Abstract
A method was developed that allows conversion of changes in maximum Ca2+-dependent fluorescence of a fixed amount of fluo-3 into volume changes of the fluo-3–containing solution. This method was then applied to investigate by confocal microscopy the osmotic properties of the sealed tubular (t-) system of toad and rat mechanically skinned fibers in which a certain amount of fluo-3 was trapped. When the osmolality of the myoplasmic environment was altered by simple dilution or addition of sucrose within the range 190–638 mosmol kg−1, the sealed t-system of toad fibers behaved almost like an ideal osmometer, changing its volume inverse proportionally to osmolality. However, increasing the osmolality above 638 to 2,550 mosmol kg−1 caused hardly any change in t-system volume. In myoplasmic solutions made hypotonic to 128 mosmol kg−1, a loss of Ca2+ from the sealed t-system of toad fibers occurred, presumably through either stretch-activated cationic channels or store-operated Ca2+ channels. In contrast to the behavior of the t-system in toad fibers, the volume of the sealed t-system of rat fibers changed little (by <20%) when the osmolality of the myoplasmic environment changed between 210 and 2,800 mosmol kg−1. Results were also validated with calcein. Clear differences between rat and toad fibers were also found with respect to the t-system permeability for glycerol. Thus, glycerol equilibrated across the rat t-system within seconds to minutes, but was not equilibrated across the t-system of toad fibers even after 20 min. These results have broad implications for understanding osmotic properties of the t-system and reversible vacuolation in muscle fibers. Furthermore, we observed for the first time in mammalian fibers an orderly lateral shift of the t-system networks whereby t-tubule networks to the left of the Z-line crossover to become t-tubule networks to the right of the Z-line in the adjacent sarcomere (and vice versa). This orderly rearrangement can provide a pathway for longitudinal continuity of the t-system along the fiber axis.
Keywords: t-system, skeletal muscle, osmotic gradient, skinned fibers, glycerol
INTRODUCTION
In skeletal muscle it is well known that the transverse tubular (t-) system supports the spread of excitation through individual muscle fibers (Melzer et al., 1995). Moreover, the t-system supports other major cellular functions such as membrane repair, water transport, and volume regulation (Krolenko and Lucy, 2001).
Importantly, this membrane system that opens to the outside, is not a static entity, but a dynamic structure as vividly demonstrated with confocal microscopy techniques by processes such as reversible vacuolation (Krolenko et al., 1995; Lännergren et al., 1999, 2000) or volume changes in response to changes in the membrane potential, cholesterol content, and osmotic pressure (Launikonis and Stephenson, 2002a).
When characterizing the properties of the t-system with respect to osmotic changes, there are advantages to studying a closed t-system. This can be achieved by mechanically removing the surface membrane (sarcolemma) of a single muscle fiber, allowing the openings of the t-system to the outside to seal off, forming a separate, closed, extracellular compartment (Stephenson and Lamb, 1993; Lamb et al., 1995; Launikonis and Stephenson, 1999, 2001, 2002b; Launikonis et al., 2003). The ability of the t-system to continue to function normally when sealed is shown by the preservation of t-system SR membrane protein coupling (Lamb and Stephenson, 1990; Posterino et al., 2000; Launikonis et al., 2003; Ørtenblad and Stephenson, 2003). As the sealed t-system prevents the free movement of solution into and out of this network, it allows the opportunity to examine directly its osmotic properties in the absence of the peripheral surface membrane (sarcolemma). This is notable because there may be important differences between the sarcolemma and the t-system, considering that they have different origins (Flucher et al., 1991). Such work separating the t-system from the surface membrane has been possible with detubulated fibers, but in these preparations the properties of the sarcolemma rather than those of the t-system were the central focus of investigation (Fujino et al., 1961; Eisenberg and Eisenberg, 1968; Gage and Eisenberg, 1969). Here we are able to study the properties of the t-system in the absence of the sarcolemma with full experimental control over the myoplasmic environment. We have already reported that the sealed t-system does not respond with reversible vacuolation when challenged with the rapid removal of glycerol (Launikonis and Stephenson, 2002b) as the intact t-system does (Krolenko et al., 1995) and in this study we examine in detail the osmotic properties of the t-system by changing the osmolality of the myoplasmic environment with glycerol and sucrose.
MATERIALS AND METHODS
Preparation of Muscle Fibers
The Animal Ethics and Experimentation Committee at La Trobe University approved the use and killing method of all animals in this study. Cane toads (Bufo marinus) or frogs (Rana pipiens) were stunned with a heavy blow to the head and killed by double pithing. The iliofibularis muscles were dissected and well blotted on filter paper (Whatman No. 1) and then placed on a layer of Sylgard 184 (Dow Chemicals) in a Petri dish and immersed in a layer of paraffin oil. Male rats (Long Evans, hooded; 3-mo-old) were killed under deep anesthesia with halothane (2% vol/vol). The extensor digitorum longus (EDL) muscles were dissected and similarly blotted and placed on a layer of Sylgard in a petri dish under a layer of paraffin oil.
Fluorescent dyes (see below) were trapped in the sealed t-system as described previously (Launikonis and Stephenson, 1999, 2001, 2002b). Briefly, single intact fibers (toad or frog) or isolated small bundles of fibers (rat) were administered a physiological solution containing fluorescent dye (see below) with a microcap (Drummond) while still under oil. The excess solution was then removed from around the fibers, which were then mechanically skinned thus trapping the fluorescent dye in the t-system as it sealed off during the “skinning” procedure (Launikonis and Stephenson, 2001). Skinned fibers were then transferred to custom-built experimental wells, which used a thin coverslip as a base. Preparations were secured to the bottom of experimental wells with pins glued parallel to the coverslip surface to reduce the distance between the preparation and the objective lens above which they were placed. Unless otherwise specified, the well contained a standard myoplasmic K+ solution (see below).
Solutions
The “dye” solutions were physiological solutions containing one of three fluorescent dyes: fluo-3 (pentaammonium salt), Oregon green and calcein; all from Molecular Probes. The fluo-3 solutions contained (mM): NaCl, 112; KCl, 3.3; MgCl2, 1; CaCl2, 2.5; fluo-3, 1; and HEPES, 20 (pH 7.4 with NaOH) for toad fibers and NaCl, 145; KCl, 3; CaCl2, 4; MgCl2, 2; fluo-3, 1; and HEPES, 10 (pH 7.4 with NaOH) for rat fibers. Note that the ionized [Ca2+] in the fluo-3 solutions for toad and rat were close to 1.5 and 3 mM, respectively, which would ensure effective saturation (>99%) of fluo-3 provided that [Ca2+] in the t-system does not decrease below ∼100 μM under the conditions of the experiments undertaken in this study. The Oregon green solutions were the same as the fluo-3 solutions except that fluo-3 was replaced by Oregon green. Calcein solutions were similar to the fluo-3 solutions containing (mM): NaCl, 115; KCl, 4.2; CaCl2, 2.1; MgCl2, 0.9; HEPES, 8.5; glucose, 8.5; calcein 8.5 (pH 7.2 with NaOH) for amphibian fibers and NaCl, 135; KCl, 5; CaCl2, 2.5; MgCl2, 1; HEPES, 10; glucose 10; calcein 10 (pH 7.2 with NaOH) for rat fibers. The osmolalities of the “dye” solutions used with the amphibian and rat muscle fibers were close to 255 and 290 mosmol kg−1, respectively.
The standard myoplasmic solution in which the amphibian fibers were incubated after mechanical skinning contained (mM): K+, 117; Na+, 36; HDTA, 49.5; free Mg2+, 1; MgATP, 7 (total ATP, 8); phosphocreatine, 10; NaN3, 1; HEPES, 60 with final pH 7.10 and CaEGTA/EGTA, 0.5 with pCa (−log10[Ca2+]) 6.7. In one experiment, a Na+ myoplasmic solution was used, which was similar to the standard K+ solution except that Na+ replaced all the K+. For rat standard myoplasmic K+ solution, [K+], [HEPES], and [CaEGTA/EGTA] were raised to 125, 90, and 1 mM, respectively, and total [Mg] was slightly adjusted to maintain 1 mM free Mg2+. The amphibian and rat standard myoplasmic solutions had osmolalities of 255 and 280 momsmol kg−1, respectively. Unless otherwise indicated, the osmolality of myoplasmic solutions was changed by simple dilution or by the addition of sucrose (Blinks, 1965). In the myoplasmic solutions set at 2,550 and 2,800 mosmol kg−1 with sucrose, the pCa was adjusted from 6.7 to > 9 to prevent a contracture caused by Ca2+ release at this osmolality (Lamb et al., 1993). In some experiments, glycerol was added to the myoplasmic solutions. The osmolality of all solutions was determined with a Vapor Pressure Osmometer (Wescor). All experiments were conducted at room temperature (23 ± 1°C).
Confocal Imaging
Coverslips with dye (fluo-3 and Oregon green)-loaded preparations were placed on the stage of an inverted Leitz laser (Ar ion; excitation wavelength 488 nm) scanning confocal microscope and images were obtained with 40× (NA 1.3) or 63× (NA 1.4) oil immersion lenses as previously described in detail (Launikonis and Stephenson, 2001). Preparations were scanned in X-Y mode, averaging eight scans line−1; all images were stored on an optical disk for later analysis.
All experiments using calcein were performed in the laboratory of Eduardo Ríos (Rush University Medical Center, Chicago). Fibers were prepared in the same manner as those loaded with fluo-3 and were imaged with the 40× (NA 1.2) water immersion lens of the MRC 1000 (Biorad Laboratories) confocal microscope (for microscope details see Ríos et al., 1999).
Image Analysis
Fluorescence images were analyzed with in-built confocal microscope software. Fluorescence intensity of the fiber, Ffiber, was obtained by subtracting the background fluorescence intensity, Fmin, from the raw fluorescence intensity of the fiber. Fmin was derived from the fluorescence intensity of the nonfiber areas in the image plane (see Fig. 2). At least 1,500 and 500 pixels were averaged to obtain Ffiber and Fmin, respectively. The initial Ffiber reading in each preparation (always in standard K+ solution) was set to 100 arbitrary units (au) and all subsequent fluorescence measurements were expressed relative to this initial reading. Images from preparations loaded with fluo-3 are presented in greyscale or the “glow” palettes while images from preparations loaded with calcein are presented in the “blue/green/red/yellow” or “green-white linear” palettes.
Figure 2.
The effect of osmolality on the sealed t-system. Confocal images of a toad-skinned fiber exposed to internal solutions of increasing osmotic strengths: 255 (A), 446 (B), and 2,550 (C) mosmol kg−1. Sucrose was added to the myoplasmic K+ solution to increase osmolality and images were taken ∼5 min after exposure to the hypertonic solutions. Note the progressive decrease in number of vesicles (Launikonis and Stephenson, 2002b) as the osmolality was increased. Microscope settings are constant for all panels. Bar, 20 μm.
