Trypsin (1 μg) was added and incubated for 3 min at 30°C, after which 10 μg soybean trypsin inhibitor was added. This solution was added in a ratio of 1:30 to the pipette solution.
Compositions of solutions used in the patch-clamp experiments are given for bath solutions in Table I, and for pipette solutions in Table II. Free calcium concentration was estimated by the WebMaxC v2.22 buffer program (Chris Patton, Stanford University, CA). For the “low-calcium” (B3; Table I) and “low-calcium-Cs” (B4) solutions in the presence of 1 mM BaCl2 free calcium was estimated to be ∼19 μM. To determine the relative permeability to halides the CsCl in the extracellular solution was substituted with the corresponding cesium salt on an equimolar basis in 4–6 experiments for each ion species tested. In experiments under biionic conditions 3 M KCl salt-agar bridge was placed between the Ag/AgCl pellet and the bath solution.
TABLE I.
Bath Solutions
| Solution | Na+ | K+ | Cs+ | Ca2+ | Mg2+ | Ba2+ | Cl− | Aspartic acid | ChTX | HEPES | EGTA | pH | pH adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | |||
| B1 | control | 145 | 6 | 0.1 | 1 | 1 | 145 | 10−4 | 10 | 7.4 | NaOH | |||
| B2 | control Cs | 140 | 0.1 | 1 | 142 | 10−4 | 10 | 7.4 | CsOH | |||||
| B3 | low Ca | 145 | 6 | 0.9 | 1 | 1 | 147 | 10−4 | 10 | 1 | 7.4 | NaOH | ||
| B4 | low Ca-Cs | 140 | 0.9 | 1 | 142 | 10−4 | 10 | 1 | 7.4 | CsOH | ||||
| B5 | Ca free | 145 | 6 | 1 | 1 | 145 | 10−4 | 10 | 1 | 7.4 | NaOH | |||
| B6 | low Cl | 140 | 0.1 | 1 | 67 | 75 | 10−4 | 10 | 7.4 | CsOH |
TABLE II.
Pipette Solutions Used
| Solution | Na+ | K+ | Cs+ | Ca2+ | Mg2+ | Cl− | MgATP | BAPTA | HEPES | EGTA | cGMP | Aspartic acid | pH | pH adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | mM | ||||
| P1 | control | 10 | 132 | 0.01 | 1 | 134 | 0.1 | 0.1 | 10 | 10−2 | 7.35 | KOH | |||
| P2 | control Cs | 140 | 0.01 | 140 | 0.1 | 0.1 | 10 | 7.35 | CsOH | ||||||
| P3 | high BAPTA | 10 | 112 | 1 | 1.44 | 117 | 0.1 | 10 | 10 | 10−2 | 7.35 | KOH | |||
| P4 | high EGTA | 10 | 112 | 1 | 1.44 | 117 | 0.1 | 10 | 11 | 10−2 | 7.35 | KOH | |||
| P5 | fixed Ca | 140 | 5 | 140 | 0.1 | 10 | 6 | 7.35 | CsOH | ||||||
| P6 | low Cl | 140 | 5 | 65 | 0.1 | 10 | 6 | 75 | 7.35 | CsOH |
Drugs and Chemicals
Adenosine 3′,5′-cyclic monophosphate (cAMP), adenosine 5′-(β,γ-imido)triphosphate tetralithium salt hydrate (AMP-PNP), ATP-γ-S, albumin, 4-aminopyridine (4-AP), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), cesium acetate (Cs-acetate), cesium bromide (CsBr), cesium chloride (CsCl), cesium fluoride (CsF), cesium iodide (CsI), guanosine 3′,5′-cyclic monophosphate (cGMP), caffeine, 4,4′-diisothiocyanatstilbene-2,2′-disulphonic acid (DIDS), dl-dithiothreitol, ethylenediaminetetraacetic acid (EDTA), ethylene glycol-O,O′-bis(2 aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA), hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES), magnesium adenosine-5′-triphosphate (MgATP), nifedipine, niflumic acid, N-methyl-D-glucamine (NMDG), papain, sodium thiocyanate (NaSCN), tetraethylammonium chloride (TEA), indanyloxyacetic acid (R(+)-IAA-94), trypsin, as well as the salts for all solutions were obtained from Sigma-Aldrich. Charybdotoxin (ChTX) and iberiotoxin (IbTX) was purchased from Latoxan. Mibefradil was obtained from Hoffman-LaRoche; β–phenyl-1,N2-etheno-8-bromoguanosine-3′-5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-Br-PET-cGMP) from BioLog Life science Institute; apamin from Alomone Labs; protein kinase G Iα and cell-permeable protein kinase G Iα inhibitor from Calbiochem; soybean trypsin inhibitor from Fluka.
Statistics
All data are given as means ± SEM. Only one experimental recording was taken from each cell, thus n is the number of cells. Statistical analysis was performed using cells from at least three different isolations. Unpaired Student's t test was used for single comparisons, and one-way analysis of variance test with Bonferroni's post-test for multiple comparisons (GraphPad Prism v. 2.01; GraphPad Software). Nonlinear regression to the Hill equation was used for the analysis of concentration-effect curves. Linear regression was used to compare estimated and experimental changes in ECl, and in experiments for determination of the relative conductance of halides.
Relative permeability was determined by measuring the shift in reversal potential (Erev) upon changing the solution on one side of the membrane from one containing chloride ions (Cl−) to another with the substitute anion (X−). The permeability ratio was estimated using the Goldman-Hodgkin-Katz equation: PX/PCl = exp(−ΔErevF/RT), where ΔErev is the difference between the reversal potential with the test anion X− and that observed with Cl−, F is Faraday's constant, R is the gas constant, and T is temperature.
RESULTS
A cGMP-sensitive Calcium-activated Inward Current in Smooth-muscle Cells
We have previously reported the presence of a calcium-activated inward current that required cyclic GMP for activation in rat mesenteric arterial smooth-muscle cells (Peng et al., 2001). Those experiments were made using the ampthotericin permeabilized-patch technique. In the present set of experiments, we were able to identify a similar current in conventional, ruptured-patch whole-cell recordings. In the presence of 10 μM cGMP in the intracellular solution (solutions B1:P1, as defined in Tables I and II), application of 10 mM caffeine evoked a transient current in ∼90% of cells, as shown in Fig. 1 A. The density of this whole-cell current at −60 mV holding potential was 7.58 ± 0.35 A F−1 (n = 57) in cells with an average capacitance of 16.2 ± 0.47 pF. The time-course of this current was similar to the time-course of the calcium elevation measured by Fura-2 in response to caffeine (unpublished data). Chelating the intracellular calcium with either 10 mM BAPTA (n = 8) or 11 mM EGTA (n = 6) (solutions B1:P3 and B1:P4, respectively) eliminated this current. This observation was consistent with the effect of 10 μM ryanodine seen in previous experiments (Peng et al., 2001) and showed that the evoked current was secondary to the calcium elevation by caffeine. In the absence of cGMP, no current or a small inward current was observed in response to caffeine application at −60 mV holding potential: current density without cGMP was 0.12 ± 0.14 A F−1 (n = 30) in cells with an average capacitance of 18.9 ± 4.1 pF.
