GABA in the Endocrine Pancreas
γ-Aminobutyric acid (GABA) is a principal nonpeptidal neurotransmitter with a well-characterized role as an inhibitor of neuronal firing in the CNS (Cherubini et al., 1991; Thomas-Reetz and De Camilli, 1994). GABA is also found in other tissues, such as the endocrine pancreas (Thomas-Reetz and De Camilli, 1994). In the neuron, GABA is synthesized in close proximity to synaptic vesicles, it is produced from glutamate by the enzyme glutamate decarboxylase (GAD) and then transported into the synaptic vesicles against a proton electrochemical gradient (Thomas-Reetz and De Camilli, 1994). Exocytosis of neuronal synaptic vesicles is triggered when voltage-sensitive calcium channels open, causing a transient increase in cytosolic calcium. GABA is consequently released into the synaptic space, where it interacts with ionotropic receptors on the postsynaptic membrane, increasing their chloride conductance and thereby inducing membrane hyperpolarization and inhibition of exocytosis (Cherubini et al., 1991; Thomas-Reetz and De Camilli, 1994). However, when the electrochemical chloride gradient across the membrane is directed outward, as in neonatal hippocampal neurons, GABA is excitatory (Cherubini et al., 1991).
The endocrine pancreas also contains high concentrations of GABA, and the 65-kD isoform of GAD (GAD 65) predominantly in the core of the islets of Langerhans, where insulin-producing β-cells are located (Sorenson et al., 1991; Thomas-Reetz and De Camilli, 1994; Chessler et al., 2002). For more than three decades the role of β-cell GABA has puzzled scientists, with interest intensifying on the discovery that GAD 65 is one of the major autoantigens in type 1 diabetes mellitus, a disease in which β-cells are systematically destroyed by an autoimmune mechanism (Yoon et al., 1999). Evidence suggests that β-cell GABA localizes to synaptic-like microvesicles (SLMVs) and is exocytosed in a regulated manner, as it is in neurons (Thomas-Reetz and De Camilli, 1994). What are the stimuli for β-cell GABA release? Which islet cell type/s are the targets of this fast-acting signaling molecule and what, if any, are its effects on hormone secretion? These important questions have remained unanswered largely because of the difficulty in obtaining quantitative amounts of secreted GABA to detect temporal changes in GABA release. A fresh approach to the detection of secreted GABA, providing both high temporal resolution and sensitivity, is presented by Braun and colleagues in this issue (Braun et al., 2004). This new methodology and its application contribute a significant step toward the characterization of the mechanisms of β-cell GABA release and offers a platform for further studies of GABA stimulus-secretion coupling and SLMV exocytosis.
SLMVs in β-cells
SLMVs have been identified in peptide-secreting endocrine cells, including those in the gut, adrenal chromaffin cells, and the pancreatic islets (Thomas-Reetz and De Camilli, 1994; Anhert-Hilger et al., 1996). These microvesicles are classified as synaptic-like because they contain membrane proteins involved in neuronal synaptic vesicle exocytosis, including synaptophysin, the vSNARE (vesicular soluble N-ethylmaleimide-sensitive factor attachment receptor) synaptobrevin-2, and the putative calcium sensor synaptotagmin I/II (Thomas-Reetz and De Camilli, 1994; Regazzi et al., 1995; Lang et al., 1997). Other proteins implicated in the regulation of exocytosis, e.g., SNAP25, syntaxin I, and Rab3, have been identified in β-cells and derived cell lines (Lang, 1999). Ultrastructural studies have previously demonstrated that β-cell SLMVs are distinct in both appearance and size from the insulin-containing large dense core vesicles (LDCVs) (Sorenson et al., 1991; Lang et al., 1997). Now, Braun et al. (2004), provide quantitative information and show that a proportion of these SLMVs appear to be localized in abundance at the plasma membrane, with some vesicles apparently docked (Braun et al., 2004). The striking similarity between β-cell SLMVs and neuronal synaptic vesicle morphology, subcellular distribution and the overlap in exocytosis-associated membrane proteins suggests that they may share a role as vehicles for storage and release of signaling molecules.