Calibration of Volume Changes in the Sealed t-system Using Fluo-3
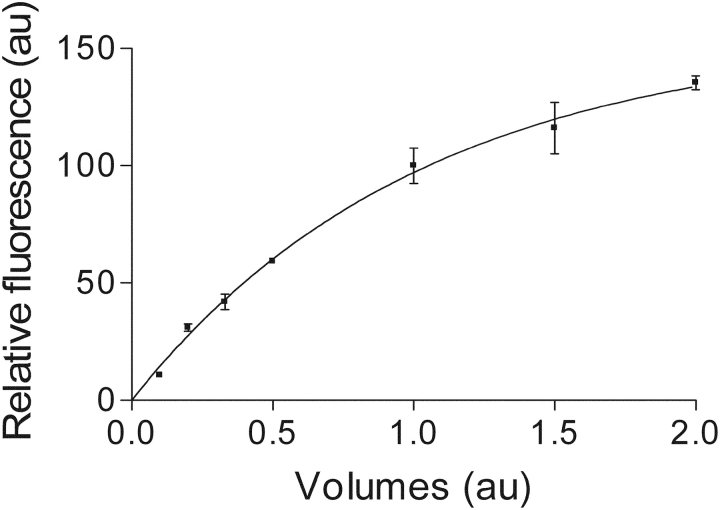
Fluo-3 is a high Ca2+-affinity dye (Kapp > 106 M−1 under prevailing physiological ionic conditions above pH 6.8; Lattanzio, 1990; Thomas et al., 2000). Consequently, it is fully saturated with Ca2+ in the presence of mM Ca2+ in the environment under all conditions to be encountered in this study and also importantly, the maximum fluorescence intensity is not sensitive to pH changes above 6.0 (Minta et al., 1989; Lattanzio, 1990; Harkins et al., 1993). Since fluo-3 carries three negative charges when complexed with Ca2+, one would expect that the maximum fluorescence intensity is sensitive to changes in ionic strength in the environment. Indeed, as shown in Fig. 1, the maximum fluorescence intensity of fluo-3 decreases as water is removed from a typical physiological Na-containing solution and increases when this solution is diluted. Therefore, a decrease in volume of the sealed t-system due to water loss will result in an increased ionic strength and lower Ffiber fluorescence intensity within the t-system lumen and vice versa. Also importantly, the maximum Ca2+-induced fluorescence intensity of fluo-3 does not appear to be affected by relatively large changes in viscosity and osmolality associated with molecules that are not electrically charged. Thus, there was no change in the fluo-3 fluorescence maximum (au) when 400 mM glycerol was added to the Na-based toad physiological solution containing 1 mM fluo-3 (100.0 ± 6.1, n = 3; vs. 105.1 ± 9.9, n = 3; for control and glycerol containing solution, respectively; t test, P > 0.5). These properties of fluo-3 make it an ideal probe to calibrate volume changes in sealed and very narrow extracellular spaces containing mM Ca2+ such as the sealed tubular system, based on ionic concentration–related changes in the maximum fluo-3 fluorescence intensity. Thus, volume changes can be directly derived from changes in the maximum fluo-3 fluorescence intensity using a calibration curve with different volumes of water added to a constant mix of solutes mimicking the composition of the extracellular environment, provided that the total amount of osmolites trapped in the sealed t-system changes little when the imbalance in osmolality across the t-system membrane is addressed by the flux of water across the t-system. Such a curve displaying the maximum fluo-3 fluorescence intensity as function of volumes of water added to the above mix of solutes was obtained by imaging droplets of fluo-3 containing solutions made from a 10-fold concentrated toad physiological solution, which contained (mM): NaCl, 1120; KCl, 30; MgCl2, 10; CaCl2, 25; fluo-3, 10; and HEPES, 200 (pH 7.4 with NaOH). For this calibration, droplets of solutions at various dilutions were put on coverslips and imaged with the 40× objective. This objective samples with a pixel size of 0.245 × 0.245 × 0.5 μm. The optical section was positioned in the region of the droplet that produced the fluorescence maximum for the given conditions. Three separate spots within the droplet (fluorescing at the maximum level) were recorded and averaged. Since the [fluo-3] varied between these solutions, the relative fluorescence intensity per pixel measured at each dilution of the concentrated physiological solution was scaled according to [fluo-3]. A control experiment where the [fluo-3] was varied in droplets of solution of the same ionic composition showed a correlation coefficient >0.999 between fluorescence intensity per pixel and [fluo-3] in solution. The relationship between maximum Ca2+-induced fluo-3 fluorescence and the water added (“volumes”) is shown in Fig. 1, where one unit of volume represents the volume of water needed to obtain the standard toad physiological solution (i.e., the composition of the sealed t-system in isotonic solution) and the corresponding fluorescence intensity has an arbitrary value of 100.
Figure 1.
Calibration curve showing the change in the maximum Ca2+-induced fluo-3 fluorescence intensity per pixel when the volume of water is altered for a constant amount of solutes corresponding to the toad physiological solution when the water volume added was 1.0 (see materials and methods). The method of calibration is described in the text. The continuous line is a function of type: y = β(1 − exp(−kx)), where β = 156 au; k = 0.9735 volumes−1, and r = 0.999.
It was therefore essential that the absolute number of ions remained relatively constant in the sealed t-system over the course of each experiment. To test this, two types of experiments were performed. In the first type of experiment Ffiber fluorescence intensity was measured after skinned toad fiber preparations were first left to equilibrate in the standard myoplasmic solution, then 5 min after the fiber transfer to an isosmotic solution where the standard myoplasmic solution was diluted 25% with an isosmotic sucrose solution and finally after the preparation was returned to the initial standard myoplasmic solution for 5 min. The ratio between Ffiber in the isosmotic but 25% diluted standard myoplasmic solution and the average value of the bracketed Ffiber values in the standard myoplasmic solution was 0.98 ± 0.02 (n = 3), which was not different from 1.0. This result shows that there must be very small changes in the total amount of osmolites and ionic concentration in the t-system over the period of observation, even when the ionic composition of the myoplasmic environment was considerably changed, and that the marked increase in t-sysvol after exposure of the skinned fiber preparation to a standard myoplasmic solution diluted 25% with distilled water (Fig. 7, A and C) could not have been the result of marked changes in the total amount of osmolites in the t-system. In the second type of experiment, mechanically skinned frog and rat fibers with calcein- or fluo-3–loaded t-system were placed in myoplasmic solutions that were made 10-fold hyperosmotic with sucrose and the results obtained with the two different dyes were compared. Note that as shown above, fluo-3 fluorescence is sensitive to changes in ionic strength, while calcein fluorescence is insensitive to changes in ionic strength but sensitive to volume changes since it is a self-quenching dye (Jayaraman et al., 2001). As shown in results, there was complete agreement between the fluo-3 and the respective calcein sets of data for both amphibian and rat fibers exposed for 5 min to a myoplasmic environment made highly hypertonic with sucrose. This directly shows that electrically charged osmolites could not have left the t-system in significant amounts over a 5-min period in the highly hyperosmotic solution to disturb the tight relationship between t-system volume and ionic concentration.
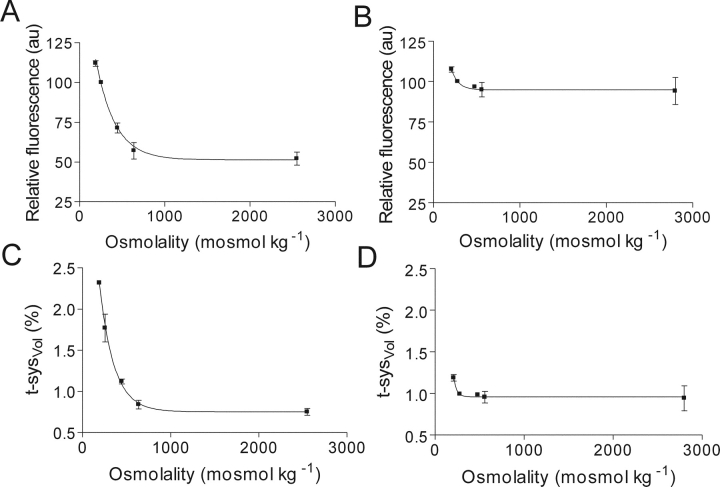
Figure 7.
Volume changes in the sealed t-system at different osmolalities. (A and B) Relative maximum Ca2+-induced fluo-3 fluorescence signal emitted from the sealed t-system of toad- and rat-skinned fibers in internal solutions of different osmolalities, respectively. (C and D) Fractional volume of the toad and rat t-system expressed as a percentage of total intact fiber volume in normal physiological solution (t-sysVol) at different osmolalities, respectively. The results in C and D were determined from the effect of changes in water content on the fluo-3 fluorescence signal (Fig. 1), which we have assumed to equate to t-system volume changes (t-sysVol) (see text). The result is from 9 (A and C) and 15 (B and D) fibers. The continuous lines are functions of type: y = βexp(−kx) + c; where, β = 148.5 au (A), 171.8 au (B), 4.95% intact fiber volume (C), and 78.9% intact fiber volume (D); k = 4.28 osmol−1 kg (A), 12 osmol−1 kg (B), 6.07 osmol−1 kg (C), and 28 osmol−1 kg (D); c = 51.3 au (A), 94.9 au (B), 0.753% intact fiber volume (C), and 0.96% intact fiber volume (D); and r = 0.969 (A), 0.541 (B), 0.999 (C), and 0.999 (D).
Effect of Glycerol on Fluo-3 Fluorescence from the Sealed t-system
We examined the effect of glycerol addition and rapid removal on the fluo-3 fluorescence signal emitted from the sealed t-system using a protocol based on that used to induce reversible vacuolation in intact fibers with glycerol (Krolenko et al., 1995). In these experiments, an initial fluorescence measurement was made by imaging the dye-loaded preparation in the presence of standard myoplasmic K+ solution. Immediately after the initial reading, the standard solution was exchanged for standard solution containing 0, 200, or 400 mM glycerol or 0, 100, or 200 mM sucrose (Fig. 8). The preparation was then imaged after 1, 5, and 20 min after the solution exchange. Immediately after the imaging at 20 min, the standard solution containing glycerol or sucrose was exchanged for standard solution only. The preparation was imaged at 1, 5, and 20 min after this exchange of solutions. These time points correspond to 21, 25, and 40 min after the initial imaging of the preparation at the start of each experiment, respectively, and are presented as such in Fig. 11. In control experiments, only standard myoplasmic K+ solution was used. The solution in the experimental well was changed at the same time points as in the glycerol/sucrose experiments. The exchange of solutions generally resulted in a small drop in fluorescence intensity emitted from the preparation, presumably by washing away any dye that had leaked from the sealed t-system.
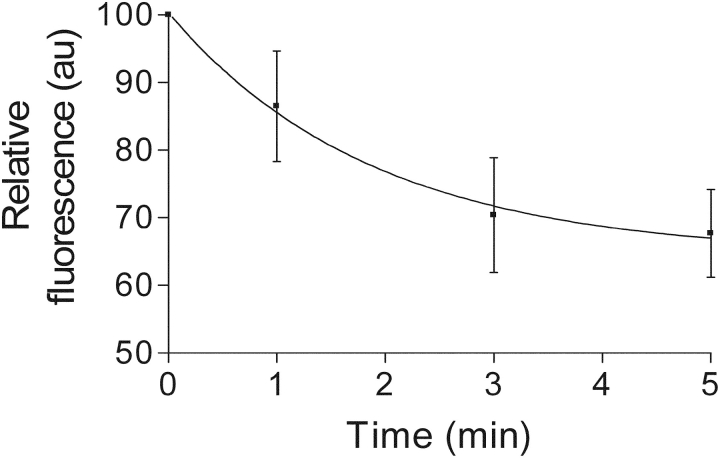
Figure 8.
50% hypotonic myoplasmic solution opens Ca2+-permeant channels in the t-system. After equilibration in standard solution, exposure to a 50% hypotonic solution caused a drop in fluo-3 fluorescence from the sealed t-system. This loss of fluorescence can be attributed to the loss of Ca2+ (see text). The loss of fluorescence signal could be fitted by a function of type: A exp(−kx) + c, where, A = 35.9 au, k = 0.53 min−1, c = 64.5 au, and r = 0.999. Result is from three fibers.
