Abstract
Developmental changes in the regulation of smooth muscle contraction were examined in urinary bladder smooth muscle from mice. Maximal active stress was lower in newborn tissue compared with adult, and it was correlated with a lower content of actin and myosin. Sensitivity to extracellular Ca2+ during high-K+ contraction, was higher in newborn compared with 3-wk-old and adult bladder strips. Concentrations at half maximal tension (EC50) were 0.57 ± 0.01, 1.14 ± 0.12, and 1.31 ± 0.08 mM. Force of the newborn tissue was inhibited by ∼45% by the nonmuscle myosin inhibitor Blebbistatin, whereas adult tissue was not affected. The calcium sensitivity in newborn tissue was not affected by Blebbistatin, suggesting that nonmuscle myosin is not a primary cause for increased calcium sensitivity. The relation between intracellular [Ca2+] and force was shifted toward lower [Ca2+] in the newborn bladders. This increased Ca2+ sensitivity was also found in permeabilized muscles (EC50: 6.10 ± 0.07, 5.77 ± 0.08, and 5.55 ± 0.02 pCa units, in newborn, 3-wk-old, and adult tissues). It was associated with an increased myosin light chain phosphorylation and a decreased rate of dephosphorylation. No difference was observed in the myosin light chain phosphorylation rate, whereas the rate of myosin light chain phosphatase–induced relaxation was about twofold slower in the newborn tissue. The decreased rate was associated with a lower expression of the phosphatase regulatory subunit MYPT-1 in newborn tissue. The results show that myosin light chain phosphatase activity can be developmentally regulated in mammalian urinary bladders. The resultant alterations in Ca2+ sensitivity may be of importance for the nervous and myogenic control of the newborn bladders.
Keywords: myosin light chain kinase, myosin light chain phosphatase, MYPT-1, nonmuscle myosin
INTRODUCTION
During development from fetal to adult life, the urinary bladder gradually establishes its adult structure and functional properties. The cellular and integrative processes controlling micturition develop gradually, and since micturition is essential, they have to be functionally coordinated during development. The micturition is a complex process involving sensory input, motor impulses, and reflex coordination, as well as receptor activation and contraction of the detrusor smooth muscle (Andersson and Hedlund, 2002; Andersson and Arner, 2004). The intrinsic smooth muscle contractile activity may be essential for efficient bladder emptying before maturation of the neural voiding mechanisms (Szell et al., 2003), and the cellular properties of the detrusor muscle are thus of key importance for appropriate bladder function. In a study by Szell et al. (2003), it was demonstrated that the coordination of the spontaneous contractile activity was altered in the rat bladder during development after birth. Although these changes might reflect developmental aspects of pacemaker cells and membrane properties, the contractile activity is also dependent on the characteristics of the contractile system and its regulation. At present, very little is known regarding the developmental aspects of these cellular processes in the urinary bladder.
During development, the motor proteins of the detrusor smooth muscle change from a fetal to an adult phenotype. Newborn and fetal bladder tissue contain slower nonmuscle myosins, which may contribute to slow contractions under special conditions (Morano et al., 2000; Lofgren et al., 2003), however the smooth muscle myosin heavy chain seems to be the predominant contractile protein after birth. The relative expression of the spliced isoforms of the smooth muscle heavy chain changes during development. In the rabbit bladder (Lin et al., 2000), the SM1/SM2 myosin heavy chain ratio gradually decreases during fetal development, and SM2 remains the dominant isoform during adult life. This change in isoforms most likely does not affect the contractile kinetics (Andersson and Arner, 2004). The myosin heavy chain containing the 7-amino acid insert (SM-B) seems to be dominant during fetal development in various rat smooth muscles (White et al., 1998). In the bladder, high levels of SM-B have been reported to be expressed throughout the development and in adults (White et al., 1998; Babu et al., 2000). In smooth muscle from chicken gizzard, the essential myosin light chains switch from the predominant LC17b type toward more of the LC17a isoform during development, which is correlated with development of a phasic contractile phenotype (Fisher et al., 1997; Ogut and Brozovich, 2000). Whether such changes also occur in mammalian muscles is to our knowledge not reported.
The contractile properties of smooth muscle are also dependent on the level of activation. The urinary bladder of the newborn rabbit is more sensitive to extracellular Ca2+ and to a cholinergic agonist compared with adult bladders (Zderic et al., 1991). This alteration was explained by differences in intracellular Ca2+ levels (Zderic et al., 1991).
Although variation in free [Ca2+] is the main regulator of smooth muscle force, modulation of the calcium sensitivity is an important additional mechanism (Somlyo and Somlyo, 2003). The sensitivity to Ca2+ can be varied by changing the activities of the myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP) (Somlyo and Somlyo, 2003) or via secondary regulatory mechanisms acting independently of changes in myosin phosphorylation. Two different forms of the MLCK (Kamm and Stull, 2001) are known to be present in smooth muscle. The “long” form, normally not expressed in adult smooth muscle tissue, is present in embryonic smooth muscle cells and nonmuscle cells. The functions of the MLCK isoforms during development of smooth muscle are not known in detail. Different MLCP isoforms are also expressed during development of smooth muscle (Khatri et al., 2001). In chicken gizzard, a comparatively fast smooth muscle, a switch in the expression of the myosin targeting subunit (MYPT-1) from the M133 isoform to the M130 form occurs at ED (embryonic day) 20 (Ogut and Brozovich, 2000; Khatri et al., 2001). Dephosphorylation of regulatory light chains was faster at ED 20 than at ED 16 in the gizzard (Ogut and Brozovich, 2000), possibly associated with the change in MLCP isoforms. Such developmental changes have not been characterized in phasic mammalian smooth muscle.
The urinary bladder of the mouse is comparatively easily isolated and examined in mechanical and biochemical experiments. We have used this tissue to address the following questions regarding the development of smooth muscle calcium regulation: (1) are the intracellular Ca2+ levels in relaxed and activated states different in newborn and adult bladder smooth muscle; (2) is the Ca2+ sensitivity of contraction different; and, if so, (3) which mechanism can explain the altered Ca2+ sensitivity?
We show, using intact isolated mouse bladder muscle strips and intracellular Ca2+ measurements, that the Ca2+ sensitivity of the contractile system is increased in newborn bladders. Experiments on chemically permeabilized preparations revealed an increased Ca2+ sensitivity associated with an increased myosin light chain phosphorylation and a lower MLCP activity in the newborn tissue. These changes were associated with a lower expression of the MYPT-1 phosphatase subunit, suggesting that a regulated expression of phosphatase is responsible for modulation of calcium sensitivity and contractility during urinary bladder development.
MATERIALS AND METHODS
Animals and Preparations
Adult (10–14 wk, weight ∼30 g), 3-wk-old (∼13 g), and newborn (0–2 d, ∼2 g) NMRI mice (B&K AB) were euthanized by cervical dislocation. The experiments were approved by the Animal Ethics Committee at Lund University.
The bladders were removed and transferred to cold HEPES-buffered solution with the following composition (in mM): NaCl 118, KCl 5, Na2HPO4 1.2, MgCl2 1.2, HEPES 24, glucose 10, and CaCl2 1.6, pregassed with 100% O2 (pH 7.4).