Figure 1.

Original recordings of whole-cell cGMP-dependent inward current in response to a 10-mM caffeine application (A), to 0.4 μM of ionomycin (B), and to 1 μM of the voltage-gated calcium channel agonist BAY K8644 (C). The holding potential in all experiments was −60 mV. Solutions were: A, B1:P1; B and C, B4:P2 with 10 μM ryanodine added to the pipette solution.
A similar current could be evoked by increasing calcium influx in the presence of ryanodine, by applying either ionomycin or the calcium channel opener, BAY-K 8644. Both treatments caused sustained elevations in intracellular calcium measured by Fura-2 (unpublished data). Bath application of 0.4 μM ionomycin (solutions B4:P2 with 10 μM ryanodine added to the pipette solution) caused only small changes in current, but subsequent application of membrane-permeable 8Br-cGMP rapidly activated a sustained inward current (Fig. 1 B) with an average amplitude of 10.3 ± 1.12 A F−1 (n = 14) at a holding potential of −60 mV in cells with an average capacitance of 16.0 ± 1.0 pF. The sequence of events were similar after application of first BAY-K 8644 and then 8Br-cGMP (Fig. 1 C); average current density here was 6.48 ± 0.41 A F−1 (n = 5) (−60 mV holding potential; cell capacitance 23.0 ± 2.85 pF). Run-down of the current in the presence of cGMP was not pronounced (unpublished data), suggesting that it did not critically depend on components in the cytoplasm that could be washed out in the whole-cell experiment.
Ion Selectivity
The inward current is unlikely to be a potassium current, since the potassium equilibrium potential (−78.5 mV) is negative to the holding potential (−60 mV). Also, the cGMP-sensitive inward current was insensitive to various K-channel antagonists: neither 100 nM charybdotoxin (n = 34) nor 100 nM iberiotoxin (n = 13), 1 μM apamin (n = 5), 10 mM 4-aminopyridine (n = 5), or 10 mM tetraethyl ammonium (n = 5) reduced the [Ca2+]i-activated inward current. In 6 of 10 experiments performed in the absence of potassium-channel blockers, a transient outward current was seen superimposed on the inward current: this outward current was most likely due to activation of a KCa channel, since it was never observed in the presence of 100 nM charybdotoxin (ChTX).
It is possible that Na+ ions carried the inward current (ENa +68 mV for B1:P1). However, neither partial nor complete replacement of extracellular sodium affected the amplitude of the current, indicating that sodium is not involved: the inward current amplitude was 8.66 ± 0.74 A F−1 (n = 6; cell capacitance 15.8 ± 0.65 pF) in the presence of 145 mM Na+ (solutions B1:P1), 8.93 ± 0.62 A F−1 (n = 4; cell capacitance 16.9 ± 2.59 pF) in the presence of 10 mM Na+ (the bath solution B1 was modified by substitution of 135 mM Na+ with NMDG; the pipette solution was P1), and 7.15 ± 0.48 A F−1 (n = 5; cell capacitance 14.8 ± 1.71 pF) in the absence of Na+ (the bath solution B1 was modified by complete substitution of Na+ with NMDG; the pipette solution P1).
Calcium ions could also potentially carry inward current (ECa +89 mV for B1:P1). However, neither 1 μM nifedipine (n = 5) nor 10 μM mibefradil (n = 5) inhibited the inward current. Furthermore, superfusing the cell for 1 min with a Ca2+-free solution before caffeine application did not affect the current: the inward current amplitude was 8.12 ± 2.12 A F−1 (n = 5; solutions B1:P1) in the control conditions and 8.79 ± 2.07 A F−1 (n = 5; cell capacitance 12.8 ± 0.46 pF) after superfusion with Ca2+-free solution (solutions B5:P1). This finding not only demonstrates that the charge carrier is not calcium ions, but it also shows that the calcium that activates the current during caffeine stimulation is derived from an intracellular pool, and that the current under these conditions is not activated secondarily to calcium influx through, e.g., store-operated calcium channels.
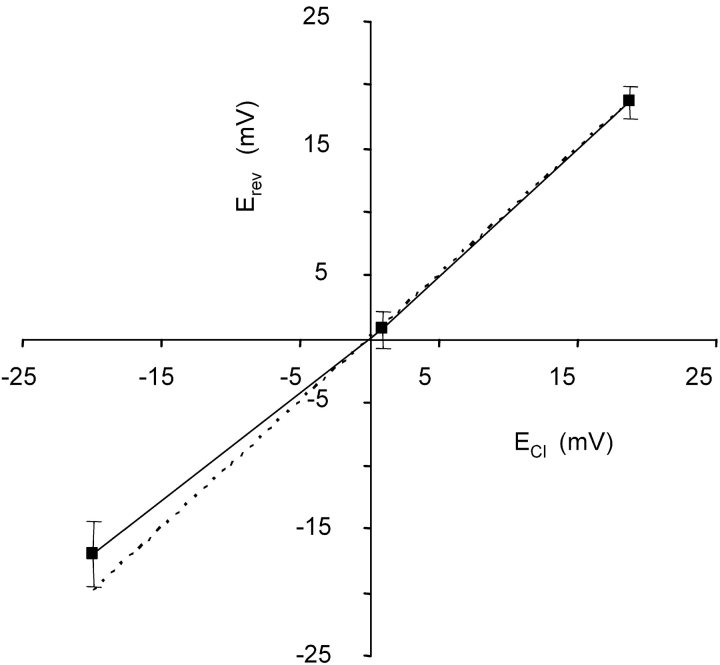
A further candidate as charge carrier for an inward current is chloride. An involvement of chloride was tested in CsCl solutions by partially replacing chloride by aspartate in either the intracellular or extracellular solution. The replacement was such that it would move the equilibrium potential ∼20 mV in either direction. This caused corresponding shifts in the reversal potential of the current (Fig. 2). The current thus was determined to be carried by chloride ions.
Figure 2.
The relation between measured reversal potential of the current and calculated equilibrium potential for chloride (ECl). The dashed line is the identity line, expected for an ideal chloride channel. Solutions were B2:P6, B2:P5, and B6:P5 from left to right points, respectively. 10 μM cGMP was added to all pipette solutions. Vertical bars indicate SEM.
Effects of Chloride Channel Blockers
Since the current appeared to be a chloride current, we examined the effect of the classical chloride channel blockers niflumic acid, IAA-94, and DIDS on the current. Fig. 3 shows the caffeine-evoked inward current before and after a 1-min preincubation with the blockers. While the current could be inhibited by relatively high concentrations of these blockers, it was less sensitive than expected for a typical calcium-activated chloride current.
Figure 3.

Concentration-dependent effects of the Cl− channel antagonists niflumic acid (A), IAA-94 (B), and DIDS (C) on the inward current response to caffeine. Solutions were B1:P1. Vertical bars indicate SEM.
Effect of Divalent Metal Ions
Since some chloride currents have been reported to be sensitive to divalent cations, we tested Ni2+, Zn2+, and Co2+ on the caffeine-activated current.