Figure 11.
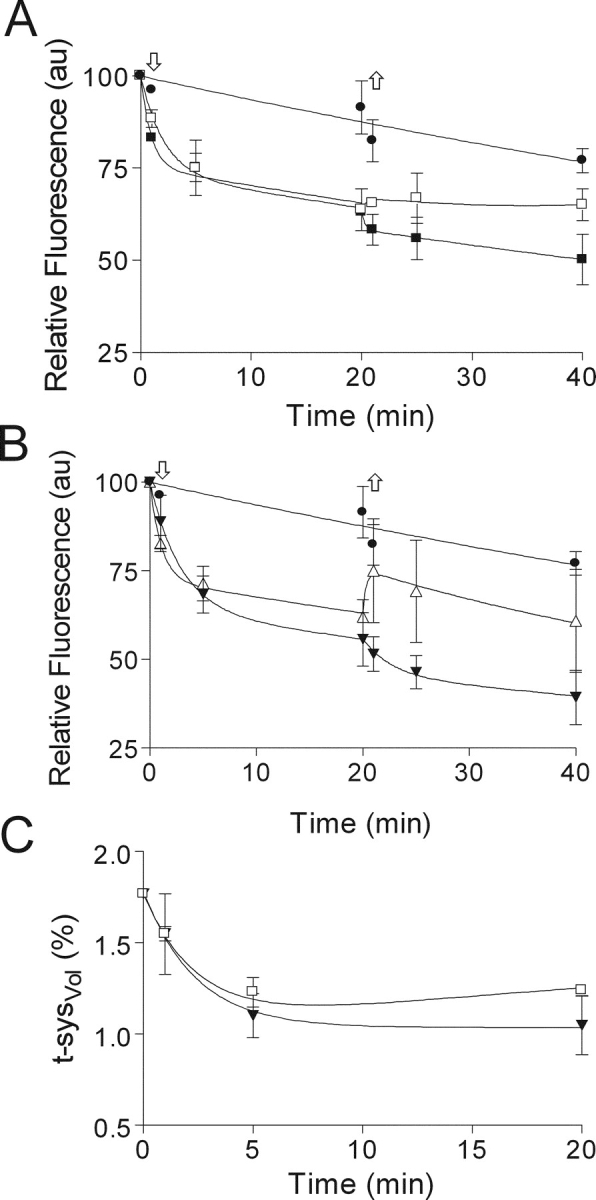
TABLE I.
| 0 ≤ t ≤ 20 min | 20 ≤ t ≤ 40 min | Corrected for dye loss 0 ≤ t ≤ 20 min |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| • | ▵ | ▾ | □ | ▪ | ▵ | ▾ | □ | ▪ | ▾ | □ | |
| k (min−1) | 0.007 ± 0.001 |
0.932 ± 0.410 |
0.348 ± 0.177 |
0.482 ± 0.316 |
1.07 ± 0.294 |
— | 0.376 ± 0.730 |
— | — | 0.421 ± 0.087 |
0.018 ± 0.002 |
| c (au) | — | 72.5 ± 2.79 |
64.1 ± 5.93 |
73.8 ± 4.07 |
75.4 ± 1.72 |
— | 45.7 ± 6.23 |
— | — | — | — |
| c (% t-sysVol) | — | — | — | — | — | — | — | — | — | 1.035 ± 0.038 |
— |
| β (au) | — | — | — | — | — | 12.36 ± 11.81 |
— | 3.9 ± 4.62 |
−4.71 ± 3.89 |
— | — |
| β (% t-sysVol) | — | — | — | — | — | — | — | — | — | 0.748 ± 0.051 |
— |
| δ | — | — | — | — | — | −0.04 ± 0.127 |
— | 0.05 ± 0.064 |
−0.001 ± 0.096 |
— | — |
| r | 0.856 | 0.867 | 0.968 | 0.916 | 0.899 | 0.245 | 0.558 | 0.078 | 0.548 | 0.998 | 0.998 |
Analysis of Results
In the text, mean values ± standard error are given; n is the number of fibers. Student's t-test was used to determine statistical significance (P) where appropriate. GraphPad software (Prism) was used to fit data to various functions.
RESULTS
t-system Volume and Structure in Skinned Fibers
Fig. 2 shows confocal images of a skinned fiber from toad with fluo-3 trapped in the sealed t-system. Fig. 2 A shows an image of a skinned fiber in standard myoplasmic K+ solution (255 mosmol kg−1) at low spatial resolution, where the banded fluorescence pattern representing the t-system is almost completely obscured by local swellings of the t-system (vesicles). We have described the phenomenon of vesicle formation in the sealed t-system of toad muscle in detail elsewhere (Launikonis and Stephenson, 2002b), pointing out that there is variability in the number of vesicles present in skinned fibers of toad (see higher resolution images in Lamb et al., 1995; Launikonis and Stephenson, 2001, 2002b; Launikonis et al., 2003). After the skinned fiber had been imaged under standard conditions, it was then exposed to anisosmotic myoplasmic solutions with osmotic concentrations of 446 and 2,550 mosmol kg−1 and allowed to equilibrate for ∼5 min before being imaged (Fig. 2, B and C, respectively). In the hypertonic solutions the banded fluorescence pattern of the t-system became more prominent compared with that in the presence of standard myoplasmic solution, as vesicles lost water and discharged their content into the t-system, suggesting that under our conditions the vesicles were in communication with the rest of the t-system (Launikonis and Stephenson, 2002b). Similarly, vacuoles that develop in intact muscle fibers during eccentric exercise were shown to maintain a direct connection with the tubular system (Yeung et al., 2002). Note also that neither the diameter nor the sarcomere spacing of the skinned fiber changed as the osmolality of the bathing solution was increased (Fig. 2).
When estimating the volume of the sealed t-system as a fraction of the total intact fiber volume (t-sysVol), we assumed an initial t-sysVol of 0.99% (t-sysVol of depolarized intact t-system; Launikonis and Stephenson, 2002a) as we expect the t-system to be depolarized by the process of skinning (Lamb and Stephenson, 1990). However, as vesicles form in skinned fibers from toad (Launikonis and Stephenson, 2002b) there must be an increase in the t-sysVol once the t-system seals. It was not possible to calculate t-sysVol in skinned fibers in a similar way as we described for intact fibers, as there is not a pool of dye solution (fluorescence maximum reference) around the fiber in equilibrium with the extracellular space within the fiber in skinned preparations as there is in intact ones (Launikonis and Stephenson, 2002a). To estimate the fraction of which regularly shaped t-tubules and vesicles contribute to the overall t-sysVol in its sealed state we compared the average of the fluorescence intensity along four lines perpendicular to the regularly shaped t-tubules (i.e., no vesicles) to the average fluorescence intensity along four random lines (through vesicles and regular t-tubules; averaging over 1,000 pixels) in images of the sealed t-system at high spatial resolution. We have previously presented such images of the sealed t-system (Figs. 6, A and E, Fig. 1 D, and Fig. 1 A in Launikonis and Stephenson, 2001, 2002b, and Launikonis et al., 2003, respectively). In the presence of standard myoplasmic K+ solution the ratio of the fluorescence intensity between the random lines and those specifically through regular t-tubules was 1.79 ± 0.17 (n = 9) and this ratio was not significantly different when measured in skinned fibers when the t-system was depolarized in the presence of a myoplasmic Na+ solution where all K+ in the standard myoplasmic solution was replaced with Na+ (1.76 ± 0.32, n = 3; t test, P > 0.5). Therefore, with the assumption that the regularly shaped t-tubules occupy 0.99% fiber volume before skinning, we used the factor 1.79 to correct for the increase in t-sysVol due to the formation of vesicles in the sealed t-system in the standard myoplasmic solution (see Fig. 4). With these assumptions, the isotonic t-sysVol in skinned fibers of toad is 1.77% of intact fiber volume in physiological solution of normal tonicity.
Figure 6.
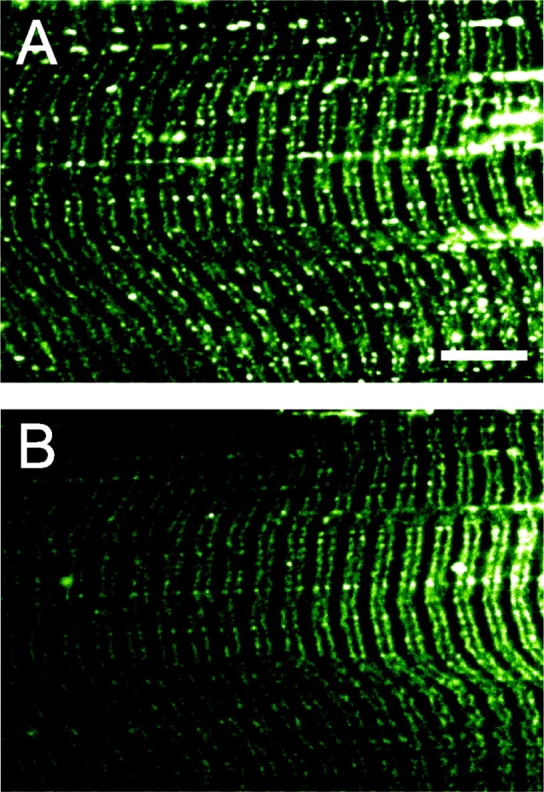
Effect of highly hypertonic solution on calcein fluorescence from the sealed t-system of a rat skinned fiber. The rat fiber was bathed in a myoplasmic solution with osmotic strengths of 280 (A) and 2800 (B) mosmol kg−1. There is little difference in the fluorescence signals in both images. Note the longitudinal connections between the t-tubules. Bar, 10 μm.
Figure 4.