GABA Is Synthesized in β-cells
An extensive body of research has demonstrated that GABA is present in high levels within pancreatic islets, and that the majority is localized to the β-cells, although small amounts are also detected in the islet mantle (for review see Sorenson et al., 1991; Gilon et al., 1991). GABA is synthesized in the cytosol from its precursor glutamate by GAD. Glutamate is generated in the mitochondria, either from the TCA-cycle intermediate α-ketoglutarate by glutamate dehydrogenase or by other transamination reactions. Glutamate is then transferred to the cytosol, where it can also be produced by glutaminolysis. GABA catabolism occurs in the mitochondria where GABA is transaminated with α-ketoglutarate by GABA transaminase to form succinate semialdehyde. This has been referred to as the GABA shunt (Sorenson et al., 1991). β-cells express high levels of GAD 65, the membrane-associated isoform of GAD, which is also linked with synaptic vesicles in neurons (Thomas-Reetz and De Camilli, 1994; Chessler et al., 2002). High levels of GAD activity have indeed been detected in islets (Sorenson et al., 1991). GAD 67, another isoform of GAD, exhibits different subcellular localization to GAD 65 in β-cells and neurons (Thomas-Reetz and De Camilli, 1994). This isoform has been identified in rat but not in human or mouse islets (Shi et al., 2000; Chessler et al., 2002). In rat, GAD 67 appears to be more abundant in the islet mantle, where glucagon-secreting α-cells and somatostatin-secreting δ-cells predominate, rather than in the β-cell containing core, where GAD 65 is exclusively localized (Chessler et al., 2002). However, it should be noted that endocrine cells might not be the only source of islet GABA in vivo and in some experimental systems, such as isolated islets and in situ perfused pancreas. The presence of GABAergic neurons, closely associated with, and indeed penetrating into the islet mantle, has been confirmed by immunocytochemistry (Sorenson et al., 1991).
Is β-cell GABA Localized within SLMVs?
Although a direct demonstration of GABA localization in β-cell SLMVs by immunogold electron microscopy is lacking, such analyses have thus far indicated that GABA is concentrated in regions enriched with SLMVs (Reetz et al., 1991) and indeed excluded from LDCVs (Braun et al., 2004, this issue). Moreover, GAD 65 clearly colocalizes with β-cell SLMV membrane proteins, as it does with synaptic vesicles in neurons, strongly supporting the notion that GABA is transported into SLMVs once synthesized (Reetz et al., 1991). Uptake studies of radio-labeled GABA by purified SLMVs of the β-cell line βTC3 have revealed an ATP-dependent proton ionophore-sensitive GABA uptake mechanism, similar to that observed in neuronal synaptic vesicles (Thomas-Reetz and De Camilli, 1994). A similar demonstration of ATP-dependent GABA uptake was made in permeabilized RIN 38 cells, a pancreatic β-cell line (Anhert-Hilger et al., 1996). The only fly in the ointment is that the GABA/glycine transporter VGAT (VIAAT), initially characterized in GABAergic neuronal cells (McIntire et al., 1997), was recently identified in rat, but not human islets (Chessler et al., 2002). Moreover, the VGAT expression pattern paralleled that of GAD 67, being more abundant in the islet mantle than the core (Chessler et al., 2002). It is possible that β-cells utilize an alternative transporter for GABA uptake into SLMVs, which displays similar properties to the neuronal vesicular transporter. Despite the lack of a molecular identity for the β-cell vesicular GABA transporter, it seems that GABA is in all probability stored in, and exocytosed from, SLMVs, as has become increasingly evident from studies of GABA secretion, discussed below.