Smooth muscle strips (∼0.5 mm wide and 3–4 mm long) were dissected from the mid-portion of the urinary bladder in the circular direction, and the mucosa was gently removed from strips taken from 3-wk-old and adult bladders. The mucosa was left intact in strips from the newborn bladders since it was very thin. Intact strips were mounted for recording of force and intracellular [Ca2+]. Preparations were chemically permeabilized for measurement of calcium sensitivity and myosin light chain phosphorylation.
Force and Intracellular Ca2+ in Intact Muscle
The muscle preparations were tied with silk tread and attached to a steel rod at one end and to a force transducer (Grass FT03C; Grass Medical Instruments) at the other end, in an open organ bath (50 ml) at 37°C. The preparations were stretched to 150% of the initial length and allowed to equilibrate for 30 min in a Krebs solution with the following composition (in mM): NaCl 118, glucose 11.5, KCl 4.7, NaHCO3 25, KH2PO4 1.2, MgCl2 1.2, and CaCl2 2.5, gassed with 95% O2 and 5% CO2 to obtain a pH of 7.4. The solution also contained the muscarinic antagonist scopolamine (10−5 M), the α1 adrenoceptor antagonist prazosin (10−6 M), and α, β-methylene ATP (10−6 M), which desensitizes the P2X receptor.
The dependence of active force on extracellular [KCl] at 2.5 mM Ca2+ was determined by performing separate contractions at increasing [K+], obtained by isosmotically replacing NaCl with KCl in the Krebs solution. Initial peak force and sustained force (after 5 min) were measured.
The intracellular calcium concentration in depolarized tissue was determined using the Fura-2 technique essentially as described previously (Lucius et al., 1998). The strips were loaded for 3–4 h at 22°C in 10 μM Fura-2 a.m. (Molecular Probes), 1% DMSO, and 0.02% Pluronic F127 in HEPES-buffered solution (composition described above). The strips were then washed in the HEPES solution for 20 min. Aluminum clips were attached to the strips, and the preparations were mounted between a force transducer and a hook in an 800-μl bath (37°C) containing Krebs solution and blockers as described above. Initially, a 5-min contraction was induced by 125 mM K+ (isosmotical replacement of NaCl with KCl) in 2.5 mM Ca2+. The muscle strip was relaxed in Ca2+-free Krebs solution. The muscle was then depolarized with 125 mM K+, and CaCl2 was added cumulatively. Force and intracellular [Ca2+] were measured for 30 s at each Ca2+ level, 5 min after the addition of CaCl2 when force had reached a plateau. The free calcium concentration was calculated from the ratio (R) between the intensities at excitation wavelengths 340 and 380 nm. At the end of the experiment, the Fura-2 signal for each preparation was calibrated as described by Lucius et al. (1998). In brief, the muscle was exposed to ionomycin in calcium-free solution for determination of the Rmin value followed by exposure to high calcium for determination of Rmax and Mn2+ for determination of background fluorescence. No significant differences were noted in the Rmin, Rmax, and background fluorescence values between the types of muscle preparations examined.
The involvement of nonmuscle myosin in force generation was studied using the selective nonmuscle myosin inhibitor Blebbistatin, (Kovacs et al., 2004) in intact preparations from newborn and adult mice. Muscle strips were mounted and activated with high K+ as described above. Blebbistatin (10−5 M) was added after an initial control contraction, and the preparations were allowed to equilibrate with the blocker for 20 min before a calcium dose–response curve was performed. Control experiments in the absence of Blebbistatin were performed in parallel. The force 5 min after addition of calcium was measured. In similar experiments, the effects of the MLCK inhibitor ML-9 (10−5 M) and of the Rho kinase inhibitor Y27632 (10−5 M) were examined. Blebbistatin and ML-9 were obtained from Calbiochem.
Force and Ca2+ Sensitivity of Chemically Permeabilized Muscle
Preparations were chemically permeabilized using 1% Triton X-100 as described previously (Arner and Hellstrand, 1985). The permeabilized preparations were mounted, using aluminum foil clips, in 0.5-ml plastic cups at 22°C in a standard solution containing (in mM) 30 TES, 4 EGTA, 2 free Mg2+, 3.2 MgATP, 12 phosphocreatine. Calmodulin (0.5 μM) and creatine kinase (0.5 mg/ml) were also added. Rigor solutions were made by omitting ATP, phosphocreatine, and creatine kinase. The pH was adjusted to 6.9 using KOH, and the ionic strength was adjusted to 150 mM using KCl. To determine the Ca2+ sensitivity, the muscles were activated in solutions with increasing Ca2+ concentrations obtained by varying the ratio of CaEGTA/EGTA. The free [Ca2+] and the composition of the solutions were calculated as described previously (Arner, 1983).
Absolute force was measured in permeabilized ATP-γ-S–activated fibers. The preparations were mounted as described above and incubated in ATP-free activating solution (pCa 4.5) with 0.5 μM calmodulin and 2 mM ATP-γ-S for 10 min. The preparations were then contracted in ATP-containing solution with 0.5 mg/ml creatine kinase. The maximal force 5 min after activation was recorded. The length and weight of the preparations were measured and absolute stress (force/area) was calculated.
Quantification of Regulatory Myosin Light Chain Phosphorylation
The extent of myosin light chain phosphorylation was determined at intermediate Ca2+ (pCa 5.8) in newborn, 3-wk-old, and adult tissue. The preparations were mounted at fixed length, and force was determined in separate preparations in parallel. The muscle preparations were quickly frozen in liquid nitrogen and fixed in 15% trichloroacetic acid containing 4 mM pyrophosphate. The preparations were washed with acetone and kept at −80°C until subjected to urea/glycerol gel electrophoresis essentially as described by Weber et al. (1999), using a Bio-Rad Laboratories MiniGel system. The proteins were transferred to PVDF membranes, and the light chains were labeled using a mouse monoclonal myosin regulatory light chain antibody (Sigma-Aldrich) and visualized using the enhanced chemiluminescence (ECL) method (Santa Cruz Biotechnology). Analysis was performed using Quantity One software (Bio-Rad Laboratories). The intensity of the bands corresponding to the unphosphorylated (LC) and phosphorylated (LCP) forms of the 20-kD myosin light chain was evaluated, and the relative myosin light chain phosphorylation was expressed as the ratio LCP/(LC+LCP).
The rate of dephosphorylation was measured in permeabilized tissue during relaxation in calcium-free standard solution after a maximal contraction in high calcium standard solution (pCa 4.5). Tissue samples from adult and newborn tissue were quickly frozen as described above at time 0 (immediately before relaxation) and after 15 s, 30 s, and 1 min in calcium-free solution (pCa 9.0). The preparations were treated and subjected to urea/glycerol gel electrophoresis as described above. The ratio of LCP/(LC+LCP) was expressed relative to the value at time 0.