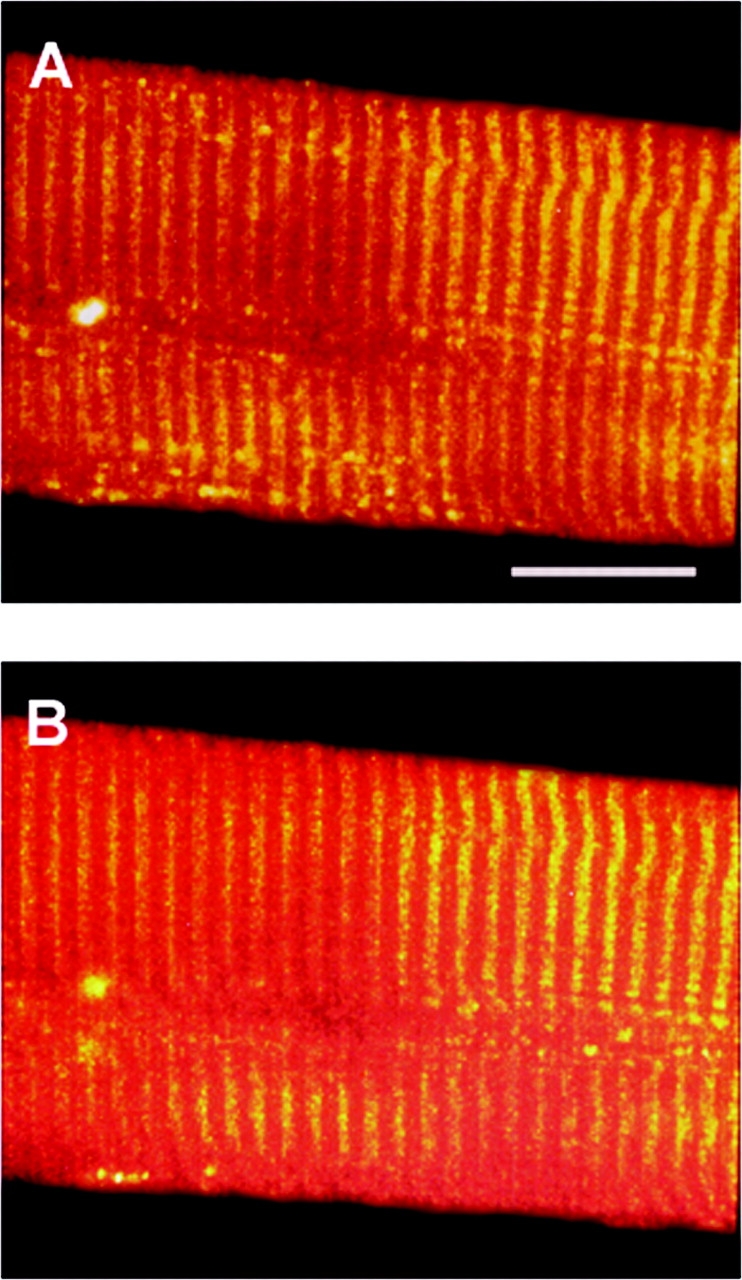
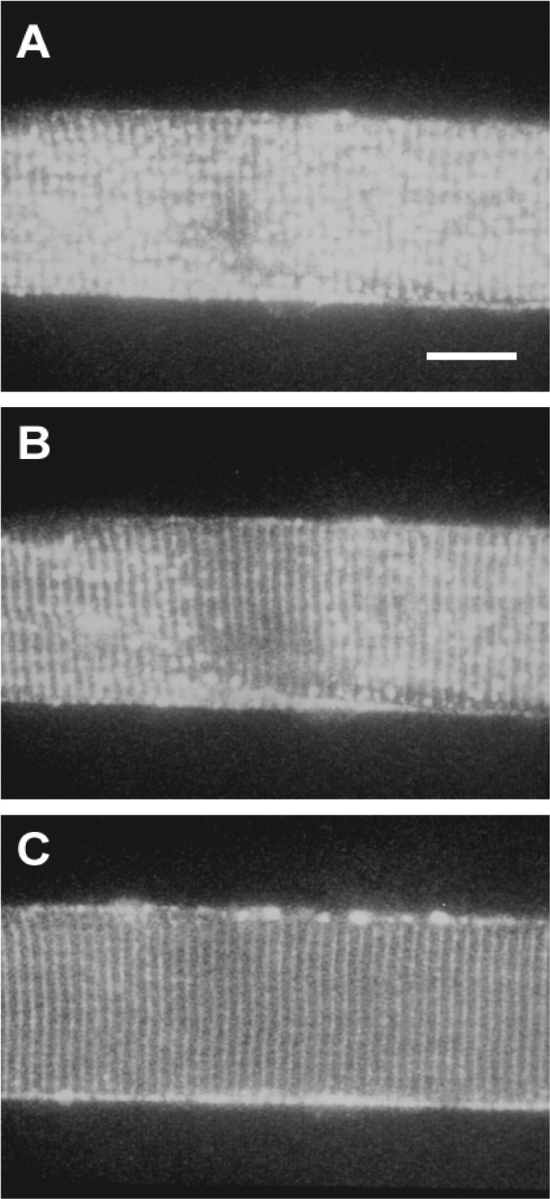
The effect of osmolality on the sealed t-system of rat fibers. Confocal images of a rat-skinned fiber exposed to myoplasmic solution of osmotic strengths: 280 (A) and 480 (B) mosmol kg−1. In B sucrose was added to the standard myoplasmic solution to increase osmolality and the image was taken after 5 min equilibration to the new solution. Note there is little difference between the images. Microscope settings are constant for both panels. Bar, 20 μm.
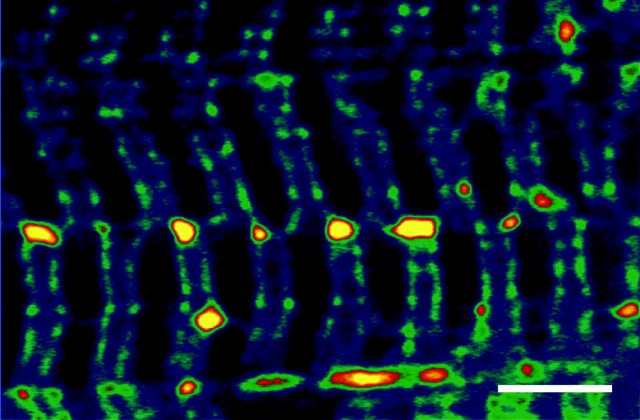
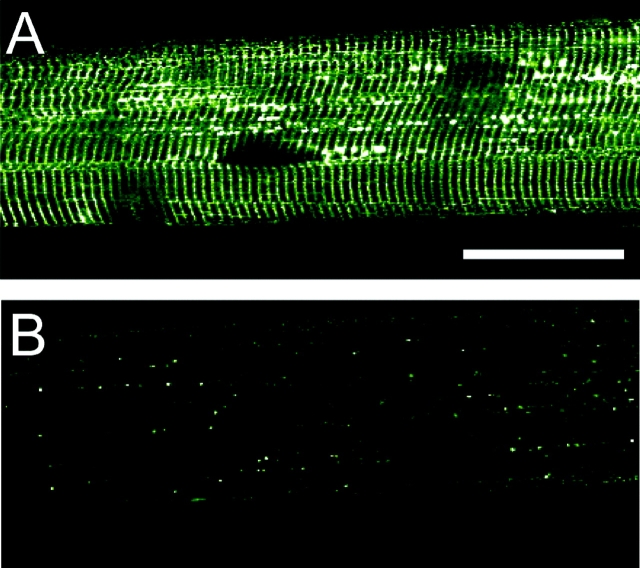
In Fig. 3 is an image of the sealed t-system of a rat skinned fiber loaded with 10 mM calcein bathed in standard myoplasmic solution. In this image the closer riws of t-tubules border the Z-lines and at a certain point in the fiber, the t-tubule on the left of the Z-lines moves to the right of the Z-lines in the adjacent sarcomeres (and vice versa). Furthermore, there appear to be elements that connect the t-tubules that border the Z-lines at the point of sarcomere misregistration, as large vesicles form at these junctions. Such t-system elements may not have the same rigid skeleton as the regular t-system, thus making them more prone to swelling. This orderly lateral shift of the t-system networks whereby t-tubule networks to the left of the Z-lines crossover to become t-tubule networks to the right of the Z-lines in the adjacent sarcomere (and vice versa) together with tubular elements that connect the t-tubule networks that border the Z-lines can provide the main pathway for longitudinal propagation of action potentials via t-system elements along the fiber axis, as shown previously by Posterino et al. (2000).
Figure 3.
Confocal image of the sealed t-system of rat loaded with calcein showing the shift of t-tubules from one sarcomere to an adjacent sarcomere. The closer rows of t-tubules border the Z-lines. Thus t-tubules to the left of the Z-lines crossover at a point of sarcomere misregistration to become t-tubules to the right of the Z-lines (at the midline of this image) and vice versa. Bar, 5 μm.
Other confocal images of skinned rat fiber with fluo-3 trapped in the sealed t-system are shown in Fig. 4. In this particular case, the double rows of the t-tubules that border the Z-lines could not be resolved. The fiber was first imaged in standard myoplasmic solution (280 mosmol kg−1; Fig. 3 A) and then 5 min after the myoplasmic solution was changed to one with an osmotic strength of 480 mosmol kg−1 (Fig. 4 B). Note there is little difference between the two images. Skinned EDL fibers of rat, in the most part, do not vesiculate upon skinning (although we do present some images here showing t-tubule swelling in isotonic solution), and we have presented this evidence elsewhere (Launikonis and Stephenson, 2002b). As the t-sysVol of intact rat fibers is very similar to that of toad fibers, we assigned the value of 0.99% to the sealed t-system of rat skinned fibers, which corresponds to the depolarized t-sysVol in intact toad fibers, as we expect the t-system to be depolarized upon skinning.
Volume Changes in the t-system as a Function of Osmolality
Volume changes in the sealed t-system can be estimated either from changes in the fluorescence intensity of calcein, a self-quenching dye, which produces a fluorescence signal independent of ionic strength (Jayaraman et al., 2001), or from changes in the fluorescence intensity of fluo-3, which is sensitive to changes in the ionic strength as shown in the materials and methods. To verify that the two methods lead to the same results for the conditions used in this study, the sealed t-system in amphibian and mammalian skeletal fibers was exposed to extreme conditions in solutions made highly hypertonic with sucrose and the results were then compared.
A representative result obtained with frog-skinned iliofibularis fibers loaded with 8.5 mM calcein is presented in Fig. 5. Fig. 5 A shows a strong fluorescence signal being emitted from the t-system in a myoplasmic solution at 255 mosmol kg−1. This fluorescence signal dropped markedly upon 5 min equilibration with a myoplasmic solution at 2,550 mosmol kg−1 (Fig. 5 B), indicating that significant quenching of the dye had occurred as the t-system was no longer clearly evident. Note that the microscope settings were held constant for imaging in both solutions for comparative purposes and that when the gain and zoom factor were increased that the t-tubules could be visualized, indicating that the t-system had not been disrupted in the solution at 2,550 mosmol kg−1 (unpublished data). On average, the fluorescence signal dropped to 41.1 ± 7.5% (n = 3) of the control value (corrected for a small amount of dye loss). Assuming a t-sysVol of 1.77% at 255 mosmol kg−1 for amphibian fibers and proportionality between volume change and calcein quenching (Jayaraman et al., 2001), this fluorescence signal translates to a t-sysVol of 0.73 ± 0.13% of intact fiber volume.
Figure 5.
Effect of highly hypertonic solution on calcein fluorescence from the sealed t-system of a frog skinned iliofibularis fiber. The frog fiber was bathed in a myoplasmic solution with osmotic strengths of 255 (A) and 2,550 (B) mosmol kg−1. Note the fluorescence signal drops significantly due to self-quenching of calcein in the presence of the highly hypertonic solution. The elliptically shaped dark regions in A probably indicate the position of nuclei in the fiber. Bar, 50 μm.
As already shown in Fig. 2, the fluorescence intensity of the fluo-3 trapped in the in the t-system of toad fibers also decreased significantly due to the net increase in ionic strength in the lumen of the t-system as water was removed. These changes in fluo-3 fluorescence intensity were converted into t-system volume changes using the calibration curve shown in Fig. 1 where different volumes of water were added to a constant mix of solutes mimicking the composition of the extracellular environment when the relative volume was equal to 1.0. From these calculations a value of 0.75 ± 0.04% (n = 6) of intact fiber volume was obtained when the osmolality was increased from 255 to 2,550 mosmol kg−1. This result is essentially the same as that obtained with calcein.
In rat fibers loaded with 10 mM calcein, there was little difference in the fluorescence signals when the osmolality was increased from 280 to 2,800 mosmol kg−1 (Fig. 6). In 2,800 mosmol kg−1 solution, the fluorescence signal dropped to only 93.2 ± 4.7% (n = 4) of the control value after the small correction for dye loss. This translates to a reduction in t-sysVol to 0.92 ± 0.05%, which is not different from the result obtained with fluo-3 (0.94 ± 0.15% intact fiber volume; n = 4).
