Abstract
The effects of organic quaternary amines, tetraethylammonium (TEA) chloride and benzyltriethylammonium (BTEA) chloride, on Na,K pump current were examined in rat cardiac myocytes superfused in extracellular Na+-free solutions and whole-cell voltage-clamped with patch electrodes containing a high Na+-salt solution. Extracellular application of these quaternary amines competitively inhibited extracellular K+ (K+ o) activation of Na,K pump current; however, the concentration for half maximal inhibition of Na,K pump current at 0 mV (K 0 Q) by BTEA, 4.0 ± 0.3 mM, was much lower than the K 0 Q for TEA, 26.6 ± 0.7 mM. Even so, the fraction of the membrane electric field dissipated during K+ o activation of Na,K pump current (λK), 39 ± 1%, was similar to λK determined in the presence of TEA (37 ± 2%) and BTEA (35 ± 2%), an indication that the membrane potential (V M) dependence for K+ o activation of the Na,K pump current was unaffected by TEA and BTEA. TEA was found to inhibit the Na,K pump current in a V M-independent manner, i.e., inhibition of current dissipated 4 ± 2% of the membrane electric field. In contrast, BTEA dissipated 40 ± 5% of the membrane electric field during inhibition of Na,K pump current. Thus, BTEA inhibition of the Na,K-ATPase is V M-dependent. The competitive nature of inhibition as well as the similar fractions of the membrane electric field dissipated during K+ o-dependent activation and BTEA-dependent inhibition of Na,K pump current suggest that BTEA inhibits the Na,K-ATPase at or very near the enzyme's K+ o binding site(s) located in the membrane electric field. Given previous findings that organic quaternary amines are not occluded by the Na,K-ATPase, these data clearly demonstrate that an ion channel–like structure provides access to K+ o binding sites in the enzyme.
Keywords: Na,K-ATPase; K+ binding; tetraethylammonium chloride; benzyltriethylammonium chloride
INTRODUCTION
The stoichiometry of ion transport by the Na,K-ATPase under physiologic conditions, 3 Na+ extruded from the cell in exchange for the uptake of 2 K+ per ATP hydrolyzed (Glynn, 1985; Rakowski et al., 1989), results in one positive charge being moved across the cell membrane per enzyme transport cycle (De Weer et al., 1988; Glynn and Karlish, 1990; Läuger, 1991; Vasilets and Schwarz, 1993; Rakowski et al., 1997). This movement of positive charge can be measured as a membrane depolarization upon inhibition of the Na,K-ATPase with cardiac glycosides or as cardiac glycoside–inhibitable, extracellular K+ (K+ o)-dependent steady-state outward current (Na,K pump current) under voltage-clamp conditions where other ionic membrane currents are blocked or inactivated (Cooke et al., 1974; Isenberg and Trautwein, 1974; Eisner and Lederer, 1980; De Weer et al., 1984; Gadsby et al., 1985; Ishizuka et al., 1996; Berlin and Peluffo, 1997). The net movement of positive charge during the enzyme transport cycle also requires that the rate of at least one reaction step is dependent on the membrane potential (Läuger, 1991). The finding that Na,K pump current is membrane potential (V M) dependent in extracellular Na+ (Na+ o) and K+ o-containing salt solutions confirmed this theoretical requirement and demonstrated that under some conditions the V M-dependent reaction(s) can become rate limiting for steady-state ion transport (Hansen et al., 1981; Läuger, 1991). In fact, Na,K pump current has a bell-shaped dependence on V M in the presence of nonsaturating concentrations of Na+ o and K+ o (Lafaire and Schwarz, 1986; Bielen et al., 1991; Rakowski et al., 1991; Sagar and Rakowski, 1994; Berlin and Peluffo, 1997). Such a biphasic relationship necessitates the presence of at least two V M-dependent reaction steps in the transport cycle (Läuger, 1991).
One V M-dependent reaction of the Na,K-ATPase was identified with Na+-dependent transport steps by demonstrating that the positive slope of the Na,K pump current-voltage relationship, observed at negative V M in Na+ o- and K+ o-containing solutions, was lost when Na+ was removed from the extracellular medium (Nakao and Gadsby, 1989; Rakowski et al., 1991; Sagar and Rakowski, 1994). The V M-dependent step underlying this behavior was characterized by trapping the Na,K-ATPase in Na+-transporting conformations (under K+ o-free conditions that promote Na+-Na+ exchange) and studying presteady-state kinetics of Na+ o-dependent charge-moving reaction steps (Nakao and Gadsby, 1986). Such experiments showed that transport of Na+ to the extracellular medium moves the equivalent of a full charge across two thirds to three quarters of the membrane dielectric (Nakao and Gadsby, 1986; Bühler et al., 1991; Fendler et al., 1993; Rakowski, 1993; Hilgemann, 1994a; Holmgren and Rakowski, 1994; Heyse et al., 1994). Furthermore, the Na+ o concentration dependence of this process provided evidence that the V M-dependent step occurs during extracellular Na+ release and binding (Gadsby et al., 1993; Wuddel and Apell, 1995). These results showed that the binding site for at least one Na+ is within the membrane electric field, i.e., the binding site in the E 2P enzyme conformation is located in an ion well exposed to the extracellular medium.
In Na+ o-free solutions, the Na,K pump current-voltage relationship showed a negative slope in the presence of nonsaturating concentrations of K+ o (Bielen et al., 1991; Rakowski et al., 1991; Sagar and Rakowski, 1994; Berlin and Peluffo, 1997; Peluffo et al., 2000). Because Na+ o-free solutions prevent Na+ o-dependent reactions from influencing the V M dependence of Na,K pump current, this negative slope demonstrated that a second V M-dependent reaction was tied to K+-dependent transport steps. Measurement of transient charge movements dependent on K+ o or extracellular Tl+ (Tl+ o), a K+ congener, under conditions promoting K+-K+ exchange showed that this reaction moved a charge through approximately one third of the membrane dielectric. The Tl+ o concentration dependence of these charge movements also demonstrated that extracellular ion binding was the V M-dependent reaction (Peluffo and Berlin, 1997). Thus, similar to Na+ o binding, K+ binds in an extracellularly facing ion well of the Na,K-ATPase.
This extracellularly-facing ion well is most often envisioned conceptually as a narrow access channel in the enzyme, analogous to the pore of an ion channel (De Weer et al., 1988; Rakowski et al., 1997). The V M dependence of ion binding reactions in this scenario occurs because the membrane electric field strength affects the probability that ions occupy the access channel. However, as first pointed out by Hilgemann (1994b), an equally valid mechanism involves rapid, V M-dependent microscopic occlusion reactions in which weakly V M-dependent ion binding/coordination reactions are accompanied by rapid rearrangements of charge in the ion-bound protein with respect to the membrane electric field. Charge rearrangement during microscopic ion occlusion reactions could involve intrinsic protein moieties and/or the bound ion; however, with this mechanism, V M dependence of ion binding reactions arise because electric field strength affects the probability of charge rearrangements during ion binding and release. The problem is that this alternative mechanism of V M-dependent ion binding reactions is experimentally indistinguishable from the access channel model as long as the proportion of the ion-bound, but not -occluded enzyme state remains very small. In fact, experimental data suggest that the fraction of Rb+ (another K+ congener) -bound, but not -occluded enzyme is indeed very low under conditions that promote K+ occlusion by the Na,K-ATPase (González-Lebrero et al., 2002). Thus, it could be very difficult to distinguish between the alternate ion binding mechanisms outlined above in the presence of transported cations such as Na+ and K+. However, if ion transport could be blocked in such a manner that a substantial fraction of the enzyme resides in an ion-bound, but not -occluded state, then these alternative scenarios for V M-dependent ion binding should be experimentally distinguishable.
Organic quaternary amines inhibit ion transport by the Na,K-ATPase in a manner that is competitive with K+ o activation of the enzyme (Kropp and Sachs, 1977) and inhibit Rb+ occlusion under conditions that would otherwise promote binding of extracellular ions (Forbush, 1988). Of particular note in the context of the present study, quaternary amines do not appear to become occluded by the enzyme (Forbush, 1988). One interpretation of these data is that organic quaternary amines inhibit ion occlusion by the Na,K-ATPase by binding at or near K+ o binding sites in the enzyme. Should this interpretation be correct, these organic amines may allow us to separate binding and occlusion reactions by the enzyme and thereby study the mechanism of V M-dependent ion binding.
This study reports the concentration and V M-dependent properties of Na,K pump current inhibition by two such quaternary amines, TEA and its related analogue, benzyltriethylammonium ion (BTEA). The results show that, while these two compounds inhibit K+ o activation of Na,K pump current in a competitive manner, the V M-dependent properties of enzyme inhibition are quite different. The implications of these results for defining the mechanism of V M-dependent extracellular ion binding are then discussed.
Preliminary communications for these data have been published previously (Berlin and Peluffo, 1998; Peluffo et al., 2001).
MATERIALS AND METHODS
Ventricular myocytes were enzymatically isolated from rat hearts and voltage-clamped with patch electrodes (1.0–1.5 MΩ) as published previously (Ishizuka et al., 1996). After establishing a whole-cell voltage clamp at a holding potential of −40 mV, cells were superfused in a Na+- and K+-free salt solution (see below) for 5–6 min to allow the contents of the patch electrode solution (see below) to exchange with the cell interior. Na,K pump current was defined as a K+ o-activated outward current that was reversibly inhibited in the presence of 1 mM ouabain. During experiments, voltage-clamped cells were exposed to various K+-containing superfusion solutions for short periods, <30 s, every 2 min to ensure that bulk cytosolic Na+ concentration was maintained at or near patch pipette Na+ concentration (115 mM) throughout the experiment. Superfusion solutions containing different K+ concentrations were applied in random order. To determine the V M dependence of Na,K pump current, step changes in V M between −100 and +40 mV were produced from the holding potential every 500 ms for 100 ms.
The time constant for settling of current after a 5-mV depolarization was typically in the 200–300 μs range without series resistance compensation and cell capacitances were in the range of 120–250 pF.
Solutions
The patch-electrode solution contained (in mM) 85 sodium sulfamate, 20 tetraethylammonium chloride, 10 ATP-Mg2+ salt, 5 pyruvic acid, 5 creatine phosphate–Tris salt, 10 EGTA–Tris salt, and 10 HEPES (pH 7.35 with NaOH). The total concentration of Na+ in this solution was 115 mM. Na+-free superfusion solutions contained the following (in mM): 145 tetramethylammonium (TMA) chloride, 2.3 MgCl2, 0.2 CdCl2, 5.5 dextrose, 10 HEPES (pH 7.4 with Tris). KCl was added to this solution in various concentrations (0.05–5 mM). Our initial experiments were performed with a superfusion solution containing 145 N-methyl-D-glucamine (NMG) instead of TMA; however, TMA blocked contaminating ionic currents more completely than NMG. Furthermore, the K+ o dependence of Na,K pump current activation was similar in the presence of NMG and TMA-containing solutions (unpublished data). Tetraethylammonium chloride and benzyltriethylammonium chloride were added to the superfusion solution with equimolar substitution for TMA chloride. All experiments were performed at 37°C. Reagents were obtained from Sigma-Aldrich.