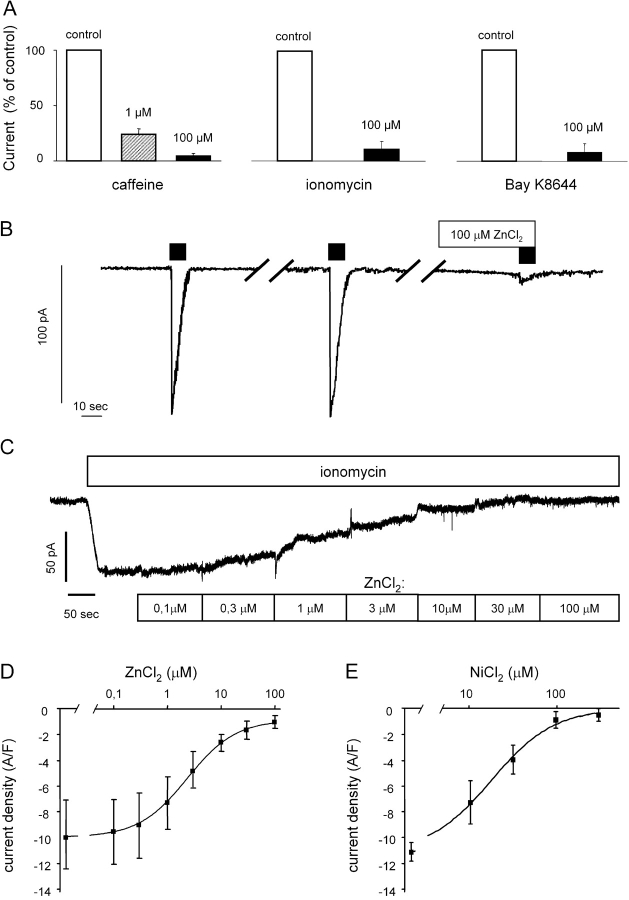
A 1-min incubation with 100 μM Zn2+ inhibited the calcium-activated inward current evoked either by a 10-mM caffeine application, by incubation with 0.4 μM ionomycin, or by an application of 1 μM BAY K 8644 (Fig. 4, A and B). Even in a concentration of 1 μM, Zn2+ strongly reduced the current (tested only with caffeine; Fig. 4 A). The effect of Zn2+ was specific for the chloride current, since KCa channel currents could still be activated by caffeine application if the experimental solutions did not contain K channel blockers (bath solution was B1 without BaCl2 and ChTX; pipette solution was P1): at +60 mV, outward current density was 159 ± 38 A F−1 (n = 4; cell capacitance 17.9 ± 3.85 pF) in the absence of Zn2+ and 177 ± 40 A F−1 (n = 4, NS; cell capacitance 17.8 ± 2.69 pF) in the presence of 100 μM Zn2+, and this was reduced to 0.6 ± 0.9 A F−1 (n = 4; cell capacitance 15.3 ± 1.45 pF) by further addition of 100 nM ChTX. Thus, the effect of Zn2+ is an inhibition of the inward current evoked by calcium release, and not an inhibition of the calcium release itself.
Figure 4.
Effect of divalent cations on the cGMP-dependent Ca2+-activated Cl− current. A shows the mean effect of ZnCl2 on the cGMP-sensitive ICl(Ca) evoked by caffeine, ionomycin, and BAY K 8644. Solutions were B1:P1, B4:P2, and B4:P2, from left to right. 10 μM cGMP was added to all pipette solutions. Vertical bars indicate SEM. Original recording in B shows two control responses to 10 mM caffeine (black bars) and one response in the presence of Zn2+. Solutions were B1:P1. The breaks in the recordings correspond to 10-min wash out periods. Trace in C shows original recordings of the currents evoked by 0.4 μM ionomycin application in solutions B4:P2 with 10 μM cGMP added to the pipette solution. The concentration of ZnCl2 in the bath was cumulatively increased. D shows concentration-response relation for Zn2+ constructed from experiments as shown in C. Vertical bars indicate SEM. E shows concentration-response relation for Ni2+ on the inward current response to caffeine. Vertical bars indicate SEM. Solutions were B1:P1.
The concentration-effect curve for the effect of Zn2+ on the inward current evoked by ionomycin in solutions B4:P2 (with 10 μM cGMP added to the pipette solution) had an EC50 for Zi2+ of 2.2 μM with a Hill coefficient of 1.0. (Fig. 4, C and D). The high Zn2+ sensitivity was used to identify this current in later experiments.
Also Ni2+ inhibited the calcium-activated inward current, although less potently then Zn2+. As shown in Fig. 4 E, the concentration-effect curve had an EC50 for Ni2+ of 27.5 μM with a Hill coefficient of 1.5. In contrast, Co2+ had little effect on the current at concentrations up to 100 μM. The current density was 6.28 ± 1.10 A F−1 under control conditions, and after 1 min in the presence of 100 μM Co2+ it was 5.70 ± 1.07 A F−1 (n = 4, NS; cell capacitance 17.8 ± 2.23 pF) when the membrane potential was held at −60 mV.
Anion Selectivity of the Current
We obtained the relative permeabilities for different anions under biionic conditions (see materials and methods) based on symmetrical CsCl solution with extracellular ionomycin. Typical current-voltage recordings are shown in Fig. 5, A–F. As is evident from the current-voltage curves, the chloride current did not show any voltage dependence or rectification. The relative permeability (PX/PCl) was calculated from the shift in Erev using the Goldman-Hodgkin-Katz equation and the relative conductance (GX/GCl) was measured for outward currents. The selectivity of the channel estimated from PX/PCl followed the sequence: SCN− > Br− > I− > Cl− > acetate > F− >> aspartate (2.85:1.39:1.25:1:0.87:0.79:0.06). However, the relative conductances exhibited a different sequence: I− > Br− > Cl− > acetate > F− > aspartate = SCN− (1.41:1.17:1:0.85:0.71:0.26:0.23). Based on these results we estimated the relative affinity of the anion for a binding site in the channel (Qu and Hartzell, 2000) as the ratio of the relative permeabilities to the relative conductances; this sequence was: SCN− >> Br− = F− = Cl− = acetate = I− ≥ aspartate.
Figure 5.
Typical current-voltage recordings under biionic conditions, representative of 4–6 experiments of each kind. ICl(Ca) was stimulated by ionomycin in solutions B4:P2 with 10 μM cGMP added to the pipette solution. Chloride was substituted with fluoride (A), bromide (B), iodide (C), thiocyanate (D), acetate (E), and l-aspartate (F).
Current Activation
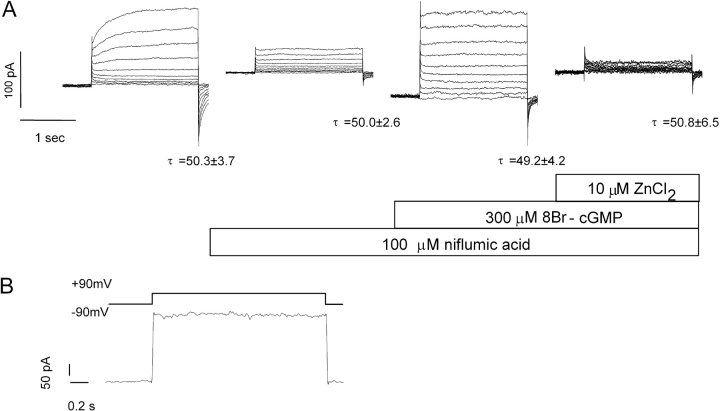
We noticed that elevating calcium via the pipette solution (900 nM free calcium in Cs+-containing solution, P5) often caused the appearance of a current even in the absence of cGMP. This was in contrast to experiments with either caffeine, ionomycin, or BAY-K 8644, where such a current was never observed. This was analyzed further in the experiment in Fig. 6 A. Here, calcium was introduced from the pipette solution, and voltage steps showed the presence of a slowly activating current, with a reversal potential close to ECl. This current was sensitive to inhibition by niflumic acid. Subsequent addition of 8Br-cGMP caused the appearance of another current without time dependence that was sensitive to inhibition by Zn2+. This protocol revealed the presence of a conventional calcium-activated Cl− current in the same cells that expressed the cGMP-dependent current.