Since the figures for both amphibian and rat t-system volume estimations in highly hypertonic myoplasmic solutions were essentially the same with either calcein or with fluo-3 (0.73 ± 0.13% vs. 0.75 ± 0.04% intact fiber volume for amphibian fibers and 0.92 ± 0.05% vs. 0.94 ± 0.15% intact fiber volume for rat fibers), one can conclude that the two methods provide the same results. This validates the assumptions made for the use of fluo-3 to estimate volume changes in the t-system, namely that little change in the total amount of electrically charged osmolites occurs in the t-system over a 5-min period even when subjected to very large osmotic forces. Note, however, that the signal to noise ratio is greater with 1 mM fluo-3 than with 8.5–10 mM calcein because there is a severalfold greater change in fluo-3 fluorescence intensity (Fig. 1) than in calcein fluorescence intensity (for calcein concentrations <10 mM that do not greatly alter the ionic and osmotic composition of the t-system under standard conditions; Jayaraman et al., 2001) with a change in volume over the same volume range. Therefore, most volume change measurements in this study were made with fluo-3 (1 mM) –loaded t-system.
As previously described (Launikonis and Stephenson, 2002b), fluo-3 intensity from the sealed t-system in toad and rat fibers decreases gradually over time in control experiments with rate constants of 0.0066 and 0.022 min−1, respectively (see e.g., Fig. 11 for toad fibers), due mainly to fluo-3 transport across the t-system (Launikonis and Stephenson, 2002b). This corresponds to a fluo-3 loss of 3.3 and 10%, respectively, after 5 min. Fig. 7, A and B, shows the summarized data for the change in the fluo-3 fluorescence intensity in toad and rat fibers after 5-min exposure to anisotonic solutions and after the application of the small correction due to fluo-3 transport across the t-system.
The t-system–related fluorescence intensity in toad fibers markedly decreased when the osmotic concentration in the myoplasmic environment was increased above 255 mosmol kg−1 and increased when the osmolality of the myoplasmic environment was decreased by 25% from 255 mosmol kg−1, consistent with water loss from the t-system and water entry into the t-system respectively (Fig. 7 A).
In contrast, there was only a very small decrease in fluorescence intensity when rat fibers were exposed to solutions of increased osmolality (Fig. 7 B), although when exposed to solutions of lowered osmolality (210 mosmol kg−1; 25% hypotonic) there was an increase in the fluorescence signal, similar to that occurring in toad fibers (data not shown; see Fig. 10 B).
Figure 10.
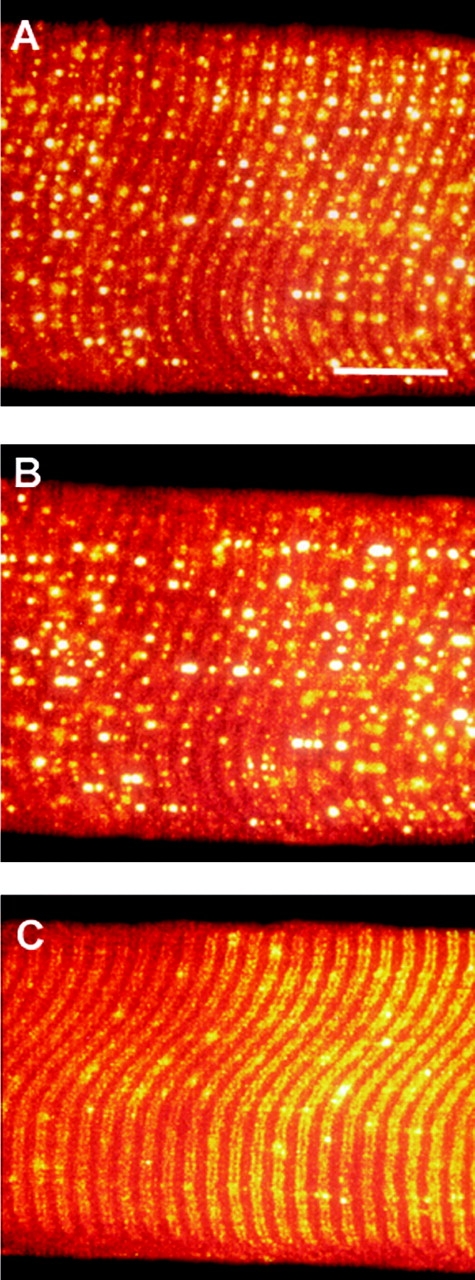
The relatively high permeability of the sealed t-system of rat for glycerol is apparent in hypotonic solution. Confocal images of a rat-skinned fiber exposed to standard myoplasmic solution of osmotic strength: 280 (A), 280 (B; 210 mM normal solutes + 70 mM glycerol), and 2,800 (C) mosmol kg−1. Images in B and C were taken after 5 min equilibration to the new solution. Note that the vesicles apparent in A become larger and brighter in B, indicating that water has moved into the sealed t-system. In C, water is drawn from the vesicles by the hypertonic solution. Bar, 20 μm.
The relative fluorescence intensities of fluo-3–loaded toad and rat fibers exposed for 5 min to anisotonic solutions are shown in Fig. 7, A and B, and these values were converted into t-sysVol values based on the calibration curve in Fig. 1 as described in materials and methods after correction for the small dye loss over 5 min (see above). The results for t-sysVol are shown in Fig. 7, C and D, assuming that the t-sysVol was 1.77 and 0.99% in the standard myoplasmic solution for toad and rat fibers, respectively (see above). From Fig. 7 C one can observe that the t-sysVol of toad skinned fibers varied from 2.32 ± 0.02% (n = 3) to 0.75 ± 0.04% (n = 6) of intact fiber volume over the range of osmolalities from 191 to 2550 mosmol kg−1, respectively. Fig. 7 C also shows that the sealed t-system could not be compressed below ∼0.75% of the total intact fiber volume as the osmotic concentration in the myoplasmic environment was increased for 5 min to 1,000 mosmol kg−1 and above. Experiments, where the skinned fibers were kept for up to 20 min in highly hyperosmotic solutions with sucrose confirmed that the t-system could not be compressed below 0.75 ± 0.05% of total intact fiber volume.
After 5 min exposure to anisosmotic solutions, the t-sysVol of rat fibers varied only little from 1.12 ± 0.04% (n = 4) to 0.94 ± 0.15% (n = 4) of intact fiber volume over the range of osmolalities from 210 to 2,800 mosmol kg−1 (Fig. 7 D). Increasing the time of exposure to highly hyperosmotic solutions for up to 20 min did not lead to further reductions in the estimated t-system volume, suggesting that the sealed t-system of rat EDL muscle fibers is relatively incompressible.
It was not possible to determine the response of the rat sealed t-system to 50% hypotonic solution (140 mosmol kg−1), as this solution caused a Ca2+-independent contracture (Stephenson, 1993), which resulted in fiber damage. Such a contracture did not occur in toad fibers exposed to 50% hypotonic solution. However, there was a drop in the fluo-3 fluorescence signal in this solution from toad fibers and this issue is addressed below.
50% Hypotonic Solutions Activate Ca2+-permeant Channels in Toad t-system
Fig. 1 predicts that an increase in t-system fluo-3 fluorescence should be observed if there is a net movement of water into the t-system (increase in t-sysVol). This was indeed the case when toad and rat fibers were placed in myoplasmic solutions that were 25% hypotonic compared with the normal solutions (Fig. 7, A and B). However, when the osmolality of the bathing solution was dropped by 50% in toad fibers from 255 to 127.5 mosmol kg−1, there was actually a decrease rather than an increase in fluo-3 fluorescence intensity. This decrease continued over a period of 5 min (Fig. 8). Clearly, the loss of fluo-3 fluorescence intensity in the sealed t-system of toad fibers when placed in a 50% hypotonic solution cannot be due to an increase in ionic strength. The only other factors that can explain this happening are marked loss of dye and/or loss of Ca2+ from the sealed t-system. To distinguish which of these two factors is mainly responsible for the drop in fluorescence, a control experiment was performed with Oregon green, a fluorescent dye that is not sensitive to Ca2+ and is much less sensitive to ionic strength than fluo-3 (over the range of 1.0–2.0 “volumes” in Fig. 1 the fluorescence intensity of Oregon green increased by <15% compared with an increase in maximum fluo-3 fluorescence intensity by >35%). Since there was no significant difference in the level of Oregon green fluorescence intensity after 5-min exposure of the toad fiber preparation with Oregon green in the sealed t-system to an isotonic or to a 50% hypotonic solution (74.6 ± 6.2% of control, n = 2 vs. 83.1 ± 6.1% of control, n = 3, respectively, t test, P = 0.42), one can conclude that the marked drop in fluo-3 fluorescence intensity when the skinned fiber preparation was exposed to 50% hypotonic solution cannot be due to significant loss of dye. This implies that [Ca2+] in the sealed t-system must have dropped to ∼1 μM to account for the decrease in fluo-3 fluorescence intensity by ∼25% (assuming a Kapp value for fluo-3 of 3.106 M−1). This would happen if Ca2+-permeant channels in the sealed t-system open and allow Ca2+ to flow out of the t-system into the myoplasmic environment down its electrochemical gradient.
Response of the Sealed and Intact Toad Fiber t-system to Osmotic Stress
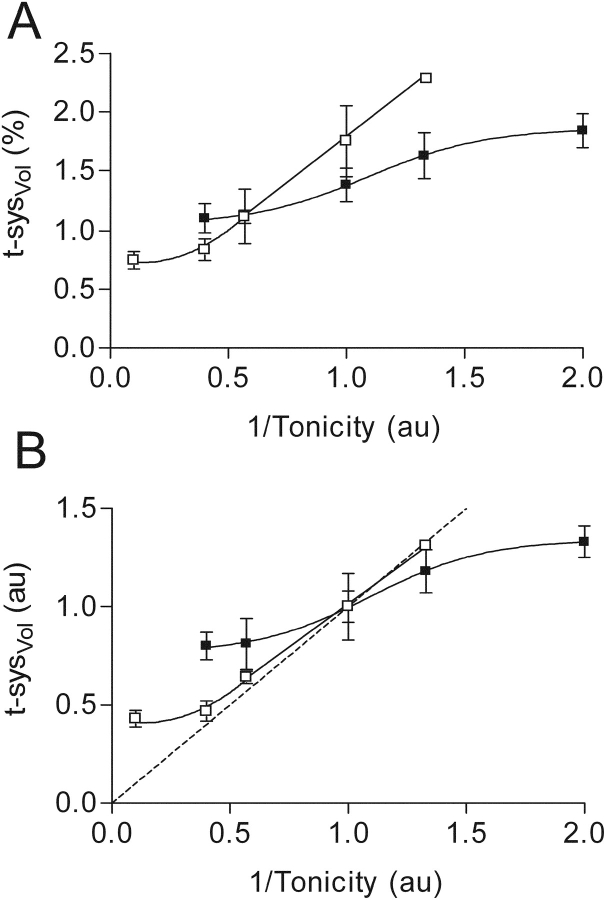
In Fig. 9 are compared the changes in t-sysVol of both intact and skinned toad fibers when the tonicity of the bathing solutions was altered. When t-sysVol is expressed as a percentage of isotonic fiber volume (Fig. 9 A), the greater ability of the sealed than of the intact t-system to change its volume over the range of tonicities is clearly shown. It is important to bear in mind that the isotonic t-sysVol is different in intact and skinned fibers (1.38 vs. 1.77%, respectively) because of the vesicle formation in the skinned toad fiber (see earlier). The intact and sealed t-systems in toad fibers are also different with respect to the t-sysVol they can be compressed to when the osmolality is increased to very high levels (∼0.9 and 0.75% isotonic intact fiber volume, respectively). Note that our estimation of the smallest possible t-sysVol in intact fibers was originally 0.9% when we interpreted the relationship between t-sysVol (relative to isotonic fiber volume) and the inverse of osmolality as linear (Fig. 4 B in Launikonis and Stephenson, 2002a), whereas the minimum t-sysVol appears to be closer to 1% and therefore this relationship should be regarded as sigmoidal (Fig. 9 A).