Statistics and Curve Fitting
Data are presented as mean ± SEM for the indicated number of cells. Current density was calculated by dividing K+ o-activated currents by cell capacitance measured as the integral of current elicited with 5-mV pulses. Indicated functions were fit to the data using a nonlinear least squares Gauss-Newton algorithm (Rossi and Garrahan, 1989) or with commercial software (SigmaPlot, SPSS).
RESULTS
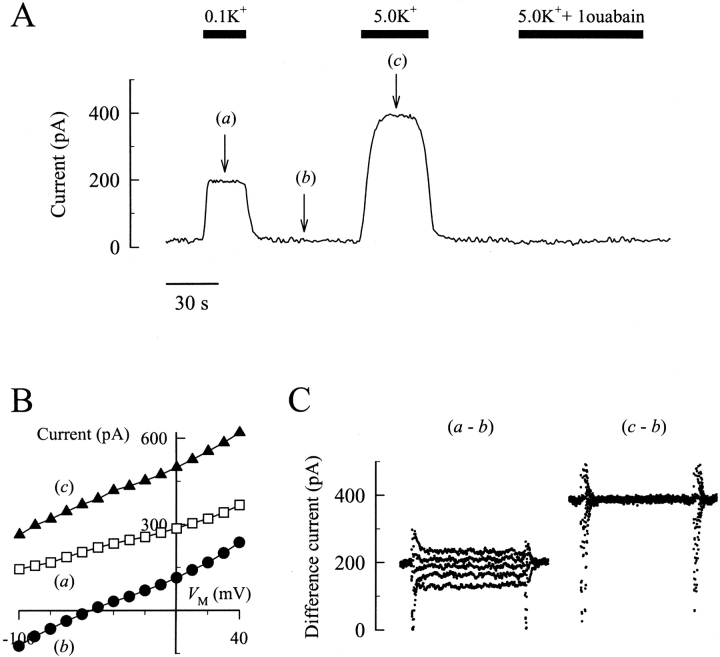
Na,K pump current has been characterized previously as a K+ o-activated, ouabain-inhibitable outward current in single cardiac myocytes (Gadsby et al., 1985; Gadsby and Nakao, 1989; Bielen et al., 1991; Stimers et al., 1991; Ishizuka et al., 1996; Berlin and Peluffo, 1997). Fig. 1 shows this outward current in Na+ o-free solutions and briefly summarizes the experimental protocol used throughout this study. After establishing a whole-cell voltage clamp, rat ventricular myocytes were initially superfused with a Na+ o- and K+ o-free salt solution. When the K+ o concentration in the superfusion solution was subsequently increased, an outward current was observed (Fig. 1 A) that was maintained throughout the period of K+ o exposure except when 1 mM ouabain was also present in the superfusion solution. To determine the V M dependence of Na,K pump current (Fig. 1 B), step changes in V M from the holding potential of −40 mV were produced at the times indicated in Fig. 1 A and K+ o-sensitive difference currents were calculated at each V M (Fig. 1 C). When a saturating K+ o concentration (5 mM) was used to activate the Na,K pump, the resulting difference current (c–b), showed very little dependence on V M in the range of −100 to +40 mV; however, when nonsaturating K+ o concentrations (0.1 mM) were used, the Na,K pump current became smaller at more positive V M (a–b), as reported previously (Rakowski et al., 1991; Sagar and Rakowski, 1994; Berlin and Peluffo, 1997; Peluffo et al., 2000).
Figure 1.
Protocol for measurement of Na,K pump current in rat cardiac myocytes. (A) Current record. A cell was held at −40 mV in a Na+ o- and K+ o-free superfusion solution. K+ and ouabain, at the indicated concentrations (in mM), were added to the superfusion solution for the periods indicated by the filled bars. The arrows indicate the time when current-V M relationships in B were determined. (B) Current-V M relationships. The displayed data are steady-state current levels averaged over the last 50 ms of each voltage step. (C) K+ o-sensitive difference currents. 0.1 mM (a–b) and 5 mM K+ o-sensitive currents (c–b) for 100-ms voltage steps are shown. Superimposed tracings (a–b) are currents in response to voltage steps to −80, −50, −20, +10, +40 mV from top to bottom, respectively. Superimposed tracings (c–b) do not show differences at any V M tested. Na,K pump current levels shown in remaining figures were measured with similar maneuvers.
The V M dependence of Na,K pump current was determined over a range of K+ o concentrations between 0.05 and 5 mM (Fig. 2 A). These data make it obvious that, as K+ o is lowered, the current-V M relationship displays a negative slope and an apparent shift to the left along the V M axis. To measure the effect of V M on Na,K pump current, the K+ o dependence of current activation was determined by fitting a Hill equation to the data at each V M. The calculated maximal current density (I max) at each V M is shown in Fig. 2 B. As can be seen in this figure, I max appeared to show little change with V M. This impression was confirmed by regression analysis that showed the slope of a line through the data as a function of V M was not significantly different than zero. For this reason, I max was averaged over all test V M to yield a value of 2.40 ± 0.10 pA/pF.
Figure 2.
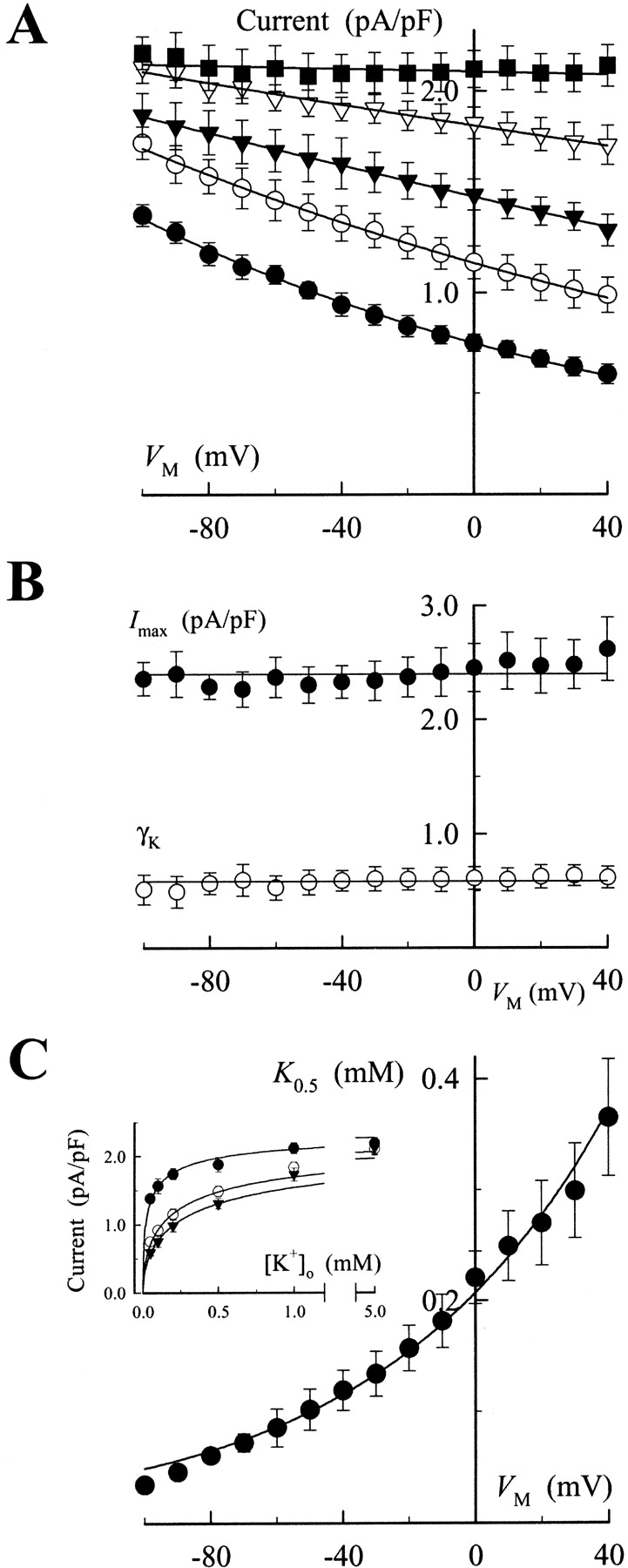
V M and K+ o concentration dependence of Na,K pump current. (A) V M dependence of Na,K pump current at various K+ o concentrations. Currents were measured as in Fig. 1 and plotted versus V M for superfusion solutions containing 0.05 (•), 0.2 (○), 0.5 (▾), 1.0 (▿), and 5.0 mM K+ o (▪). The curves were fit to the data by eye. (B) The V M dependence of I max and γK. A Hill equation was fit to the data in A at each V M. The resulting values of I max and γK along with their standard errors are shown. The solid lines are the average parameter values calculated over all test V M. Note: the units of the y-axis differ for I max and γK. (C) V M dependence of the apparent affinity for K+ o activation of Na,K pump current. The K 0.5 values derived from fitting the data at each V M with a Hill equation are plotted versus V M. The solid curve is the best-fit function to Eq. 1 with K 0 0.5 = 204 μM and λK = 0.39. (Inset) K+ o dependence of Na,K pump current at −100 (•), 0 (○) and +40 mV (▾) is shown. The curves were generated by solving Eq. 6 () at various K+ o concentrations using the parameter values determined from the fitting procedure described in the text.
This fitting procedure also yielded the Hill coefficient (γK) for K+ o activation of Na,K pump current. Similar to I max, the value of γK was found to show no trend with V M (Fig. 2 B), so γK at all 15 V M tested were averaged to yield a value of 0.58 ± 0.07. This value is similar to that measured for K+ o activation of Na,K pump current under zero extracellular Na+ conditions by Sagar and Rakowski (1994) in oocytes and by Peluffo et al. (2000) in HeLa cells expressing heterologous Na,K-ATPase.
