Abstract
ROMK channels are regulated by internal pH (pHi) and extracellular K+ (K+ o). The mechanisms underlying this regulation were studied in these channels after expression in Xenopus oocytes. Replacement of the COOH-terminal portion of ROMK2 (Kir1.1b) with the corresponding region of the pH-insensitive channel IRK1 (Kir 2.1) produced a chimeric channel (termed C13) with enhanced sensitivity to inhibition by intracellular H+, increasing the apparent pKa for inhibition by ∼0.9 pH units. Three amino acid substitutions at the COOH-terminal end of the second transmembrane helix (I159V, L160M, and I163M) accounted for these effects. These substitutions also made the channels more sensitive to reduction in K+ o, consistent with coupling between the responses to pHi and K+ o. The ion selectivity sequence of the activation of the channel by cations was K+ ≅ Rb+ > NH4 + >> Na+, similar to that for ion permeability, suggesting an interaction with the selectivity filter. We tested a model of coupling in which a pH-sensitive gate can close the pore from the inside, preventing access of K+ from the cytoplasm and increasing sensitivity of the selectivity filter to removal of K+ o. We mimicked closure of this gate using positive membrane potentials to elicit block by intracellular cations. With K+ o between 10 and 110 mM, this resulted in a slow, reversible decrease in conductance. However, additional channel constructs, in which inward rectification was maintained but the pH sensor was abolished, failed to respond to voltage under the same conditions. This indicates that blocking access of intracellular K+ to the selectivity filter cannot account for coupling. The C13 chimera was 10 times more sensitive to extracellular Ba2+ block than was ROMK2, indicating that changes in the COOH terminus affect ion binding to the outer part of the pore. This effect correlated with the sensitivity to inactivation by H+. We conclude that decreasing pHI increases the sensitivity of ROMK2 channels to K+ o by altering the properties of the selectivity filter.
Keywords: ROMK2, IRK1, renal K secretion, Xenopus oocytes, transmembrane helix
INTRODUCTION
ROMK channels from the kidney are regulated by intracellular pH; acidification of the cytoplasm below pH 7.0 results in a sharp decrease in whole-cell conductance and in single-channel open probability through a cooperative process with Hill coefficients greater than two (Tsai et al., 1995; Fakler et al., 1996; Choe et al., 1997; McNicholas et al., 1998; Chanchevalap et al., 2000). This response probably accounts for decreases in renal K secretion known to occur during acidosis (Malnic et al., 2000). A lysine residue near the NH2 terminus (K61 in ROMK2), presumably within the first transmembrane-spanning domain, is critical for this response, and may be the site whose ionization state controls the channel's function (Fakler et al., 1996; Choe et al., 1997; Schulte et al., 1999). Other amino acids, both in the NH2 terminus and the COOH terminus, have been shown to modulate the response by shifting the apparent pKa (Choe et al., 1997; Schulte et al., 1999; Chanchevalap et al., 2000).
These channels can also be closed by decreasing the concentration of K+ in the extracellular fluid (Doi et al., 1996; Sackin et al., 2001). This is a property also shared by some voltage-gated K channels in which slow, “C-type” inactivation is promoted by low extracellular K+ concentrations (Lopez-Barneo and Aldrich 1993; Liu et al., 1996; Starkus et al., 1997). In ROMK, the effects of extracellular K+ and intracellular pH are interdependent; decreasing K+ o makes the channels more sensitive to pHi (Doi et al., 1996; Sackin et al., 2003). Furthermore, mutation of the same critical lysine (K61 of ROMK2) abolishes K+ o sensitivity (Schulte et al., 2001; Sackin et al., 2003).
Interactions between pH and K+ effects may be explained by conformational changes that propagate from the inside aspect of the channel protein to the outside or vice versa (Schulte et al., 2001). An alternative mechanism has also been proposed in which the effects of external cations are exerted through occupancy of the selectivity filter, and are coupled to intracellular events through changes in the accessibility of the filter to internal K+ (Zhou et al., 2001). According to this scheme, lowering intracellular pH could close the channels from the inside, prevent entry of intracellular K+ to the selectivity filter, and make the channels more sensitive to external K+ removal (Sackin et al., 2003).
In this study we report that the pHi and K+ o responses of ROMK2 are also modulated by the COOH-terminal portion of the second transmembrane domain. Interactions of these sites with NH2-terminal portions of the protein may determine the stability of the open and/or closed states of the channels. We have used a ROMK2/IRK1 chimera that has enhanced sensitivity to pHi and K+ o and also exhibits strong inward rectification to examine the possible coupling mechanisms. Our results suggest that blocking access of internal K+ to the pore is not sufficient to explain the observed coupling between pHi and K+ o. We propose that reducing intracellular pH alters the ion-binding properties of the selectivity filter.
MATERIALS AND METHODS
Construction of Chimeras and Point Mutants of ROMK2
Chimeras were constructed using the splicing by overlap extension method as described in (Choe et al., 1999) using parts of either ROMK2 (EMBL/GenBank/DDBJ accession no. L29403) or IRK1 (EMBL/GenBank/DDBJ accession no. X73052). Chimeras used in this study are illustrated schematically in Fig. 1 . Chimera C13 was originally designed to have the cytoplasmic COOH-terminal portion of the channel from IRK1, with the rest of the protein from ROMK2. In fact, based on the X-ray crystal structures of the KcsA and bacterial Kir channels (Doyle et al., 1998; Kuo et al., 2003), the portion of this chimera coming from IRK1 probably includes the COOH-terminal portion of the second transmembrane domain. Chimera C107 has its extracellular domain—between the two transmembrane domains—from IRK1, while the rest of the protein is from ROMK2. C25 has both NH2- and COOH-terminal tails from IRK1. Point mutations in ROMK2 and the chimeras were engineered with a PCR QuikChange site-directed mutagenesis kit (Stratagene), with primers synthesized by QIAGEN. Nucleotide sequences were checked using an ABI Prism 377XL automated DNA sequencer at the Biomedical Resource Center of Cornell University.
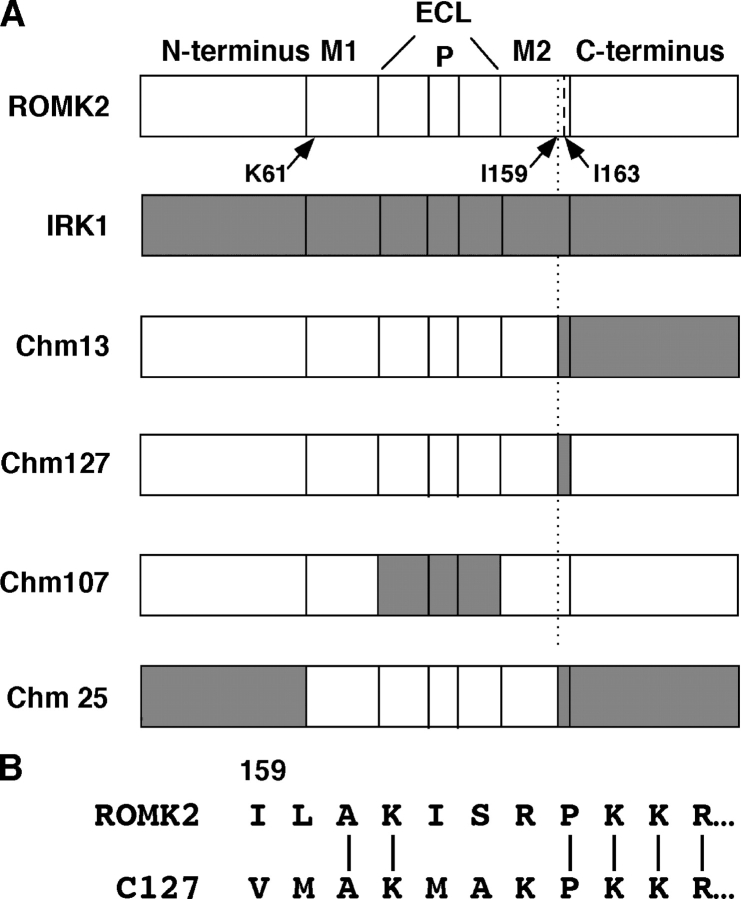
Figure 1.
ROMK2/IRK1 chimeras. (A) Each protein is divided into the cytoplasmic NH2 and COOH terminus, the two transmembrane domains M1 and M2, and the extracellular loop (ECL), which includes the pore region (P) containing the selectivity filter. Light segments represent components from ROMK2; shaded segments represent components from IRK1. Arrows indicate the positions of the three point mutations K61M, I159V, and I163M. (B) Sequence comparison of ROMK2 and C127.
The precise definition of the chimeras can be seen in Table I .
TABLE I.