Figure 6.
Activation characteristics of Ca2+-activated Cl− currents in mesenteric artery smooth-muscle cells. (A) A cGMP-independent current was activated by depolarization in the presence of 900 nM Ca2+ in the pipette solution (solutions were B2:P5). This current showed slow activation in response to a depolarizing step (left) and was inhibited by 100 μM niflumic acid (second panel). In the presence of niflumic acid, addition of 300 μM 8Br-cGMP revealed the presence of another inward current (third panel), which was inhibited by 10 μM Zn2+ (right). (B) Superfusion with 0.4 μM ionomycin (solutions B4:P2 with 10 μM cGMP added to the pipette solution) caused appearance of only the cGMP-sensitive current, which activated immediately and deactivated without a tail current.
Inspection of the tail currents in Fig. 6 A showed the expected tail current of the conventional calcium-activated current (first panel), but the possible increase in tail current after application of 8Br-cGMP is not expected for a current lacking time and voltage dependence. This observation prompted us to examine the tail current more closely in experiments using ionomycin to elevate intracellular calcium (since this procedure only activated the cGMP-dependent current). As shown in Fig. 6 B, under these conditions an instantly activating current was seen that lacked tail current. The cGMP-dependent current thus seems to be instant-on, instant-off; the reason for the apparent increase in tail current in Fig. 6 A is unknown.
Cyclic GMP Dependence
As mentioned above, hardly any inward current could be evoked by caffeine in the absence of cGMP. Increasing concentrations of cGMP increased the amplitude of the current (Fig. 7 A; solutions B1:P1 with the concentrations of cGMP shown in Fig. 7 A added to the pipette solution). The concentration-effect curve was quite steep, with a Hill coefficient of ∼3.3 and an EC50 of ∼6.4 μM. Based on this concentration-effect relation, we routinely used 10 μM internal cGMP to activate the current near-maximally.
Figure 7.
Characterization of the effects of cGMP. (A) Concentration-effect relation for cGMP on the caffeine-activated inward current. EC50 is 6.4 μM, the Hill coefficient is 3.3. Solutions were B1:P1 with addition of different cGMP concentrations to the pipette solution. (B) Concentration-effect relation for inhibition of the inward current induced by caffeine by the competitive cGMP antagonist, Rp-8-Br-PET-cGMP. Note near-complete inhibition of current at high antagonist concentration. The EC50 corresponds to 37 μM, and the Hill coefficient is 2.3. Solutions were B1:P1. (C) Inhibition of the caffeine-induced inward current by a membrane-permeable peptide inhibitor of PKG (Dostmann et al., 2000). Solutions were B2:P2 with 10 μM cGMP added to the pipette solution. (D) Comparison of effects of cGMP and cAMP on the caffeine-induced inward current. Even the 10-fold higher concentration of cAMP activated only a fraction of the current, and this was inhibited by the cGMP antagonist. Solutions were B1:P1 with either 10 μM cGMP or 100 μM cAMP added to the pipette solution. (E) Inhibition of the caffeine-induced inward current by the nonhydrolyzable ATP analogue, AMP-PNP. The bath solution was B1. The pipette solution was P1 (containing 10 μM cGMP), in which ATP was replaced by the indicated concentration of AMP-PNP. (F) Activation of the membrane current by irreversible phosphorylation with ATP-γ-S and by intracellular application of the active subunit of PKG. Both agents cause the activation of the zinc-sensitive current. Solutions were B2:P5. In A–F the number of experiments (n) is indicated near each point or bar. Vertical bars indicate SEM.
The effects of cGMP were further investigated pharmacologically. The current evoked in the presence of 10 μM cGMP was antagonized by the competitive cGMP antagonist, Rp-8-Br-PET-cGMP (Fig. 7 B), as well as by the membrane-permeable PKG inhibitor developed by Dostmann et al. (2000) (Fig. 7 C). Current activation was selective for cGMP: even in 10-fold higher concentrations than required for cGMP, the activation by cAMP was only 10% of maximal, and the cAMP effect was inhibited by the cGMP antagonist, Rp-8-Br-PET-cGMP (Fig. 7 D).
The possible involvement of a kinase in the activation of the current was investigated by replacing the ATP normally present in the intracellular solution by the nonhydrolyzable AMP-PNP. As shown in Fig. 7 E, in the presence of 3 mM AMP-PNP <20% of the current could be evoked by caffeine. The lack of effect of the competitive inhibitor AMP-PNP in lower concentration (0.1 μM) may indicate sufficient production of ATP within the cell to sustain current activation.
Further support for the involvement of a kinase in activating the current was obtained by studying the effects of ATP-γ-S, which can replace ATP as a kinase substrate. The thiol group of ATP-γ-S is ineffective as a substrate for phosphatases (Yount, 1975), leading to what functionally appears as irreversible phosphorylation. Including ATP-γ-S in the pipette solution caused the appearance of an inward current over the first few minutes after breaking into the cell (Fig. 7 F). When this current had stabilized, addition of 8Br-cGMP had no further effect (not depicted). The current was inhibited by 100 μM Zn2+. Thus, ATP-γ-S activated the cGMP-dependent current as would be expected if a kinase was involved.
We also examined whether PKG was capable of activating the current, using the active subunit of PKG isolated as described in materials and methods. When this was added to a pipette solution without cGMP, activation of a Zn2+-sensitive current was observed after breaking into the cell (Fig. 7 F). Activation was not seen if ATP was replaced by 3 mM AMP-PNP in the pipette solution (unpublished data). Adding 8Br-cGMP in the presence of active PKG did not enhance the current further (unpublished data). This demonstrates that the current can be activated by PKG.