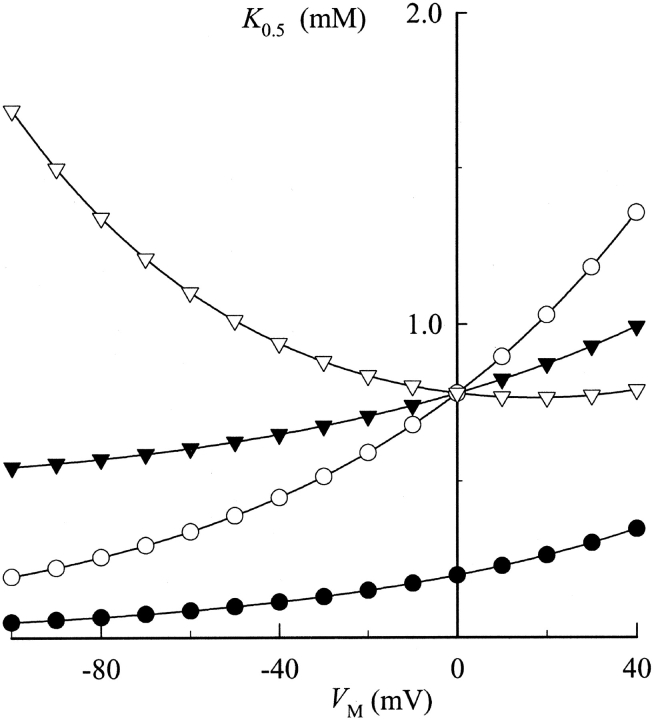
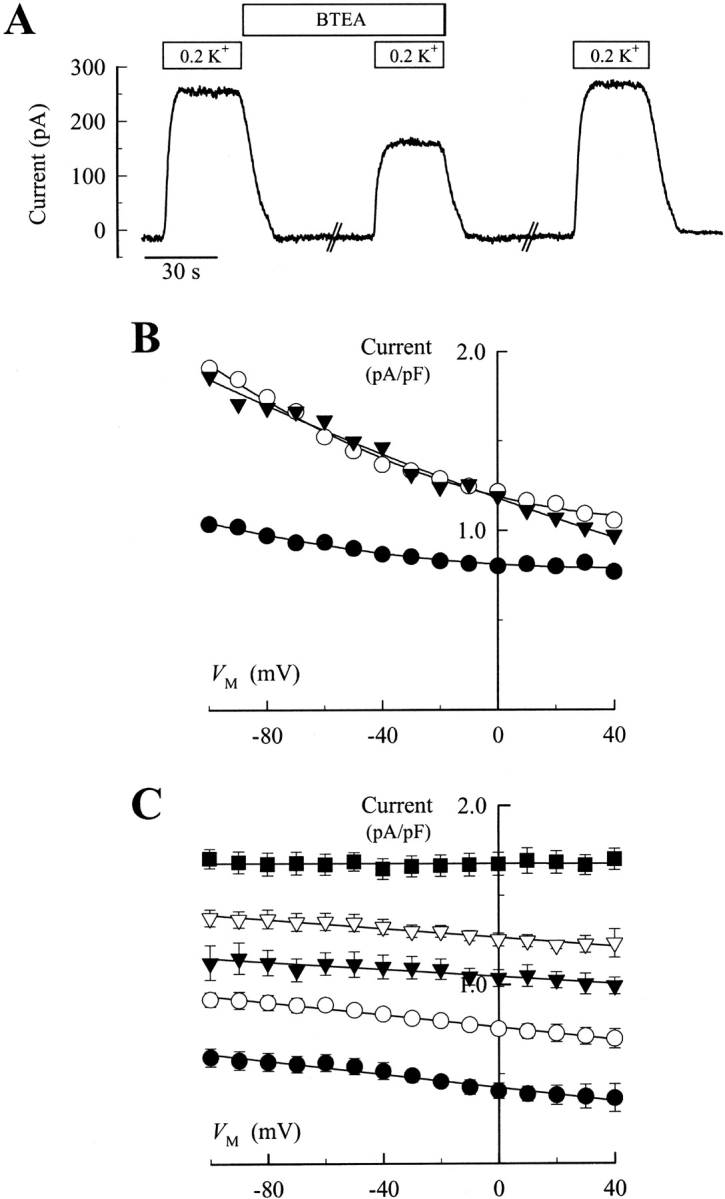
By comparison, the concentration of K+ o needed to yield a similar level of current activation increased at more positive V M, as is reflected by an increase in the K+ o concentration required for half-maximal activation (K 0.5) from 35 ± 4 to 365 ± 53 μM in going from −100 to +40 mV, respectively (Fig. 2 C). Thus, as reported previously (Sagar and Rakowski, 1994; Peluffo et al., 2000), the apparent affinity for K+ o activation of the Na,K pump is decreased as the cell interior is made more positive, consistent with the idea that K+ binding occurs in an extracellularly facing ion well.
Under the conditions of the present experiments, high intracellular and zero extracellular Na+, Na,K pump current can be described by a pseudo two-state model that includes one lumped reaction step as well as a second V M- and K+ o-dependent reaction (Hansen et al., 1981; Sagar and Rakowski, 1994; Peluffo et al., 2000). Using this model, the following equation,
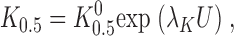 |
(1) |
can be derived to determine the K 0.5 at 0 mV (K 0 0.5) and the portion of the membrane electric field (λK) dissipated during K+ o-dependent reactions. The term “U” is the unitless form of voltage, zFV M /RT, where z is equal to 1, the net charge moved during the ion transport cycle. Fitting Eq. 1 to the data in Fig. 2 C yields values of K 0 0.5 and λK of 204 ± 4 μM and 0.39 ± 0.01 (n = 15), respectively. This value of K 0 0.5 and the fact that K+ o-dependent reactions dissipate approximately one third of the membrane dielectric are consistent with our previous data (Berlin and Peluffo, 1997; Peluffo et al., 2000).
The two-step fitting procedure described above for the data in Fig. 2 allowed for the determination of four parameters, I max, K 0 0.5, γK, and λK, that describe the K+ o concentration and V M dependence of Na,K pump current under the conditions of these experiments. To determine the utility of this fitting procedure, Eq. 6 in the was solved at each V M and K+ o concentration tested using these parameter values. The calculated current densities were then compared with the experimental data. Using the entire set of experimentally measured current densities, the root mean square error of the calculated current densities was found to be 0.0057 pA/pF. Similarly, the sum percent error of the calculated current densities, defined as
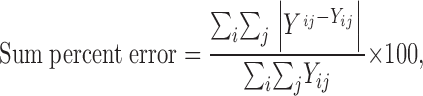 |
where Y ^ ij and Yij are the calculated and measured current densities, respectively, at the i th K+ o concentration and j th V M, was determined to be 3.5%. Both error values show that differences between calculated and measured currents are quite small, an indication that our fitting procedure provides an acceptable description of the data. The accuracy of the fitting procedure can also be inferred by comparing the experimental data to curves determined by solving Eq. 6 () using the fitted parameters values for I max, K 0 0.5, γK, and λK. As can be seen in the inset of Fig. 2 C, the calculated curves closely follow the experimentally measured current densities at −100, 0, and +40 mV. Given the outcome of these tests, we used a similar two-step fitting procedure when analyzing subsequent data.
Effect of Extracellular Tetraethylammonium ions on Na,K Pump Current
Extracellular TEA has been shown to inhibit activity (Zemková et al., 1988), ion transport (Sachs and Conrad, 1968), and current generation by the Na,K-ATPase (Eckstein-Ludwig et al., 1998). For this reason, our initial characterization of the effects of organic quaternary amines began with TEA. Experiments were performed in the same manner as outlined in Fig. 1 A except that immediately after establishing a whole-cell voltage-clamp, the cells were superfused in the Na+ o- and K+ o-free salt solution that also contained TEA at the indicated concentrations. TEA was substituted in equimolar fashion for TMA, which itself does not affect the Na,K pump (unpublished data). Fig. 3 A shows that 25 mM TEA decreased the 0.1 mM K+ o-activated outward current at all V M tested. Similar to control conditions (0 TEA), the current-V M relationship displayed a negative slope.
Figure 3.
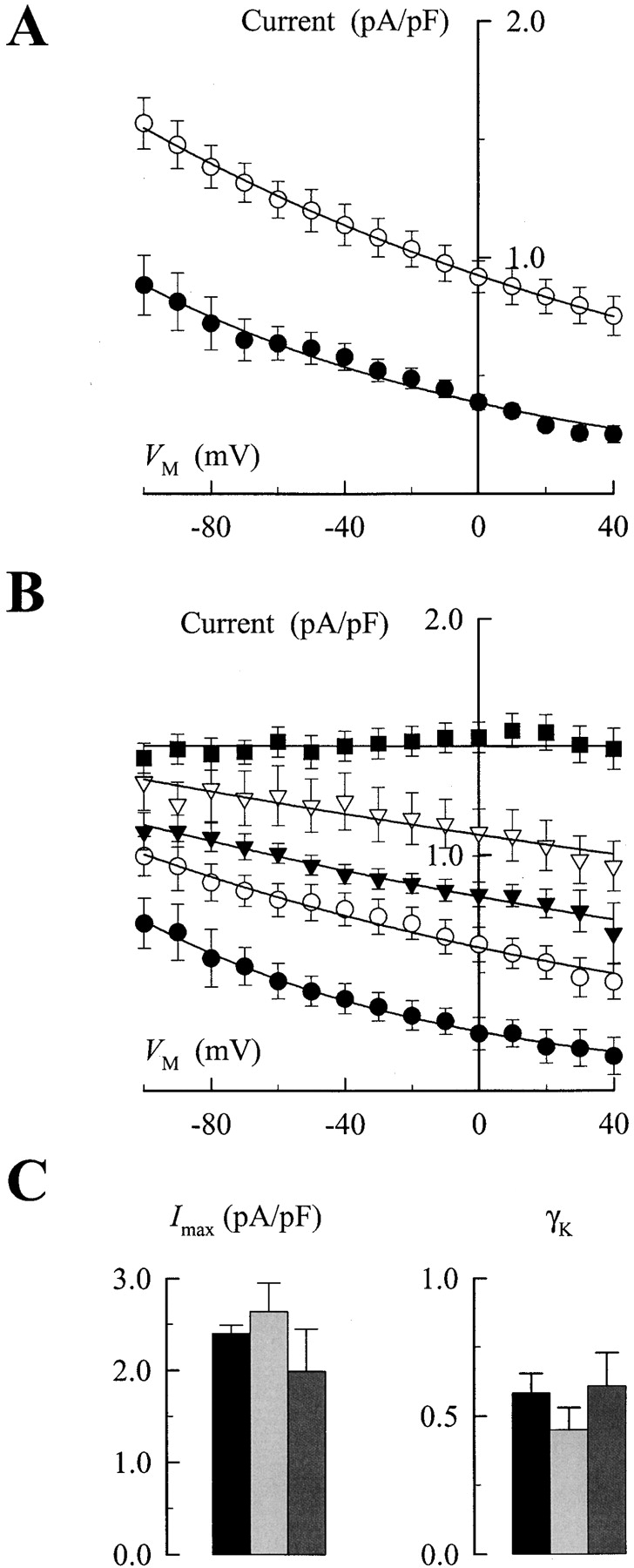
Effect of TEA on the V M and K+ o concentration dependence of Na,K pump current. (A) V M dependence of 0.1 mM K+ o-activated Na,K pump current measured in the presence (•) and absence of 25 mM TEA (○). Curves were fit to the data by eye. (B) V M dependence of Na,K pump current in the presence of 25 mM TEA. Na,K pump current densities are plotted as in Fig. 2 A for superfusion solutions containing 0.05 (•), 0.2 (○), 0.5 (▾), 1.0 (▿), and 5.0 mM K+ o (▪). Curves were fit to the data by eye. (C) Average I max (left) and γK values (right) plus SEM calculated in the presence of 0 (black), 5 (light gray), and 25 mM TEA (dark gray).