Definition of Chimeras
| ROMK2 residues | IRK1 residues | |
|---|---|---|
| C13 | NH2-terminal to A158 | V178 to COOH-terminal |
| C25 | V68 to A158 | NH2-terminal to V86 V178 to COOH-terminal |
| C107 | NH2-terminal to Y89 A137 to COOH-terminal |
L109 to I155 |
| C127 | NH2-terminal to A158 P166 to COOH-terminal |
V178 to K184 |
Expression of Channels
Plasmids were linearized with NotI restriction enzyme and transcribed in vitro with T7 RNA polymerase in the presence of the GpppG cap using mMESSAGE mMACHINE kit (Ambion). Synthetic cRNA was dissolved in water and stored at −70°C before use. Stage V-VI oocytes were obtained by partial ovariectomy of female Xenopus laevis (NASCO), anesthetized with tricaine methanesulfonate (1.5 g/L, adjusted to pH 7.0). Oocytes were defolliculated by incubation in OR2 solution (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, adjusted to pH 7.5 with NaOH) containing 2 mg/ml collagenase type II and 2 mg/ml hyaluronidase type II (Sigma-Aldrich) for 90 min and (if necessary) another 90 min in a fresh enzyme solution at 23°C. Oocytes were injected with 0.5–1 ng of cRNA and incubated at 19°C in 2× diluted Leibovitz medium (Life Technologies) for 1–4 d before measurements were made.
Whole-cell Experiments
Whole-cell conductances were measured in intact oocytes using a two-electrode voltage clamp (TEV200; Dagan Corp.). Unless otherwise stated, current-voltage relations were determined over a range of 120 mV using 13 voltage command pulses (50-ms duration, 10-mV increments) centered around a holding potential close to the oocyte's resting potential.
pH titration curves in intact oocytes were obtained by changing the extracellular pH also with permeant acetate-buffered solutions to control intracellular pH (Tsai et al., 1995; Choe et al., 1997). The relation between intracellular and extracellular pH was calculated from a previous calibration with ROMK2-expressing oocytes: pHi = 0.595•pHo + 2.4 (Choe et al., 1997).
Cut-open Oocyte Experiments
The cut-open oocyte preparation was used in those experiments requiring control of intracellular pH to values more alkaline than 7.2. This consisted of a vaseline-gap oocyte chamber fabricated by RE Weiss, following the original design (Perozo et al., 1992; Taglialatela et al., 1992; Costa et al., 1994), but modified for internal perfusion of the oocyte via a multibarrel pipette (200-μm tip diameter), fabricated by fusing four or five silica capillaries inside a 1.5 mm OD soda lime hematocrit tube. The silica capillaries were connected to separate pH reservoirs driven by a variable speed syringe pump. Switching between internal perfusion solutions was accomplished without generation of pressure transients.
After mounting the oocyte in the multicompartment chamber, but before inserting the voltage microelectrode, the multibarrel perfusion pipette was inserted midway through the bottom of the oocyte from below the chamber and perfusion was begun with an internal solution of pH 8.0 or 9. Internal pH was successively decreased to 8, 7.5, 7, 6.5, and 6 at a constant extracellular pH of 7.8. At oocyte perfusion rates of 50 μl/h, ∼5 min were required for equilibration of the oocyte at each new internal pH. Cut-open oocytes were maintained in the open-circuit condition except for periodic voltage pulses (at 5-min intervals) that were used to generate current-voltage relations at different internal pHs.
Voltage Jump Experiments
To study the effects of holding potential on channel activity, the oocyte membrane voltage was clamped either to the calculated K+ reversal potential (EK) or to depolarized potentials, usually 50 mV positive to EK. Inward conductance was measured as the difference in current measured by 20-ms pulses to voltages 40 and 60 mV negative to EK.
Barium Block Experiments
The affinity of the channel for Ba2+ was evaluated by measuring the normalized whole-cell current at different extracellular Ba2+ concentrations and voltages using the equation:
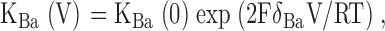 |
where KBa (V) is the voltage-dependent inhibition constant and δBa is the fraction of the electric field at the site of Ba2+ block. In these experiments the voltage pulse protocol spanned the range of −200 to +20 mV.
Solutions
Extracellular solutions for two-electrode voltage clamp experiments
(1) For pH titrations: 55 mM KCl, 55 KAcetate, 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, pH adjusted with KOH.
(2) For Ko titrations: 110 mM NaCl +KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, pH adjusted with NaOH.
(3) For ion substitution experiments: 110 mM NaCl + (KCl, RbCl, NH4Cl), 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, pH adjusted with NaOH. K+, Rb+, and NH4 + were added to the extracellular solution by iso-osmotic replacement of Na+. The permeant ion concentrations were increased monotonically from 1 mM to avoid acid loads which might result from sudden decreases in NH4 + concentration.
Extracellular solutions for cut-open oocyte experiments
100 mM KCl, 5 mM NaCl, 1 mM MgCl2, 2 mM CaCl2, buffered to a constant pH of 7.8 with NaOH.
Intracellular solutions for internal perfusion of cut-open oocyte experiments
100 mM KCl, 5 mM NaCl, 1 mM EGTA, 0.5 mM MgATP, 100 μM DTT, pH buffered between 6.0 and 9.0 with NaOH.
RESULTS
pHi Sensitivity of Chimera C13
The initial observation of this study was that a chimera (C13), formed by substituting the COOH terminus of IRK1 into ROMK2, was much more sensitive to changes in pH than was the parent ROMK2 channel. IRK1 itself is insensitive to pH over the range of 6.0 to 7.5. Typical current traces are illustrated in Fig. 2 . Internal pH was controlled with external permeant acetate buffers. Under these conditions, pHi changes in parallel with pHo (Tsai et al., 1995; Choe et al., 1997). Whole-cell ROMK2 currents show very little rectification and little sensitivity to pH between 8.2 and 7.4. In contrast, the C13 chimera was a strong inward rectifier, as reported previously (Taglialatela et al., 1994; Yang et al., 1995), and was very sensitive to changes in pH in this range.
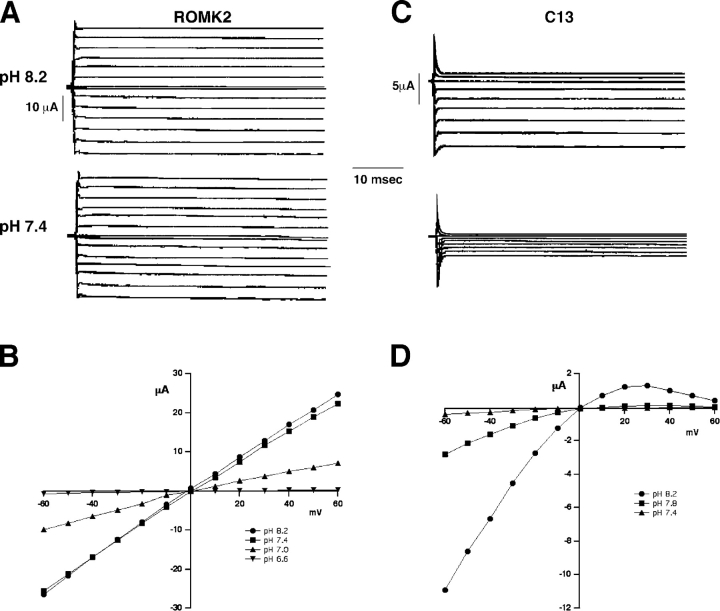
Figure 2.
pH dependence of ROMK2 and C13. (A and C) Current traces for ROMK2 and C13 at different extracellular/intracellular pH. External solutions contained 55 mM KCl and 55 mM K acetate adjusted to the indicated pH values. Holding potential was 0 mV. Currents were measured at test potentials of −60 to +60 mV. Reducing pHo from 8.2 to 7.4 had little effect on ROMK2 currents (A) but markedly reduced the inward currents through the C13 chimera (C). (B and D) Steady-state I-V relationships for ROMK2 (B) and C13 (D) at different pH.
I-V curves for ROMK2 and C13 at different pHs are shown in Fig. 2 (bottom). ROMK2 conductance was independent of voltage, but strongly dependent on pHi between 6.8 and 6.3 (pHo between 7.4 and 6.6). Normalized inward conductances are summarized in Fig. 3 A. Half-maximal conductance for ROMK2 occurred at pHo ∼6.9, corresponding to an apparent pKa for the inhibition process of ∼6.5 (Choe et al., 1997). In contrast, C13 currents were almost completely inhibited at pHi of 6.8. It is impossible to reliably estimate the apparent pKa for C13 with this procedure using intact oocytes, since maximal conductance appears to occur at pHi ≥ 8.5, which is above the range that can be controlled with permeant acetate buffers.