Calcium-activated Chloride Current in Pulmonary Artery Myocytes
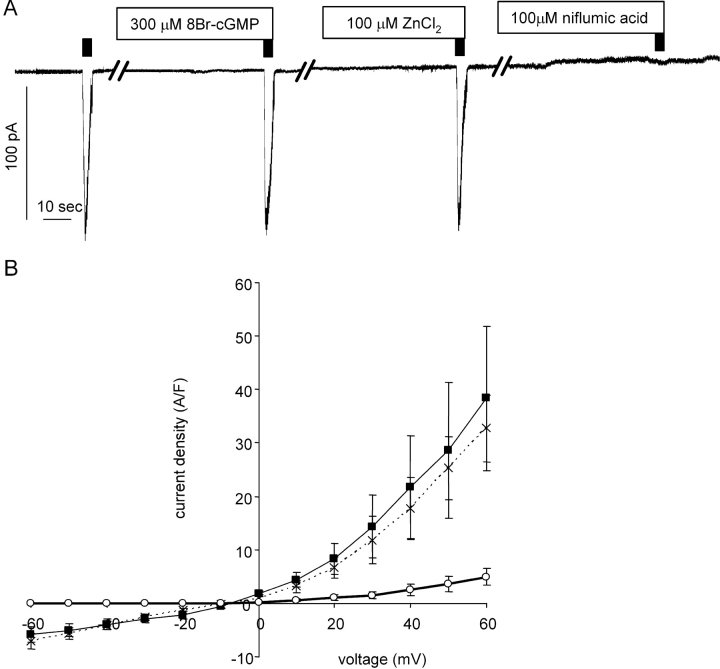
To confirm that the characteristics of the cGMP-dependent current are not merely a reflection of our experimental conditions, we also recorded the previously well-characterized ICl(Ca) from pulmonary arteries (Salter et al., 1995; Greenwood and Large, 1999; Greenwood et al., 2001). This current could be evoked by caffeine in the absence of cGMP (Fig. 8 A, solution B1:P1 without cGMP in the pipette solution). The density of the whole-cell current at −60 mV in the control condition was 6.16 ± 0.74 A F−1 (n = 14; capacitance 18.5 ± 1.5 pF) and was not enhanced by 300 μM 8Br-cGMP (5.91 ± 1.06 A F−1, n = 5; capacitance 19.8 ± 1.8 pF). Zn2+ at a concentration of 100 μM, which eliminates the cGMP-dependent ICl(Ca) of mesenteric artery myocytes, did not change the current in the pulmonary artery myocytes (current density 6.93 ± 1.53 A F−1, n = 5; capacitance 15.8 ± 1.6 pF). Also in contrast to the cGMP-dependent current in the mesenteric artery smooth-muscle cells, the current in the pulmonary artery myocytes was inhibited (n = 5) by 100 μM niflumic acid (Fig. 8).
Figure 8.
Ca2+-activated Cl− currents recorded in smooth-muscle cells from pulmonary arteries. Original recordings in A show stimulations by 10 mM caffeine (as indicated with black bars) under control conditions (solutions were B1:P1 without cGMP in the pipette solution) and in the presence of 8Br-cGMP, ZnCl2, and niflumic acid as indicated. Breaks in the records correspond to 10 min wash periods. B shows the mean current densities of the Ca2+-activated Cl− current in control conditions (filled squares, thin line) in the presence of 100 μM ZnCl2 (crosses, dashed line) and in the presence of 100 μM niflumic acid (open circles, thick line). Experimental protocol was as in A. Vertical bars indicate SEM.
The current-voltage relations were obtained for the current evoked by caffeine using a ramp protocol (Fig. 8 B). The current exhibited outward rectification as previously described for ICl(Ca) in pulmonary arteries (Large and Wang, 1996; Greenwood et al., 2001).
DISCUSSION
In the present work we have characterized a cGMP-dependent, Ca2+-activated inward current that we have previously demonstrated (Peng et al., 2001) in vascular smooth-muscle cells from rat mesenteric small arteries.
The most prominent channel activated by calcium release in vascular smooth-muscle is the large-conductance calcium-activated potassium channel (ZhuGe et al., 1998; Smani et al., 2001). Such a current was also observed in the present study, but at holding potentials of −60 mV the dominating current was an inward current. Using a combination of ion substitution experiments and blockers it was possible to conclude that the current was a Cl− current since the current reversal potential closely followed the Cl− equilibrium potential, whereas extracellular removal of either Na+ or Ca2+ had no effect on the current.
The pharmacological and biophysical characteristics of this Ca2+-activated, cGMP-dependent Cl− current were investigated and will be discussed in relation to the previously described Ca2+-activated Cl− current in smooth muscle (Wang et al., 1992; Large and Wang, 1996; Greenwood and Large, 1999).
cGMP Dependence
The cGMP-dependent ICl(Ca) was evoked by calcium release from the SR in response to caffeine application, through influx of Ca2+ induced by ionomycin or BAY K 8644, and by direct activation with high-calcium pipette solution. In every case the cGMP dependence was apparent and the EC50 value of ∼6 μM (determined with caffeine induced activation) is similar to that of cyclic nucleotide–gated channels elsewhere (Müller et al., 1998, 2001), and substantially lower than that for cGMP modulation of calcium-activated potassium currents (Schubert and Nelson, 2001). Recalculation of data on cGMP content in mesenteric arteries (Liu et al., 2002) with data on smooth-muscle cell water content (Aalkjaer and Mulvany, 1983) suggest that concentrations in the range 2–4 μM may be physiological. This agrees well with the concentration range over which the present channel is regulated. Since the current was elicited by four different means of increasing intracellular Ca2+ it is unlikely that the role of cGMP is due to an effect on the Ca2+ release or Ca2+ kinetics. In particular since ionomycin could elicit the current in the presence of ryanodine it is unlikely that cGMP acts through modification of Ca2+ release from the SR. Therefore, a more likely site of action of cGMP is on the channel or a channel-related protein. Moreover, since current activation seemed to require phosphorylation, was sensitive to PKG inhibitors, and could be evoked by PKG, we believe that the effect of cGMP on the current is mediated through PKG.
There is no previous report on a cGMP-dependent ICl(Ca) in smooth muscle. In cat tracheal smooth-muscle, cGMP inhibits a Ca2+-activated Cl− current, an effect not mediated by G kinase (Waniishi et al., 1998). That current further differs from the present current in having a higher sensitivity to Cl−-channel blockers and being insensitive to 500 μM Zn2+. Indirect evidence from rat aorta, based on the influence of the chloride gradient on contractile responses to noradrenaline in the presence and absence of endothelium, also points to an inhibitory role of cGMP on chloride currents (Lamb and Barna, 1998). In portal vein smooth-muscle cells, both enhancement and inhibition of the volume-sensitive chloride current by cGMP have been reported (Ellershaw et al., 2000). Cyclic AMP generally has been reported to activate chloride channels, especially the CFTR (Chen et al., 2001), but had no effect on the present current. Interestingly, the epithelial CFTR channel was also shown to be activated by 100 μM cGMP via PKG (Lin et al., 1992). However, that channel had a different permeability sequence from the one studied here, and could also be activated by PKA.
In addition to the cGMP-dependent current, a cGMP-independent current was detected in the smooth-muscle cells from the rat mesenteric arteries, and this was the only current detected in the smooth-muscle cells from pulmonary arteries. This indicates that not all vascular beds may have a prominent cGMP-dependent ICl(Ca) and also shows that the cGMP dependence was not a consequence of our experimental approach. The fact that the conventional ICl(Ca) was more clearly detected with high calcium in the pipette than after caffeine application may indicate that the two currents differ in their calcium sensitivity or are differently affected by calcium release.