To describe the inhibitory effects of TEA on Na,K pump current completely, the experiments shown in Fig. 2, measuring K+ o and V M dependence of Na,K pump current, were repeated in the presence of various TEA concentrations. The effect of 25 mM TEA on the K+ o and V M dependence of Na,K pump current was examined in eight cells. The resulting data, shown in Fig. 3 B, appeared to be quite similar to currents recorded under control conditions (Fig. 2 A) in the sense that, with 5 mM K+ o, the current displayed little V M dependence but, at lower K+ o concentrations, a negative slope in the current-V M relationship was still obvious. Just as in Fig. 3 A, however, the current densities at nonsaturating K+ o concentrations were smaller in the presence of 25 mM TEA.
These data were fit with a Hill equation at each V M to calculate parameter values for I max, γK, and K 0.5. As in Fig. 2, the values for I max and γK showed no dependence on V M so they were averaged for all 15 V M tested to yield values of 1.99 ± 0.46 pA/pF and 0.61 ± 0.12, respectively. Similar experiments and analyses were performed with 13 cells exposed to 5 mM TEA (not depicted) and the calculated average values for I max and γK were 2.64 ± 0.31 pA/pF and 0.45 ± 0.08, respectively. These parameter values, along with those determined in the absence of TEA, are summarized in Fig. 3 C.
The calculations above led to two conclusions. First, the values of I max in the presence of 5 and 25 mM TEA are not significantly different than I max in the absence of the quaternary amine. In agreement with previous reports, these results suggest that TEA inhibition of Na,K pump current is competitive with K+ o activation of ion transport (Sachs and Conrad, 1968). Second, the values of γK were likewise not significantly different in the presence and absence of TEA. Given this lack of effect, the three mean values of γK were averaged and the result (0.55) was used to fix γK during simultaneous fitting of the data in Fig. 4, as discussed below.
Figure 4.
Effect of TEA on the apparent K+ o affinity for activation of Na,K pump current. Na,K pump current was measured in the presence of 0 (•), 5 (○), and 25 mM TEA (▾) and K 0.5 values were calculated at each V M as described in the text. Curves are functions determined by simultaneously fitting all the data to Eq. 2 with γK fixed at a value of 0.55. The resulting parameter values for K 0 0.5, K 0 Q, γQ, λK, and λQ are listed in the text.
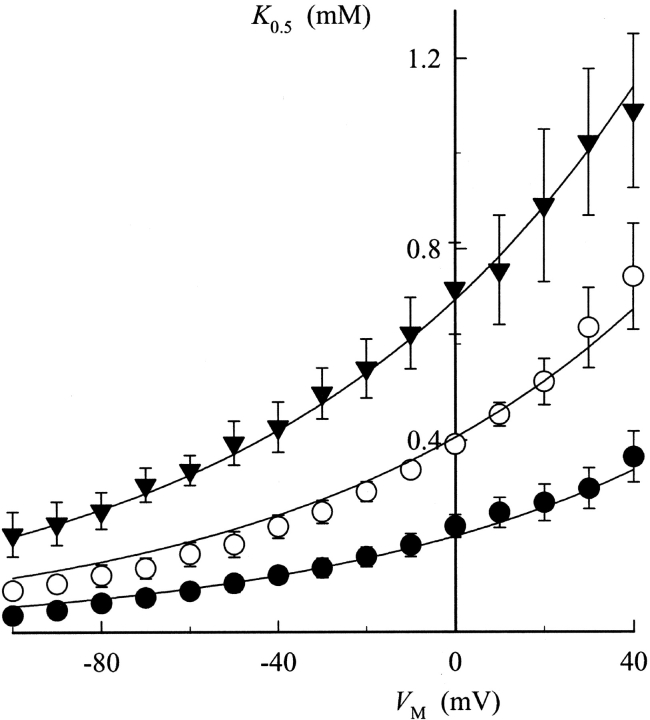
Fig. 4 shows the K 0.5 values calculated at each V M and then plotted against V M for each TEA concentration. The K 0.5 values in Fig. 2 C are plotted again for purposes of comparison. These data show that TEA produces a concentration-dependent increase in K 0.5 at all V M. As in control experiments, the apparent affinity for K+ o activation of Na,K pump current was decreased at more positive V M, i.e., K+ o activation still appears to be V M-dependent in the presence of TEA.
To determine how TEA affects the V M dependence of Na,K pump activation by K+ o, the data in Fig. 4 were fit with an equation derived from a pseudo three-state model for the Na,K pump exposed to an extracellular inhibitor that is competitive with K+ o for activation of the enzyme (see ):
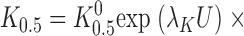 |
(2) |
 |
where [Q] and K Q 0 are the quaternary amine concentration and the amine concentration for half-maximal inhibition at 0 mV, respectively, and γQ and λQ are the Hill coefficient and the portion of the membrane dielectric dissipated during Na,K pump current inhibition, respectively. This equation has six parameters that can be varied to optimize the fit to the data. However, information regarding one of these parameters, γK, is not available in the plotted data. Even so, as mentioned above and shown in Fig. 3 C, γK is independent of V M and TEA concentration. This finding allows γK to be fixed, in this case, at its average value of 0.55, while the other five parameters are varied to fit Eq. 2 to the data in Fig. 4 as a function of V M and amine concentration.
Solid curves in Fig. 4 are the best-fit functions produced by simultaneously fitting Eq. 2 to the entire displayed dataset. The calculated values of K 0.5 0 and λK in the presence of TEA were 201 ± 6 μM and 0.37 ± 0.02, respectively. These parameter values were not significantly different than those calculated in the absence of TEA with Eq. 1 (in Fig. 2 C). Furthermore, these calculations showed that K Q 0 for TEA was 26.6 ± 0.7 mM. The value of λQ, 0.04 ± 0.02, was not significantly different than zero, so that it is clear that TEA binding does not occur in the membrane electric field, i.e., TEA inhibition is V M independent. These results are consistent with those of Eckstein-Ludwig et al. (1998), who also found that TEA block of Na,K pump currents in oocytes is not V M dependent. The results in Fig. 4 are also consistent with the predictions of a pseudo three-state model for the Na,K-ATPase in the presence of a V M-independent blocker that inhibits the enzyme in a competitive manner with K+ o (see and Fig. 9).
The value of γQ calculated with Eq. 2 was equal to 0.45 ± 0.02. This value of γQ is significantly less than one, suggesting that negative cooperativity, possibly between multiple TEA molecules, occurs during inhibition of the Na,K-ATPase. This value is also similar to that for γK (0.55).
Effect of Extracellular Benzyltriethylammonium Ions on Na,K Pump Current
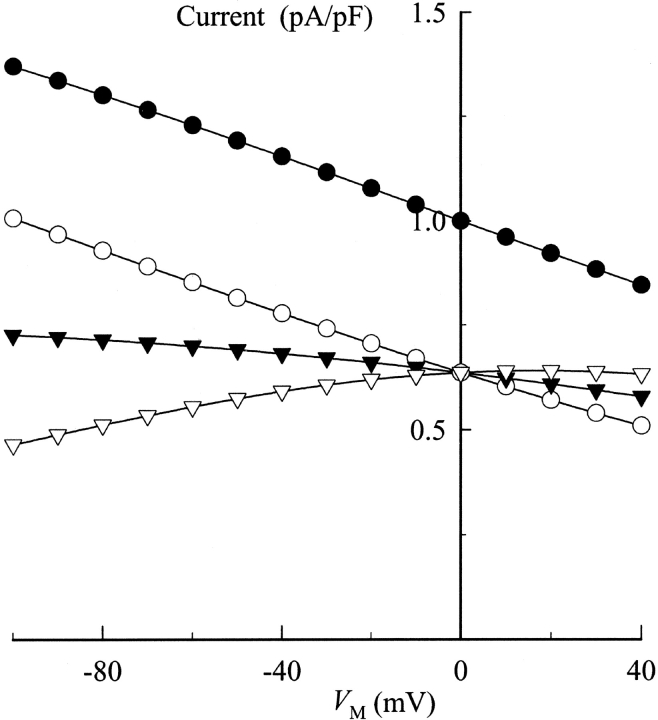
In studying the effects of organic amines on Rb+ occlusion by the Na,K-ATPase, Forbush (1988) noted that quaternary amines containing a benzyl side chain had the highest apparent affinities for slowing release of occluded Rb+ from the enzyme. Therefore, to determine if these benzyl-containing quaternary amines inhibited Na,K pump current in a similar manner to TEA, we examined the effects of a benzylic derivative of TEA, BTEA, on Na,K pump current. At concentrations where TEA had little effect on current, BTEA decreased Na,K pump current amplitude by nearly 50% (Fig. 5 A). Like TEA, current inhibition by BTEA was rapidly reversible at all V M (Fig. 5, A and B). Most interestingly, the V M dependence of current in the presence of BTEA looked quite different than current in the presence of TEA. Inspection of Figs. 3 A and 5 B shows that BTEA appeared to inhibit current more effectively at negative V M, whereas TEA more effectively blocked current at positive V M. Thus, the effect of V M on Na,K pump current in the presence of BTEA appears to be different than that observed with TEA, a V M-independent blocker of the Na,K-ATPase.
Figure 5.
Effect of BTEA on Na,K pump current. (A) Current record showing the reversibility of BTEA inhibition. BTEA (2 mM) and 0.2 mM K+ o were added to Na+ o- and K+ o-free superfusion solution as indicated. Note the breaks in the current record. In this experiment, the myocyte was incubated with BTEA-containing, K+ o-free solution for 1.5 min before current activation with 0.2 mM K+ o. Likewise, 0.2 mM K+ o-activated current was measured 1.5 min after washout of BTEA-containing solution. (B) Effect of BTEA on the V M dependence of 0.2 mM K+ o-activated Na,K pump current. Displayed current-V M relationships were determined before (○), during (•), and after superfusion with 2 mM BTEA-containing solution (▾). (C) V M dependence of Na,K pump current in the presence of 5 mM BTEA. Na,K pump current densities are plotted as in Fig. 2 A for superfusion solutions containing 0.05 (•), 0.2 (○), 0.5 (▾), 1.0 (▿), and 5.0 mM K+ o (▪). Curves were fit to the data by eye.