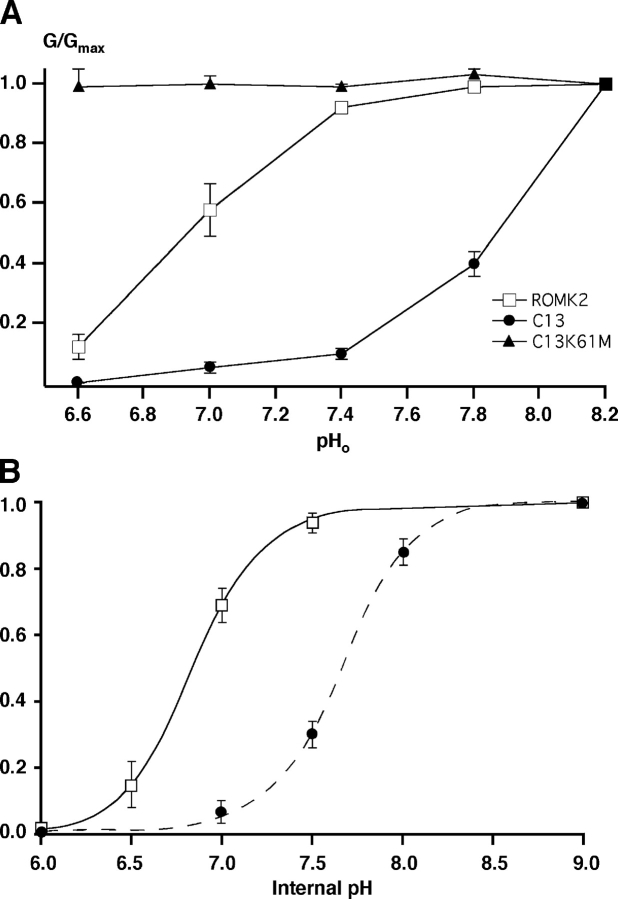
Figure 3.
pH dependence of inward conductance through ROMK2, C13, and ROMK2 K61M. (A) Conductance was measured in experiments similar to those shown in Fig. 2. Values were normalized to those at pH 8.2 and plotted as a function of external pH. Data represent means ± SEM for 4–6 oocytes. (B) Conductance measured in cut-open oocytes, expressing either ROMK2 or C13. Data were normalized to values obtained at pHi = 9. ROMK2: open squares, solid line, pKa = 6.8 ± .03, Hill coefficient 2.2 ± 0.3 (n = 4). C13: solid circles, dashed line, pKa = 7.7 ± .04, Hill coefficient 2.3 ± 0.3 (n = 7).
To confirm these observations and make a more quantitative comparison we measured the effect of internal pH in cut-open, perfused oocytes where pHo was held constant and pHi varied directly over a wider range. Normalized inward conductances for ROMK2 and the C13 chimera are compared in Fig. 3 B. Both ROMK2 and C13 currents could be abolished by perfusion with low pH solution. The apparent pKa values were 6.8 ± 0.03 (n = 4) for ROMK2 and 7.7 ± 0.04 (n = 5) for C13 in 100 mM K solutions. Hence, the COOH-terminal substitution shifted the pH response of the channel by 0.9 ± 0.05 pH units in an alkaline direction. These results are in good agreement with those obtained in intact oocytes using the permeant buffer method. Since the latter approach could be employed much more routinely we used it in subsequent experiments to further define the role of the COOH terminus in the pH response.
To see if the responses of C13 were mediated by the same mechanism as those of ROMK, we mutated the K61 residue to methionine, the corresponding amino acid in IRK1. This substitution was previously shown to abolish the response to pHi in ROMK2 (Fakler et al., 1996; Choe et al., 1997). It had a similar effect on C13 (Fig. 3 A), suggesting that the change in pKi in this chimera was the result of a shift in sensitivity of the same pH-sensing mechanism, rather than the addition of a separate pH sensor.
Specific Sites Conferring Altered pHi Sensitivity
To determine whether the critical amino acids for pH sensing are in the transmembrane domain or the cytoplasmic portion of the channel, we constructed the C127 chimera, in which eight amino acids at the end of the putative second transmembrane region (M2) of ROMK2 were replaced by their corresponding IRK1 residues (see Fig. 1). C127 channels displayed small, but measurable currents, whose pH sensitivity was virtually identical to that of C13 (Fig. 4) , indicating that this region was crucial to the shift in pH sensitivity of C13. C127, unlike C13, was not a strong inward rectifier. This is consistent with previous identification of acidic amino acids in the cytoplasmic tail as principal determinants of strong rectification in these channels (Taglialatela et al., 1994; Yang et al., 1995). The single-channel conductance of C127 was 38 ± 3 pS in 110 mM K+, similar to that of ROMK2 (38 pS) but larger than that of C13 (20 pS; Choe et al., 2000). Thus, the smaller conductance of C13 vs. ROMK2 is also conferred by the cytoplasmic parts of the COOH terminus.
Figure 4.
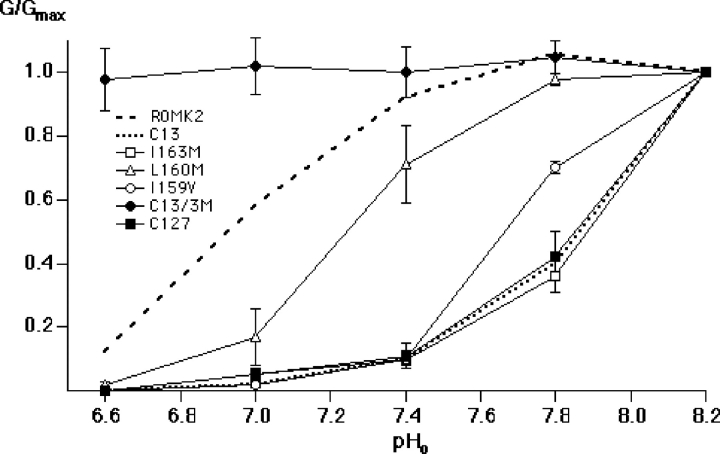
pH dependence of inward conductance through the ROMK2 mutants I163M, L160M, and I159V and the C13 mutant “C13-3M” (C13 V159I/M160L/M163I). Conductance was measured and plotted as in Fig. 3 A. Data represent means ± SEM for 4–5 oocytes. Dashed and dotted lines show data for ROMK2 and C13, respectively, from Fig. 3 A.
Since C127 and ROMK2 differ by only five amino acids (Fig. 1), point mutations were made to assess the importance of each of these amino acids to the pH sensitivity of the channel. Two mutations, S164A and R165K, had the same response to pH as did ROMK2 (unpublished data). The other three amino acids shifted the pH response curve toward more alkaline values (Fig. 4). The strongest of these was the I163M mutation, which mimicked the pH sensitivity of both C13 and C127. The position of this residue within the structure of the transmembrane part of the protein is shown in Fig. 13 (see discussion). Another mutation, I159V, also shifted the pH titration curve to the right. The effects of these two mutations were not additive, since both are contained within the C127 and C13 chimeras, whose responses to pH were similar to that of the point mutant I163M. A third mutation, L160M, produced a smaller increase in pH sensitivity.
Figure 13.
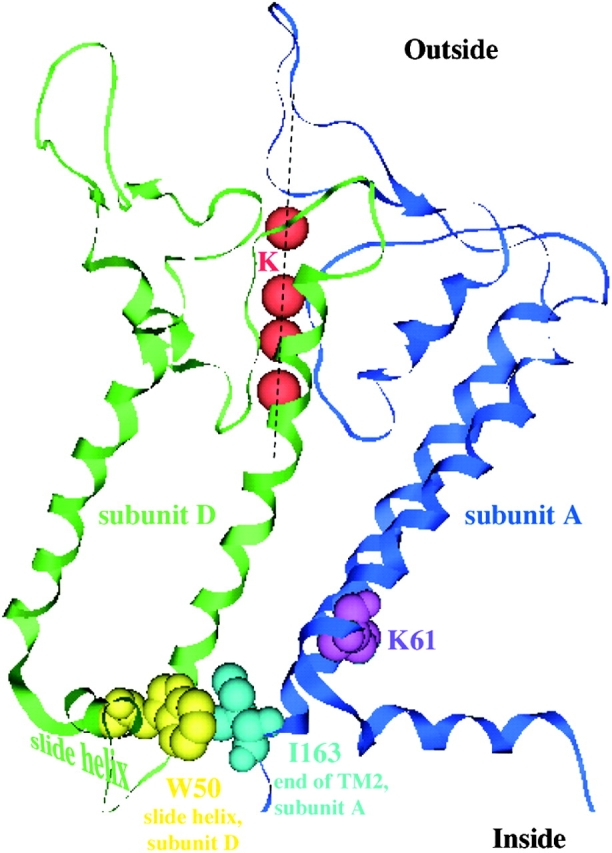
Possible interactions between I163 at the end of the second transmembrane helix of subunit A with residues (e.g., W50) on the slide helix of subunit D. The relevant residues for Kir 1.1 pH gating (space-filled) are superimposed on the crystal structure of a KirBac1.1. Only 2 of 4 KirBac1.1 subunits are shown.
To examine whether converse mutations in the C13 chimera shift the pH response back to that of ROMK2, we made a triple mutant (C13-3M) in which three residues of C13 (V159, M160, and M163) were replaced with the corresponding amino acids of ROMK2. The pH sensitivity of this mutant channel was either abolished or shifted to values even more acid than that of ROMK2 (Fig. 4). Thus, the shift was in the predicted direction but its magnitude was larger than expected. We did not investigate this phenomenon further.