ICl(Ca) has been reported to be more stable in experiments using the permeabilized patch technique than in conventional whole-cell experiments, where it tends to show significant run-down (Janssen and Sims, 1992; Large and Wang, 1996). This suggests the presence of a soluble factor of importance for maintaining channel activity. In our previous study (Peng et al., 2001) using permeabilized patches, the current studied here could be activated even after some time in the absence of 8Br-cGMP. In the present study using conventional whole-cell experiments the current was not detectable 5 min after breaking the membrane in the absence of cGMP. This suggests that the permeabilized cells retained some endogenous cGMP, which was sufficient for partial activation of the current. However, we did not observe such a pronounced run-down in the pulmonary myocytes, where the current was totally insensitive to cGMP. This makes it unlikely that the run-down of the conventional ICl(Ca) can be explained by washout of cGMP.
Relative Anion Selectivity
The relative permeability for all Ca2+-activated chloride channels depends on the ionic radius, and although slightly variable the typical sequence is I− > Br− > Cl− > F− (Amédée et al., 1990; Arreola et al., 1996; Clapp et al., 1996; Frings et al., 2000). The permeabilities obtained in our experiments under biionic conditions, while not identical, were similar (Br− > I− > Cl− > F−). The sequence Br− > I− > Cl− > F− is typical for a system with an I−/Cl− selectivity ratio close to 1, while the sequence I− > Br− > Cl− > F− is characteristic for systems with an I−/Cl− ratio higher than 1 (Wright and Diamond, 1977). Our data had I−/Cl− ratios of 1.25 (permeability) and 1.41 (conductance), and the obtained selectivity sequences thus fit the theoretical predictions well (Wright and Diamond, 1977). The data are consistent with selectivity being primarily based on energy of hydration.
Thiocyanate had a high permeability but a low conductance through the channel, indicating that it may bind with high affinity within the channel. Thiocyanate shows a similar high affinity for the GABA receptor of spinal neurons (Bormann et al., 1987). It also shows high affinity for the Xenopus oocyte calcium-activated chloride channel (Qu and Hartzell, 2000), although both its relative permeability and conductance in the oocyte channel are somewhat higher than in our data.
The fact that both cGMP-dependent and cGMP-independent Cl− currents were present in the same smooth-muscle cells opens the possibility that the data for anion selectivity for the cGMP-dependent current might be contaminated by the cGMP-independent current. We never observed the conventional, cGMP-independent current in experiments using ionomycin to elevate [Ca2+]i under control conditions, and therefore used this protocol in the substitution experiments. It is possible that a contaminating current may have become visible in the presence of a substitute ion. However, none the substitutes used here give substantially larger currents than chloride through the conventional, cGMP-independent current (Qu and Hartzell, 2000), with the possible exception of aspartate. Even though we cannot entirely exclude a contribution of the conventional current to the permeability experiments, we therefore believe any such contribution to be small.
Effect of Cl− Channel Blockers
There are no specific high-affinity blockers for Ca2+-activated Cl− channels available presently. Niflumic acid has been shown in several studies to be an effective inhibitor in micromolar concentrations (Pacaud et al., 1989; Hogg et al., 1994; Lamb et al., 1994; Kirkup et al., 1996). The cGMP-dependent ICl(Ca) was sensitive to both niflumic acid, DIDS, and IAA-94, but the potency of these drugs was low. However, some Ca2+-activated Cl− channel clones are either insensitive or have a low sensitivity to niflumic acid (Cunningham et al., 1995). This has been suggested to be due to a dual effect of niflumic acid to simultaneously enhance and block ICl(Ca) by binding to an external site, probably close to the mouth of the chloride channel (Piper et al., 2002). Whether this can explain the low sensitivity of the cGMP-dependent ICl(Ca) to niflumic acid is unknown. The fact that not only niflumic acid, but also DIDS and IAA-94 exhibited a low potency could suggest that the structure of the channel may differ from other Ca2+-activated Cl− channels. The experiments in Figs. 6 and 8 demonstrate clearly the difference in sensitivity to niflumic acid between the cGMP-dependent and -independent ICl(Ca).
Cation Sensitivity
ICl(Ca) is known to be sensitive to block by several multivalent cations, such as gadolinium, lanthanum, cadmium, cobalt, and nickel applied to the extracellular side of the membrane, whereas Zn2+ has little effect (Tokimasa and North, 1996; Waniishi et al., 1998). Lack of sensitivity to Zn2+ was also found in our experiments on pulmonary arterial myocytes (Fig. 8) and for the cGMP-independent ICl(Ca) in mesenteric artery smooth-muscle cells. In contrast, the cGMP-dependent ICl(Ca) had a high sensitivity to block by Zn2+ and was unaffected by cobalt. This pattern is known for voltage-gated chloride channels (Kurz et al., 1997; Chen, 1998), and suggests that the cGMP-dependent ICl(Ca) in this respect is more like these chloride channels.
Voltage and Time Dependence
The cGMP-dependent ICl(Ca) did not display the voltage dependence commonly seen for other ICl(Ca) (Ishikawa and Cook, 1993; Arreola et al., 1996; Large and Wang, 1996; Greenwood et al., 2001). The voltage dependence of ICl(Ca) depends on the calcium concentration and at very high intracellular calcium concentrations little rectification of ICl(Ca) is seen in parotid glands (Arreola et al., 1996). However, in some experiments we fixed intracellular calcium at 900 nM where we found no rectification, while the cGMP-insensitive current in the same experiments exhibited outward rectification (Fig. 6). Even though the voltage independence was unexpected, similar observations have previously been reported for ICl(Ca) in hepatocytes (Koumi et al., 1994) and in neuronal tissue (Krause and Welsh, 1990; Hallani et al., 1998).
Comparison with other Channels
The cGMP-dependent ICl(Ca) is similar to the classical, cGMP-independent ICl(Ca) in terms of being calcium dependent, but distinct in terms of lack of voltage and time dependence, and in terms of its sensitivity to pharmacological inhibitors. Its high sensitivity to inhibition by divalent cations seems reminiscent of voltage-activated Cl− channels. However, many of these characteristics are similar to what has been reported recently for members of the bestrophin family (Qu et al., 2003; Tsunenari et al., 2003). Whether this similarity is only superficial or points to structural similarities remains to be determined.
Physiological Role
Since the chloride equilibrium potential of mesenteric artery smooth-muscle cells is about −30 mV (Videbaek et al., 1990; Chipperfield and Harper, 2000), opening of these channels in a resting smooth-muscle with a membrane potential in the range of −50 to −60 mV will cause depolarization, calcium influx, and contraction (Pacaud et al., 1992; Wang et al., 1992; Lepretre et al., 1994; Kirkup et al., 1996). In the arteries studied here, calcium release is not essential for depolarization in response to noradrenaline, and the transmembrane gradient for chloride has little influence on the membrane potential at steady-state, whether at rest or during maximal activation (Nilsson et al., 1998). The cGMP-dependent ICl(Ca) therefore is not likely to be important for the tonic contractile response to receptor activation. In contrast, the current has characteristics that could suggest that it has importance for smooth-muscle synchronization as we have suggested previously (Peng et al., 2001). Synchronization is essential for coordinating oscillations in [Ca2+]i in vasomotion, and we have suggested that this is brought about by changes in membrane potential subsequent to released intracellular calcium activating the cGMP-dependent current (Peng et al., 2001). In this way this current may generate intermittent depolarizations maintaining synchrony between the individual smooth-muscle cells in the vessel wall. At the same time, by promoting continuous variations in tone, formation of the latch state is prevented, enabling the smooth-muscle to respond rapidly to, e.g., neural stimuli. Being regulated by two important pathways, [Ca2+]i and NO/cGMP, this current is potentially important in integrating endothelial and smooth-muscle control of vascular function.