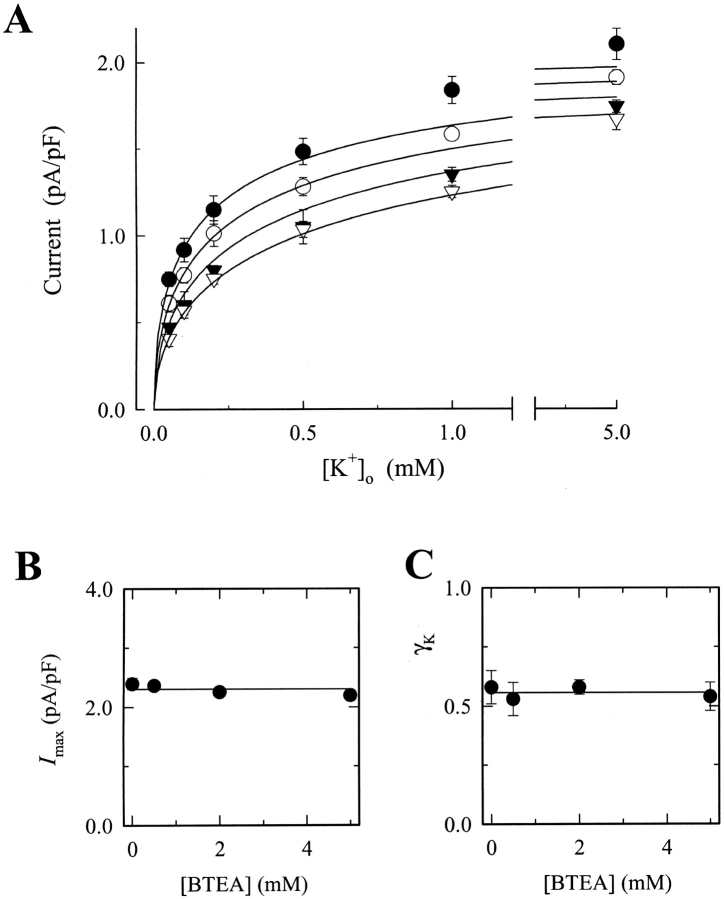
To examine the properties of BTEA block more fully, experiments in Figs. 1 and 2 were repeated in the presence of various BTEA concentrations from 0.5 to 5 mM. Fig. 5 C shows the K+ o and V M dependence of Na,K pump current measured in the presence of 5 mM BTEA. The notable feature of Fig. 5 B, i.e., the relatively shallow slope of the current-V M relationship in the presence of BTEA, is observed at all K+ o examined, quite unlike the current-V M relationship of Na,K pump current in the absence of BTEA (Fig. 2 A). The concentration dependence of BTEA effects on Na,K pump is summarized in Fig. 6 A, which shows current density measured at 0 mV in the presence of different BTEA concentrations and plotted as a function of K+ o concentration. BTEA reduced Na,K pump current density in a concentration-dependent manner.
Figure 6.
Effect of BTEA on the K+ o concentration dependence of Na,K pump current at 0 mV. (A) K+ o dependence of Na,K pump current at 0 mV in the presence of 0 (•), 0.5 (○), 2 (▾), and 5 mM BTEA (▿). Data were collected from 14, 8, 12, and 10 cells, respectively. Curves result from the solution of Eq. 5 () at each BTEA concentration using the average I max and γK values calculated for the entire BTEA dataset (see B and C, respectively) along with K 0 0.5, K 0 Q and γQ values in the text. (B) Effect of BTEA on maximal Na,K pump current density. Data points are the average I max and SEM determined at each BTEA concentration. For some points, SEM is smaller than the symbol. The line is the average value of the displayed data points. (C) Effect of BTEA on the Hill coefficient for K+ o activation of Na,K pump current. Data points are the average γK and SEM determined at each BTEA concentration. The line is the average value of the displayed data points. See the text for details.
Similar to the analytical procedure shown in Fig. 3, the data at each V M and BTEA concentration were fit with a Hill equation. This fitting procedure showed that both I max and γK were V M independent at all BTEA concentrations tested (not depicted). As a result, average values of I max and γK for the 15 V M tested were calculated and plotted in Fig. 6, B and C, respectively. Over the concentration range tested, BTEA had no significant effect on I max or γK, an indication that this quaternary amine is a competitive inhibitor of Na,K pump current. These average values of I max and γK were, therefore, averaged again to yield values of 2.30 pA/pF and 0.56, respectively.
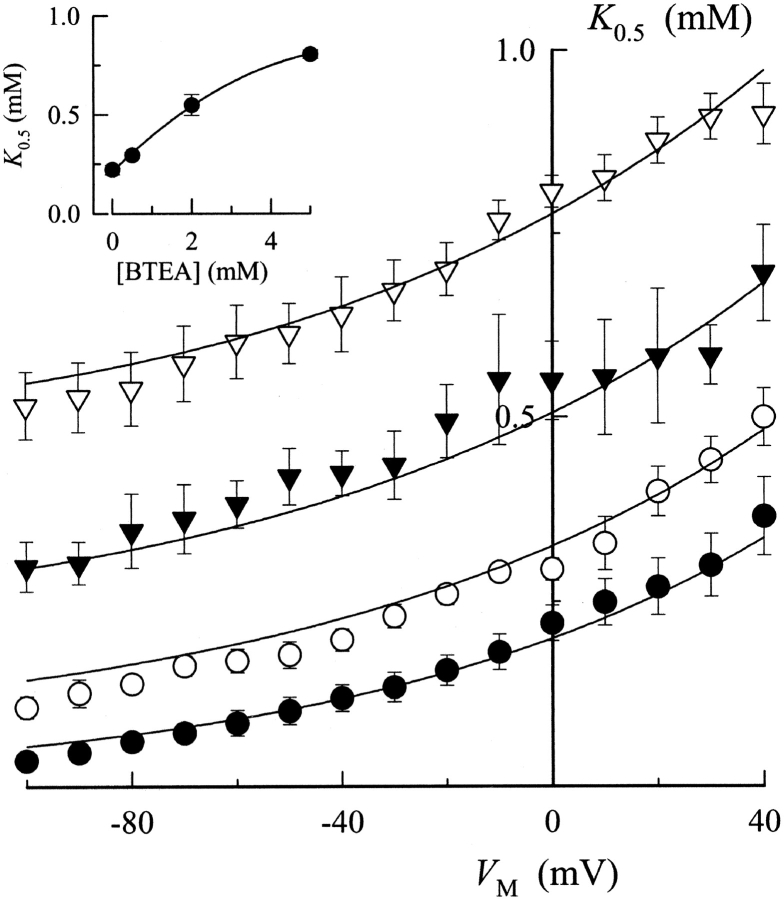
Fitting the Na,K pump current data obtained at each V M with Hill equations also showed the V M and BTEA dependence of K 0.5 values (Fig. 7). BTEA produced a concentration-dependent increase in K 0.5 at all V M tested. The unusual feature of the data was the apparent parallel shift of K 0.5 to higher values with BTEA, irrespective of V M. This type of behavior is actually predicted for a V M-dependent inhibitor of the Na,K-ATPase (see ). By simultaneously fitting Eq. 2 to all data in Fig. 7 with γK fixed at 0.56, K 0 0.5 and λK for current activation, 200 ± 10 μM and 0.35 ± 0.02, were found to be unchanged in the presence of BTEA. On the other hand, the K 0 Q for block of current activation was calculated to be 4.0 ± 0.3 mM, a value almost sevenfold lower than that for TEA. Thus, consistent with the results of Forbush (1988), the benzylic side chain increased the potency for block of ion transport. These same calculations showed that λQ for BTEA was 0.40 ± 0.05, a demonstration that a sizable fraction of the membrane dielectric was dissipated during BTEA block. In other words, BTEA inhibited the Na,K-ATPase in a V M-dependent manner. In addition, λQ was not significantly different than λK. The implications of this result are presented in the discussion.
Figure 7.
Effect of BTEA on the V M dependence of apparent K+ o affinity for activation of Na,K pump current. K 0.5 values calculated at each V M are plotted for Na,K pump current measured in the presence of 0 (•), 0.5 (○), 2 (▾), and 5 mM BTEA (▿). Curves are the functions determined by simultaneously fitting all the data to Eq. 2 with γK = 0.56. The resulting parameter values for K 0 0.5, K 0 Q, γQ, λK, and λQ are listed in the text. (Inset) K 0.5 at 0 mV plotted as a function of BTEA concentration. The curve through the data points was fit by eye.
To demonstrate that the calculated parameters provided a reasonable description of the data, the values of these parameters were used to solve Eq. 5 () at each BTEA concentration. The resulting curves (Fig. 6 A) showed that the predicted values were very close to the experimental data points.
Given the average value of γK above, 0.56, Eq. 2 predicts that the value of K 0.5 (at any V M) will increase monotonically in a parabolic-like manner with BTEA concentration if γQ equals 1. The inset of Fig. 7 does show that K 0.5 (at 0 mV) increases monotonically with BTEA; however, the slope of the relationship between K 0.5 and BTEA becomes somewhat less steep at the higher amine concentrations tested. Such a nonlinear relationship between K 0.5 and amine concentration is predicted by Eq. 2 if γQ < 1. In fitting Eq. 2 to the data, the value of γQ was found to be 0.56 ± 0.03. By plotting Na,K pump current density versus BTEA concentration at each K+ o, γQ was found to be independent of K+ o concentration (not depicted). These results indicate that BTEA inhibits the Na,K-ATPase with negative cooperativity.
DISCUSSION
The main finding of this study is that the organic quaternary amine, BTEA, inhibits Na,K pump current in a V M-dependent manner, whereas tetraethylammonium ion blocks Na,K pump current in a V M-independent manner. To the best of our knowledge, V M-dependent block has not been reported previously for a Na,K-ATPase inhibitor. Therefore, to understand the mechanism of such block, we first need to review the effects of quaternary amines on ion binding and occlusion by the Na,K-ATPase as well as the V M dependence of ion binding reactions themselves. In brief, the basis of our conclusion that quaternary amines produce V M-dependent block resides in the fitting of experimental data to a pseudo three-state model that has two premises: (1) K+ o binding to the Na,K-ATPase is V M dependent, and (2) quaternary amine inhibition is competitive with K+ o activation. Evidence supporting these premises is reviewed below.