Figure 9.
The ability of the intact (▪) and sealed (□) t-system of toad fibers to change its volume in response to osmotic stress. t-sysVol expressed in absolute (A) and relative (B) terms as a function of the inverse of tonicity, where 1 unit of tonicity is 255 mosmol kg−1. The continuous lines represent the best fitted curves to the data points for t-sysVol as a function of tonicity in both cases. The intact t-system data is from Launikonis and Stephenson (2002a). The broken line in B represents the theoretical relationship between osmolality and volume in a system that acts as a perfect osmometer (i.e., t-sysVol ∞ 1/tonicity). Note also that the isotonic t-sysVol is 1.38 and 1.77% in intact and skinned fibers (A), respectively, but both have been assigned the value of 1 in B for comparative purposes.
To determine the ability of the sealed and intact t-system to change volume in relation to the change in bathing solution tonicity, t-sysVol is expressed in relative units against 1/tonicity (Fig. 9 B). This figure shows that the sealed t-system is able to change its volume close to the isosmotic line at 1/tonicity values >0.4. This behavior is very similar to the change in volume of the intact fiber over this range of tonicities (Blinks, 1965). In contrast, the t-system in intact fibers does not change its volume in proportion to the change in the osmotic concentration in the external environment because it remains in communication with the external environment.
Effect of Glycerol on the Sealed t-system of Rat
Since the results in Fig. 7 show that the rat t-system is relatively incompressible in hypertonic solution but can take up water when the myoplasmic solution was made 25% hypotonic, to examine its permeability to glycerol we exposed the rat sealed t-system to a 25% hypoosmotic solution with 70 mM added glycerol (210 mM normal solutes + 70 mM glycerol = 280 mosmol kg−1 (isoosmotic)). If glycerol were freely permeable, glycerol would act like water in the solution, causing an influx of glycerol followed by water into the sealed t-system and consequently an increase in fluo-3 fluorescence. However, if glycerol was not permeable or only very slowly permeable, then the myoplasmic solution would remain isotonic with the t-system lumen and there would be no net flux of water and no change in fluo-3 fluorescence. An example of this experiment is shown in Fig. 10. After 5-min exposure, the 25% hypoosmotic solution plus 70 mM glycerol caused an increase in t-system fluorescence compared with the standard solution. On average, t-system fluorescence increased to 114.6 ± 6.6% (n = 5; one-sided paired t test, P < 0.05) of the initial reading in standard solution. This shows that glycerol equilibrates within 5 min because the result is similar to the fluorescence increase in 25% hypotonic solution without glycerol (Fig. 4 D) and suggests that most of the glycerol equilibrated across the rat t-system within this time frame.
Effect of Glycerol on the Sealed t-system of Toad Fibers
In contrast to the rat fibers where glycerol appears to be relatively permeant across the t-system, experiments with toad fibers show that glycerol is rather impermeant across the t-system of amphibian muscle. The experiments on toad fibers are summarized in Fig. 11. We used a protocol similar to that employed by Krolenko et al. (1995) to elicit t-system vacuoles in intact fibers. This protocol was designed not only to determine the relative permeability of the t-system to glycerol, but also to examine the mechanisms behind vacuolation of the intact t-system.
In the presence of 200 and 400 mM added glycerol there was a large reduction in the intensity of the fluo-3 fluorescence signal emitted from the sealed t-system compared with controls. This reduction in the fluorescence signal persisted for 20 min indicating that glycerol is much less permeable than water, thus causing an efflux of water from the sealed t-system and a subsequent increase in ionic strength in the sealed tubules resulting in the fluo-3 fluorescence decrease. In preparations exposed to 200 mM glycerol, its removal from the myoplasmic medium sees a gradual recovery of the fluorescence signal toward the control values but no such recovery was observed in preparations previously exposed to 400 mM glycerol (Fig. 11 A; see discussion).
To help determine the permeability of glycerol across the sealed t-system membrane of toad we used sucrose in an experiment with the same protocol as that in Fig. 11 A. The summary of results is shown in Fig. 11 B. As expected, the fluorescence signal from the t-system was reduced by the presence of 100 or 200 mM sucrose. Upon removal of sucrose (immediately after the measurement at 20 min) there was an increase in the average fluorescence signal from the preparations previously exposed to 100 mM but not 200 mM sucrose (see discussion).
A further complexity in the kinetics in the fluorescence signal change in Fig. 11, A and B, is the response to the addition of sucrose or glycerol is the crossing-over of the curves before reaching equilibrium after ∼5 min. The shrinkage of the t-tubules (before equilibrium) would be dependent on the rate of glycerol/sucrose diffusion into the preparation, on the rate water is drawn out the t-tubules, and on the rate the t-tubular skeleton can change conformation to adjust to the new osmotic environment. In Fig. 11 A, at 1 min after addition of the hyperosmotic solution, shrinkage is faster in 400 mM glycerol than in 200 mM glycerol as it would normally be expected. In Fig. 11 B, however, the shrinkage appears to be smaller at 1 min after the addition of the 200 mM sucrose solution than after the addition of the 100 mM sucrose solution. Such a situation would occur if the interface between the 200 mosmol kg−1 sucrose solution and the standard solution in the preparation would be more stable than the interface between the 100 mosmol kg−1 sucrose solution and the standard solution as can happen with discontinuous sucrose gradients.
In Fig. 11 C are shown the effects of 200 mM glycerol and sucrose on estimated t-sysVol, which have been derived from the fluorescence signals in Fig. 11, A and B (corrected for fluo-3 loss from the t-system), and the calibrations in Figs. 1 and 7. The effect of 200 mM sucrose on t-sysVol shows a steady decrease, which plateaus after ∼5 min and remains constant. This result indicates that sucrose is indeed membrane impermeant, as a steady level is reached and maintained. The rate constant for the decrease of t-sysVol (ke) under these conditions is an effective (combined) rate constant for (a) sucrose diffusion into the skinned fiber preparation (but not into the t-system) from the surrounding medium, (b) water efflux from the sealed t-system, and (c) mechanical changes in the t-system structures that eventually determine the t-system volume. The data points for sucrose in Fig. 11 C could be well fitted by an exponential equation of type:
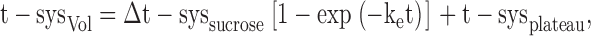 |
(1) |
where t-sysplateau represents t-sysVol at equilibration = 1.035% of intact fiber volume, Δt-syssucrose represents the difference between the initial and final t-sysVol = 0.748% of intact fiber volume, and ke represents the effective rate constant = 0.421 min−1.
The effect of 200 mM glycerol on t-system volume changes (Fig. 11 C) appears to be in a first approximation similar to that of 200 mM sucrose, implying that glycerol was rather impermeant to the t-system. Nonetheless, one can use the data points in Fig. 11 C to estimate the t-system permeability for glycerol considering that in contrast to sucrose, glycerol will gradually cross the tubular membranes and enter the t-system and this will reverse the osmotic gradient until eventually, the t-system volume returns to its initial value. Thus, in the presence of 200 mM glycerol there will be first a decrease in t-system volume associated with the loss of water due to the osmotic gradient generated by the presence of glycerol in the bathing environment but, as glycerol diffuses into the t-system, there will be a return of the t-system volume to its initial value as the osmotic gradient changes direction and water moves back into its lumen. The change in t-system volume in this case can be modeled by an expression containing two exponentials, one associated with a decrease in volume due to water loss caused by the presence of glycerol in the bathing environment and the other associated with an increase in volume due to glycerol entry and water influx into the t-system:
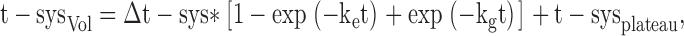 |
(2) |
where, Δt-sys represents the volume by which the t-sysVol decreases in the presence of 200 mM of an impermeant osmolite like sucrose; ke represents the effective rate constant for the decrease in t-sysVol as a result of the addition of 200 mM osmolite to the solution; kg is the rate constant for the volume changes associated with glycerol entry into the sealed t-system; and t-sysplateau represents the final t-sysVol after addition of 200 mM of an impermeant substance. Assuming that the values for ke, Δt-sys and t-sysplateau were those estimated from fitting Eq. 1 to the data points for 200 mM sucrose in Fig. 11 C (see also Fig. 7 C), the value of kg (0.018 ± 0.001 min−1) was then estimated by fitting Eq. 2 to the data points for glycerol in Fig. 11 C. Since ke is associated with osmolite diffusion into the preparation (but not into the sealed t-system) and with t-system volume changes subsequent to water fluxes across the t-system, and since ke is ∼20 fold greater than kg, it follows that the rate of glycerol entry across the t-system must be the dominant factor in determining the value of kg.
The rate of glycerol entry into the t-system of toad fibers can also be calculated in a different way from results in Fig. 11 C, where it is shown that after toad fibers were equilibrated with 200 mM glycerol for 20 min, the t-sysVol was compressed from 1.77 to 1.22% equivalent intact fiber volume. From these data one can calculate the [glycerol] in the t-system after 20 min, assuming that at this moment in time the content of the t-system was in quasiosmotic equilibrium with the environment. Thus, from the t-system volume ratio of 1.77/1.22 one can calculate that the solutes normally found in the t-system would contribute 1.77/1.22 × 255 = 370 mosmol kg−1 to the osmotic concentration in the t-system lumen, because the osmotic concentration associated with these solutes inside the sealed t-system should be inversely proportional to the t-sysVol (Figs. 7 and 9). Therefore, the concentration of normal solutes inside the t-system was 370 mosmol kg−1. Considering now that the osmolality of the t-system lumen should be close to that of the bathing solution (455 mosmol kg−1), it follows that [glycerol] in the t-system was close to 455 – 370 = 85 mosmol kg−1. Since the amount of glycerol in the t-system after 20 min represents 29.3% (85.4 mM at t-sysVol 1.22%) of all of the glycerol in the t-system at equilibrium (200 mM at t-sysVol 1.77%) one can estimate that the apparent rate constant (k g) for the diffusion of glycerol into the t-system was 0.017 min−1 (0.293 = 1 − exp(−kg*20 min)), which is essentially the same value as that obtained from fitting the data points in Fig. 11 C. Since the volume of the intact fiber is 98.23/1.77 greater than that of the sealed t-system, the estimated rate constant for glycerol entry into an intact fiber across the t-system is 1.77/98.23*0.017 = 3 × 10−4 min−1, which is very small indeed.
DISCUSSION
During the course of these experiments we observed t-tubules of rat fibers “crossing-over” from one sarcomere to an adjacent sarcomere (Fig. 3). This involves the t-tubule to the left of the Z-line becoming the t-tubule to the right of the Z-line in the adjacent sarcomere and vice versa. We are not aware of this trait of the mammalian skeletal muscle t-system being described previously. The only similar proposal has been made by Peachey and Eisenberg (1978), where they describe a helical arrangement of the t-system in frog skeletal muscle fibers.
This interesting observation of t-tubule “crossing-over”, as well as the presence of more longitudinal elements in the mammalian than amphibian t-system (Launikonis and Stephenson, 2002b), means the mammalian t-system is connected much more intimately and frequently along the fiber axis than the amphibian t-system. This observation is consistent with the better longitudinal propagation of action potentials in mammalian than in amphibian fibers (Posterino et al., 2000; Launikonis and Ríos, 2004).
