Abstract
The hyperpolarizing receptor potential of ciliary photoreceptors of scallop and other mollusks is mediated by a cGMP-activated K conductance; these cells also express a transient potassium current triggered by depolarization. During steady illumination, the outward currents elicited by voltage steps lose their decay kinetics. One interesting conjecture that has been proposed is that the currents triggered by light and by depolarization are mediated by the same population of channels, and that illumination evokes the receptor potential by removing their steady-state inactivation. Exploiting the information that has become available on the phototransduction cascade of ciliary photoreceptors, we demonstrated that the same downstream signaling elements are implicated in the modulation of voltage-elicited currents: direct chemical stimulation both at the level of the G protein and of the final messenger that controls the light-dependent channels (cGMP) also attenuate the falling phase of the voltage-activated current. Application of a protein kinase G antagonist was ineffective, suggesting that a cGMP-initiated phosphorylation step is not implicated. To ascertain the commonality of ionic pathways we used pharmacological blockers. Although millimolar 4-aminopyridine (4-AP) suppressed both currents, at micromolar concentrations only the photocurrent was blocked. Conversely, barium completely and reversibly antagonized the transient voltage-activated current with no detectable effect on the light-evoked current. These results rule out that the same ionic pores mediate both currents; the mechanism of light modulation of the depolarization-evoked K current was elucidated as a time-dependent increase in the light-sensitive conductance that is superimposed on the inactivating K current.
Keywords: photoreceptors; CNG channel; potassium ion channels; gating, ion channels; blockers, potassium channel
INTRODUCTION
In the eyes of several marine mollusks, a distinct class of photoreceptors is found, with properties that markedly diverge from those of classical invertebrate visual receptors: the light-sensing structure is ciliary, rather than microvillar (Miller, 1958; Barber et al., 1967), the receptor potential is hyperpolarizing (McReynolds and Gorman, 1970; Mpitsos, 1973), and the transduction cascade involves mobilization of cGMP instead of signaling via the phosphoinositide pathway (Gomez and Nasi, 1995). The ion channels that underlie the photocurrent in these sensory cells have been characterized (Gomez and Nasi, 1994a), and are of special interest because they share functional properties with two superfamilies of ion channel proteins believed to have evolved from a common ancestor, namely, cyclic nucleotide–gated channels (CNGCs) and voltage-dependent potassium channels (Guy et al., 1991; Kaupp, 1991; Yau, 1994). On the one hand, they are activated by cGMP and, like other members of the CNGC family, are blocked by the organic antagonists l-cis-diltiazem and 3,4-dichlorobenzamil (Gomez and Nasi, 1997) and exhibit a pronounced outward rectification arising from voltage-dependent blockade by extracellular Ca and Mg (Nasi and Gomez, 1999). On the other hand, their pore is K selective (Gorman et al., 1982; Gomez and Nasi, 1994a), and they are susceptible to blockade by K channel antagonists, especially 4-aminopyridine (4-AP) (Gomez and Nasi, 1994b). As such, these channels seemingly bridge the gap between these two canonical classes of proteins.
The evolutionary kinship of CNGCs to voltage-dependent channels naturally raises the question of how a chemically controlled mechanism for channel opening was acquired, and whether the gating structures implicated are homologous to those operated by transmembrane potential in their ancestral cousins. In voltage-dependent potassium channels, seminal experiments by Armstrong (1971) on pore-blocker accessibility had indicated that the main gate seems to be located near the inner vestibule of the permeation pathway, a notion subsequently corroborated in cloned Shaker channels using cysteine scanning mutagenesis (Liu et al., 1997; Holmgren et al., 1998). This structural arrangement is consistent with more recent crystallographic data on a voltage-gated bacterial K channel (Jiang et al., 2003), which show a constriction formed by bundling together of multiple α-helices near the intracellular side, and has been suggested to be a design feature of considerable generality, though subject to some variations (for review see Swartz, 2004). In addition, other structural motifs participate in modulating ionic fluxes through the pore of voltage-gated channels: these include a cytosolically located tethered “ball-and-chain” (Armstrong et al., 1973), comprising the initial stretch of residues at the amino terminus of the polypeptide that mediates the rapid inactivation observed in a variety of channels known as N-type inactivation (Hoshi et al., 1990). Moreover, molecular motions involving residues in the pore loop can collapse the external entrance to the pore and quench ionic current, and are responsible for the slower C-type inactivation (Liu et al., 1996).