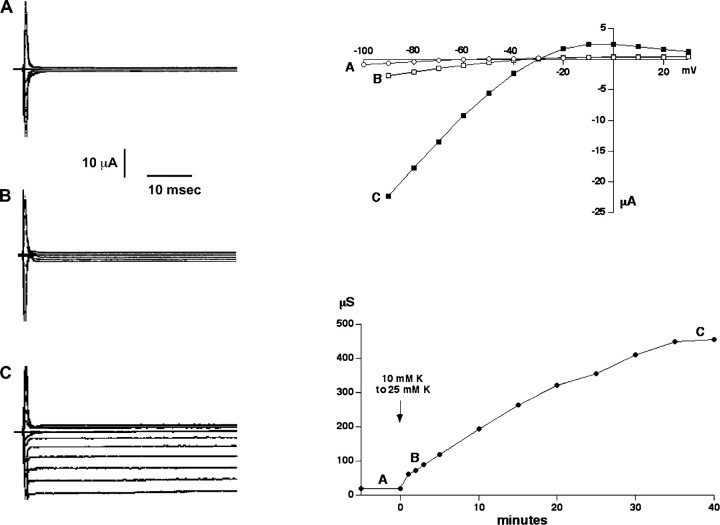
Ko Sensitivity of Chimera C13
Because of the previously observed linkage between the effects of pHi and K+ o, we assessed the effects of the COOH-terminal substitutions on the response of ROMK2 to reductions in K+ o. Fig. 5 illustrates the K+ o dependence of inward C13 currents. When external K+ o was ≤10 mM, C13-expressing oocytes had low conductances that were barely distinguishable from background. Raising K+ o from 10 to 25 mM elicited the slow increase in C13 conductance shown in Fig. 5. Several observations suggest that this conductance change reflects an activation of the channels, similar to that reported previously for ROMK2 (Sackin et al., 2001), rather than a simple increase in the availability of permeant ions. First, the time course is very slow compared with that required to change the solution completely (<1 min). Second, outward currents measured just positive to the reversal potential were also increased (Fig. 5). Finally, the C13-K61M construct showed no indications of K+ o-dependent gating. When K+ o was increased changes in conductance occurred rapidly, as fast as the solution could be exchanged, and were subsequently stable (unpublished data).
Figure 5.
Effect of external K+ o on inward conductance through C13. (Left) Current traces for a C13-expressing oocyte in 10 mM K+ (A), 1 min after increasing K+ to 25 mM (B) and 40 min after the solution change (C). (Bottom right) Time course of inward conductance. (Top right) I-V curves at times A–C.
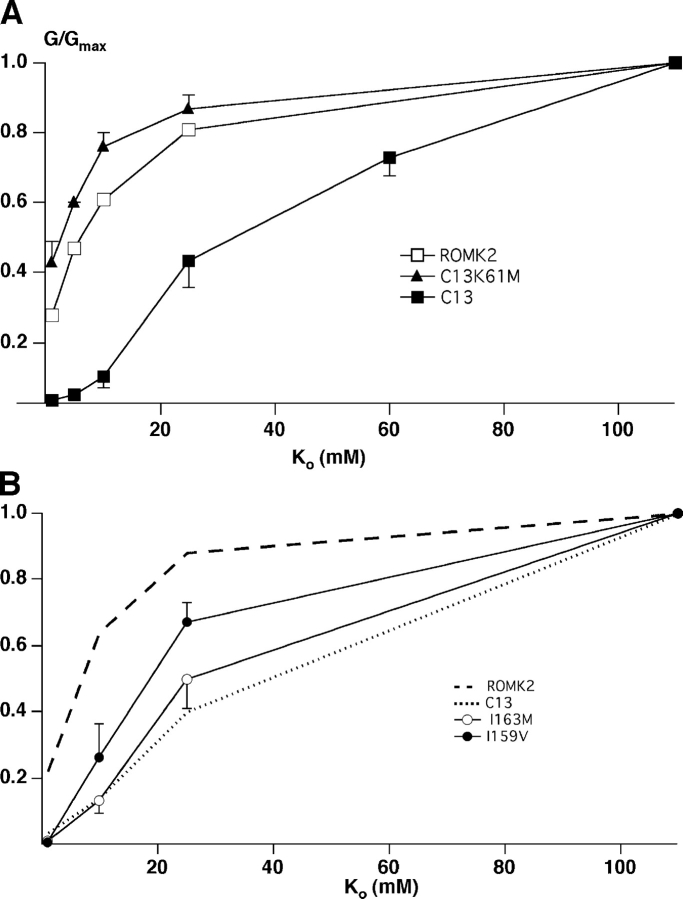
The activation of C13 required higher concentrations of K+ than did ROMK2, in which the conductance was nearly maximal at 10 mM Ko (Fig. 6) . A K+ conductance was measurable in most cases even in 1 mM Ko +, implying that some ROMK2 channels were open under these conditions. Therefore, the conductance increases in this set of experiments presumably reflected both an increase in single-channel current arising from increased permeant ion concentration as well as an activation of the channels.
Figure 6.
Steady-state concentration-conductance relationships for ROMK2, C13, C13–3M (A), and for I163M and I159V (B). Oocytes were perfused with solutions containing KCl concentrations of 1–110 mM, substituting for NaCl. Data represent means ± SEM for 5–11 oocytes.
As with pH sensitivity, point mutations at the end of the second transmembrane region could also account for the differences in K+ o sensitivity between C13 and ROMK2. As shown in Fig. 6, the K+ o dependence of ROMK2-I163M was almost identical to that of C13. The I159V mutation also shifted the K+ o conductance curve but to a somewhat small extent. Finally, the C13-3M mutant had a K+ o dependence that was nearly identical to that of ROMK2 (Fig. 6 A). These observations provide further evidence for the tight coupling between the responses to pHi and K+ o; mutants that are more sensitive to closure by low pHi are also more sensitive to closure by removal of K+ o.
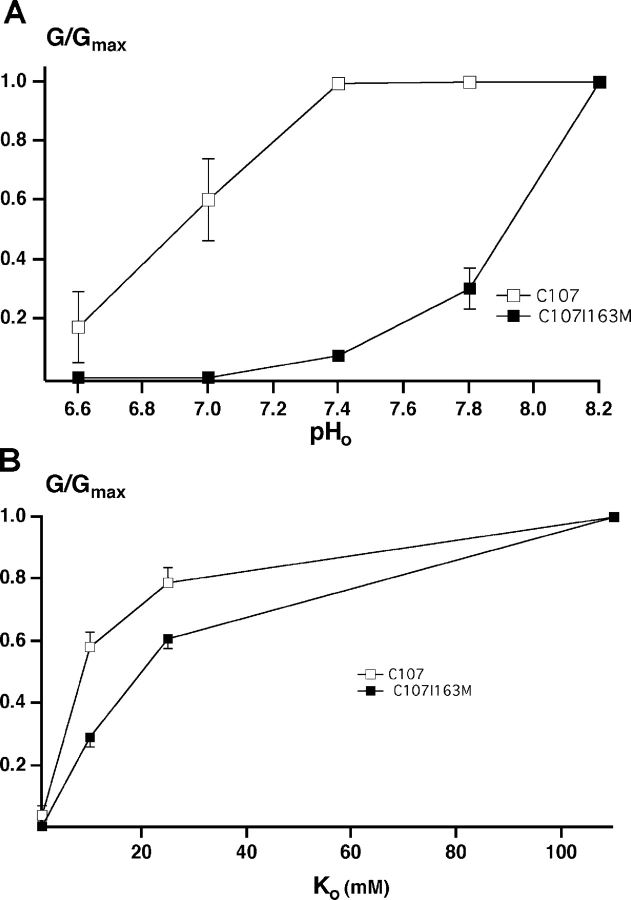
In principle, the COOH-terminal substitutions could affect the machinery for sensing either pHi or K+ o. To distinguish these possibilities we investigated another chimera (C107) in which the entire extracellular loop of ROMK2 was replaced by that of IRK1 (see Fig. 1). This chimera has both the pore region and the flanking extracellular domains of IRK1. Since substitution of either the pore region or the COOH-terminal flanking region between P and M2 strongly reduced the sensitivity to external K+ removal (Sackin et al., 2001), we expected C107 channels to remain open at low K+ o. We then made the point mutation I163M on the background of C107. We predicted that if the mutation had a primary effect on the pHi response then the effect should still be present in the C107 chimera.
The responses of C107 and C107-I163M to pHi and to K+ o are shown in Fig. 7 . As expected, C107 conductance was inhibited by lowering pHi. Reducing K+ o to 1 mM decreased conductance but did not appear to induce channel closure as outward currents remained high, and increasing K+ o from 1 mM to higher concentrations produced rapid increases in currents and not the slow increase shown in Fig. 5. The I163M mutation shifted the pH response as it did in ROMK2 (Fig. 4). The C107-I163M mutation also produced a shift in the response to K+ o relative to C107. However, this was smaller than the corresponding shift produced by ROMK2-I163M relative to ROMK2 (Fig. 6). These results are most consistent with a primary effect of the I163M mutation on the pHi response.
Figure 7.
Effect of pH and Ko + on inward conductance through C107 and C107 I163M. (A) pH dependence. (B) Ko + dependence. Data represent means ± SEM for five oocytes.
Fig. 13 illustrates a model of the structure of the lower part of the transmembrane region, based on the structure of the bacterial inward rectifier channel (KirBac) reported by Doyle and colleagues (Kuo et al., 2003), showing the positions of the amino acids identified as being important in the pH response. Our working hypothesis is that this response entails a closure of the pore near its cytoplasmic end (see discussion).