K+ o binding is the V M-dependent reaction during ion transport by the Na,K-ATPase: Evidence for the V M dependence of a K+ o-dependent reaction step during ion transport by the Na,K-ATPase is shown by experiments, similar to those in Fig. 1, which display a K+ o-dependent negative slope in the current-V M relationship of Na,K pump current (Bielen et al., 1991; Rakowski et al., 1991; Sagar and Rakowski, 1994; Berlin and Peluffo, 1997; Peluffo et al., 2000). To attribute this V M dependence of Na,K pump current to K+ o-dependent reactions, experiments were performed under conditions that prevent other V M-dependent reaction steps from regulating the kinetics of the ion transport cycle. The concentration of cytosolic Na+ in this study, set by the patch-electrode solution containing 115 mM Na+, is high enough to saturate cytosolic-facing Na+ binding sites of the Na,K-ATPase whose apparent Na+ affinity, under turnover conditions similar to our own, is ∼10 mM (Nakao and Gadsby, 1989). With saturating concentrations, cytosolic Na+ binding reactions are not rate limiting. Therefore, the V M-dependent properties of these reactions cannot be observed. In addition, experiments were also performed in the absence of extracellular Na+, conditions that render extracellular Na+ release reactions by the Na,K-ATPase irreversible so that enzyme turnover will not depend on the V M dependence of these reactions (Läuger, 1991). Our observation that Na,K pump current density measured in the presence of saturating K+ o concentrations (5 mM) is almost independent of V M (Figs. 1 C and 2 A) also confirms the point that V M-dependent reaction steps do not control enzyme turnover under these conditions, thereby allowing us to conclude that any V M dependence of intracellular K+-dependent reactions (e.g., Hansen et al., 2002) is not apparent under our experimental conditions. Thus, a K+ o-dependent step is the only V M-dependent reaction that can limit enzyme turnover in our experiments.
The mechanism of the K+ o- and V M-dependent reaction was identified in our previous work (Peluffo and Berlin, 1997) by trapping the Na,K-ATPase in K+-transporting conformations, i.e., those that promote K+–K+ exchange, and then measuring the Tl+ o dependence of presteady-state charge movements during perturbations of V M. The disappearance of these charge movements at saturating Tl+ o concentrations demonstrated that the V M-dependent reaction is extracellular Tl+ (or K+) binding.
Quaternary Amines Competitively Block K+ Transport by the Na,K-ATPase
The competitive nature of quaternary amine inhibition of Na,K pump current is demonstrated by our data showing that maximal Na,K pump current density is unchanged (Figs. 3 C and 6 B) while the apparent affinity for K+ o activation of current is significantly decreased (Figs. 4 and 7). Previous studies have also shown comparable effects of extracellular TEA (Sachs and Conrad, 1968) and tetrapropylammonium ion (TPA) on Na+ and K+ transport mediated by the Na,K-ATPase in red blood cells (Kropp and Sachs, 1977) and TEA on Na,K pump currents in oocytes (Eckstein-Ludwig et al., 1998). Thus, we conclude that our results are consistent with previous reports that quaternary amines inhibit K+ o-dependent activation of ion transport by the Na,K-ATPase in a competitive manner.
Our own data and previous reports, therefore, suggest that the premises of the pseudo three-state model (see ) are valid. As a result, we concluded that BTEA inhibits Na,K pump current in a V M-dependent manner. On the other hand, TEA appears to block Na,K pump current in a V M-independent manner. The occurrence of V M-dependent block and the effect of quaternary amines on ion occlusion by the Na,K-ATPase provide evidence to distinguish between the two alternative mechanisms posited in the literature (Hilgemann, 1994b) to explain V M-dependent ion binding in the Na,K-ATPase. The basis for distinguishing between these alternative mechanisms is discussed below.
The most detailed study of quaternary amine block of ion transport (Kropp and Sachs, 1977) suggested that TPA is a dead-end inhibitor with highest affinity for the Na,K-ATPase without bound K+, slightly decreased affinity when the enzyme is bound with a single K+ and a very much lower affinity for the doubly K+-bound enzyme. This behavior was characterized in Na+ o-free conditions, similar to the present study, and a hyperbolic relationship between K+ o concentration and ion transport rate was observed in the presence and absence of amine. A linear Dixon plot of ion transport rate versus TPA concentration was interpreted as showing that binding of a single quaternary amine was sufficient to inhibit the Na,K-ATPase. In the present experiments, the Hill coefficient (γK) for K+ o activation of Na,K pump current in the absence of TEA or BTEA was 0.58, an indication of negative cooperativity for K+ o activation of enzyme turnover. Interestingly, the Hill coefficient (γQ) for quaternary amine inhibition of current had a similar value, whether the amine was TEA or BTEA. Negative cooperativity suggests that more than one amine may be involved in inhibiting Na,K pump current. Furthermore, since the Hill coefficient for TEA and BTEA inhibition of current is independent of K+ o, this negative cooperativity is unlikely to be due to a quaternary amine–K+ interaction.
Effect of Amines on K+ Occlusion by the Na,K-ATPase
Binding and occlusion of extracellular K+ by the Na,K-ATPase appears to be an ordered process in which only one of the two K+ binding sites is accessible from the extracellular medium (Forbush, 1987; González-Lebrero et al., 2002). This postulate rests principally on data showing that, in the presence of Mg2+ and inorganic phosphate (Pi), K+ and K+ congeners slow the release kinetics of ∼50% of the Rb+ occluded by the Na,K-ATPase, without affecting the rapid release of the remaining occluded Rb+ (Glynn et al., 1985; Forbush, 1987). Similar results were obtained whether Rb+ was occluded without other ligands or in the presence of Na+, Mg2+, and micromolar ATP concentrations (González-Lebrero et al., 2002). Forbush (1987) interpreted these data as showing that Rb+ bound to one K+ site, the “fast” site, that could rapidly release Rb+ to the extracellular medium and to a second K+ site, the “slow” site, that could only be occupied by Rb+ that had first bound to the fast site. As a result, when the first K+ binds at the fast site, the second K+ ion cannot bind until the first bound K+ shifts to the second slow site. Ordered binding of Rb+ to the fast and slow K+ sites also led to ordered release from these sites. The conditions of these experiments, i.e., including Mg2+ and Pi during Rb+ release, suggested that release was to the extracellular side of the enzyme.
The accessibility of the fast K+ site to the extracellular medium also appears to be restricted by a flickering or leaky gate (Forbush, 1987; González-Lebrero et al., 2002). The strongest data supporting this concept is that the affinity with which extracellular Rb+ blocks release of bound Rb+ is similar to the affinity for Rb+ occlusion by the Na,K-ATPase (Forbush, 1987). Since the kinetics of extracellular K+ binding reactions are very rapid (Peluffo and Berlin, 1997), such a similarity in Rb+ blocking and occlusion affinities could only occur if access to K+ binding sites is rate limiting. In other words, the gate is poised strongly toward the closed position. This gating process might be analogous to the rapid opening and closing kinetics observed in ion channels (Hille, 2001) and with palytoxin-modified Na,K-ATPase (Kim et al., 1995; Artigas and Gadsby, 2003).
Organic quaternary amines, and in fact a variety of amine-containing compounds, are interesting because, like extracellular K+ congeners, they can block the release of Rb+ from the slow K+ site without affecting release of Rb+ from the fast K+ binding site (Forbush, 1988). Quaternary amines, however, differ from K+ in one important respect. The amine concentrations required to block Rb+ occlusion are far higher than those required to block release of occluded Rb+ (Forbush, 1988). These data have been interpreted as showing that extracellular quaternary amines have access to the fast K+ binding site in the enzyme, but that they do not become occluded like K+. Forbush (1988) envisioned the action of quaternary amines as getting a “foot in the door”, thereby preventing the closing of the occlusion gate. Thus, quaternary amines appear to bind to sites in the Na,K-ATPase without becoming occluded.
Quaternary Amine Binding Sites in the Na,K-ATPase
The experiments of Forbush (1988) showing that quaternary amines slow the release of 86Rb+ from Na,K-ATPase, do not necessarily demonstrate that the amines bind to the fast K+ binding site in the enzyme. In a similar manner, competitive inhibition of enzyme activation is not sufficient to demonstrate that amines and K+ bind at the same site (Segel, 1975).
Our data show that inhibition of Na,K pump current by TEA or BTEA displays a Hill coefficient significantly less than 1. One implication of this result is that the quaternary amines are capable of interacting at two binding sites. Certainly, our finding that TEA inhibits Na,K pump in a V M-independent manner while BTEA produces V M-dependent block shows that the amines can act at sites with different locations in the membrane electric field; however, the relationship between electrical and physical distances is unknown. Even so, inhibition of Na,K pump current by BTEA dissipates 40% of the membrane electric field, and activation of Na,K pump current by extracellular K+ dissipates a similar fraction of the membrane electric field (35–39%) in our experiments. Given that BTEA is a competitive inhibitor of the enzyme (Fig. 6), the simplest explanation for our data is that this amine is blocking at or very near a K+ binding site located within the membrane dielectric.
With the Hill coefficient for current block by BTEA being less than one, we can also speculate that BTEA is capable of interacting with a second site, possibly the same site located outside of the membrane electric field at which TEA inhibits Na,K pump current. However, if BTEA interacts at two sites in the Na,K-ATPase, an interesting problem arises as to why TEA does not also produce V M-dependent block of the enzyme. In fact, we cannot rule out this possibility because TEA may interact with the K+ site located in the electric field with much lower affinity than BTEA. In this regard, we performed a few experiments using 100 mM extracellular TEA (unpublished data), and the data gave some indication that V M-dependent block occurred, specifically, a small decrease in the slope of the K 0.5-V M relationship in the presence of 100 mM TEA. We did not investigate this point because any V M-dependent effects of TEA do not change our mechanistic interpretation of V M-dependent inhibition of the Na,K-ATPase, and we were concerned that high TEA concentrations were having additional effects on the cells under study. In any case, if the amines are interacting at two sites in the Na,K-ATPase, one located in the membrane electric field, the other outside of the field, this result does not itself demonstrate that the amines are binding at the slow and fast K+ sites identified by Forbush (1987), since we cannot conclude whether an amine binding site located outside of the membrane electric field is a K+ binding site; however, such a possibility is not incompatible with our data.