A tantalizing scheme has been proposed for the gating mechanism of the photocurrent in ciliary photoreceptors (Shimatani and Katagiri, 1995): the light-sensitive K conductance would be comprised of transient voltage-gated K channels akin to IA, and illumination would generate the photoresponse by removing their steady-state inactivation. The seminal observation was that membrane depolarization in the dark elicits an inactivating outward current, but if a similar voltage stimulation is applied during illumination the current becomes sustained. Moreover, application of 4-AP at millimolar concentrations suppresses both the depolarization-activated current and the photocurrent (Shimatani and Katagiri, 1995). The appeal of this conjecture is twofold: first, the explicit identity of the allosteric transitions triggered by the internal messenger for light transduction and of the proposed “moving parts” of the gating machinery; second, the evolutionary thread linking homologous functions in distantly related ion channels that have come to subserve entirely disparate functions. In the present report we examined whether a common signaling pathway is responsible for the modulation of the voltage-gated currents and the activation of the light-dependent current, and addressed in a systematic way the hypothesis that the same population of ion channels is implicated in the two cases. Preliminary aspects of this work were previously presented in abstract form (Gomez and Nasi, 2000b).
MATERIALS AND METHODS
Pecten irradians (bay scallop) were obtained from the Aquatic Resources Division of the Marine Biological Laboratory. The techniques for enzymatically isolating viable ciliary photoreceptors and performing whole-cell patch-clamp recording have been described in detail previously (Gomez and Nasi, 1994a). Cells plated in a flow chamber were continuously perfused with artificial sea water (ASW) containing (in mM) 480 NaCl, 10 KCl, 10 CaCl2, 49 MgCl2, 10 HEPES, 5.5 d-glucose, pH 7.75. The “intracellular” solution used to fill thin-wall borosilicate patch pipettes contained 100 mM KCl, 200 mM K-glutamate, 22 mM NaCl, 5 mM Mg ATP, 10 mM HEPES, 1 mM EGTA, 100 μM GTP, and 300 mM sucrose, pH 7.3 (electrode resistance 2–4 MΩ, in ASW). Series resistance was routinely compensated, and current signals were low-pass filtered at 1.5–2 kHz (−3 dB) with a Bessel 4-pole filter, before digitizing at 3–5 kHz sampling rate with 12-bit resolution.
Chemical Stimulation
The slowly hydrolyzable cyclic nucleotide analogue 8-bromo cyclic guanosine monophosphate (8-Br-cGMP) was purchased from Alexis and from Sigma-Aldrich. The PKG inhibitor KT5823 was from Calbiochem. l-cis-diltiazem was obtained form Gödecke. Apamin, guanosine 5′-O-[3-thiotriphosphate] (GTP-γ-S), and 4-AP (>99% pure) were from Sigma-Aldrich. A battery of toxins targeting different potassium channels was obtained from Alomone Labs. Intracellular application of test substances was performed by dissolving them in the internal solution and dialyzing them via the patch pipette. Rapid extracellular application entailed pressure ejection from a glass micropipette (3–4 μm tip diameter) positioned ∼50 μm from the target cell. The puffer pipette was connected to a solenoid-operated valve and a precision regulator to apply pressurized nitrogen. Alternatively, the solution in the entire recording chamber was exchanged with a flowthrough system.
Light Stimulation
The optical stimulators consisted of a 100-W tungsten-halogen light source (Oriel Corporation), a condenser, an infrared absorbing filter, an electromechanical shutter (Vincent Associates), and collimating and field lenses and filters. An adjustable pinhole or an iris diaphragm placed in a conjugated image plane restricted the illuminated region on the recording chamber to a disc ∼200 μm in diameter. The output beam was combined with that of the microscope illuminator via a beam splitter prism placed above the condenser, as previously described (Nasi, 1991). Broadband light was used in all the experiments (515–670 nm), determined the combination of a heat-absorbing filter and an edge filter (Schott Glass Technologies) interposed in the light path. Effective light intensity was calibrated in vivo by matching the photocurrent amplitude to that obtained with monochromatic light (500 nm, 3-cavity interference filter; Ditric Optics), the intensity of which was measured with a radiometer (UDT Hawthorne; model 370). Light intensity is expressed in terms of equivalent photon flux at 500 nm (Gomez and Nasi, 1994a, 1995). Calibrated neutral-density filters (Melles Griot) provided controlled light attenuation. During experimental manipulations, the cells were viewed with a Newvicon TV camera (model WV-1550; Panasonic) using a near-infrared long-pass filter for illumination (λ > 780 nm; Andover Corporation). The infrared illuminator was turned off for several minutes before testing light responses.