Selectivity of the Ko-activation Site
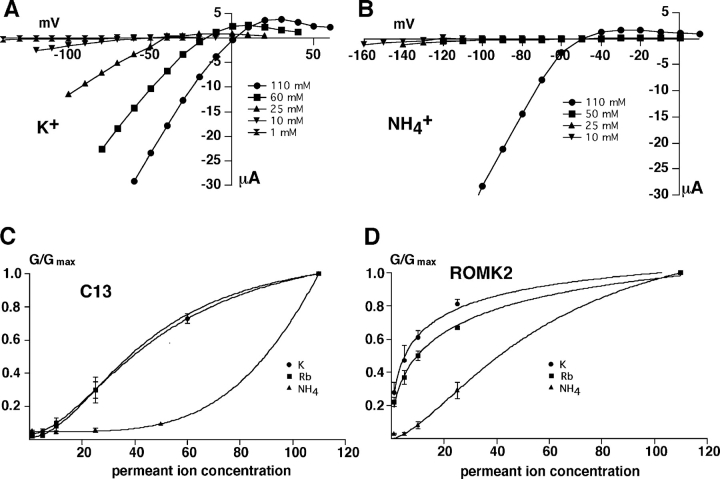
In contrast, the most obvious site of action of external K+ is within the pore itself. Doi et al. (1996) showed that Rb+ can substitute for K+ in the activation process, consistent with an effect within the permeation pathway. Since the permeability of ROMK2 to NH4 + is about one tenth that to K+ or Rb+ (Chepilko et al., 1995; Choe et al., 2000), we reasoned that NH4 + should also be able to activate the channels, but would require higher concentrations. We first made use of the C13 chimera in which channel inhibition with low K+ o was more complete. Current-voltage relations with different concentrations of extracellular K+ and NH4 + are shown in Fig. 8, A and B , respectively. Using the linear region of the I-V relationship for inward currents, the concentration dependence of C13 conductance was plotted as a function of ion concentration for K+, Rb+, and NH4 + (Fig. 8 C), yielding a selectivity series for the activation process: K+ ≅ Rb+ > NH4 + >> Na+. In these experiments, extracellular K+ and Rb+ activated the channels with a threshold of 10–25 mM, whereas in the presence of NH4 + more than 50 mM was required to produce a significant conductance. Na+ did not activate at all. The selectivity could not be quantified more precisely because maximal activation with NH4 + was probably not achieved even at 110 mM. Qualitatively, however, this sequence matches that of the selectivity for ion permeability of ROMK2 and C13 channels measured by changes in the reversal potential (Chepilko et al., 1995; Choe et al., 2000).
Figure 8.
Activation of C13 and ROMK2 channels by extracellular K+, Rb+, and NH+ 4. Oocytes were incubated in medium containing 1 mM permeant ion + 109 mM NaCl until a steady-state conductance was achieved. Permeant ion concentrations were then increased in steps up to 110 mM, substituting for Na+. Conductance was normalized to maximal values measured at the 110 mM concentration. (A and B) Current-voltage relationships for C13 with increasing concentrations of K+ (A) or NH4 + (B). (C) Conductance-concentration relationships for C13. (D) Conductance-concentration relationships for ROMK2. Smooth curves are drawn through the data points and have no theoretical meaning. Data represent the means ± SEM for 4–6 oocytes.
ROMK2 had a similar selectivity sequence for activation (Fig. 8 D), although the concentrations required were lower for each of the ions. This finding provides additional support for the idea that the activating ions exert their effects within the pore. The simplest interpretation is that occupancy of the pore by permeant or blocking ions prevents collapse or closure of the selectivity filter itself.
Channels Can be Inactivated by Blocking the Pore from the Inside
The interactions between the effects of intracellular pH and extracellular K+ can be described by a three-state scheme in which protonation of an internal site closes the channel (closed(H,K) state). The channel enters a second, more stable closed state (closed(H,0)) when K+ o is decreased. This state is accessible from the closed(H,K) state but not from the open state:
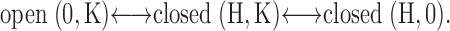 |
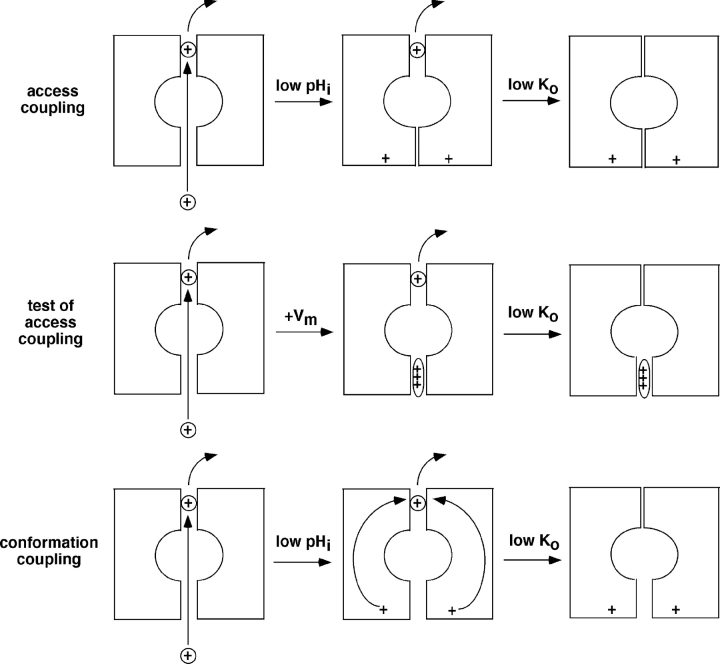
One simple way to account for this scheme is to postulate two distinct channel gates (Doi et al., 1996; Sackin et al., 2003). When the channels are protonated, a gate between the selectivity filter and the cytoplasm is closed: (closed(H,K)). This stops ion flow and prevents access of intracellular K+ to the selectivity filter. If K+ o is also reduced, occupancy of the selectivity filter by K+ is reduced and an outer gate, comprised of the filter itself, can shut. Such a collapse of the selectivity filter in response to a reduction of permeant ion concentration was demonstrated in x-ray crystal structures of KcsA channels (Zhou et al., 2001). Thus, occupancy of the pore by K+, coming from either the inside or the outside, is required to keep the pore open. If an inner gate regulating access of K+ to the channels from the inside is closed, the channels should become more sensitive to removal K+ o.
To examine whether impeding access to the selectivity filter from the inside promotes closure, we applied a positive voltage to the strongly rectifying C13 channels. C13 has the COOH terminus of IRK1 (Kir2.1) which confers a high affinity for internal blocking cations including Mg2+ and polyamines (Matsuda et al., 1987; Vandenberg, 1987; Ficker et al., 1994; Lopatin et al., 1994). Applying a transmembrane voltage more positive than EK facilitates this block, greatly reducing outward currents through the channel (see Fig. 2). The model (Fig. 9) predicts that this block should mimic the effects of closing an internal gate, by preventing access of intracellular K+ to the selectivity filter, leading to slow closure of the channels.
Figure 9.
Schematic representation of two possible mechanisms of coupling between inner and outer channel gates, and an experimental test of coupling through restricted K+ access.
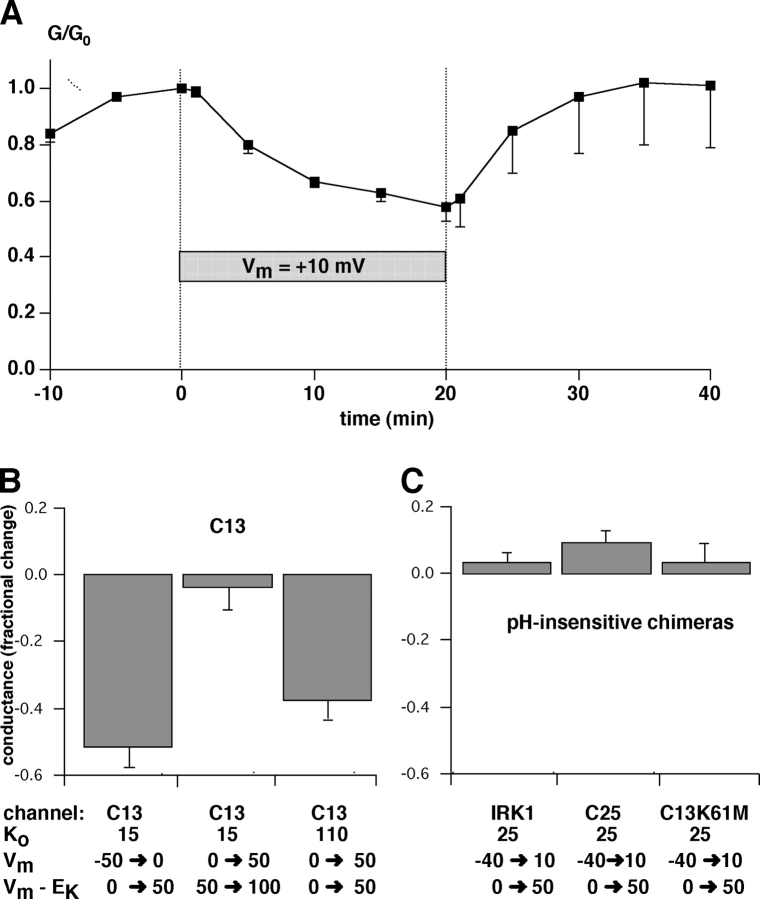
The effects of chronic depolarization on the channels were examined by measuring inward conductance with intermittent 20-ms pulses to negative voltages. Such a pulse protocol was long enough to open the channels and allow a conductance measurement, but short enough to prevent changes due to the slow gating events. Depolarization of the C13 chimera from its reversal potential of −40 mV (in 25 mM K) to +10 mV produced a negligible outward holding current but slowly diminished the inward conductance, indicating channel closure (Fig. 10 A). The time course of this inhibition was similar to that seen during removal of external K+ (Doi et al., 1996; Sackin et al., 2001)
Figure 10.