Volume Changes in the Sealed t-system
This study shows that maximum fluo-3 Ca2+–induced fluorescence is sensitive to changes in the ionic strength of its environment and that it is very well suited in conjunction with confocal microscopy to measure changes in the ionic strength and associated volume changes in membrane-bound structures of much smaller dimensions than the optical resolution of the confocal microscope. The results unequivocally show that the estimated volume of the sealed t-system of toad muscle fibers changed in proportion to the reciprocal of tonicity of the myoplasmic environment (Fig. 8) for a broad range of osmolalities (190–638 mosmol kg−1) when the skinned fiber preparation was exposed to an anisosmotic solution for 5 min. This result in itself validates the method of t-system volume measurement because one could not obtain such behavior where the sealed t-system volume changed isosmotically with the tonicity of the extracellular solution unless the assumption that the total amount of solutes in the t-system changed little over the period of time when the measurements were made. Further support for the validity of the method is provided by (a) experiments in which sucrose was used under isosmotic conditions to reduce the myoplasmic ionic strength and ion concentration (see materials and methods) when even after 20 min in the presence of a myoplasmic solution made hyperosmotic with sucrose, the t-system volume shrunk to a value that was very close to the predicted value if there was no change in the total amount of osmolites within the sealed t-system over this time period (Fig. 10); and (b) in parallel experiments with the ionic strength independent dye, calcein, which yielded the same calculated t-sysVols in highly hypertonic solutions as fluo-3 (Figs. 5 and 6).
The osmotic behavior of the t-system of toad fibers when the myoplasmic osmolality was changed between 190 and 638 mosmol kg−1 is very similar to that displayed by intact frog fibers when the osmolality of the external environment was changed over the same range (Blinks, 1965). This behavior is explained by water movements across the membranes of a closed compartment, since in such a system water is drawn in to or out of the compartment depending on the osmotic gradient across the membranes. As the amount of water in the closed compartment is incompressible, the net volume of water within that compartment effectively determines the volume of the compartment. This is in distinct contrast to the behavior of an open compartment such as the t-system in an intact fiber (Fig. 9; Launikonis and Stephenson, 2002a), where extracellular fluid is able to flow relatively freely in and out of the t-system and the change in t-sysVol in hypotonic and hypertonic solutions is basically dependent on the change in total fiber volume (Blinks, 1965) and on the forces exerted on the tubular membranes (Fig. 9; Launikonis and Stephenson, 2002a).
There was a lower limit to which the sealed t-system volume could change in both toad and rat fibers in response to increased myoplasmic osmolality. Thus, the sealed t-system could not be compressed within 5 min by more than ∼45–55% in toad fibers (Fig. 7 C) and by more than 5% in rat fibers (Fig. 7 D) under conditions of high osmolality (>1,000 mosmol kg−1). These lower limits remained essentially unchanged even after waiting for 20 min in myoplasmic solutions that were 10 times hyperosmotic compared with standard myoplasmic solutions. Note that under such conditions all vesicles associated with the t-system are compressed and the pattern of the t-system becomes very regular (Figs. 2, 6, and 10). A possible explanation for the limit to which the t-system can be compressed at high osmolalities is that the tubules possess a structural skeleton that acts as a shell preventing them from collapsing and becoming compressed below a certain volume. Indeed, there is evidence that the t-system contains glycoproteins (Dörrscheidt-Käfer, 1979) and proteoglycans (Davis and Carlson, 1995) that can form the basis of the tubular skeleton that must also be responsible for maintaining the t-system at fixed locations at the level of the sarcomere. Also the greater rigidity of the t-system in the rat than in toad muscle is most likely due to the smaller diameter and presence of tethers in t-tubules of rat muscle (Dulhunty, 1984), which would allow it to withstand greater pressures without deformation for the same mechanical tubular skeleton characteristics. The tubular skeleton would be lined on the myoplasmic side by the tubular membrane, which, if permeant to water but not to solutes, would need to withstand large hydrostatic pressure differences to halt osmosis in face of the large osmotic gradient. This is entirely possible if the tubular skeleton would act as a surface (S-) layer supporting the lipid bilayer of the tubular membrane, because it is known that under such circumstances the mechanical stability of the supported lipid bilayer can be increased (Schuster and Sleytr, 2002). Assuming that the supported lipid bilayer can withstand a maximal induced tension of ∼4.3 mN/m when the pressure gradient is exerted from the lipid bilayer-faced side of the structure (Schuster and Sleytr, 2002), one can calculate that the lipid bilayer would be able to withstand a hydrostatic pressure difference of 1 MN m−2 (∼10 atmospheres) if the diameter of the pores in the supporting surface layer was <17.2 nm (pressure difference * area of the pore < surface tension * circumference of the pore). The size of such “pores” in the tubular skeleton is likely much smaller than 17 nm considering that the diameter of the t-tubules themselves is in the order of 10–50 nm (Dulhunty, 1984). It is also possible that the permeability of the tubular membrane for water decreases markedly as the stress on the lipid bilayer increases (Soveral et al., 1997), so that the system is far from reaching osmotic equilibrium after 20 min exposure to very high osmolality solutions.
Another possible explanation for restricting the volume to which the t-system can shrink under hypertonic conditions would be that the t-tubules come to osmotic equilibrium because of nonideal osmotic behavior of proteins lining its walls. However, if this were the case it is difficult to see how the ionic environment in tubules of either rat or toad fibers to which fluo-3 should be sensitive appears not to have changed as the osmotic concentration around the tubules was increased several fold above 1,000 mosmol kg−1 (Fig. 7).
In absolute terms the sealed t-system of toad fibers in standard solution appears to have a volume that is greater than that displayed by intact fibers due to the formation of vesicles that remain in communication with the t-system (see text related to Fig. 2). We have calculated that the vesicles that appear upon skinning in toad fibers increase the t-sysVol by nearly 30% (from 1.38 to 1.77% intact fiber volume). There is variability in the number of vesicles appearing in confocal images in various regions of the fiber, but generally all skinned fibers from toad formed vesicles in their t-tubular network. However, the increase in t-sysVol upon skinning is only a relatively small fraction of the potential increase in the t-system volume because it has been reported that the t-system in amphibian skeletal muscle fibers can increase up to 10–15% of intact fiber volume when fully vacuolated (Krolenko and Lucy, 2001).
Activation of Ca2+ Channels in the Toad t-system by Hyposmotic Changes
The conclusion derived in results that exposure of the sealed toad t-system to 50% hyposmotic solutions causes Ca2+ loss from the t-system is further supported by results summarized in Fig. 11. In these experiments, preparations exposed for 20 min to standard myoplasmic solutions containing, in addition, 100 mM sucrose or 200 mM glycerol, could sustain a significant increase in the fluo-3 fluorescence signal from the sealed t-system upon their return to the standard myoplasmic solution, but this was not the case for preparations exposed for 20 min to myoplasmic solutions with 200 mM sucrose or 400 mM glycerol. The removal of 100 mM sucrose or 200 mM glycerol causes an increase in t-sysVol of ∼25% (1.32–1.77% and 1.22–1.54%, respectively, as calculated from Figs. 7 and 11). This is consistent with the result in Fig. 7, where decreasing the osmolality of the myoplasmic solution by 25% caused an increase in t-sysVol by 30% (1.77–2.30%; Fig. 7), with no net loss of t-system ions and thus an increase in the fluorescence signals. In contrast, when 200 mM sucrose or 400 mM glycerol were removed from the myoplasmic environment after 20 min equilibration, there was a marked decrease in fluo-3 fluorescence. This again indicates that a 40–50% decrease in osmolality causes the opening of ion channels in the t-system that allow Ca2+ to escape from the t-system.
We must note here that changing the myoplasmic osmolality not only changes t-sysVol but will also affect SR volumes. Thus, one mechanism that would cause loss of t-system ions in myoplasmic solutions made >40% hypotonic would involve store-operated Ca2+-channels (SOCs) that become activated as a result of decrease in [Ca2+] in the SR. We have recently shown that SOCs are functional in the sealed t-system of toad fibers (Launikonis et al., 2003) and observed marked SR Ca2+ loss upon transfer of the preparation from a solution of normal osmolality to a 50% hypotonic solution (unpublished data).
The other mechanism of t-system Ca2+ loss would involve the opening of stretch-activated ionic channels (Wong and Chase, 1986; Wong et al., 1990; Filipovic and Sackin, 1991; Sackin, 1995) that would become activated when a significant volume of water enters the t-system, stretching its membranes. Such stretch-activated ionic channels that are permeant for Ca2+ play an important role in cell volume regulation (Wong et al., 1990) and are known to exist in skeletal muscle fibers (Franco-Oberon and Lansman, 1994). Since the volume of the t-system is known to increase by many times more than 50% in vacuolated muscle fibers, this increase in t-system volume would be expected to activate the stretch-activated ion channels in the t-system (Krolenko and Lucy, 2001) and cause the muscle fiber to contract. However, no contraction is observed under such conditions (Krolenko and Lucy, 2001), suggesting that it is the SOC channels rather than the stretch-activated channels that are responsible for Ca2+ loss from the t-system under more severe hypotonic conditions.
Permeability of the Sealed t-system to Glycerol
This study has conclusively shown that there is a significant difference between the permeability of the t-system of rat and toad skeletal muscle fibers to glycerol. Analysis of fluorescence signals emitted from the sealed t-system of rat fibers suggests that glycerol permeates and equilibrates across the t-system membrane on a timescale of seconds to minutes (Fig. 10). In contrast, the same concentration of glycerol could not equilibrate across the t-system membrane of toad fibers within 20 min (Fig. 11). This implies that there must be at least one order of magnitude difference in the rates of permeation of glycerol across the t-system of toad and rat fibers. Since the lower volume/surface area ratio in rat than toad sealed t-system (Dulhunty, 1984; Launikonis and Stephenson, 2002a) can account for only a factor of ∼2 for differences between the rates of glycerol permeation across the t-system, it follows that the permeability of the t-system membrane for glycerol is larger in rat than in toad fibers.
From Fig. 1 in Krolenko and Lucy (2001), one can estimate a rate constant for glycerol entry into intact frog fibers (kg) of 0.08 min−1. Taking into consideration that the volume to surface area of the sarcolemma (without the t-system) is ∼25 μm for a fiber of 100 μm in radius, this translates into an apparent permeability coefficient of the sarcolemma for glycerol of ∼66 nm s−1, which is similar to the permeability coefficient for glycerol of lipid bilayers (Orbac and Finkelstein, 1980; Paula et al., 1996). In contrast, the permeability coefficient of the t-system for glycerol in toad fibers is estimated at only 0.014 nm s−1, considering the rate constant of glycerol entry in the t-system measured in this study (0.018 min−1) and a volume to surface ratio of ∼50 nm (Dulhunty, 1984; Launikonis and Stephenson, 2002a). Thus, our results indicate that the t-system of anuran fibers is rather impermeant to glycerol compared with the sarcolemma and show that the t-system differs from the sarcolemma not only with respect to permeability coefficients to ions (e.g., Cl−, K+; Gage and Eisenberg, 1969; Dulhunty, 1979; Coonan and Lamb, 1998), but also with respect to permeability coefficients of uncharged molecules like glycerol. The larger difference with respect to glycerol permeability of the t-system and sarcolemma in amphibian muscle than in mammalian muscle is most likely due to the different distribution of aquaglycerolporins (Borgnia et al., 1999), which are members of the aquaporin family of channels that are able to pass water and glycerol. The aquaporins play a major role in osmoregulation and therefore they regulate water movement across the surface membranes.