Regulated GABA Release from β-cells
Synaptic vesicle exocytosis in neurons occurs by regulated rather than constitutive exocytosis, in response to a transient rise in cytosolic calcium (Thomas-Reetz and De Camilli, 1994). Is β-cell GABA also released by a regulated process? Studies of GABA release have until now required large amounts of starting material, usually cell lines, to obtain sufficient amounts of secreted GABA for quantitative analysis. These studies have revealed that in the β-cell line RIN 38, and the neuroendocrine cell line BON, GABA secretion can be stimulated by high concentrations of extracellular K+, or the calcium ionophore A23187, both dependent on the presence of extracellular calcium (von Blankenfeld et al., 1995; Anhert-Hilger et al., 1996). GABA release from BON cells was also stimulated twofold by the cAMP-raising agent IBMX, a phosphodiesterase inhibitor (John et al., 1998). This suggests that, like insulin secretion, GABA is released in response to an increase in cytosolic calcium, as a result of membrane depolarization, which triggers the opening of voltage-gated calcium channels. Other studies in MIN6 cells, a widely used mouse β-cell line, have shown that GABA release can be stimulated up to threefold by mastoparan, a wasp venom peptide that activates G-proteins and stimulates LDCV exocytosis (Ohara-Imaizumi et al., 2001). Moreover, mastoparan-stimulated GABA release was attenuated by the tetanus toxin C1 light chain, a neurotoxin that cleaves synaptobrevin-2. In contrast, overexpression of the t-SNARES SNAP 25 or syntaxin IA inhibited mastoparan-induced insulin secretion but not GABA release (Ohara-Imaizumi et al., 2001). These findings imply that regulated GABA release involves similar but not identical mechanisms to those of insulin secretion. Both of these processes appear to involve machinery implicated in neurotransmitter release.
The notion that β-cell GABA release is a regulated process has been reinforced by Braun et al. (2004) using dispersed rat islets. The authors employed an adenoviral system to overexpress the GABAA receptor, a GABA-activated Cl− channel, as a novel detection system for GABA release. To this end, whole-cell patch-clamped β-cells were used as biosensors for their own secreted GABA, detected as fluctuations in current (current transients). The authors show that GABA release occurred in response to membrane depolarization, dependent on the entry of extracellular calcium through Cd2+-sensitive (voltage-gated) channels. The release of caged calcium in GABAA receptor overexpressing β-cells also induced an increase in transient currents associated with receptor activation. These findings demonstrate that calcium entry through voltage-gated channels, and not membrane depolarization itself, can trigger GABA release. Interestingly, when the concentration of calcium within the pipette solution was increased from 3–25 μM, a twofold increase in GABAA-mediated current changes and a similar increase in total membrane capacitance was observed. As the authors state, an increase in cell capacitance is largely reflective of exocytosis of LDCVs rather than SLMVs (<2% of total increase). However, this does not necessarily indicate that the same calcium sensor is involved in GABA (SLMV) and LDCV release, as the sensors identity and detailed calcium-dose response relationships remain to be established. It was also noted that depolarization-induced current changes rapidly desensitized, but recovered after a 2-min break in trains of depolarizations. This supports a vesicular origin for GABA, perhaps within readily releasable and reserve SLMV pools, compatible with their ultrastructural findings. In agreement with the stimulatory effect of IBMX on GABA release from neuroendocrine cell lines (John et al., 1998), the presence of forskolin, an adenylate cyclase activator, increased the number of depolarization-induced GABA release events detected (Braun et al., 2004). Therefore, cAMP acts as a potentiator not only of LDCV, but also SLMV exocytosis (Lang, 1999).
Does High Glucose Stimulate β-cell GABA Release?
An important question when considering the role of GABA as an intraislet paracrine signaling molecule must be whether islet GABA secretion is stimulated, as insulin, or inhibited during post-prandial hyperglycemia. Glucose metabolism stimulates LDCV exocytosis by depolarizing the plasma membrane, which promotes calcium influx through voltage-gated calcium channels (Lang, 1999). Many authors who believe GABA secretion is stimulated by glucose have relied on an early paper demonstrating an increase in GABA release from the β-cell line βTC6 only apparent after a long (total of 12 h) exposure to high glucose (Gaskins et al., 1995). However, such a prolonged exposure to high glucose may have altered the expression of the enzymes involved in GABA metabolism, or SNARES involved in SMLV exocytosis. Moreover, a concurrent increase in glucose-induced insulin secretion was not demonstrated, questioning the validity of this cell line as a model for primary β-cells. In another paper where the widely accepted β-cell model, MIN6, was used, no significant increase in GABA release was observed after a 2-h incubation in high glucose, contrasting with a twofold stimulation of insulin secretion (Nagamatsu et al., 1999), also reported in a later study (Hayashi et al., 2003).