Effect of a depolarizing holding potential on the inward conductance of strongly rectifying channels. (A) Time course of inward conductance changes with C13. Oocytes were superfused with solution containing 25 mM K. Inward conductances were measured between −60 and −80 mV with a holding potential of −40 mV, close to EK and the resting membrane potential. After a steady-state was achieved, the holding potential was changed to +10 mV, and the conductance continued measured at the same test potentials. After 20 min the holding potential was returned to −40 mV. Data represent means ± SEM for five oocytes. (B) Summary of experiments similar to those shown in A using C13 under different initial conditions, including initial holding potential and K+o concentration. (C) Summary of experiments similar to those shown in A using different pH-insensitive channels. The plots in B and C show fractional changes in conductance in response to a 50-mV depolarization. Data represent means ± SEM for 4–6 oocytes.
The voltage effect appears to depend on channel block rather than voltage per se. As shown in Fig. 10 B, with K+ o = 15 mM a similar slow inhibition was observed when the voltage was shifted from −50 to 0 mV, i.e., From EK to EK + 50 mV. A further depolarization from 0 to +50 mV had little effect. However, with K+ o = 110 mM the same voltage shift of 0 to +50 mV (again from EK to EK + 50 mV) produced inhibition over a 20-min time course. Thus, the voltage effect correlates better with changes in V-EK rather than with the absolute membrane potential. This is similar to the voltage dependence of block by intracellular cations, which also depends on V-EK rather than on V.
Block from the Inside Is Not Sufficient to Inactivate the Channels
To see if blocking the channels from the inside is sufficient to close the channels, we repeated these maneuvers using channels in which regulation by pHi is removed through mutation of the K61 residue. One of these is a chimera, C25, with the transmembrane and extracellular regions of ROMK2, but the NH2 and COOH termini of IRK1 (Choe et al., 2000). The second construct (C13-K61M) has the ROMK2 sequence except for the COOH terminus of IRK1 and the point mutation K61M. If blocking access of the selectivity filter to intracellular K were sufficient to cause closure, then the voltage-dependent effects would be independent of the pH gate and should be observed in these channels.
Application of a depolarizing voltage to either C25 or C13-K61M, under the same conditions used to inactivate C13, blocked outward conductance but produced no observable effect on inward conductance as long as external K was maintained above 10 mM (Fig. 10 B). IRK1 itself was also insensitive to voltage. We conclude that simple depolarization-induced block of the pore from the inside does not by itself produce inactivation and hence does not account for coupling between internal pH and external K under these conditions. However, when external K+ was decreased to ≤1 mM, strong depolarizations could reduce inward conductance in both C25 and C13-K61M (Sackin et al., 2004).
Properties of Outer Pore Are Altered by Intracellular Domains
An alternative mechanism for coupling internal pH and external K could be through propagated changes in protein conformation (Schulte et al., 2001). According to this view, either protonation of the pH sensor or the closure of the channel in response to this protonation alters the properties of the K+ o-sensitive gate. To test this, we measured the affinity of the channel for extracellular Ba2+, which binds to the KcsA channel at the inner end of the selectivity filter (Jiang and MacKinnon, 2000). It is likely that the site of binding to ROMK2 is similar, since mutations in this region of the pore alter the sensitivity for Ba2+ block and these changes account for the differences in the affinities of ROMK2 and IRK1 for Ba2+ (Zhou et al., 1996).
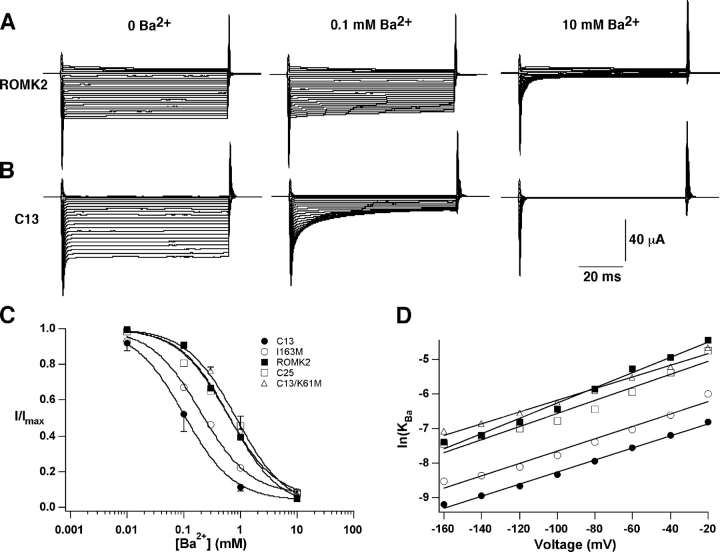
As shown in Fig. 11 , KBa, the Ba2+ concentration required for 50% block of conductance measured using the two-electrode voltage clamp, is ∼10-fold lower for C13 channels than for ROMK2. The effect is evident at all membrane voltages tested, and the voltage dependence of block is not dramatically different for the two channels. This observation suggests that changes in the COOH terminus can alter the properties of the outer pore.
Figure 11.
Ba2+ block of K+ channels. Oocytes were superfused with solution containing 110 mM KCl. BaCl2 was then added to the solution at concentrations of 0.01, 0.1, 1, and 10 mM. Currents were measured at voltages between −20 and −160 mV under each condition and were normalized to values obtained in the absence of Ba2+. (A and B) Typical traces for ROMK2 (A) and C13 (B) -expressing oocytes with zero, submaximal (0.1 mM), and near-maximal (10 mM) Ba2+. (C) Dose–response relationships of different channels at a voltage of −160 mV. Data represent means ± SEM (shown for representative data points) of 4–7 oocytes. (D) KBa values as a function of voltage. Mean values obtained from best-fits to the data points to the equation: KBa (V) = KBa(0)exp(2FδBaV/RT). Values of KBa(0) (mM) and δBa were: ROMK2 (13.8; 0.27) C13 (1.4; 0.21) C25 (7.8; 0.21) C13K61M (10.0; 0.19) ROMK2I163M (2.8; 0.23).
The change in Ba2+ sensitivity correlates better with pH dependence of the channel rather than with the primary structure of the COOH terminus per se. As shown above, the IRK1 COOH terminus of the C13 chimera increases the sensitivity of the channel to both internal acidity (Figs. 2 and 3) and to external Ba2+ (Fig. 11). Both effects are mimicked by the point mutant I163M in ROMK2, which has the same sensitivity to internal pH (Fig. 3) and almost the same sensitivity to external Ba2+ (Fig. 11) as the C13 chimera.
Conversely, abolishing the pH sensor of C13, using either a point mutation (C13-K61M) or by replacing the entire NH2 terminus with that of IRK1 (C25), decreases KBa to levels similar to those of ROMK2 (Fig. 11). Thus, channels that have increased pH sensitivity, and increased likelihood of protonation and closure, also have increased sensitivity to external Ba2+.
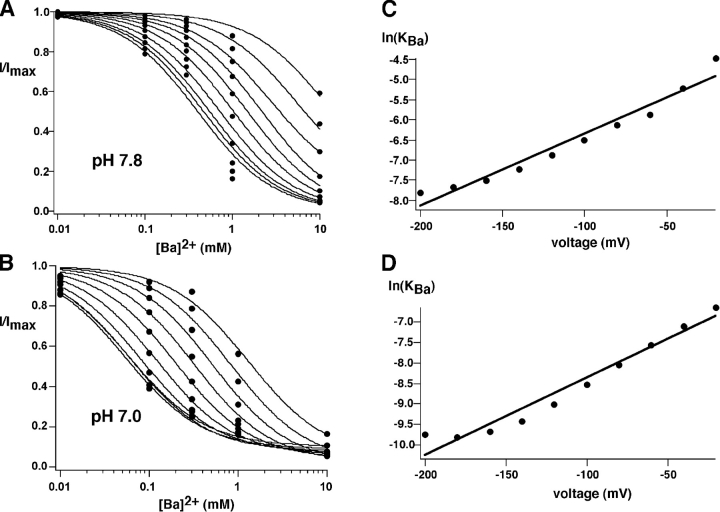
To directly assess whether the change in Ba2+ affinity correlates with protonation of the internal pH sensor, we measured KBa in ROMK2 channels at two different intracellular pHs, using the two electrode voltage clamp and permeant acetate buffers. Lowering extracellular pH to 7.0 decreased KBa to levels similar to that seen with either C13 or ROMK2-I163M (Fig. 12) . Again, the effect was primarily on the intrinsic inhibition constant rather than the voltage dependence of block. In Fig. 12, C and D, the apparent inhibition constant appeared to become less voltage-dependent at large negative voltages. This was seen at both pH values and does not affect the overall conclusion that the pH effect is independent of voltage. These results suggest that titration of the pH sensor by intracellular protons can influence at least one fundamental property of the selectivity filter, namely block by Ba2+.
Figure 12.