Implications of VM-dependent Block by BTEA for the Mechanism of VM-dependent K+ o Binding by the Na,K-ATPase
As mentioned above, our previous work has demonstrated that a V M-dependent step during K+ transport by the Na,K-ATPase is extracellular K+ binding, and that this reaction is followed by a slower electroneutral reaction (Peluffo and Berlin, 1997). The Albers-Post reaction model for the Na,K-ATPase (Glynn, 1985) would suggest this slow electroneutral reaction is probably occlusion and enzyme dephosphorylation. Scheme I summarizes this reaction sequence without specifying the stoichiometry of K+ binding or the phosphorylation
SCHEME I.
state of the enzyme. Given Rb+ occlusion data suggesting the existence of a flickering gate, the underlying reason for slow occlusion kinetics is that the gate opens with low probability (Forbush, 1987; González-Lebrero et al., 2002). Data showing rapid gating of the palytoxin-modified enzyme (Kim et al., 1995; Artigas and Gadsby, 2003), analogous to the opening and closing of the gate in an ion channel, might suggest that Scheme I could be modified so that rapid ion binding is followed by a rapidly reversible gating reaction and then slower ion occlusion (Scheme II). Hilgemann (1994b) predicted a similar reaction sequence and suggested that the gating step (to form E{K}) would be a microscopic occlusion reaction that precedes the formation of a stable ion-occluded enzyme intermediate. With such a reaction scheme, Hilgemann (1994b) also predicted that as long as the fraction of K+-bound but not -occluded enzyme (EK conformation) is small, it would not be practical to distinguish whether the V M-dependent reaction is ion binding or rapid gating of the ion-bound enzyme.
SCHEME II.
The first issue then is whether the concentration of EK in Scheme II is likely to be small during K+ binding and occlusion. In Rb+ occlusion conditions, González-Lebrero et al. (2002) calculated the equilibrium constants for ion binding and occlusion to show that the reaction equilibrium is displaced almost entirely toward the occluded state (E(K) conformation), a point made quite obvious by the ability to occlude Rb+ in the presence of Na+, Mg2+, and micromolar concentrations of ATP (Glynn and Richards, 1982). Therefore, at least in the forward direction, fast gating must very much favor the closed gate conformation.
In the reverse direction, there is no direct information about the equilibrium position of the ion binding reaction under the conditions of our experiments. Indirectly, one could infer that the bound conformation would rapidly decay to the unbound state because the K 0.5 for equilibrium Rb+ occlusion in the absence of other ligands is much smaller than the Rb+ concentration that produces half-maximal rate of occlusion (González-Lebrero et al., 2002). In Scheme II, this difference in K 0.5 values would suggest that either the formation of the Rb+-bound but not -occluded state and/or the closed gate conformation of the enzyme is disfavored. However, as pointed out above, the gating reaction is strongly poised in the closed direction (González-Lebrero et al., 2002), so that only the binding reaction can realistically be disfavored. Thus, a reasonable conclusion is that the K+-bound but not -occluded conformation of the enzyme exists only in very small quantities.
Organic quaternary amines make it possible to distinguish whether the ion binding or gating reactions are V M dependent. Forbush (1988) showed that these amines bind to the Na,K-ATPase but are not occluded, and he interpreted these data as showing that closing of the flickering gate is blocked. If this is the case, the enzyme can accumulate to a significant degree in the amine-bound (but not -occluded) conformation. Under these conditions, it should be possible to distinguish whether binding or gating is the V M-dependent reaction. If gating is V M dependent then amine block of enzyme cycling should be V M independent. Conversely, if binding is V M dependent, one could anticipate that amine block could also be V M dependent. The present data clearly demonstrate that the latter is possible. In addition, we found that the fraction of the membrane dielectric dissipated during BTEA inhibition is similar to that dissipated during K+ o activation of enzyme cycling. These data suggest that the binding step is the major V M-dependent reaction while conformational changes associated with ion binding move little charge in the membrane electric field. This V M dependence of ion binding will occur as K+ moves from the extracellular medium to its binding sites in the enzyme, implying that a high-field access channel exists in the Na,K-ATPase. Thus, these data provide strong evidence for an ion channel–like structure in the Na,K-ATPase.
Summary
In this study, we have demonstrated that the organic quaternary amine, BTEA, is a competitive, V M-dependent blocker for K+ o activation of Na,K pump current under conditions where K+ o binding is the only V M-dependent reaction controlling enzyme turnover. Given previous data concerning amine inhibition of ion transport and occlusion by the Na,K-ATPase as well as our understanding of V M-dependent K+ o binding by this enzyme, the present results indicate that BTEA is binding at a K+ site located in the membrane electric field. Furthermore, these data demonstrate that this binding site must be located in an access channel–like structure in the enzyme. Thus, quaternary amines should provide a valuable tool to probe the structure of K+ binding sites and the access channel of the Na,K-ATPase.
Acknowledgments
The authors wish to thank the technical assistance of Palak Raval Nelson and Marguerite Schmid, and Dr. Dawn A. Lott for helpful discussions on validating our fitting procedures.
This work was supported by the National Institutes of Health (J.R. Berlin) and the American Heart Association (R.D. Peluffo).
Olaf S. Andersen served as editor.
APPENDIX
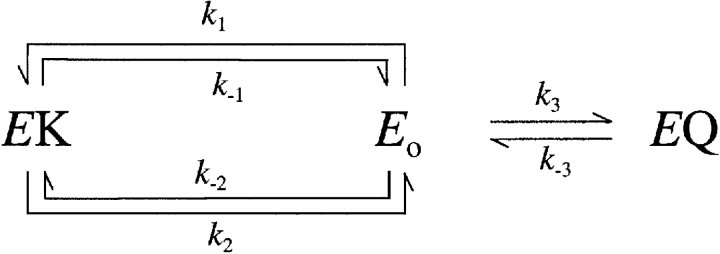
In the absence of quaternary amines, Na,K pump current measured in Na+ o-free solution and saturating cytosolic Na+ concentrations can be described with a pseudo two-state model in which a single reaction step is V M dependent (Sagar and Rakowski, 1994). To model the effect of quaternary amines on Na,K pump current, the following properties of quaternary amines are assumed: (a) inhibition is competitive with K+ o activation, (b) inhibition prevents the enzyme from supporting ion transport, i.e., no current arises from inhibited enzymes, (c) the amine binds with unknown stoichiometry, and (d) inhibition can be V M dependent. With these assumptions, Scheme III describes possible reactions of the Na,K-ATPase where the enzyme is assumed to occur in three forms, a K+-bound form (EK), an ion-free form equivalent to E 2P (E o), and an amine-bound form (EQ). Stoichiometry of K+ binding to the enzyme is not shown in this scheme.
SCHEME III.
Voltage dependence is assigned arbitrarily to the binding steps to E o (k 1 for K+ o and k 3 for Q) rather than dissociation steps (k −1 and k −3, respectively). If the backward running of the enzyme under our conditions is assumed to be negligible, i.e., k −2 ∼0, Scheme III can be solved for the concentration of EK under steady-state conditions to be as in Eq. 3
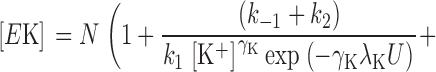 |
(3) |
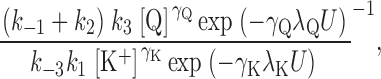 |
where N is the total concentration of Na,K-ATPase proteins involved in ion transport, i.e., N = [E o] + [EK] + [EQ], and [K+] is the K+ o concentration. Assuming that Na,K pump current (I) is equal to zFk 2[EK], where z = 1 charge per cycle, Eq. 3 can be substituted into this expression to yield:
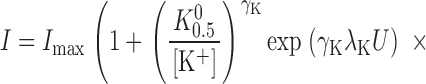 |
(4) |
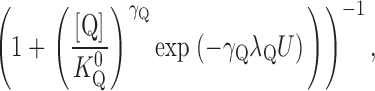 |
where
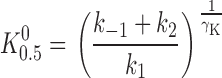 |
(4a) |
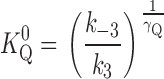 |
(4b) |
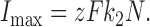 |
(4c) |
Na,K pump current (I) in Eq. 4 depends on [K+], the amine concentration ([Q]) and V M, all experimentally controllable parameters. Fig. 8 shows the results of a simulation in which the amine is assumed to act in a V M-independent fashion, i.e., λQ = 0. Simulated current is activated at constant [K+] (0.2 mM) in the absence and presence of the inhibitory quaternary amine. Note the similarity to Fig. 3 A, inhibition of Na pump current by TEA. Like the experimental data, the V M-independent inhibitor appears to block simulated Na,K pump current more effectively at positive V M. However, when the simulation is run with a V M-dependent blocker (λQ = 0.4 and 0.75), the V M dependence of the current progressively changes so that the inhibitor appears to block current more effectively at negative V M, similar to the data shown in Fig. 5 B.
Figure 8.
Simulations showing the effect of quaternary amine inhibition on the V M dependence of Na,K pump current. Eq. 4 was solved with the following parameter values: K 0 0.5 = 0.2 mM, K 0 Q = 4 mM, [Q] = 5 mM, λK = 0.37, γK = 0.56, γQ = 0.6, and λQ = 0 (○), 0.4 (▾), and 0.75 (▿). The control curve (•) was calculated with [Q] = 0 mM.
Eq. 4 can also be expressed in the more familiar format of a Hill equation:
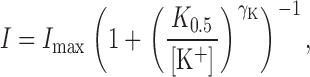 |
(5) |
where K 0.5 is expressed as in Eq. 2. Simulations for the effect of V M-independent and -dependent blockers on the K 0.5-V M relationship are shown in Fig. 9. If inhibition of Na pump current is V M independent (λQ = 0), the K 0.5 for K+ o will simply be scaled up from the curve obtained in the absence of inhibitor. On the other hand, with V M-dependent inhibition, the curves progressively flatten out and change curvature as the ion-well depth for the inhibitor is increased (λQ = 0.4 and 0.75). Again, the simulations with λQ = 0 and 0.4 are qualitatively similar to the V M dependence of K 0.5 values observed in the presence of TEA (Fig. 4) and BTEA (Fig. 7), respectively.
Figure 9.
Simulations showing the effect of quaternary amine inhibition on the V M dependence of K 0.5. Eq. 2 was solved with the parameter values used in Fig. 8 where λQ = 0 (○), 0.4 (▾), and 0.75 (▿). The control curve (•) was calculated with [Q] = 0 mM.
In the absence of organic amines, Scheme III reduces to a pseudo two-state model. In this case, Eq. 4 simplifies to
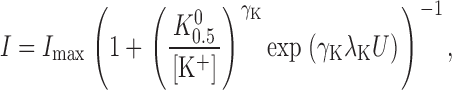 |
(6) |
and the expression for K 0.5 becomes Eq. 1, which is used to fit the data in Fig. 2 C.
Abbreviations used in this paper: BTEA, benzyltriethylammonium ion; NMG, N-methyl-D-glucamine; TMA, tetramethylammonium ion; TPA, tetrapropylammonium ion.