A mere handful of studies have addressed the question of glucose-stimulated GABA secretion in primary pancreatic preparations. Increased GABA release in the presence of high glucose was detected using pieces of rabbit pancreas (Gerber and Hare, 1980). After 15 min, a twofold increase in secreted GABA was observed. However, a convincing demonstration of glucose-induced insulin secretion was again lacking in this study. In contrast, a later investigation showed inhibition (40%) of GABA release from reaggregated rat β-cells after a 2-h (or 24-h) culture period in high when compared with low glucose conditions. A threefold increase in insulin secretion was observed in parallel (Winnock et al., 2001). In agreement with this finding, a second study demonstrated a twofold increase in GABA release from rat islets after 30 min when islets were transferred from high to low glucose conditions (Hayashi et al., 2003). A concurrent decrease in insulin secretion was confirmed.
The overall trend from these studies is not clear and so the effect of glucose on GABA release remains inconclusive. However, the function of GABA in the CNS as a fast-acting signaling molecule should be considered. All of the aforementioned studies examined GABA release after a relatively long incubation time (minutes to hours), which might be inappropriate for analyzing physiologically relevant GABA secretion. The new approach by Braun et al. (2004) enables GABA exocytosis to be monitored in real-time over milliseconds to seconds. Although glucose-induced GABA secretion was not demonstrated directly by Braun et al. (2004) it was inferred by the ability of a depolarization-induced calcium rise to elicit GABA secretion. Their GABA biosensor approach could be used to directly substantiate the effect of glucose by patch clamp monitoring of membrane currents in “sniffer” cells, overexpressing the GABAA receptor, but located adjacent to normal β-cells. “Sniffer” cells would not be anticipated to sense glucose as they would be patched in whole-cell mode. Superfusion with high glucose could stimulate GABA release from control cells, and in turn activate the GABAA receptors on the “sniffer” cells.
GABA Receptor Expression in the Endocrine Pancreas
There are two distinct types of GABA receptors in the CNS, the ionotropic GABAA (a chloride channel) and GABAC receptors, and the metabotropic GABAB receptor. GABAB is a G-protein coupled receptor that can suppress presynaptic neurotransmitter release. This involves the direct inhibition of voltage-gated calcium channels, or the reduction of postsynaptic neuron firing by indirectly activating Kir3-type potassium channel subunits and inhibiting adenylate cyclase via G-protein subunits (Kaupmann et al., 1998). It is widely accepted that GABAA receptor is expressed in the islet. However, exactly which islet cell types express this receptor is still to be elucidated. In whole human islets, the α2, β3 and γ1 subunits of the GABAA receptor were detected by RT-PCR, a more limited spectrum than in the brain (α1–6, β1–3, γ1–2), perhaps indicative of only one pharmacologically distinct receptor or expression in just one cell type (Yang et al., 1994). In the rat islet, however, antibodies against α1 and 2 and β1 and γ2 all strongly stained all cell types (von Blankenfeld et al., 1995). Immunostaining of guinea pig pancreatic islets indicated that GABAA receptors are expressed in α and δ, but not β-cells (Rorsman et al., 1989). GABAB receptors have been detected by both RT-PCR and Western blotting in whole human islets and MIN6 cells (Brice et al., 2002). In view of the apparent discrepancies between species, detailed islet cell-type specific molecular profiling of GABA receptors should be performed.
GABA Action on Islet Hormone Release
The existence of large amounts of GABA in β-cells, the likelihood that it is released from SLMVs in a regulated manner, and the presence of GABA receptors on neighboring islet cells strongly suggests a signaling role for GABA within the microorgan. Does GABA act as a modulator of islet hormone release by auto and/or paracrine actions? In vivo, insulin is secreted from β-cells and somatostatin from δ-cells during hyperglycaemia, whereas α-cells are active in the hypoglycaemic state. The evidence presented below advocates that insulin secretion may be inhibited by GABA. In the perfused rat pancreas, where islet microcirculation is preserved, glucose-induced insulin secretion was inhibited in a dose-dependent manner by both GABA and baclofen (a GABAB-specific agonist), indicative of GABA action by the GABAB receptor (Gu et al., 1993). The underlying mechanism for this could be the attenuation of the glucose-evoked increase in cytosolic calcium, as seen in isolated β-cells (Gu et al., 1993). In a separate study, muscimol (a GABAA receptor agonist) had no effect on glucose-induced insulin secretion in the perfused rat pancreas, despite a concurrent inhibition of somatostatin release (Robbins et al., 1981). These findings indicate that GABAA receptors may be expressed on delta but not β-cells, in agreement with receptor localization studies in guinea pig islets discussed above (Rorsman et al., 1989). Surprisingly, however, GABA had no effect on arginine-stimulated somatostatin release from isolated guinea pig islets (Rorsman et al., 1989). This discrepancy between results from isolated islets and perfused pancreata has also been seen for insulin secretion. In studies of rat islets, GABA, muscimol, or baclofen had no effect on insulin or somatostatin release (Gilon et al., 1991). Such findings are consistent with the concept that GABA may act as an autocrine inhibitor of insulin secretion, at least in rodents.