It may seem surprising that cGMP promotes an inward current leading to depolarization and vasoconstriction rather than the vasodilatation normally associated with increased cGMP. However, it should be pointed out that the net effect of elevating cGMP in mesenteric small arteries is hyperpolarization and relaxation. How these apparently opposing effects of cGMP on membrane events are orchestrated under physiological conditions is currently unknown.
In summary, the present study characterized a novel Ca2+-activated Cl− channel with an obligatory requirement for cGMP in its activation by intracellular calcium. This channel has distinct properties reminiscent of characteristics of both classical Ca2+-activated and voltage-gated Cl− channels. The channel is characterized by a high sensitivity to inhibition by Zn2+ but a low sensitivity to inhibition by conventional chloride channel blockers and exhibits no rectification, and is thus different from the ICl(Ca) of pulmonary artery myocytes (Greenwood et al., 2001; this study). Further molecular characterization of the channel(s) responsible for this current could provide insight into structural elements of importance for Cl− channel function and for regulation of vascular function in general.
Acknowledgments
This work was supported by the Danish Medical Research Council (grant no. 9802388) and the Danish Heart Foundation (grant no. 98-1-2-12A-22593). The Water and Salt Research Center at the University of Aarhus is established and supported by the Danish National Research Foundation (Danmarks Grundforskningsfond).
Lawrence G. Palmer served as editor.
References
- Aalkjaer, C., and M.J. Mulvany. 1983. Sodium metabolism in rat resistance vessels. J. Physiol. 343:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amédée, T., W.A. Large, and Q. Wang. 1990. Characteristics of chloride currents activated by noradrenaline in rabbit ear artery cells. J. Physiol. 428:501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola, J., J.E. Melvin, and T. Begenisich. 1996. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J. Gen. Physiol. 108:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, M.J., and A. Galione. 1988. Cytosolic calcium oscillators. FASEB J. 2:3074–3082. [DOI] [PubMed] [Google Scholar]
- Bormann, J., O.P. Hamill, and B. Sakmann. 1987. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. 385:243–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton, F.C., S. Ohya, B. Horowitz, and I.A. Greenwood. 2002. Comparison of the properties of CLCA1 generated currents and ICl(Ca) in murine portal vein smooth-muscle cells. J. Physiol. 539:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., T.C. Hwang, and K.D. Gillis. 2001. The relationship between cAMP, Ca2+, and transport of CFTR to the plasma membrane. J. Gen. Physiol. 118:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T.Y. 1998. Extracellular zinc ion inhibits ClC-0 chloride channels by facilitating slow gating. J. Gen. Physiol. 112:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield, A.R., and A.A. Harper. 2000. Chloride in smooth muscle. Prog. Biophys. Mol. Biol. 74:175–221. [DOI] [PubMed] [Google Scholar]
- Clapp, L.H., and A.M. Gurney. 1991. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp. Physiol. 76:677–693. [DOI] [PubMed] [Google Scholar]
- Clapp, L.H., J.L. Turner, and R.Z. Kozlowski. 1996. Ca2+-activated Cl− currents in pulmonary arterial myocytes. Am. J. Physiol. 270:H1577–H1584. [DOI] [PubMed] [Google Scholar]
- Cunningham, S.A., M.S. Awayda, J.K. Bubien, I.I. Ismailov, M.P. Arrate, B.K. Berdiev, D.J. Benos, and C.M. Fuller. 1995. Cloning of an epithelial chloride channel from bovine trachea. J. Biol. Chem. 270:31016–31026. [DOI] [PubMed] [Google Scholar]
- Dostmann, W.R., M.S. Taylor, C.K. Nickl, J.E. Brayden, R. Frank, and W.J. Tegge. 2000. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Iα inhibit NO-induced cerebral dilation. Proc. Natl. Acad. Sci. USA. 97:14772–14777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellershaw, D.C., I.A. Greenwood, and W.A. Large. 2000. Dual modulation of swelling-activated chloride current by NO and NO donors in rabbit portal vein myocytes. J. Physiol. 528:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings, S., D. Reuter, and S.J. Kleene. 2000. Neuronal Ca2+-activated Cl− channels-homing in on an elusive channel species. Prog. Neurobiol. 60:247–289. [DOI] [PubMed] [Google Scholar]
- Greenwood, I.A., and W.A. Large. 1999. Modulation of the decay of Ca2+-activated Cl− currents in rabbit portal vein smooth muscle cells by external anions. J. Physiol. 516:365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, I.A., J. Ledoux, and N. Leblanc. 2001. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J. Physiol. 534:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, H., M.J. Mulvany, and H. Nilsson. 1993. Rhythmic contractions of isolated small arteries from rat: influence of the endothelium. Acta Physiol. Scand. 148:153–163. [DOI] [PubMed] [Google Scholar]
- Gustafsson, H., and H. Nilsson. 1994. Rhythmic contractions in isolated small arteries of rat: role of K+ channels and the Na+,K+-pump. Acta Physiol. Scand. 150:161–170. [DOI] [PubMed] [Google Scholar]
- Hallani, M., J.W. Lynch, and P.H. Barry. 1998. Characterization of calcium-activated chloride channels in patches excised from the dendritic knob of mammalian olfactory receptor neurons. J. Membr. Biol. 161:163–171. [DOI] [PubMed] [Google Scholar]
- Hogg, R.C., Q. Wang, and W.A. Large. 1994. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br. J. Pharmacol. 112:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa, T., and D.I. Cook. 1993. A Ca2+-activated Cl− current in sheep parotid secretory cells. J. Membr. Biol. 135:261–271. [DOI] [PubMed] [Google Scholar]
- Janssen, L.J., and S.M. Sims. 1992. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J. Physiol. 453:197–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup, A.J., G. Edwards, M.E. Green, M. Miller, S.D. Walker, and A.H. Weston. 1996. Modulation of membrane currents and mechanical activity by niflumic acid in rat vascular smooth muscle. Eur. J. Pharmacol. 317:165–174. [DOI] [PubMed] [Google Scholar]
- Koumi, S., R. Sato, and T. Aramaki. 1994. Characterization of the calcium-activated chloride channel in isolated guinea-pig hepatocytes. J. Gen. Physiol. 104:357–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, K.H., and M.J. Welsh. 1990. Voltage-dependent and Ca2+-activated ion channels in human neutrophils. J. Clin. Invest. 85:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz, L., S. Wagner, A.L.J. George, and R. Rudel. 1997. Probing the major skeletal muscle chloride channel with Zn2+ and other sulfhydryl-reactive compounds. Pflugers Arch. 433:357–363. [DOI] [PubMed] [Google Scholar]