References
- Artigas, P., and D.C. Gadsby. 2003. Na+/K+-pump ligands modulate gating of palytoxin-induced ion channels. Proc. Natl. Acad. Sci. USA. 100:501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin, J.R., and R.D. Peluffo. 1997. Mechanism of electrogenic reaction steps during K+ transport by the Na,K-ATPase. Annals NY Acad. Sci. 834:251–259. [DOI] [PubMed] [Google Scholar]
- Berlin, J.R., and R.D. Peluffo. 1998. Inhibition of Na,K pump current in cardiac myocytes by organic quaternary amines. Biophys. J. 74:A338. [Google Scholar]
- Bielen, F.V., H.G. Glitsch, and F. Verdonck. 1991. Dependence of Na+ pump current on external monovalent cations and membrane potential in rabbit cardiac Purkinje cells. J. Physiol. 442:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler, R., W. Sturmer, H.-J. Apell, and P. Läuger. 1991. Charge translocation by the Na+,K+-pump: I. Kinetics of local field changes studied by time-resolved fluorescence measurements. J. Membr. Biol. 121:141–161. [DOI] [PubMed] [Google Scholar]
- Cooke, I.M., G. LeBlanc, and L. Tauc. 1974. Sodium pump stoichiometry in Aplysia neurons from simultaneous current and tracer measurements. Nature. 251:254–256. [DOI] [PubMed] [Google Scholar]
- De Weer, P., R.F. Rakowski, and D.C. Gadsby. 1984. Current generated by backward-running electrogenic Na pump in squid giant axons. Nature. 309:450–452. [DOI] [PubMed] [Google Scholar]
- De Weer, P., D.C. Gadsby, and R.F. Rakowski. 1988. Voltage dependence of the Na-K pump. Annu. Rev. Physiol. 50:225–241. [DOI] [PubMed] [Google Scholar]
- Eckstein-Ludwig, U., J. Rettinger, L.A. Vasilets, and W. Schwarz. 1998. Voltage-dependent inhibition of the Na+,K+ pump by tetraethylammonium. Biochim. Biophys. Acta. 1372:289–300. [DOI] [PubMed] [Google Scholar]
- Eisner, D.A., and W.J. Lederer. 1980. Characterization of the electrogenic sodium pump in cardiac Purkinje fibers. J. Physiol. 303:441–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler, K., S. Jaruschewski, A. Hobbs, W. Albers, and J.P. Froehlich. 1993. Pre-steady-state charge translocation in Na,K-ATPase from eel electric organ. J. Gen. Physiol. 102:631–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush, B. 1987. Rapid release of 42K or 86Rb from two distinct transport sites on the Na,K-pump in the presence of Pi or vanadate. J. Biol. Chem. 262:11116–11127. [PubMed] [Google Scholar]
- Forbush, B. 1988. The interaction of amines with the occluded state of the Na,K-pump. J. Biol. Chem. 263:7979–7988. [PubMed] [Google Scholar]
- Gadsby, D.C., and M. Nakao. 1989. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J. Gen. Physiol. 94:511–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby, D.C., J. Kimura, and A. Noma. 1985. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 315:63–65. [DOI] [PubMed] [Google Scholar]
- Gadsby, D.C., R.F. Rakowski, and P. De Weer. 1993. Extracellular access to the Na,K pump: pathway similar to ion channel. Science. 260:100–103. [DOI] [PubMed] [Google Scholar]
- Glynn, I.M. 1985. The Na+, K+-transporting adenosine triphosphatase. The Enzymes of Biological Membranes, Vol. 3. A.N. Martonosi, editor. Plenum Press, New York. 35–114.
- Glynn, I.M., and D.E. Richards. 1982. Occlusion of rubidium ions by the sodium-potassium pump: its implications for the mechanism of potassium transport. J. Physiol. 330:17–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn, I.M., and S.J.D. Karlish. 1990. Occluded cations in active transport. Annu. Rev. Biochem. 59:171–205. [DOI] [PubMed] [Google Scholar]
- Glynn, I.M., J.L. Howland, and D.E. Richards. 1985. Evidence for the ordered release of rubidium ions occluded within the Na,K-ATPase of mammalian kidney. J. Physiol. 368:453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Lebrero, R.M., S.B. Kaufman, M.R. Montes, J.G. Nørby, P.J. Garrahan, and R.C. Rossi. 2002. The occlusion of Rb+ in the Na+/K+-ATPase. J. Biol. Chem. 277:5910–5921. [DOI] [PubMed] [Google Scholar]
- Hansen, U., D. Gradmann, D. Sanders, and C.L. Slayman. 1981. Interpretation of current-voltage relationships for “active” ion transport systems: I. Steady-state reaction-kinetic analysis of Class-I mechanisms. J. Membr. Biol. 63:165–190. [DOI] [PubMed] [Google Scholar]
- Hansen, P.S., K.A. Buhagiar, B.Y. Kong, R.J. Clarke, D.F. Gray, and H.H. Rasmussen. 2002. Dependence of Na+-K+ pump current-voltage relationship on intracellular Na+, K+, and Cs+ in rabbit cardiac myocytes. Am. J. Physiol. 283:C1511–C1521. [DOI] [PubMed] [Google Scholar]
- Heyse, S., I. Wuddel, H.-J. Apell, and W. Stürmer. 1994. Partial reactions of the Na,K-ATPase: determination of rate constants. J. Gen. Physiol. 104:197–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann, D.W. 1994. a. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 263:1429–1432. [DOI] [PubMed] [Google Scholar]
- Hilgemann, D.W. 1994b. Flexibility and constraint in the interpretation of Na+/K+ pump electrogenicity: What is an access channel? The Sodium Pump. E. Bamberg and W. Schoner, editors. Steinkopff/Darmstadt. New York. 507–516.
- Hille, B. 2001. Ion Channels of Excitable Membranes. 3rd ed. Sinauer Associates, Sunderland, MA.
- Holmgren, M., and R.F. Rakowski. 1994. Pre-steady-state transient currents mediated by the Na/K pump in internally perfused Xenopus oocytes. Biophys. J. 66:912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg, G., and W. Trautwein. 1974. The effect of dihydroouabain and lithium ions on the outward current in cardiac Purkinje fibers. Pflugers Arch. 350:41–54. [DOI] [PubMed] [Google Scholar]
- Ishizuka, N., A.J. Fielding, and J.R. Berlin. 1996. Na pump current can be separated into ouabain-sensitive and -insensitive components in single rat ventricular myocytes. Jap. J. Physiol. 46:215–223. [DOI] [PubMed] [Google Scholar]
- Kim, S.Y., K.A. Marx, and C.H. Wu. 1995. Involvement of the Na,K-ATPase in the induction of ion channels by palytoxin. Naunyn-Schmiedeberg's Arch Pharmacol. 351:542–554. [DOI] [PubMed] [Google Scholar]
- Kropp, D.L., and J.R. Sachs. 1977. Kinetics of the inhibition of the Na-K pump by tetrapropylammonium chloride. J. Physiol. 264:471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaire, A.V., and W. Schwarz. 1986. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J. Membr. Biol. 91:43–51. [DOI] [PubMed] [Google Scholar]
- Läuger, P. 1991. Electrogenic Ion Pumps. Sinauer Associates, Sunderland, MA.
- Nakao, M., and D.C. Gadsby. 1986. Voltage dependence of Na translocation by the Na/K pump. Nature. 323:628–630. [DOI] [PubMed] [Google Scholar]
- Nakao, M., and D.C. Gadsby. 1989. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J. Gen. Physiol. 94:539–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo, R.D., and J.R. Berlin. 1997. Electrogenic K+ transport by the Na+-K+ pump in rat cardiac ventricular myocytes. J. Physiol. (Lond.). 501:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo, R.D., J.M. Argüello, and J.R. Berlin. 2000. The role of Na,K-ATPase α subunit serine 775 and glutamate 779 in determining the extracellular K+ and membrane potential-dependent properties of the Na,K-pump. J. Gen. Physiol. 116:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo, R.D., Y. Hara, and J.R. Berlin. 2001. Voltage dependence of inhibition of the cardiac Na,K-pump forward cycling by quaternary amines. Biophys. J. 80:37a. [Google Scholar]
- Rakowski, R.F. 1993. Charge movement by the Na/K pump in Xenopus oocytes. J. Gen. Physiol. 101:117–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski, R.F., D.C. Gadsby, and P. De Weer. 1989. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J. Gen. Physiol. 93:903–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski, R.F., L.A. Vasilets, J. LaTona, and W. Schwarz. 1991. A negative slope in the current-voltage relationship of the Na+/K+ pump in Xenopus oocytes produced by reduction of external. J. Membr. Biol. 121:177–187. [DOI] [PubMed] [Google Scholar]
- Rakowski, R.F., D.C. Gadsby, and P. De Weer. 1997. Voltage dependence of the Na/K pump. J. Membr. Biol. 155:105–112. [DOI] [PubMed] [Google Scholar]
- Rossi, R.C., and P.J. Garrahan. 1989. Steady-state kinetic analysis of the Na+/K+-ATPase. The effects of adenosine 5′-[β,γ-methylene]triphosphate on substrate kinetics. Biochim. Biophys. Acta. 981:85–94. [DOI] [PubMed] [Google Scholar]
- Sachs, J.R., and M.E. Conrad. 1968. Effect of tetraethylammonium on the active cation transport system of the red blood cell. Am. J. Physiol. 215:795–798. [DOI] [PubMed] [Google Scholar]
- Sagar, A., and R.F. Rakowski. 1994. Access channel model for the voltage dependence of the forward-running Na+/K+ pump. J. Gen. Physiol. 103:869–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel, I.H. 1975. Enzyme Kinetics. Wiley & Sons, New York.
- Stimers, J.R., S. Liu, and M. Lieberman. 1991. Apparent affinity of the Na/K pump for ouabain in cultured chick cardiac myocytes. J. Gen. Physiol. 98:815–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilets, L.A., and W. Schwarz. 1993. Structure-function relationships of cation binding in the Na+/K+-ATPase. Biochim. Biophys. Acta. 1154:201–222. [DOI] [PubMed] [Google Scholar]
- Wuddel, I., and H.-J. Apell. 1995. Electrogenicity of the sodium transport pathway in the Na,K-ATPase probed by charge-pulse experiments. Biophys. J. 69:909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemková, H., J. Teisinger, and F. Vyskocil. 1988. Inhibition of the electrogenic Na,K pump and Na,K-ATPase activity by tetraethylammonium, tetrabutylammonium, and Apamin. J. Neurosci. Res. 19:497–503. [DOI] [PubMed] [Google Scholar]