There is evidence to suggest that in some species GABA can inhibit the secretion of the blood-glucose–elevating hormone glucagon. In studies of isolated islets, GABA has been shown to inhibit arginine-induced glucagon release in guinea pig islets (Rorsman et al., 1989) and glucagon release from mouse islets (Gilon et al., 1991). In the latter experiment, the same result was obtained with muscimol, but not bacolfen, indicative of GABA action via the GABAA receptor. Importantly, muscimol was also found to inhibit arginine-induced glucagon secretion in the perfused rat pancreas (Gilon et al., 1991). In the αTC6 cell-line, glucagon secretion was inhibited by GABA in a dose-dependent manner, in low (1 mM) but not in high (10 mM) glucose (Gaskins et al., 1995). Under the latter condition, glucagon secretion appeared to be already maximally inhibited. These findings lead us to the interesting possibility that GABA might act as a paracrine inhibitor of glucagon secretion in high glucose conditions, as previously proposed (Rorsman et al., 1989).
GABA as a Paracrine Regulator of Glucagon Secretion
In conditions where β-cell insulin secretion is stimulated by nutrients, glucagon release is suppressed. In vivo, this situation occurs during hyperglycaemia, but is known to be deranged in the diabetic state where insulin secretion is impaired. The concept of paracrine regulation of glucagon secretion by secretory products from β-cell LDCVs, has recently been addressed (Ishihara et al., 2003). Candidate paracrine inhibitors include insulin and zinc (Ishihara et al., 2003), the latter cocrystallizes with insulin in LDCVs. To also qualify as a paracrine inhibitor during hyperglycemia, GABA must be released under high glucose conditions.
Given the ability of GABA to hyperpolarize the plasma membrane of neuronal cells, a similar effect on the α-cell plasma membrane could be envisaged to have an inhibitory effect on exocytosis of glucagon-containing LDCVs. Indeed GABA hyperpolarization of the plasma membrane and concurrent inhibition of spontaneous calcium spiking has been demonstrated with the cell-attached patch clamp technique in isolated guinea pig α-cells (Rorsman et al., 1989). It is noteworthy that the fundamentals of voltage-gated calcium influx–dependent LDCV exocytosis are similar in α- and β-cells (Ishihara et al., 2003). Indirect evidence for a hyperpolarization effect of GABA on stimulated rat β-cells has been reported (Gu et al., 1993).
Evidence for paracrine suppression of glucagon secretion by GABA during glucose stimulation remains controversial. The GABAA receptor antagonist bicuculline was unable to prevent high glucose-associated inhibition of arginine-induced glucagon secretion in the perfused rat pancreas (Gilon et al., 1991). This finding is significant in view of the preserved islet microcirculation in the perfused pancreas set-up (see below). Only one study has demonstrated an antagonistic effect of bicuculline (50%) on high glucose-associated inhibition of glucagon secretion in the guinea pig islet (Rorsman et al., 1989). However, the action of bicuculline on glucagon release in low glucose conditions was not explored, and indeed a stimulatory effect of bicuculline on somatostatin release from the same islets exposed to low glucose and arginine was observed (Rorsman et al., 1989). This raises the possibility that interstitial GABA may have acted as an inhibitor of glucagon secretion in both low and high glucose conditions. It is clear, therefore, that the actions of GABA on islet hormone release need to be elucidated.