Content of MYPT-1, Myosin, and Actin
The amounts of the contractile proteins actin and myosin and of the regulatory phosphatase subunit MYPT-1, were estimated by SDS-PAGE gel electrophoresis and Western blot. Intact bladder tissue was weighed and homogenized in a sample buffer solution (50 μl buffer/mg tissue) containing 25 mM Tris-HCl (pH 6.8), 10% glycerol, 5% mercaptoethanol, 1 mg/ml bromophenol blue, and 2% SDS. The samples were boiled for 2 min and then briefly centrifuged.
8% polyacrylamide gels were performed to quantify the contents of myosin and actin. A standard with known concentration of skeletal muscle actin was used to enable quantification of the myosin and actin contents. Three different amounts of standard and sample were loaded on each gel. The proteins were stained with Coomassie blue, and the protein bands were analyzed using Quantity One software.
For MYPT-1, the proteins were separated on 10% polyacrylamide gels (two gels with identical samples were run in parallel) and blotted onto PVDF membranes. The regulatory light chains (LC) were labeled on one gel as described above. For MYPT-1 detection on the parallel gel, a goat polyclonal antibody was used (Santa Cruz Biotechnology). Analysis was performed using Quantity One software. The relative content of MYPT-1/LC was calculated.
Estimations of MLCP and MLCK Activities
The half-time for relaxation (τ1/2) in Ca2+-free solution was measured during relaxation at pCa 9.0 after a maximal contraction (pCa 4.5) in Triton-permeabilized muscle strips from newborn and adult mice.
The MLCP activity was estimated by measuring the rate of dephosphorylation after treatment with ATP-γ-S. ATP-γ-S leads to thiophosphorylated light chains, which are slowly dephosphorylated by MLCP (Jaworowski et al., 1999). Muscle strips were first fully thiophosphorylated for 15 min in ATP-free Ca2+-containing rigor solution with 2 mM ATP-γ-S and 0.5 μM calmodulin and then contracted in Ca2+-free standard solution. This resulted in a maximal contraction. The subsequent slow force decay during 1 h was considered a measure of the MLCP activity. Control experiments showed that the rate of relaxation was significantly slower in the presence of the phosphatase inhibitor microcystin LR (30 nM), showing that the force decay was due to MLCP activity. Force values after 60 min of dephosphorylation in the absence and presence of microcystin LR were evaluated.
To estimate the MLCK activity, preparations were mounted as described above and treated for 3 min in a rigor solution without ATP, phosphocreatine, and creatine kinase. The solution contained high [Ca2+] (pCa 4.5), 0.5 μM calmodulin, and 2 mM ATP-γ-S. After wash in Ca2+-free rigor solution, the preparations were contracted with ATP-containing standard solution. The ATP-γ-S activation was repeated using 15 min treatment followed by a second contraction in ATP-containing solution. Then, the muscles were maximally activated for 30 min in ATP-γ-S and in the presence of the phosphatase inhibitor microcystin LR (30 nM), before the third contraction. Since the phosphatase activity is low toward thiophosphorylated light chains, the resulting force induced by ATP after each period in ATP-γ-S was taken as a measure of the extent of myosin light chain phosphorylation occurring during the treatment periods.
In one series of experiments, newborn and adult urinary bladder tissue were mounted for force recording and permeabilized with Staphylococcus Aureus α-toxin (Calbiochem, EMD Bioscience) 5,000–10,000 units for 60 min as described previously (Bonnevier et al., 2004). Force was recorded at suboptimal (pCa 5.5) and maximal (pCa 4.5) [Ca2+], using the solutions described above (no creatine kinase was added).
Statistics
Values are shown as mean ± SEM with the number of observations shown in parenthesis. When more than one group was compared, Bonferroni corrections of P values in the Student's t test were performed. Statistical analysis and curve fitting were performed using Sigma Plot and Sigma Stat for Windows (SPSS Science).
RESULTS
Relations between Force and [Ca2+]
In an initial series of experiments, the dependence of force on level of depolarization was examined in experiments at varied extracellular [K+]. The muscles were activated at 2.5 mM CaCl2 in separate contractions at increasing KCl concentrations. Force at the peak and during the sustained phase (5 min after activation) were recorded. The dependence of both peak and sustained force on [KCl] was similar in all groups of tissue. The concentration of KCl to elicit half-maximum tension was ∼50 mM KCl (peak force: newborn, 51 ± 3.6, n = 7; 3 wk, 44 ± 1.7, n = 6; adult, 53 ± 3.5, n = 5; sustained force: newborn, 56 ± 4.6, n = 7; 3 wk:, 48 ± 1.9, n = 6; adult, 47 ± 1.0 mM, n = 5). The responses saturated at concentrations >80 mM KCl for the adult tissue, whereas maximal force was obtained at higher KCl in the newborn (at or >100 mM KCl). In subsequent experiments we therefore used 125 mM KCl to ensure maximal depolarization.
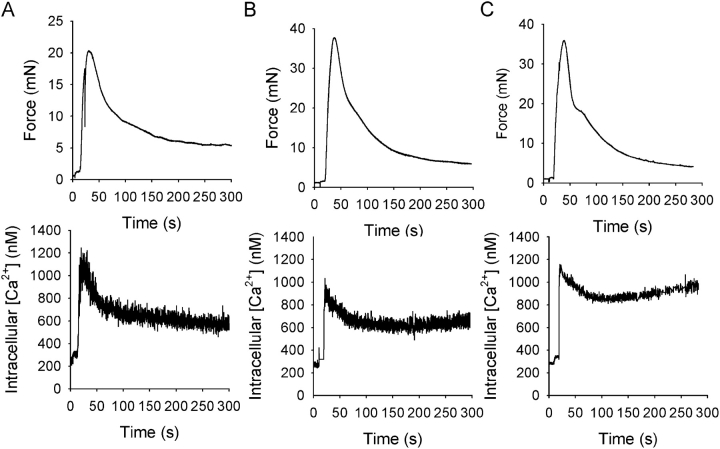
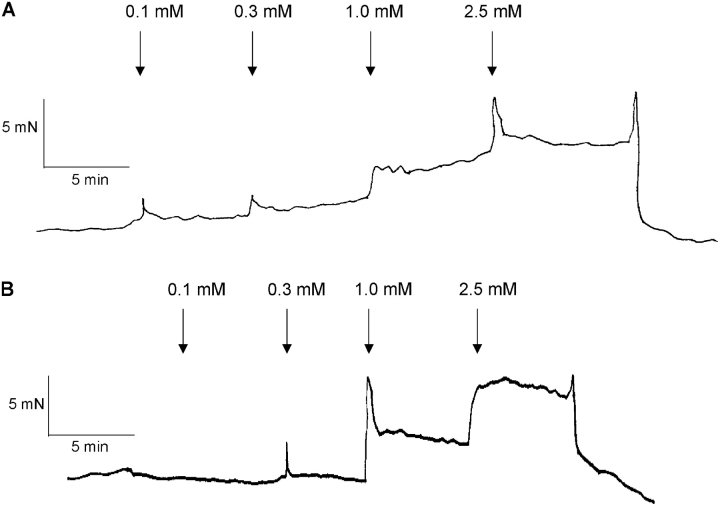
Fig. 1 shows original recordings of force and intracellular [Ca2+] in urinary bladder muscle strips from newborn, 3-wk-old, and adult mice. Activation by depolarization (125 mM KCl) was associated with a rapid increase in intracellular [Ca2+] and contraction. Since the initial peaks in intracellular [Ca2+] and force reflect a nonsteady state situation where force and [Ca2+] levels are out of phase, we chose to measure the Ca2+ sensitivity during the sustained, tonic, phase of contraction where both force and intracellular [Ca2+] levels have stabilized. To examine the relationship between extracellular [Ca2+], intracellular [Ca2+], and force, muscles were activated by depolarization with 125 mM KCl and exposed to increasing extracellular [Ca2+] (0.1, 0.3, 1.0, and 2.5 mM). Fig. 2 shows original recordings of force in depolarized muscles at different [Ca2+]. In the newborn muscle strips, force developed at lower extracellular [Ca2+].