- Lamb, F.S., and T.J. Barna. 1998. The endothelium modulates the contribution of chloride currents to norepinephrine-induced vascular contraction. Am. J. Physiol. 275:H161–H168. [DOI] [PubMed] [Google Scholar]
- Lamb, F.S., K.A. Volk, and E.F. Shibata. 1994. Calcium-activated chloride current in rabbit coronary artery myocytes. Circ. Res. 75:742–750. [DOI] [PubMed] [Google Scholar]
- Large, W.A., and Q. Wang. 1996. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am. J. Physiol. 271:C435–C454. [DOI] [PubMed] [Google Scholar]
- Lepretre, N., J. Mironneau, S. Arnaudeau, Z. Tanfin, S. Harbon, G. Guillon, and J. Ibarrondo. 1994. Activation of alpha-1A adrenoceptors mobilizes calcium from the intracellular stores in myocytes from rat portal vein. J. Pharmacol. Exp. Ther. 268:167–174. [PubMed] [Google Scholar]
- Lin, M., A.C. Nairn, and S.E. Guggino. 1992. cGMP-dependent protein kinase regulation of a chloride channel in T84 cells. Am. J. Physiol. 262:C1304–C1312. [DOI] [PubMed] [Google Scholar]
- Liu, H., J.M. Ledingham, I. Mullaney, and R. Laverty. 2002. Endothelial function in mesenteric resistance arteries from the genetically hypertensive rat. Clin. Exp. Pharmacol. Physiol. 29:405–411. [DOI] [PubMed] [Google Scholar]
- Matchkov, V., C. Aalkjaer, and H. Nilsson. 2003. A novel cGMP-dependent calcium-activated chloride current from the mesenteric resistance artery. J. Gen. Physiol. 122:40a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchkov, V., H. Peng, C. Aalkjaer, and H. Nilsson. 2001. The ion channel of vasomotion. Eur. J. Physiol. 441:R137. [Google Scholar]
- Müller, F., W. Bonigk, F. Sesti, and S. Frings. 1998. Phosphorylation of mammalian olfactory cyclic nucleotide-gated channels increases ligand sensitivity. J. Neurosci. 18:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, F., M. Vantler, D. Weitz, E. Eismann, M. Zoche, K.W. Koch, and U.B. Kaupp. 2001. Ligand sensitivity of the 2 subunit from the bovine cone cGMP-gated channel is modulated by protein kinase C but not by calmodulin. J. Physiol. 532:399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, H., L.M. Videbaek, C. Toma, and M.J. Mulvany. 1998. Role of intracellular calcium for noradrenaline-induced depolarization in rat mesenteric small arteries. J. Vasc. Res. 35:36–44. [DOI] [PubMed] [Google Scholar]
- Pacaud, P., G. Loirand, G. Gregoire, C. Mironneau, and J. Mironneau. 1992. Calcium-dependence of the calcium-activated chloride current in smooth muscle cells of rat portal vein. Pflugers Arch. 421:125–130. [DOI] [PubMed] [Google Scholar]
- Pacaud, P., G. Loirand, J.L. Lavie, C. Mironneau, and J. Mironneau. 1989. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 413:629–636. [DOI] [PubMed] [Google Scholar]
- Peng, H., V. Matchkov, A. Ivarsen, C. Aalkjaer, and H. Nilsson. 2001. Hypothesis for the initiation of vasomotion. Circ. Res. 88:810–815. [DOI] [PubMed] [Google Scholar]
- Petkov, G.V., F. Fusi, S. Saponara, H.S. Gagov, G.P. Sgaragli, and K.K. Boev. 2001. Characterization of voltage-gated calcium currents in freshly isolated smooth muscle cells from rat tail main artery. Acta Physiol. Scand. 173:257–265. [DOI] [PubMed] [Google Scholar]
- Piper, A.S., I.A. Greenwood, and W.A. Large. 2002. Dual effect of blocking agents on Ca2+-activated Cl− currents in rabbit pulmonary artery smooth muscle cells. J. Physiol. 539:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Z., and H.C. Hartzell. 2000. Anion permeation in Ca2+-activated Cl− channels. J. Gen. Physiol. 116:825–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Z., R.W. Wei, W. Mann, and H.C. Hartzell. 2003. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl− currents. J. Biol. Chem. 278:49563–49572. [DOI] [PubMed] [Google Scholar]
- Salter, K.J., J.L. Turner, S. Albarwani, L.H. Clapp, and R.Z. Kozlowski. 1995. Ca2+-activated Cl− and K+ channels and their modulation by endothelin-1 in rat pulmonary arterial smooth muscle cells. Exp. Physiol. 80:815–824. [DOI] [PubMed] [Google Scholar]
- Schubert, R., U. Krien, and H. Gagov. 2001. Protons inhibit the BKCa channel of rat small artery smooth muscle cells. J. Vasc. Res. 38:30–38. [DOI] [PubMed] [Google Scholar]
- Schubert, R., and M.T. Nelson. 2001. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol. Sci. 22:505–512. [DOI] [PubMed] [Google Scholar]
- Smani, T., S. Iwabuchi, J. Lopez-Barneo, and J. Urena. 2001. Differential segmental activation of Ca2+-dependent CI− and K+ channels in pulmonary arterial myocytes. Cell Calcium. 29:369–377. [DOI] [PubMed] [Google Scholar]
- Tokimasa, T., and R.A. North. 1996. Effects of barium, lanthanum and gadolinium on endogenous chloride and potassium currents in Xenopus oocytes. J. Physiol. 496:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunenari, T., H. Sun, J. Williams, H. Cahill, P. Smallwood, K.W. Yau, and J. Nathans. 2003. Structure-function analysis of the bestrophin family of anion channels. J. Biol. Chem. 278:41114–41125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbaek, L.M., C. Aalkjaer, A.D. Hughes, and M.J. Mulvany. 1990. Effect of pinacidil on ion permeability in resting and contracted resistance vessels. Am. J. Physiol. 259:H14–H22. [DOI] [PubMed] [Google Scholar]
- Wang, Q., R.C. Hogg, and W.A. Large. 1992. Properties of spontaneous inward currents recorded in smooth muscle cells isolated from the rabbit portal vein. J. Physiol. 451:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniishi, Y., R. Inoue, H. Morita, N. Teramoto, K. Abe, and Y. Ito. 1998. Cyclic GMP-dependent but G-kinase-independent inhibition of Ca2+-dependent Cl− currents by NO donors in cat tracheal smooth muscle. J. Physiol. 511:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, R.E., A.B. Lee, A.D. Shcherbatko, T.M. Lincoln, A. Schonbrunn, and D.L. Armstrong. 1993. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 361:263–266. [DOI] [PubMed] [Google Scholar]
- Wright, E.M., and J.M. Diamond. 1977. Anion selectivity in biological systems. Physiol. Rev. 57:109–156. [DOI] [PubMed] [Google Scholar]
- Yount, R.G. 1975. ATP analogs. Adv. Enzymol. Relat. Areas Mol. Biol. 43:1–56. [DOI] [PubMed] [Google Scholar]
- Yuan, X.J. 1997. Role of calcium-activated chloride current in regulating pulmonary vasomotor tone. Am. J. Physiol. 272:L959–L968. [DOI] [PubMed] [Google Scholar]
- ZhuGe, R., S.M. Sims, R.A. Tuft, K.E. Fogarty, and J.V. Walsh, Jr. 1998. Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J. Physiol. 513:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]