Islet GABA and Glutamate as Paracrine Effectors
Evidence suggests that the LDCVs of α-cells contain glutamate. This is suggested by the colocalization of the glutamate transporters VGLUT1 and VGLUT2 with glucagon-positive secretory granules and L-glutamate (Hayashi et al., 2003). Glutaminase, usually enriched in glutaminergic nerve endings, was also detected in α-cells (Inagaki et al., 1995). L-glutamate is the major excitatory neurotransmitter in the CNS, and like GABA, is stored and released from synaptic vesicles. Activation of ionotropic L-glutamate receptors has been shown to stimulate insulin secretion from rat islets at permissive glucose concentrations (Inagaki et al., 1995), whereas GABA release was evoked at low glucose concentrations (Hayashi et al., 2003). Glutamate is also present in β-cells, generated from glucose, and thought to participate as an intracellular cofactor in nutrient-stimulated insulin secretion (Maechler and Wollheim, 1999). The colocalization of glutamate with glucagon could suggest that any intraislet glutamate paracrine signaling activity occurs during hypoglycaemia, as supported by the observation that glutamate release from islets is greater in low rather than high glucose conditions (Hayashi et al., 2003). In contrast, the distinct partitioning of GABA and insulin within the β-cell to the SLMVs and LDCVs, respectively, confers possibilities for tailored stimulatory and inhibitory signaling pathways for exocytosis.
Paracrine signaling in the in situ islet is dictated by the direction of the microcirculation. It is generally believed that the intraislet portal system places the α and δ-cells, situated in the islet mantle, downstream of the β-cells in the core. This is supported by the observation that glucagon secretion is suppressed when β-cells are activated and α-cell hyperfunction in the absence of β-cells (Ishihara et al., 2003). What roles can be envisaged for the two candidate signaling molecules GABA and glutamate in the short-term regulation of islet hormone secretion? Under conditions where both SLMV and LDCV exocytosis is stimulated, GABA could act as an autocrine inhibitor of insulin secretion. In this situation, GABA may well act as a paracrine inhibitor of glucagon and somatostatin release (see Fig. 1). Glutamate, however, is a conceptually less attractive paracrine regulator of β-cell vesicular exocytosis in the light of our current understanding of islet microcirculation. We anticipate that these outstanding questions of islet physiology, including the puzzle of islet GABA function, will be resolved with the aid of the many biophysical and genetic tools now available, in particular the biosensor approach to detecting GABA release.
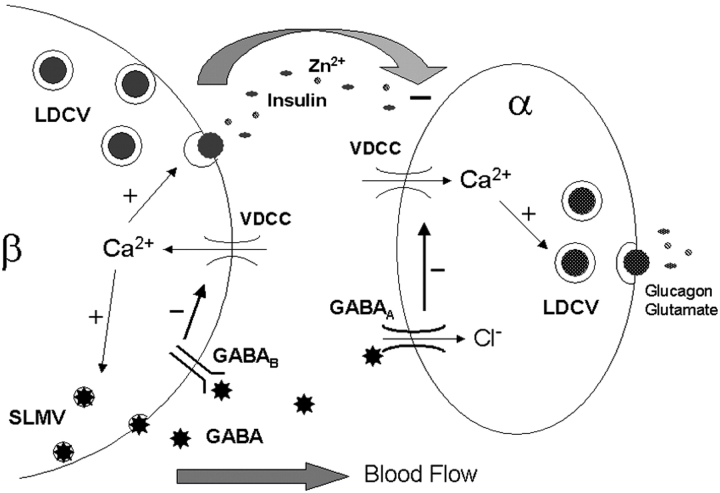
Figure 1.
Schematic diagram illustrating regulated GABA release from islet β-cell SLMVs, and the putative pathways by which it inhibits LDCV secretion in α- and β-cells. Calcium influx through voltage-dependent calcium channels (VDCC) stimulates GABA release. Secreted GABA interacts with metabotropic receptors (GABAB) and ionotropic receptors (GABAA) on recipient β- and α-cells, respectively. Receptor activation inhibits calcium entry through VDCCs, in the case of GABAA by hyperpolarizing the plasma membrane. Reduced calcium influx attenuates LDCV release in both cell types. The depicted α-cell could also represent a δ-cell, in which case somatostatin would be secreted from LDCVs in place of glucagon and glutamate. Insulin, zinc, and other products released from β-cell LDCVs probably also act as paracrine inhibitors of α-cell glucagon release.
Acknowledgments
We thank Dr. P. Maechler and Dr. S. Theander for critical reading of the manuscript.
Olaf S. Andersen served as editor.