Figure 1.
Original recordings showing force (top) and intracellular [Ca2+] transients (bottom) following activation with 125 mM KCl at 2.5 mM Ca2+ in intact urinary bladder preparations from newborn (A), 3-wk-old (B), and adult (C) mice. Mean values of intracellular [Ca2+] before initial contraction, 327 ± 58.9, 335 ± 31.2, and 469 ± 52.8 nM; at peak of contraction, 871 ± 105, 944 ± 118, and 1142 ± 239 nM; and during the sustained phase, 496 ± 35.0, 837 ± 132, and 916 ± 139 nM, in newborn, 3-wk-old, and adult mice, respectively (n = 4–5).
Figure 2.
Original recording of force in a newborn (A) and an adult (B) intact bladder muscle strip. The muscles were depolarized with 125 mM KCl and exposed to increased extracellular Ca2+ concentrations.
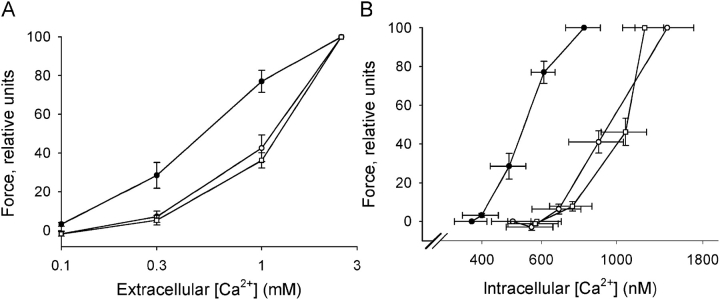
Fig. 3 shows summarized data regarding relationships between extracellular [Ca2+], force, and intracellular [Ca2+]. The dependence of force on extracellular [Ca2+] was shifted toward lower Ca2+ concentrations in the urinary bladder from the newborn mice, suggesting an increased Ca2+ sensitivity (Fig. 3 A). The half-maximal tension was observed at 0.57 ± 0.01 in newborn (n = 4), 1.14 ± 0.12 in 3 wk old (n = 5), and 1.31 ± 0.08 mM [Ca2+] in adult (n = 4) mice. The value of the newborn mice was significantly lower than both 3-wk-old and adult mice (P < 0.05, Bonferroni correction). Fig. 3 B shows force relative to intracellular [Ca2+]. The relationship for the newborn bladders was shifted toward lower intracellular [Ca2+]. Half-maximal force was obtained at 532 ± 38 and at 1002 ± 124 nM [Ca2+] (P < 0.05) in tissue from newborn (n = 4) and older mice (3-wk-old and adult mice, n = 9), respectively. These data show that the increased sensitivity to extracellular [Ca2+] of tissue from newborn mice reflects an altered dependence of force on intracellular [Ca2+], i.e., an increased Ca2+ sensitivity of the activation of the contractile proteins.
Figure 3.
Force relative to extracellular [Ca2+] (A) during contractions initiated by increasing [Ca2+] at 125 mM KCl in intact urinary bladder preparations of newborn (filled circles), 3-wk-old (open circles), and adult (open squares) mice. B shows force relative to intracellular [Ca2+]. Intracellular [Ca2+] in Ca2+-free solution (at 125 mM KCl) was 373 ± 40.9, 487 ± 80.7, and 582 ± 105 nM in newborn, 3-wk-old, and adult mice (n = 3–5).
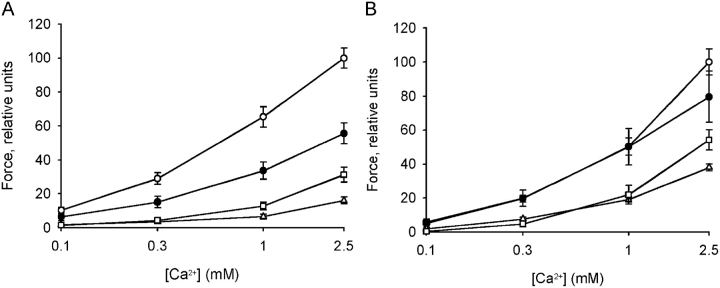
Fig. 4 shows the force in K+-depolarized muscles at different extracellular calcium concentrations. The force values were normalized to control force at 2.5 mM Ca2+ in the absence of Blebbistatin, ML-9, and Y27632. Newborn bladders had an increased sensitivity to calcium (c.f. Fig. 3 A). Blebbistatin did not influence the contraction of the adult tissue. In contrast, it inhibited force in the newborn tissue by ∼55%. This inhibitory effect was similar at all calcium concentrations, showing that inhibition by Blebbistatin does not influence calcium sensitivity. Y27632 and ML-9 inhibited contraction in both adult and newborn tissue. The effects of Y27632 and ML-9 were larger in the newborn tissue compared with adult tissue.
Figure 4.
Force relative to extracellular [Ca2+] during contractions initiated by increasing [Ca2+] at 125 mM KCl in intact urinary bladder preparations of newborn (A) and adult (B) mice. Effects of Blebbistatin (10 μM, filled circles), ML-9 (10 μM, triangles), and Y27632 (10 μM, squares) were compared with contractions in control tissue (open circles), n = 6–9.