Effect of pH on Ba2+ block of ROMK2 channels. Data were obtained in experiments similar to those shown in Fig. 5 using pHo 7.8 (A) and 7.0 (B). Best fits to the data using the equation described in Fig. 6 gave values of KBa(0) = 10.4 mM (pH 7.8) and 1.5 mM (pH 7.0). (C and D) Voltage dependence of block at pH 7.8 (C) and 7.0 (D). Values of δBa were 0.23 (pH 7.8) and 0.24 (pH 7.0), respectively.
The selectivity for K+ versus Rb+ is also thought to be a property of the selectivity filter and might change in response to alterations in the cytoplasmic domains. To test for changes in selectivity we measured the conductance ratio GRb/GK at different pH values in oocytes expressing ROMK2. We previously showed that the difference in the relative conductance to Rb+ and K+ in ROMK2 and IRK1 was attributable to the P-region (Choe et al., 2000). There was a small but consistent decrease in GRb/GK from 0.67 ± 0.02 to 0.60 ± 0.03 when the external pH was reduced from 7.8 to 7.0 in the presence of an acetate buffer (15 paired experiments). The mean change in the ratio—0.07 ± 0.02—was statistically significant (P = 0.008) although much less dramatic and less convincing than the change in Ba2+ affinity.
DISCUSSION
Modulation of pH Sensitivity by the COOH Terminus
Previous work has implicated the lysine residue (K61) in the NH2-terminal portion of ROMK as being critical for the response of the channel to internal pH (Fakler et al., 1996; Choe et al., 1997; Schulte et al., 1999). In the pH-insensitive channel IRK1 this residue is a methionine, and the K61M mutant of ROMK is also insensitive to pH. The pH-sensing mechanism may involve the titration of this lysine (Schulte et al., 1999), even though this implies that the pKa would be shifted from its value in free solution by several pH units. Alignment of ROMK2 with the KirBac1.1 channel, whose structure was recently elucidated (Kuo et al., 2003), suggests that the ROMK2-K61 residue is within the first transmembrane segment of the protein. The hydrophobic environment would increase the free energy cost of protonating the ɛ-amino group and could account, at least in part, for the shift in the apparent pKa.
In addition to K61, several other amino acids in the cytoplasmic NH2- and COOH-terminal domains influence the pH response. When the T51 residue is replaced by a negatively charged amino acid the response shifts toward more alkaline values, while positively charged substitutions have the opposite effects (Choe et al., 1997). This amino acid position is located in the “slide helix” in KirBac1.1 (Kuo et al., 2003), close to the membrane–cytoplasm interface. In addition, two positively charged arginines in the COOH terminus influence pH gating of ROMK (Schulte et al., 1999). These residues may also contribute to the shift of the pKa of K61 toward the physiological range. Several histidines in the COOH terminus also influence the pH response (Chanchevalap et al., 2000). Since the charge on these residues will vary with pHi in the physiological range, they may contribute to the sensing of pH. Finally, phosphorylation of serine residues in the NH2- and COOH-terminal segments by PKA shifts the pKa value to more acidic values (Leipzinger et al., 2000). This may mediate the activation of the channels by PKA by relieving inhibition of the channels by internal protons. In all of these cases, mutations shifted the pH response, rather than abolishing it or creating a new response. Furthermore, each of these substitutions involves a change in charge on the amino acid side-chain, suggesting that the mechanism of interaction with the pH sensor could be electrostatic.
In this study, replacing amino acids at the end of the second transmembrane had a similar effect on the sensitivity of ROMK channels to internal pH. The apparent pKa for the process of closing the channels is shifted by ∼0.9 pH units in the C13 chimera or the I163M mutant compared with ROMK2. In this case, however, the mutation does not involve a change in charge since both amino acids are neutral. Indeed, the substitution is a conservative one, without a large effect on the hydrophobic index or on the α-helix–forming propensity of the site. One interpretation is that the side chains of I163 and I159 of ROMK2 interact specifically with other residues within the protein to stabilize either the open or closed state of the channel. Alignment of ROMK2 and KirBac1.1 channels shows that one possible interaction is between I163 and residue W50 on the NH2-terminal “slide helix”, which is located at the membrane–cytoplasm interface. These two side chains are in close proximity, at least in the presumed closed state corresponding to the KirBac structure (Fig. 13) . This possible interaction is given only as an example. At this point we do not know how important the proximity of these two amino acid side-chains might be, or what other pairings might occur with I159 or with either of these residues in the open state of the channel. However, such interactions might explain the striking effect of the M2 mutations on pH sensitivity.
The processes which gate other channels of the Kir family are also affected by amino acid substitutions in M2. In the G-protein–regulated K channel (GIRK or Kir3.x) several mutations in this helix produced an increase in the agonist-independent Po, although changes in other domains, including the pore helix and M1, had similar effects (Sadja et al., 2001; Yi et al., 2001). In ATP-gated K channels (Kir6.2) substitutions at the cytoplasmic end of M2 increased Po in the absence of ATP and also increased the K1/2 for ATP inhibition (Tucker et al., 1998; Enkvetchakul et al., 2000). Again, these effects were not specific for this region as they could also be produced by mutations nearer to the extracellular end of M2. Alignment of the sequences of the three channels did not reveal any particular consensus sites for these gating effects. One difference between the KATP and ROMK2 channels is that mutations in the M2 region of Kir6.2 generally produced an increased Po, i.e., they favored the open state, whereas in ROMK2 they favored the closed state. Mutations in GIRK also increased Po but that is to be expected since they were identified in screens for constitutive channel activity. Finally, a recent study of GIRK4 channels identified the COOH-terminal end of M2 containing the residues corresponding to those studied here as the part of the channel that transduces the effects of Gβγ subunits in opening the channel gate (Jin et al., 2002).
We assume as a working hypothesis that decreased intracellular pH closes ROMK2 channels at the intracellular end of the pore. We have no direct evidence for this idea, which is based mainly on the location of the elements that seem to be crucial for this type of gating. It is consistent with the well accepted models of voltage-gated K+ channels, as well as hyperpolarization-activated cation channels, in which the activation gate is placed at the cytoplasmic end of the pore (Armstrong 1971; Holmgren et al., 1997; Shin et al., 2001). Recent studies of altered accessibility of intracellular solutes and blocker trapping during ATP-dependent gating of Kir6.2 channels are also consistent with placement of the gate at this point (Phillips et al., 2003; Phillips and Nichols, 2003).
Interactions between the pH Sensor and the Ko Sensor
ROMK channels can also be inactivated by lowering extracellular K+ to 1 mM or less (Schulte et al., 2001; Sackin et al., 2003). Previous work suggested that the site of action of external K+ to keep the channels open is within the permeation path, probably near the outer mouth of the pore (Schulte et al., 2001; Sackin et al., 2003). Our results lend support to this idea. The ability of extracellular cations to open ROMK2 (and C13) channels correlates well with their relative permeabilities measured as changes in reversal potential. The simplest explanation of this correlation is that both permeability and activation reflect the occupancy of the selectivity filter by the permeant ions.
This would imply the existence of two gates in series. Such a system has also been postulated in the case of KcsA channels, where there is structural evidence for conformational changes closing the selectivity filter in response to reduction in K+ in addition to the presumed channel closure at the helix bundle crossing near the cytoplasmic end of the pore (Zhou et al., 2001). Furthermore, gating of these channels by pH can produce mechanical movement at both ends of the pore (Perozo et al., 1999).
Coupling of pH and K Effects
There is an interaction between these responses; lowering extracellular K shifts the apparent pKa for channel inactivation to more alkaline values. A minimal, undoubtedly oversimplified model with two closed states (see results) is consistent with the available data (Doi et al., 1996; Sackin et al., 2003). The essential feature is that protonation of critical amino acids closes the channels and also allows them to enter a second, more stable closed state when external K+ is low. The enhanced sensitivity of the mutants and chimeras to lowering cell pH implies that the COOH terminus (or the I163M mutation) shifts the equilibrium from the open to the first closed state. The greater sensitivity to removal of Ko results from more channels' being available to enter the second closed state.
Two plausible mechanisms for these interactions have been suggested, and we have examined each of them experimentally. First, closure of the inner gate could make the outer gate more sensitive to extracellular K+ by preventing access of internal K to the selectivity filter (Zhou et al., 2001). Results shown in Fig. 10, A and B, are consistent with this model. However, in similar experiments with channels without pH sensors (IRK1, C25, C13-K61M), blocking access of internal K+ to the selectivity filter did not promote slow inhibition of the channels (Fig. 10 C). It is possible to inactivate these channels using more extreme conditions, with external [K+] less than 1 mM (Sackin et al., 2004). Thus, blocking the channels from the inside in the presence of very low Ko + may be sufficient to close the channels. However with [K+] ≥ 10 mM, internal block does not mimic the effect of raising intracellular H+, suggesting that other interactions are essential for the coupling process.
In the second hypothesis, protonation of the pH sensor both closes the first gate and alters the properties of the second (Schulte et al., 2001). We have shown that changes in the protonation state of the cytoplasmic pH sensor influences the pore region, particularly with respect to Ba2+ affinity. We do not know how the observed decrease in KBa might relate to a greater sensitivity to external K+ removal. Rather, we regard this finding as a proof of principle that alterations in ion (Ba2+) binding near the selectivity filter can be affected by alterations in the pH sensor or the pH gate of the channel.