References
- Anhert-Hilger, G., A. Stadtbaumer, C. Strubing, H. Scherubl, G. Schultz, E.-O. Riecken, and B. Wiedenmann. 1996. γ-Aminobutyric acid secretion from pancreatic neuroendocrine cells. Gastroenterology. 110:1595–1604. [DOI] [PubMed] [Google Scholar]
- Braun, M., A. Wendt, B. Birnir, J. Borman, L. Eliasson, J. Galvanovskis, J. Gromada, H. Mulder, and P. Rorsman. 2004. Regulated exocytosis of GABA-containing synaptic-like microvesicles in pancreatic β cells. J. Gen. Physiol. 123:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice, N.L., A. Varadi, S.J.H. Ashcroft, and E. Molnar. 2002. Metabotropic glutamate and GABAB receptors contribute to the modulation of glucose-stimulated insulin secretion in pancreatic β cells. Diabetologia. 45:242–252. [DOI] [PubMed] [Google Scholar]
- Cherubini, E., J.L. Gaiarsa, and Y. Ben-Ari. 1991. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 14:515–519. [DOI] [PubMed] [Google Scholar]
- Chessler, S.D., W.T. Simonson, I.R. Sweet, and L.P. Hammerle. 2002. Expression of the vesicular inhibitory amino acid transporter in pancreatic islet cells. Diabetes. 51:1763–1771. [DOI] [PubMed] [Google Scholar]
- Gaskins, H.R., M.E. Baldeon, L. Selassie, and J.L. Beverly. 1995. Glucose modulates γ-aminobutyric acid release from the pancreatic βTC6 cell line. J. Biol. Chem. 270:30286–30289. [DOI] [PubMed] [Google Scholar]
- Gerber, J.C., and T.A. Hare. 1980. GABA in peripheral tissues: presence and actions in endocrine pancreatic function. Brain Res. Bull. 5:341–346. [Google Scholar]
- Gilon, P., G. Bertrand, M.M. Loubatières-Mariani, C. Remacle, and J.C. Henquin. 1991. The influence of γ-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology. 129:2521–2529. [DOI] [PubMed] [Google Scholar]
- Gu, X.-H., T. Kurose, S. Kato, K. Masuda, K. Tsuda, H. Ishida, and Y. Seino. 1993. Suppressive effect of GABA on insulin secretion from the pancreatic β-cells in the rat. Life Sci. 52:687–694. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., H. Yamada, S. Uehara, R. Morimoto, A. Muroyama, S. Yatsushiro, J. Takeda, A. Yamamoto, and Y. Moriyama. 2003. Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J. Biol. Chem. 278:1966–1974. [DOI] [PubMed] [Google Scholar]
- Inagaki, N., H. Kuromi, T. Gonoi, Y. Okamoto, H. Ishida, Y. Seino, T. Kaenko, T. Iwanaga, and S. Seino. 1995. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 9:686–691. [PubMed] [Google Scholar]
- Ishihara, H., P. Maechler, A. Gjinovci, P.L. Herrera, and C.B. Wollheim. 2003. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat. Cell Biol. 5:330–335. [DOI] [PubMed] [Google Scholar]
- John, M., B. Wiedenmann, M. Kruhoffer, K. Andermann, I. Ankorina-Stark, E. Schlatter, G. Anhert-Hilger, W.-G. Forssmann, and M. Kuhn. 1998. Guanylin stimulates regulated secretion from human neuroendocrine pancreatic cells. Gastroenterology. 114:791–797. [DOI] [PubMed] [Google Scholar]
- Kaupmann, K., B. Malitschek, V. Schuler, J. Heid, W. Froestl, P. Beck, J. Mosbacher, S. Bischoff, A. Kulik, R. Shigemoto, et al. 1998. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 396:683–687. [DOI] [PubMed] [Google Scholar]
- Lang, J. 1999. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 259:3–17. [DOI] [PubMed] [Google Scholar]
- Lang, J., M. Fukuda, H. Zhang, K. Mikoshiba, and C.B. Wollheim. 1997. The first C2 domain of synaptotagmin is required for exocytosis of insulin from pancreatic β-cells: action of synaptotagmin at low micromolar calcium. EMBO J. 16:5837–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler, P., and C.B. Wollheim. 1999. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 402:685–689. [DOI] [PubMed] [Google Scholar]