Ca2+ Sensitivity of Force in Permeabilized Muscle
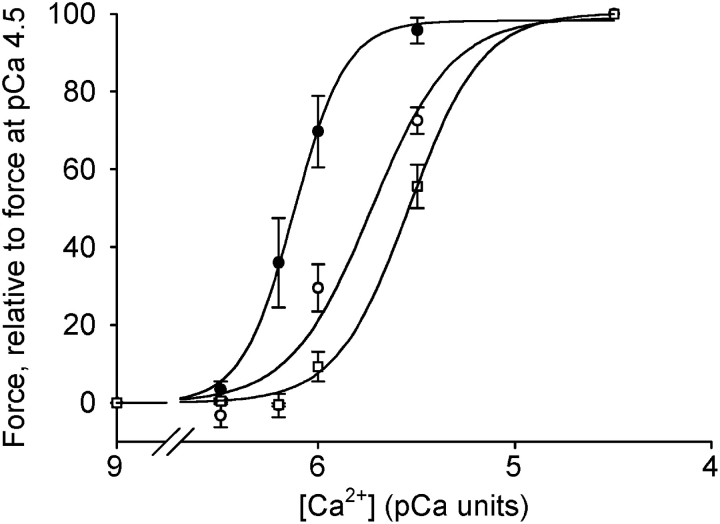
To examine the Ca2+ sensitivity at stable [Ca2+], chemically permeabilized preparations were used. Fig. 5 shows original recordings of force from permeabilized urinary bladder preparations from a newborn (A) and an adult (B) mouse at increasing free [Ca2+]. In the preparation from the newborn mouse, force developed at lower [Ca2+] compared with the preparation from the older mouse. Fig. 6 shows the summarized data for active force at different free [Ca2+]. The relation between force and [Ca2+] was significantly shifted toward lower [Ca2+] in the tissue from newborn mice. The data were analyzed using a dose–response equation (Force = Ch /(Ch + EC50 h); where C = Ca2+ concentration, h = a dimensionless constant determining the steepness of the relation, and EC50 = the concentration giving half-maximal force). EC50 (pCa units) was significantly (P < 0.05, Bonferroni correction) lower in tissue from newborn mice (6.10 ± 0.07), compared with both 3-wk-old (5.77 ± 0.08) and adult (5.55 ± 0.02) mice (n = 6). In separate experiments, we determined maximal active stress (force/cross-sectional area) in permeabilized preparations. The stress was significantly lower in newborn compared with adult tissue (13.9 ± 1.8 and 21.5 ± 2.2 mN/mm2, n = 10, P < 0.05). To examine if the difference in stress was due to altered expression of contractile proteins, we performed quantitative SDS gel electrophoresis on the tissues. The contents of actin and myosin per tissue wet weight were significantly lower in newborn tissue compared with adult (actin: 7.8 ± 1.0 and 13.2 ± 1.2 μg/mg tissue, P < 0.05; myosin: 3.2 ± 0.4 and 4.5 ± 0.3 μg/mg tissue, P < 0.01, n = 5–7).
Figure 5.
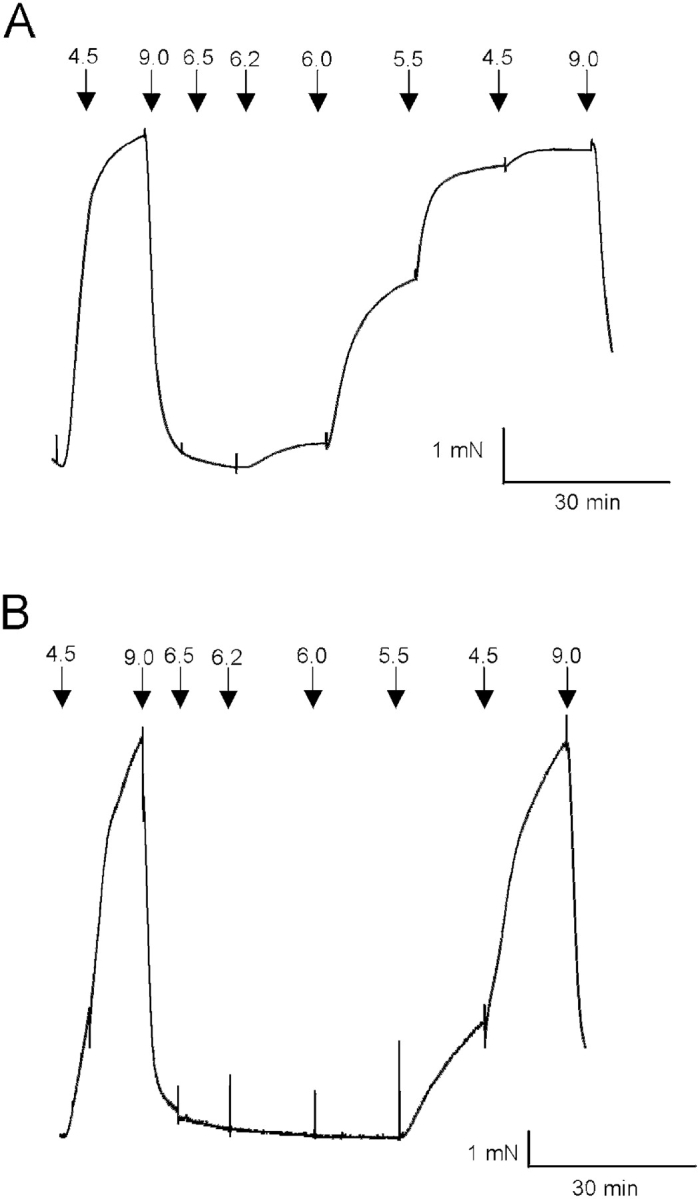
Original recordings of force in newborn (A) and adult (B) permeabilized bladder preparations. Arrows show changes to solutions with different [Ca2+] (pCa units).
Figure 6.
Measurements of force at different [Ca2+] in Triton-permeabilized bladder preparations from newborn (filled circles), 3-wk-old (open circles), and adult (open squares) mice. Force is measured relative to the force developed at pCa 4.5 (n = 6).
Quantification of LC20 Phosphorylation
The increased force at low [Ca2+] in the newborn tissue was associated with an increased myosin light chain phosphorylation. Force at intermediate [Ca2+] (pCa 5.8) relative to maximal (pCa 4.5) was ∼75% in the newborn, ∼40% in 3 wk, and ∼25% in adult preparations (compare Fig. 6). The relative myosin light chain phosphorylation, LCP/(LC+LCP), at this intermediate [Ca2+] was significantly (P < 0.05, Bonferroni correction) higher in the preparations of the newborn, compared with 3-wk-old and adult animals (newborn, 17.1 ± 2.0; 3 wk, 7.6 ± 1.2; adult, 6.2 ± 0.8%, n = 4). There were no differences in basal phosphorylation (pCa 9.0) (2.5 ± 0.8 and 2.9 ± 1.2%) and maximal relative phosphorylation (pCa 4.5) (37.7 ± 0.7 and 35.7 ± 1.2%) in newborn and adult mice (n = 4–5).
MLCK and MLCP Activities
To explore the mechanism underlying the increased Ca2+ sensitivity and myosin light chain phosphorylation, the rate of relaxation and phosphatase and kinase activities were estimated in newborn and adult tissue (Fig. 7). The relaxation from maximal contraction, when Ca2+ was decreased from pCa 4.5 to pCa 9, was significantly slower in the preparations from newborn compared with adult mice (Fig. 7, A and B). The rate of dephosphorylation was slower in the newborn tissue (Fig. 7 C).
Figure 7.