In summary, we present evidence that channel inactivation induced by low pHi involves parts of the second transmembrane domain at or near its cytoplasmic end. The crucial residues identified could participate in the opening or closure of an inner pH-sensitive gate. Removal of extracellular K+ can also inactivate the channels through effects which are exerted on the selectivity filter within the outer part of the pore. This is consistent with the existence of a separate gate or gating process which is coupled to the first. This coupling involves a propagated change in the protein in which changes in the properties of the selectivity filter are triggered by an intracellular pH-sensor.
Acknowledgments
We thank Dr. Eric Walters for his assistance in drawing Fig. 13.
This work was supported by National Institutes of Health grants DK27847 (L.G. Palmer) and DK46950 (H. Sackin). Anke Dahlmann was supported by fellowship Da536/1-1 from the Deustche Forschungsgemeinschaft.
Michael D. Cahalan served as guest editor.
References
- Armstrong, C.M. 1971. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axon. J. Gen. Physiol. 58:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanchevalap, S., Z. Yang, N. Cui, Z. Qu, G. Zhu, C. Liu, L.R. Giwa, L. Abdulkadir, and C. Jiang. 2000. Involvement of histidine residues in proton sensing of ROMK1 channel. J. Biol. Chem. 275:7811–7817. [DOI] [PubMed] [Google Scholar]
- Chepilko, S., H. Zhou, H. Sackin, and L.G. Palmer. 1995. Permeation and gating properties of a cloned renal K+ channel. Am. J. Physiol. 268:C389–C401. [DOI] [PubMed] [Google Scholar]
- Choe, H., L.G. Palmer, and H. Sackin. 1999. Structural determinants of gating in inward-rectifier K+ channels. Biophys. J. 76:1988–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe, H., H.S. Sackin, and L.G. Palmer. 2000. Permeation properties of inward-rectifier potassium channels and their molecular determinants. J. Gen. Physiol. 115:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe, H., H. Zhou, L.G. Palmer, and H. Sackin. 1997. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance and gating. Am. J. Physiol. 273:F516–F529. [DOI] [PubMed] [Google Scholar]
- Costa, A., J. Patrick, and J. Dani. 1994. Improved technique for studying ion channels expressed in Xenopus oocytes, including fast superfusion. Biophys. J. 67:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, T., B. Fakler, J.H. Schultz, U. Schulte, U. Brändle, S. Weidemann, H.P. Zenner, F. Lang, and J.P. Ruppersberg. 1996. Extracellular K+ and intracellular pH allosterically regulate renal Kir1.1 channels. J. Biol. Chem. 271:17261–17266. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., J.M. Cabral, R.A. Pfuetzerl, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Enkvetchakul, D., G. Loussouarn, E. Makhina, S.L. Shyng, and C.G. Nichols. 2000. The kinetic and physical basis of KATP channel gating: toward a unified molecular understanding. Biophys. J. 78:2334–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler, B., J. Schultz, J. Yang, U. Schulte, U. Brandle, H.P. Zenner, L.Y. Jan, and J.P. Ruppersberg. 1996. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J. 15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Ficker, E., M. Taglialatela, B.A. Wible, C.M. Henley, and A.M. Brown. 1994. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 266:1068–1072. [DOI] [PubMed] [Google Scholar]
- Holmgren, M., P.L. Smith, and G. Yellen. 1997. Trapping of organic blockers by closing of voltage-dependent K+ channels: Evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., and R. MacKinnon. 2000. The barium site in a potassium channel by x-ray crystallography. J. Gen. Physiol. 115:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, T., L. Peng, T. Mirshani, T. Rohacs, K.W. Chan, R. Sanchez, and D.E. Logothetis. 2002. The βγ subunits of G proteins gate a K+ channel by pivoted bending of a transmembrane segment. Mol. Cell. 10:469–481. [DOI] [PubMed] [Google Scholar]
- Kuo, A., J.M. Gulbis, J.F. Antcliff, T. Rahman, E.D. Lowe, J. Zimmer, J. Cuthbertson, F.M. Ashcroft, T. Ezaki, and D.A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- Leipzinger, J., G.G. MacGregor, G.J. Cooper, J. Xu, S.C. Hebert, and G. Giebisch. 2000. PKA site mutations of ROMK2 channels shift the pH dependence to more alkaline values. Am. J. Physiol. 279:F919–F926. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M.E. Jurman, and G. Yellen. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867. [DOI] [PubMed] [Google Scholar]
- Lopatin, A.N., E.N. Makhina, and C.G. Nichols. 1994. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 372:366–369. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo, J., and R.W. Aldrich. 1993. Effects of external cations and mutations on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71. [PubMed] [Google Scholar]
- Malnic, G., S. Muto, and G. Giebisch. 2000. Regulation of potassium excretion. The Kidney: Physiology and Pathophysiology. D.W. Seldin and G. Giebisch, editor. Lippincott, Williams & Wilkins, Philadelphia, PA. 1575–1613.
- Matsuda, H., A. Saigusa, and H. Irisawa. 1987. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 325:156–159. [DOI] [PubMed] [Google Scholar]
- McNicholas, C.M., G.G. MacGregor, L.D. Islas, Y. Yang, S.C. Hebert, and G. Giebisch. 1998. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am. J. Physiol. 275:F972–F981. [DOI] [PubMed] [Google Scholar]
- Perozo, E., D.M. Cortes, and L.G. Cuello. 1999. Structural rearrangements underlying K+ channel activation gating. Science. 285:73–78. [DOI] [PubMed] [Google Scholar]
- Perozo, E., D.M. Papazian, E. Stefani, and F. Bezanilla. 1992. Gating currents in Shaker K+ channels. Implications for activation and inactivation models. Biophys. J. 62:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, L.R., D. Enkvetchakul, and C.G. Nichols. 2003. Gating dependence of inner pore access in inward rectifier K+ channels. Neuron. 37:953–962. [DOI] [PubMed] [Google Scholar]
- Phillips, L.R., and C.G. Nichols. 2003. Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore. J. Gen. Physiol. 122:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin, H., L.G. Palmer, and M. Krambis. 2004. Potassium dependent slow inactivation of Kir1.1 (ROMK) channels. Biophys. J. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin, H., S. Syn, L.G. Palmer, H. Choe, and E. Walters. 2001. Regulation of ROMK by extracellular cations. Biophys. J. 80:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin, H., A. Vasilyev, L.G. Palmer, and M. Krambis. 2003. Permeant cations and blockers modulate pH gating of ROMK channels. Biophys. J. 84:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadja, R., K. Smadja, N. Alagen, and E. Reuveny. 2001. Coupling Gβγ-dependent activation to channel opening via pore elements in inwardly rectifying potassium channels. Neuron. 29:669–680. [DOI] [PubMed] [Google Scholar]
- Schulte, U., H. Hahn, M. Konrad, N. Jeck, C. Derst, K. Wild, S. Weidemann, P. Ruppersberg, B. Fakler, and J. Ludwig. 1999. pH gating of ROMK (Kir1.1) channels; Control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc. Natl. Acad. Sci. USA. 96:15298–15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte, U., S. Weidemann, J. Ludwig, J. Ruppersberg, and B. Fakler. 2001. K-dependent gating of Kir1.1 channels is linked to pH gating through a conformational change in the pore. J. Physiol. 534:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, K.S., B.S. Rothberg, and G. Yellen. 2001. Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. J. Gen. Physiol. 117:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus, J.G., L. Kuschel, M.D. Rayner, and S.H. Heinemann. 1997. Ion conduction through C-type inactivated Shaker channels. J. Gen. Physiol. 110:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela, M., L. Toro, and E. Stefani. 1992. Novel voltage clamp to record small, fast currents from ion channels expressed in Xenopus oocytes. Biophys. J. 61:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela, M., B.A. Wible, R. Caporaso, and A.M. Brown. 1994. Specification of pore properties by the carboxyl terminus of inwardly rectifying K+ channels. Science. 264:844–847. [DOI] [PubMed] [Google Scholar]
- Tsai, T.D., M.E. Shuck, D.P. Thompson, M.J. Bienkowski, and K.S. Lee. 1995. Intracellular H+ inhibits a cloned rat kidney outer medulla K+ channel expressed in Xenopus oocytes. Am. J. Physiol. 268:C1173–C1178. [DOI] [PubMed] [Google Scholar]
- Tucker, S.J., F.M. Gribble, P. Proks, S. Trapp, T.J. Ryder, T. Haug, F. Reimann, and F.M. Ashcroft. 1998. Molecular determinants of K-ATP channel inhibition by ATP. EMBO J. 17:3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg, C.A. 1987. Inward rectificatin of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc. Natl. Acad. Sci. USA. 84:2560–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Y.N. Jan, and L.Y. Jan. 1995. Control of rectification and permeation in residues in two distinct domains in an inward rectifier K+ channel. Neuron. 14:1047–1054. [DOI] [PubMed] [Google Scholar]
- Yi, B.A., Y.-F. Lin, Y.N. Jan, and L.Y. Jan. 2001. Yeast screen for constitutively active mutant G protein-activated potassium channels. Neuron. 29:657–667. [DOI] [PubMed] [Google Scholar]
- Zhou, H., S. Chepilko, W. Schutt, H. Choe, L.G. Palmer, and H. Sackin. 1996. Mutations in the pore region of ROMK enhance Ba2+ block. Am. J. Physiol. 271:C1949–C1956. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]