- McIntire, S.L., R.J. Reimer, K. Schuske, R.H. Edwards, and E.M. Jorgensen. 1997. Identification and characterisation of the vesicular GABA transporter. Nature. 389:870–876. [DOI] [PubMed] [Google Scholar]
- Nagamatsu, S., T. Watanabe, Y. Nakamichi, C. Yamamura, K. Tsuzuki, and S. Matsushima. 1999. α-Soluble N-ethylmaleimide-sensitive factor attachment protein is expressed in pancreatic β cells and functions in insulin but not γ-aminobutyric acid secretion. J. Biol. Chem. 274:8053–8060. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi, M., Y. Nakamichi, S. Ozawa, H. Katsuta, H. Ishida, and S. Nagamatsu. 2001. Mastoparan stimulates GABA release from MIN6 cells: relationship between SNARE proteins and mastoparan action. Biochem. Biophys. Res. Commun. 289:1025–1030. [DOI] [PubMed] [Google Scholar]
- Reetz, A., M. Solimena, M. Matteoli, F. Folli, K. Takei, and P. De Camilli. 1991. GABA and pancreatic β-cells: colocalisation of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 10:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi, R., C.B. Wollheim, J. Lang, J.M. Theler, O. Rossetto, C. Montecucco, K. Sadoul, U. Weller, M. Palmer, and B. Thorens. 1995. VAMP-2 and cellubrevin are expressed in pancreatic β-cells and are essential for Ca2+-but not for GTP γ S-induced insulin secretion. EMBO J. 14:2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, M.S., L.H. Grouse, R.L. Sorenson, and R.P. Elde. 1981. Effect of muscimol on glucose-stimulated somatostatin and insulin release from the isolated, perfused rat pancreas. Diabetes. 30:168–171. [DOI] [PubMed] [Google Scholar]
- Rorsman, P., P.-O. Berggren, K. Bokvist, H. Ericson, H. Mohler, C.-G. Ostenson, and P.A. Smith. 1989. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 341:233–236. [DOI] [PubMed] [Google Scholar]
- Shi, Y., J. Kanaani, V. Menard-Rose, Y.-H. Ma, P.-Y. Chang, D. Hanahan, A. Tobin, G. Grodsky, and S. Baekkeskov. 2000. Increased expression of GAD65 and GABA in pancreatic β-cells impairs first-phase insulin secretion. Am. J. Physiol. Endocrinol. Metab. 279:E684–E694. [DOI] [PubMed] [Google Scholar]
- Sorenson, R.L., D.G. Garry, and T.C. Brelje. 1991. Structural and functional considerations of GABA in islets of Langerhans. Diabetes. 40:1365–1374. [DOI] [PubMed] [Google Scholar]
- Thomas-Reetz, A.C., and P. De Camilli. 1994. A role for synaptic vesicles in non- neuronal cells: clues from pancreatic β-cells and from chromaffin cells. FASEB J. 8:209–216. [DOI] [PubMed] [Google Scholar]
- von Blankenfeld, G., J. Turner, G. Anhert-Hilger, M. John, M.O.K. Enkvist, F. Stephenson, H. Kettenmann, and B. Wiedenmann. 1995. Expression of functional GABAA receptors in neuroendocrine gastropancreatic cells. Pflugers Arch. 430:381–388. [DOI] [PubMed] [Google Scholar]
- Winnock, F., Z. Ling, R. De Proft, S. Dejonghe, F. Schuit, F. Gorus, and D. Pipeleers. 2001. Correlation between GABA release from rat islet β-cells and their metabolic state. Am. J. Physiol. Endocrinol. Metab. 282:E937–E942. [DOI] [PubMed] [Google Scholar]
- Yang, W., A.A. Reyes, and N.C. Lan. 1994. Identification of the GABAA receptor subtype mRNA in human pancreatic tissue. FEBS Lett. 346:257–262. [DOI] [PubMed] [Google Scholar]
- Yoon, J.W., C.S. Yoon, H.W. Lim, Q.Q. Huang, Y. Kang, K.H. Pyun, K. Hirasawa, R.S. Sherwin, and H.S. Jun. 1999. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in β cells. Science. 284:1183–1187. [DOI] [PubMed] [Google Scholar]