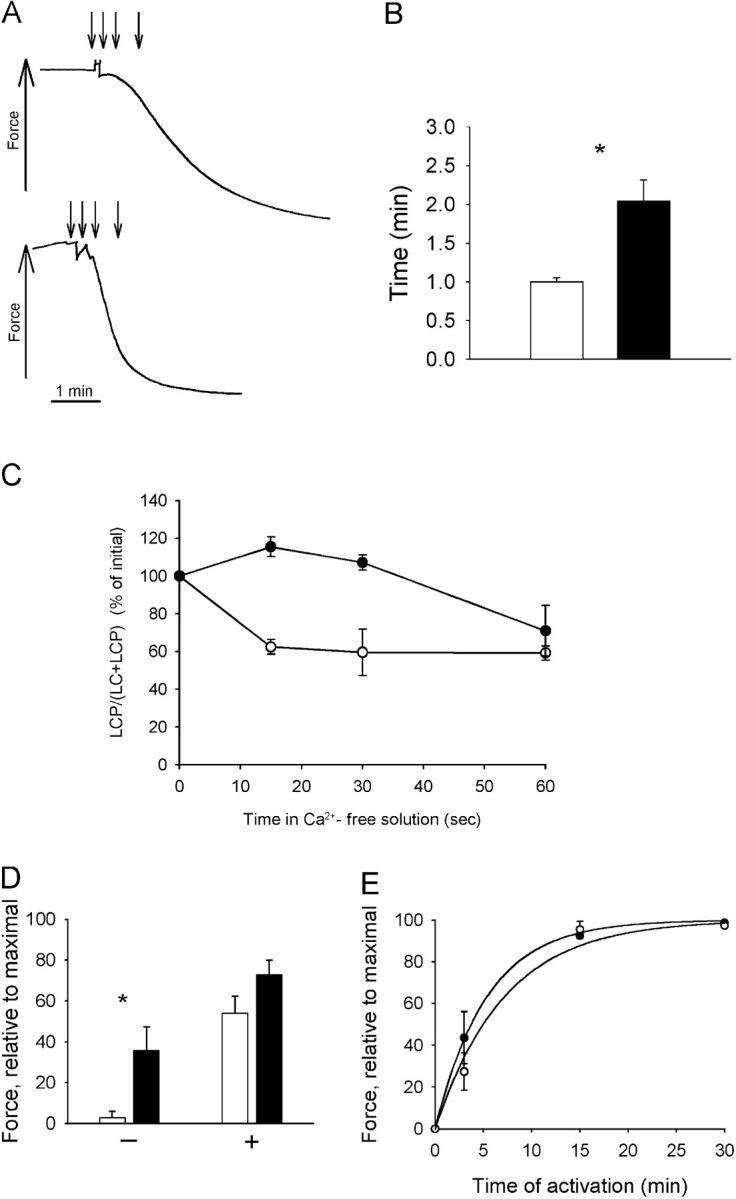
Rates of relaxation, dephosphorylation, and phosphorylation in newborn (filled symbols and bars) and adult (open symbols and bars) urinary bladder tissue. (A) Original traces of relaxation of permeabilized muscle in pCa 9.0 solution following activation and contraction in pCa 4.5 solution (newborn, top; adult, bottom). The time points 0, 15, 30, and 60 s after change of solution are indicated by errors. (B) Half time for relaxation, τ1/2 (n = 6–7 in each group) determined in experiments as shown in A. C shows myosin light chain (LC) phosphorylation at different time points (indicated in A) during relaxation (n = 4 in each group). (D) Force in thiophosphorylated muscles 1 h after maximal activation (pCa 4.5) in the presence (+) or absence (−) of microcystin LR in permeabilized bladders. Data are normalized to the initial maximal force (n = 6). (E) Force after different time in activating solution, containing ATP-γ-S in permeabilized newborn and adult mice bladders. Force is normalized to that after activation with ATP-γ-S for 30 min in high [Ca2+] (pCa 4.5) and microcystin LR. Curve fits to the mean values using an exponential function are inserted in the diagram (rates: newborn 0.14, adult 0.18 min−1, n = 5–7).
To examine the rates of dephosphorylation and phosphorylation under conditions when the rates are slower, we used ATP-γ-S thiophosphorylated muscles. Fig. 7 D shows the rate of relaxation in thiophosphorylated muscle. The measured force after 1 h (without microcystin LR) was significantly higher in newborn tissue compared with adult muscle. The relaxation of thiophosporylated muscle was slowed in the presence of microcystin LR, showing that the relaxation was due to activity of the phosphatase. The ratio of force after 1 h divided by force after 1 h plus microcystin LR was significantly higher (P < 0.05) in newborn compared with adult mice (0.51 ± 0.17 and 0.042 ± 0.02, n = 6), indicating a major difference in the phosphatase activity.
Fig. 7 E shows the extent of force after an incubation period in a solution with [Ca2+] and ATP-γ-S. This is an estimate of the rate of myosin light chain phosphorylation, corresponding to the MLCK activity. The values are normalized to the force obtained at maximal [Ca2+] in the presence of a phosphatase inhibitor (microcystin LR). As seen in the diagram, the rate of thiophosphorylation (measured as resulting force) was similar (rate constant: 0.26 ± 0.07 and 0.16 ± 0.05 min−1, n = 5 and 7) in newborn and adult tissue, suggesting that the MLCK activities are not markedly different.
In the experiments above, Triton X-100 was used, which enables full permeabilization of cellular membranes, and thus avoiding potential influence from sarcoplasmic reticulum or membrane-associated signaling. However, to exclude that the increased Ca2+ sensitivity of the permeabilized newborn urinary bladder tissue was due to the skinning procedure, we also performed one series of experiments using membrane permeabilization with Staphylococcus aureus α-toxin. Force at intermediate [Ca2+] (pCa 5.5), relative to that at optimal [Ca2+] (pCa 4.5), was significantly (P < 0.01) higher in the newborn urinary bladder compared with the adult (newborn, 46 ± 5.6%, n = 4; adult, 12 ± 3.3%, n = 4), which is consistent with the results from the Triton-permeabilized preparations.
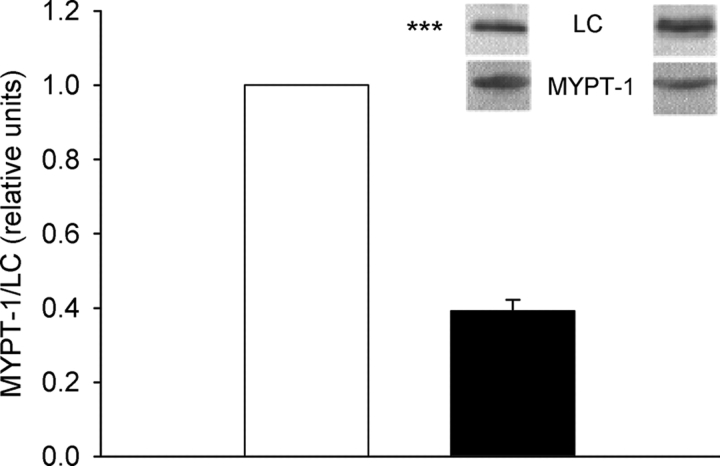
To determine the tissue content of phosphatase, we measured the amount of MYPT-1 relative to the regulatory light chains (LC) using semiquantitative Western blots. The MYPT-1/LC ratio was significantly lower in newborn tissue compared with the adult (Fig. 8). Since the myosin content was lower in the newborn tissue, the absolute MYPT-1 concentration in the tissue would be even lower than indicated by the MYPT-1/LC ratio.
Figure 8.
MYPT-1 and myosin regulatory light chain (LC) ratio in adult (open bar) and newborn (filled bar) tissue (n = 7). Samples from adult and newborn tissue were analyzed in parallel and subjected to Western blot analysis using antibodies against the regulatory light chain and the MYPT-1 subunit. The ratio MYPT-1/LC chemiluminescent signal of the adult tissue was set to 1. Photos of Western blots are presented above the bar diagram (left photos, adult; right, newborn).