Regarding the mammalian fibers, results in this paper show that the t-system of rat fibers are much more permeant to glycerol than the t-system of toad fibers. Since glycerol equilibration in the sealed t-system of rat fibers occurs on a time scale of seconds to minutes, the rate constant for glycerol equilibration must be >0.6 min−1. This value of 0.6 min−1 would translate into a rate constant for glycerol permeation at the level of the intact fiber of 0.006 min−1, considering that the volume of the t-system is only 1% of the total fiber volume. A rate of 0.006 min−1 would still be one order of magnitude smaller than the expected rate of glycerol equilibration in intact fibers. Also considering that some aquaporins are localized at the level of the sarcolemma and not in the t-system (Frigeri et al., 1998), it is possible that, like in the amphibian t-system, the permeability to glycerol of the t-system membrane in mammalian fibers may also be considerably smaller than that of the sarcolemma.
The notable differences with respect to vacuole formation between mammalian and amphibian muscles (Davey et al., 1980; Krolenko et al., 1995; Lännergren et al., 1999, 2000) can be related to differences in permeability of the t-system to various uncharged molecules and ions between the two classes of animals as shown in this study.
In summary, this study shows that the t-system membranes have exceptional properties with regard to glycerol transport. How such low t-system membrane permeability for glycerol can be achieved remains to be determined, but a possibility would be that the tubular lining of the toad t-system forms a coat that covers the lipid bilayer and is impermeant to glycerol.
Concluding Remarks
We have shown that the mechanically skinned muscle fibers are a viable means of studying transport processes across the t-system in situ with full experimental control over the intracellular environment. The results show that there are large differences with respect to t-system behavior to changes in myoplasmic osmotic concentration and glycerol movement across the t-system in rat and toad muscle fibers that can explain previous observations regarding reversible vacuolation that is thought to be important to a number of processes in muscle fibers and other cells (see Krolenko and Lucy, 2001).
Acknowledgments
We are grateful to Eduardo Ríos (Rush University Medical Center, Chicago) for allowing us to perform several experiments in his laboratory.
We also thank the ARC and NHMRC of Australia for financial support.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: EDL, extensor digitorum longus; SOC, store-operated Ca2+-channel.
References
- Blinks, J.R. 1965. Influence of osmotic strength on cross-section and volume of isolated single muscle fibres. J. Physiol. 177:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgnia, M., S. Nielsen, A. Engel, and P. Agre. 1999. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 68:425–458. [DOI] [PubMed] [Google Scholar]
- Coonan, J.R., and G.D. Lamb. 1998. Effect of transverse-tubular chloride conductance on excitability in skinned skeletal muscle fibres of rat and toad. J. Physiol. 509:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey, D.F., A.F. Dulhunty, and D. Fatkin. 1980. Glycerol treatment in mammalian skeletal muscle. J. Membr. Biol. 53:223–233. [DOI] [PubMed] [Google Scholar]
- Davis, A.K., and S.S. Carlson. 1995. Proteoglycans are present in the transverse tubule system of skeletal muscle. Matrix Biol. 14:607–621. [DOI] [PubMed] [Google Scholar]
- Dörrscheidt-Käfer, M. 1979. Excitation-contraction coupling in frog sartorius and the role of surface charge due to the carboxyl group of sialic acid. Pflugers Arch. 380:171–179. [DOI] [PubMed] [Google Scholar]
- Dulhunty, A.F. 1979. Distribution of potassium and chloride permeability over the surface and T-tubule membrane of mammalian skeletal muscle. J. Membr. Biol. 45:293–310. [DOI] [PubMed] [Google Scholar]
- Dulhunty, A.F. 1984. Heterogeneity of t-tubule geometry in vertebrate skeletal muscle fibres. J. Muscle Res. Cell Motil. 5:333–347. [DOI] [PubMed] [Google Scholar]
- Eisenberg, B.R., and R.S. Eisenberg. 1968. Selective disruption of the sarcotubular system in frog sartorius fibres. A quantitative study with exogenous peroxidase as a marker. J. Cell Biol. 39:451–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic, D., and S. Sackin. 1991. A calcium-permeable stretch-activated cation channel in renal proximal tubule. Am. J. Physiol. 260:F119–F129. [DOI] [PubMed] [Google Scholar]
- Flucher, B.E., M. Terasaki, H. Chin, T.J. Beeler, and M.P. Daniels. 1991. Biogenesis of transverse tubules in skeletal muscle in vitro. Dev. Biol. 145:77–90. [DOI] [PubMed] [Google Scholar]
- Franco-Oberon, A., Jr., and J.B. Lansman. 1994. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J. Physiol. 481:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri, A., G.P. Nicchia, J.M. Verbavatz, G. Vaenti, and M. Svelto. 1998. Expression of aquaporin-4 in fast-twitch mammalian skeletal muscle. J. Clin. Invest. 102:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino, M., T. Yamaguchi, and K. Suzuki. 1961. Glycerol effect” and the mechanism linking excitation of the plasma membrane with contraction. Nature. 192:1159–1161. [DOI] [PubMed] [Google Scholar]
- Gage, P.W., and R.S. Eisenberg. 1969. Ionic conductances of the surface and transverse tubular membranes of frog sartorius fibers. J. Gen. Physiol. 53:279–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins, A.B., N. Kurebayashi, and S.M. Baylor. 1993. Resting myoplasmic free calcium in frog skeletal muscle fibers estimated with fluo-3. Biophys. J. 65:865–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, S., Y. Song, and A.S. Verkman. 2001. Airway surface liquid osmolality measured using fluorophore-encapsulated liposomes. J. Gen. Physiol. 117:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolenko, S.A., W.B. Amos, and J.A. Lucy. 1995. Reversible vacuolation of the transverse tubules of frog skeletal muscle: a confocal fluorescence microscopy study. J. Muscle Res. Cell Motil. 16:401–411. [DOI] [PubMed] [Google Scholar]
- Krolenko, S.A., and J.A. Lucy. 2001. Reversible vacuolation of T-tubules in skeletal muscle: mechanisms and implications for cell biology. Int. Rev. Cytol. 202:243–298. [DOI] [PubMed] [Google Scholar]
- Lamb, G.D., P.R. Junankar, and D.G. Stephenson. 1995. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J. Physiol. 489:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, G.D., and D.G. Stephenson. 1990. Calcium release in skinned fibres of the toad by transverse tubule depolarization or by direct stimulation. J. Physiol. 423:495–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, G.D., D.G. Stephenson, and G.J. Stienen. 1993. Effects of osmolality and ionic strength on the mechanism of Ca2+ release in skinned skeletal muscle fibres of the toad. J. Physiol. 464:629–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren, J., J.D. Bruton, and H. Westerblad. 1999. Vacuole formation in fatigued single muscle fibres from frog and mouse. J. Muscle Res. Cell Motil. 20:19–32. [DOI] [PubMed] [Google Scholar]
- Lännergren, J., J.D. Bruton, and H. Westerblad. 2000. Vacuole formation in fatigued skeletal muscle fibres from frog and mouse: effects of extracellular lactate. J. Physiol. 526:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio, F.A., Jr. 1990. The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem. Biophys. Res. Commun. 171:102–108. [DOI] [PubMed] [Google Scholar]
- Launikonis, B.S., M. Barnes, and D.G. Stephenson. 2003. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. USA. 100:2941–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis, B.S., and E. Ríos. 2004. Ca2+ release events in mechanically skinned skeletal muscle fibres induced by spontaneous action potentials. Biophys. J. 86:578a. [Google Scholar]
- Launikonis, B.S., and D.G. Stephenson. 1999. Effects of β-escin and saponin on the transverse-tubular system and sarcoplasmic reticulum membranes of rat and toad skeletal muscle. Pflugers Arch. 437:955–965. [DOI] [PubMed] [Google Scholar]
- Launikonis, B.S., and D.G. Stephenson. 2001. Effects of membrane cholesterol manipulation on excitation-contraction coupling in skeletal muscle of the toad. J. Physiol. 534:71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis, B.S., and D.G. Stephenson. 2002. a. Tubular system volume changes in twitch fibres from toad and rat skeletal muscle assessed by confocal microscopy. J. Physiol. 538:607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis, B.S., and D.G. Stephenson. 2002. b. Properties of the vertebrate skeletal muscle tubular system as a sealed compartment. Cell Biol. Int. 26:921–929. [DOI] [PubMed] [Google Scholar]
- Melzer, W., A. Herrmann-Frank, and H.C. Lüttgau. 1995. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1241:59–116. [DOI] [PubMed] [Google Scholar]
- Minta, A., J.P. Kao, and R.Y. Tsien. 1989. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 264:8171–8178. [PubMed] [Google Scholar]
- Orbac, E., and A. Finkelstein. 1980. The nonelectrolyte permeability of planar lipid bilayer membranes. J. Gen. Physiol. 75:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula, S., A.G. Volkov, A.N. Van Hoek, T.H. Haines, and D.W. Deamer. 1996. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys. J. 70:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey, L.D., and B.R. Eisenberg. 1978. Helicoids in the T system and striations of frog skeletal muscle fibres seen by high voltage electron microscopy. Biophys. J. 22:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino, G.S., G.D. Lamb, and D.G. Stephenson. 2000. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J. Physiol. 527:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørtenblad, N., and D.G. Stephenson. 2003. A novel signalling pathway originating in mitochondria modulates rat skeletal muscle membrane excitability. J. Physiol. 548:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin, H. 1995. Mechanosensitive channels. Annu. Rev. Physiol. 57:333–353. [DOI] [PubMed] [Google Scholar]
- Schuster, B., and U.B. Sleytr. 2002. The effect of hydrostatic pressure on S-layer-supported lipid membranes. Biochim. Biophys. Acta. 1563:29–34. [DOI] [PubMed] [Google Scholar]
- Soveral, G., R.T.I. Macey, and T.F. Moura. 1997. Membrane stress causes inhibition of water channels in brush border membrane vesicles from kidney proximal tubule. Biol. Cell. 89:275–282. [PubMed] [Google Scholar]
- Stephenson, D.G. 1993. The length sensor in the sarcomere is functional in the virtual absence of Ca2+ (pCa > 10). XXXII Congress of IUPS. 25:69.4. [Google Scholar]
- Stephenson, D.G., and G.D. Lamb. 1993. Visualisation of the transverse tubular system in isolated intact and in mechanically skinned muscle fibres of the cane toad by confocal laser scanning microscopy. J. Physiol. 459:15P. [Google Scholar]
- Ríos, E., M.D. Stern, A. Gonzalez, G. Pizarro, and N. Shirokova. 1999. Calcium release flux underlying Ca2+ sparks of frog skeletal muscle. J. Gen. Physiol. 114:31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D., S.C. Tovey, T.J. Collins, M.D. Bootman, M.J. Berridge, and P. Lipp. 2000. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 28:213–223. [DOI] [PubMed] [Google Scholar]
- Wong, S.M.E., and H.S. Chase, Jr. 1986. Role of intracellular calcium in cellular volume regulation. Am. J. Physiol. 250:C841–C852. [DOI] [PubMed] [Google Scholar]
- Wong, S.M.E., M.C. DeBell, and H.S. Chase, Jr. 1990. Cell swelling increases intracellular free [Ca] in cultured toad bladder cells. Am. J. Physiol. 258:F292–F296. [DOI] [PubMed] [Google Scholar]
- Yeung, E.W., C.D. Balnave, H.J. Ballard, J.P. Bourreau, and D.G. Allen. 2002. Development of T-tubular vacuoles in eccentrically damaged mouse muscle fibres. J. Physiol. 540:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]