DISCUSSION
We show that the contractile system of smooth muscle in urinary bladder from newborn mice has an increased sensitivity to Ca2+ compared with that of older animals. This property is associated with an increased myosin light chain phosphorylation, a lower MLCP activity, and a decreased expression of the MLCP subunit MYPT-1.
Contraction of the detrusor smooth muscle of the urinary bladder is regulated by the intracellular free Ca2+ concentration in a similar manner as in other smooth muscles. The detrusor muscle is a comparatively fast and phasic muscle where sustained depolarization results in a rapid phasic contractile response with a low sustained component (Andersson and Arner, 2004). We have examined the Ca2+ dependence of force during the sustained phase of depolarization-induced contractions, where both intracellular [Ca2+] and force levels have stabilized. Since the intracellular activation/deactivation pathways via MLCK and MLCP are modulated by a complex signaling network, primarily activated following receptor activation, we performed the experiments on intact muscle with the muscarinic, purinergic, and α-adrenergic receptors blocked. In the newborn tissue, the contents of actin and myosin were lower, which was reflected in a lower active stress generation. In addition, part of the contractile response was due to activity of nonmuscle myosin, as suggested the inhibition by Blebbistatin. These results are consistent with previous reports using smooth muscle–deficient mice (Morano et al., 2000; Lofgren et al., 2003). We find evidence for an increased sensitivity of force to extracellular Ca2+ in bladder muscle from newborn mice. This result is similar to observations in urinary bladder from newborn rabbits (Zderic et al., 1991), activated with a muscarinic agonist. In principle, the increased sensitivity to extracellular Ca2+ concentration could reflect an increased level of intracellular activator Ca2+, due to altered Ca2+ influx, release, or elimination processes. Zderic et al. (1991) reported that that Ca2+ influx was increased in bethanecol-activated bladder muscle of newborn rabbits. In contrast, we find that the increased sensitivity to extracellular Ca2+ in the depolarized muscle of newborn mice is not associated with increased intracellular [Ca2+] levels, showing that the altered dependence on extracellular [Ca2+] reflects a change in the intracellular Ca2+ sensitivity. Since the sustained phase of the depolarization-induced contractions in bladder is completely blocked by the Ca2+ channel antagonist nifedipine (Zderic et al., 1994), and thus primarily reflects influx of Ca2+, our results thus exclude major alterations in the voltage-dependent L-type channel Ca2+ influx in urinary bladder muscle from newborn mice. Although part of the contractile response in the newborn mouse bladder was due to nonmuscle myosin, inhibition of this contractile component with Blebbistatin inhibited force in the newborn tissue with an equal relative amount at all examined calcium concentrations. This suggests that the difference in calcium sensitivity between newborn and adult tissue is mainly due to the calcium sensitivity of the activation/deactivation systems rather than a selective activation of the nonmuscle contractile system at low calcium. The responses of both adult and newborn tissue were inhibited by the Rho kinase inhibitor Y27632 and MLCK inhibitor ML-9. The effects of both these compounds were slightly larger in the newborn tissue, but their effects relative to each other were similar. These results suggest that both Rho kinase and MLCK are involved in the activation of sustained contractions in both adult and newborn tissue. It is possible that kinases other than the classical MLCK contribute to contractile activation in newborn smooth muscle tissue, which could be consistent with recent data from MLCK-deficient mice (Somlyo et al., 2004).
A novel observation is that the dependence of force on free [Ca2+] is shifted toward lower concentrations in the newborn mouse bladder. The increased Ca2+ sensitivity is also evident in gently permeabilized preparations (α-toxin treated) at fixed Ca2+ concentrations. To explore the cellular mechanisms of this increased Ca2+ sensitivity of the contractile system activation, we performed further experiments on Triton X-100 permeabilized muscle. In these preparations, the main modulatory mechanisms associated with receptor activation, e.g., Rho/Rho-kinase and protein kinase C, are absent, whereas the Ca2+-calmodulin–dependent myosin light chain phosphorylation, via MLCK, and the relaxation via MLCP are operational. In this model, we find a leftward shift in the Ca2+–force relationship of newborn bladders, suggesting that the increased Ca2+ sensitivity is associated with changes at the level of the contractile proteins and not primarily with alterations in receptor-mediated sensitization.
We find that the increased Ca2+ sensitivity is associated with increased myosin light chain phosphorylation. To examine the MLCK activity, we used an experimental model, where ATP-induced force after treatment with Ca2+/calmodulin and ATP-γ-S is considered to reflect the MLCK-induced (thio)phosphorylation. The rate of MLCK activity was not markedly different in newborn and adult mice, although a small difference cannot be excluded. Both the rate of relaxation following a Ca2+-induced contraction in the presence of ATP, the rate of dephosphorylation of the light chains, and the rate of relaxation of thiophosphorylated muscle were significantly slower in newborn bladder tissue. These results show that the MLCP activity is lower in newborn tissue, which provides a mechanism for the increased Ca2+ sensitivity.
The urinary bladder phosphatase is of the PP1M type (Shirazi et al., 1994) and is composed of three parts: the catalytic (PP1c), the regulatory (MYPT-1), and the 20-kD subunits (Somlyo and Somlyo, 2003). An additional regulatory peptide (CPI-17) modulates the activity and is of key importance for PKC-induced inhibition of the MLCP (Kitazawa et al., 2000). Since CPI-17 is lost during Triton permeabilization (Kitazawa et al., 1999), alterations in this regulatory component are not involved in the lower MLCP activity of newborn bladders. The MYPT subunit is considered to be involved in the regulation of the MLCP activity. Phosphorylation of a Thr site on MYPT-1, by e.g., Rho-kinase and zip-like kinase, has been shown to affect the MLCP activity (Borman et al., 2002; Somlyo and Somlyo, 2003). As discussed above, these mechanisms cannot be primarily involved in the lower MLCP activity of newborn bladders since the increased Ca2+ sensitivity is evident in the Triton-permeabilized preparations. Differential expression of alternatively spliced MYPT isoforms has been found during development in chicken smooth muscle (Ogut and Brozovich, 2000; Khatri et al., 2001). During early embryonic stages of the gizzard, the isoform lacking a leucine zipper sequence and reactivity to cGMP-dependent kinase is expressed (Khatri et al., 2001). Similar expression pattern was not found in the slower tonic muscles of the chicken aorta and of the mammalian (rat) aorta. The phasic bladder muscle is probably more similar to the phasic gizzard muscle. We find evidence that the tissue expression of the MYPT-1 isoform is significantly reduced in the newborn tissue, both when related to the phosphatase substrate (myosin light chain) and tissue wet weight. We do not have any information regarding the MYPT isoform pattern in the newborn mouse bladder. It is possible that the altered MLCP activity reflects a developmentally regulated expression of MLCP subunits in addition to a decreased total content of the enzyme, as suggested by the decreased MYPT-1 content in newborn tissue.
In conclusion, the contractile system of the urinary bladders of newborn mice has an increased sensitivity to Ca2+ caused by a lowered MLCP activity. This property would influence the bladder contractility during a time period immediately after birth, and probably also during the fetal period. The adult Ca2+ sensitivity is almost fully established within 3 wk after birth. The increased sensitivity to Ca2+ may enable contractions at lower intracellular [Ca2+], e.g., as a compensation for a less developed sarcoplasmic reticulum or not fully functional Ca2+ influx pathways in the newborn smooth muscle (Zderic et al., 1994). Since the MLCP is the main target for receptor-mediated sensitization pathways, the lower activity could also influence the receptor-mediated responses.
Acknowledgments
This study was supported by grants from the Swedish Research Council (04x-8268), the Medical Faculty, Lund University, and the Research School for Pharmaceutical Science at Lund University, Sweden.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: ED, embryonic day; LC, light chain; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase.
References
- Andersson, K.E., and A. Arner. 2004. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 84:935–986. [DOI] [PubMed] [Google Scholar]
- Andersson, K.E., and P. Hedlund. 2002. Pharmacologic perspective on the physiology of the lower urinary tract. Urology. 60:13–20. [DOI] [PubMed] [Google Scholar]
- Arner, A. 1983. Force-velocity relation in chemically skinned rat portal vein. Effects of Ca 2+ and Mg2+ Pflugers Arch. 397:6–12. [DOI] [PubMed] [Google Scholar]
- Arner, A., and P. Hellstrand. 1985. Effects of calcium and substrate on force-velocity relation and energy turnover in skinned smooth muscle of the guinea-pig. J. Physiol. 360:347–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu, G.J., D.M. Warshaw, and M. Periasamy. 2000. Smooth muscle myosin heavy chain isoforms and their role in muscle physiology. Microsc. Res. Tech. 50:532–540. [DOI] [PubMed] [Google Scholar]
- Bonnevier, J., R. Fassler, A.P. Somlyo, A.V. Somlyo, and A. Arner. 2004. Modulation of Ca2+ sensitivity by cyclic nucleotides in smooth muscle from protein kinase G-deficient mice. J. Biol. Chem. 279:5146–5151. [DOI] [PubMed] [Google Scholar]
- Borman, M.A., J.A. MacDonald, A. Muranyi, D.J. Hartshorne, and T.A. Haystead. 2002. Smooth muscle myosin phosphatase-associated kinase induces Ca2+ sensitization via myosin phosphatase inhibition. J. Biol. Chem. 277:23441–23446. [DOI] [PubMed] [Google Scholar]
- Fisher, S.A., M. Ikebe, and F. Brozovich. 1997. Endothelin-1 alters the contractile phenotype of cultured embryonic smooth muscle cells. Circ. Res. 80:885–893. [DOI] [PubMed] [Google Scholar]
- Jaworowski, A., N. Ozturk, and A. Arner. 1999. Inhibition of force and shortening in smooth muscle by vanadate. Pflugers Arch. 438:224–231. [DOI] [PubMed] [Google Scholar]
- Kamm, K.E., and J.T. Stull. 2001. Dedicated myosin light chain kinases with diverse cellular functions. J. Biol. Chem. 276:4527–4530. [DOI] [PubMed] [Google Scholar]
- Khatri, J.J., K.M. Joyce, F.V. Brozovich, and S.A. Fisher. 2001. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J. Biol. Chem. 276:37250–37257. [DOI] [PubMed] [Google Scholar]
- Kitazawa, T., M. Eto, T.P. Woodsome, and D.L. Brautigan. 2000. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J. Biol. Chem. 275:9897–9900. [DOI] [PubMed] [Google Scholar]
- Kitazawa, T., N. Takizawa, M. Ikebe, and M. Eto. 1999. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in triton X-100-demembranated rabbit arterial smooth muscle. J. Physiol. 520:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, M., J. Toth, C. Hetenyi, A. Malnasi-Csizmadia, and J.R. Sellers. 2004. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279:35557–35563. [DOI] [PubMed] [Google Scholar]
- Lin, V.K., J.B. Robertson, I.L. Lee, P.E. Zimmern, and J.D. McConnell. 2000. Smooth muscle myosin heavy chains are developmentally regulated in the rabbit bladder. J. Urol. 164:1376–1380. [PubMed] [Google Scholar]
- Lofgren, M., E. Ekblad, I. Morano, and A. Arner. 2003. Nonmuscle myosin motor of smooth muscle. J. Gen. Physiol. 121:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucius, C., A. Arner, A. Steusloff, M. Troschka, F. Hofmann, K. Aktories, and G. Pfitzer. 1998. Clostridium difficile toxin B inhibits carbachol-induced force and myosin light chain phosphorylation in guinea-pig smooth muscle: role of Rho proteins. J. Physiol. 506:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano, I., G.X. Chai, L.G. Baltas, V. Lamounier-Zepter, G. Lutsch, M. Kott, H. Haase, and M. Bader. 2000. Smooth-muscle contraction without smooth-muscle myosin. Nat. Cell Biol. 2:371–375. [DOI] [PubMed] [Google Scholar]
- Ogut, O., and F.V. Brozovich. 2000. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am. J. Physiol. Cell Physiol. 279:C1722–C1732. [DOI] [PubMed] [Google Scholar]
- Shirazi, A., K. Iizuka, P. Fadden, C. Mosse, A.P. Somlyo, A.V. Somlyo, and T.A. Haystead. 1994. Purification and characterization of the mammalian myosin light chain phosphatase holoenzyme. The differential effects of the holoenzyme and its subunits on smooth muscle. J. Biol. Chem. 269:31598–31606. [PubMed] [Google Scholar]
- Somlyo, A.P., and A.V. Somlyo. 2003. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83:1325–1358. [DOI] [PubMed] [Google Scholar]
- Somlyo, A.V., H. Wang, N. Choudhury, A.S. Khromov, M. Majesky, G.K. Owens, and A.P. Somlyo. 2004. Myosin light chain kinase knockout. J. Muscle Res. Cell Motil. 25:241–242. [DOI] [PubMed] [Google Scholar]
- Szell, E.A., G.T. Somogyi, W.C. de Groat, and G.P. Szigeti. 2003. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285:R809–R816. [DOI] [PubMed] [Google Scholar]
- Weber, L.P., J.E. Van Lierop, and M.P. Walsh. 1999. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J. Physiol. 516(Pt 3):805–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, S.L., M.Y. Zhou, R.B. Low, and M. Periasamy. 1998. Myosin heavy chain isoform expression in rat smooth muscle development. Am. J. Physiol. 275:C581–C589. [DOI] [PubMed] [Google Scholar]
- Zderic, S.A., J. Hypolite, J.W. Duckett, H.M. Snyder III, A.J. Wein, and R.M. Levin. 1991. Developmental aspects of bladder contractile function: sensitivity to extracellular calcium. Pharmacology. 43:61–68. [DOI] [PubMed] [Google Scholar]
- Zderic, S.A., U. Sillen, G.H. Liu, M.C. Snyder III, J.W. Duckett, C. Gong, and R.M. Levin. 1994. Developmental aspects of excitation contraction coupling of rabbit bladder smooth muscle. J. Urol. 152:679–681. [DOI] [PubMed] [Google Scholar]