RESULTS
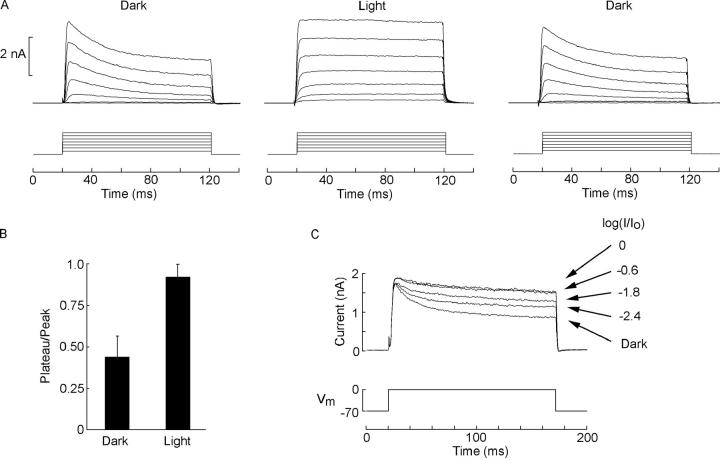
We first set out to replicate in our experimental system (namely, enzymatically isolated photoreceptors from Pecten irradians) the results that Shimatani and Katagiri (1995) had obtained in the intact retina of the Japanese scallop Patinopecten yessoensis. Dark-adapted ciliary Pecten photoreceptors also exhibit a prominent voltage-activated outward current, a component of which (from now on referred to, noncommittally, as IK) exhibits a distinct inactivation, as well as a sustained component. Fig. 1 A (left) illustrates a typical family of traces evoked by 100-ms-long depolarizing steps at 10-mV increments, delivered in the dark from a holding potential of −70 mV. The current peaks in 5–10 ms and decays to a plateau with a time constant in the range of 15–25 ms, both parameters inversely related to the size of the depolarizing voltage step. Repetition of the same stimulation protocol during application of a steady background light of near-saturating intensity (3.47 × 1014 photons·cm−2·s−1) results in currents completely devoid of decay kinetics, presenting instead a near-rectangular appearance (Fig. 1 A, middle); after a 1-min period of dark adaptation, the original time course was fully restored (right). The phenomenon proved highly reproducible (n = 17). A simple criterion to compare quantitatively the extent of the falling phase of the response in the dark vs. in the light is the ratio plateau:peak of the current, measured at some fixed depolarization within the range that produces a prominent current decay (e.g., −10 to 0 mV). The mean values obtained in the two conditions, plotted in Fig. 1 B, were 0.45 ± 0.13 (standard deviation) and 0.92 ± 0.07, respectively; the difference was highly significant (P < 10−10, t test for paired measurements). Fig. 1 C illustrates the dependency of this modulatory effect on light intensity. A voltage step of fixed amplitude (from −70 to 0 mV) was administered in the dark and in the presence of light of different intensities. The loss of the decay phase of the current was graded with photostimulation, (n = 3). The intensity range over which light modulates the time course of the voltage-gated outward current is the same as the operating range for the photocurrent. In summary, the basic observations made in intact retinas of Patinopecten are fully corroborated in isolated photoreceptors of Pecten.
Figure 1.
Light modulates the time course of the outward current evoked by voltage steps. (A) Depolarization-activated outward currents were measured in the dark from a holding potential of −70 mV. Membrane voltage was stepped at 10-mV increments. The currents, carried by potassium, exhibit a characteristic inactivation (left). The time course of this current is dramatically altered by applying steady illumination (middle), which virtually eliminates the decay kinetics leaving a sustained current. After a brief (1-min) period of dark adaptation, repetition of the voltage stimulation produced again the characteristic inactivating time course. (B) Comparison of the extent of the decay (ratio of plateau amplitude:peak amplitude) for the current elicited by a depolarization to −10 mV in the dark and in the light, pooled for 17 cells. (C) Dependency of the modulation of current decay on the intensity of stimulating light. A depolarizing step of fixed amplitude (−70 to 0 mV) was applied in the dark and during sustained illumination of different intensities; light attenuation is indicated in logarithmic units (Io, the unattenuated beam, was 13.8 × 1014 photons·cm−2·s−1).
Since the original phenomenon was described, a handful of studies have outlined the basic characteristics of the light-transduction pathway of ciliary visual receptors, suggesting that a Go is the likely downstream effector of rhodopsin photoisomerization, and that the light-sensitive conductance is activated via mobilization of cGMP, possibly involving regulation of a guanylate cyclase (Gomez and Nasi, 1995; Kojima et al., 1997; Gomez and Nasi, 2000a). If the apparent loss of inactivation of IK is the mechanism that is also responsible for the activation of IL, the signaling cascade must also be common, and therefore chemical stimulation of the phototransduction pathway should mimic the modulatory effects of light on depolarization-activated currents.
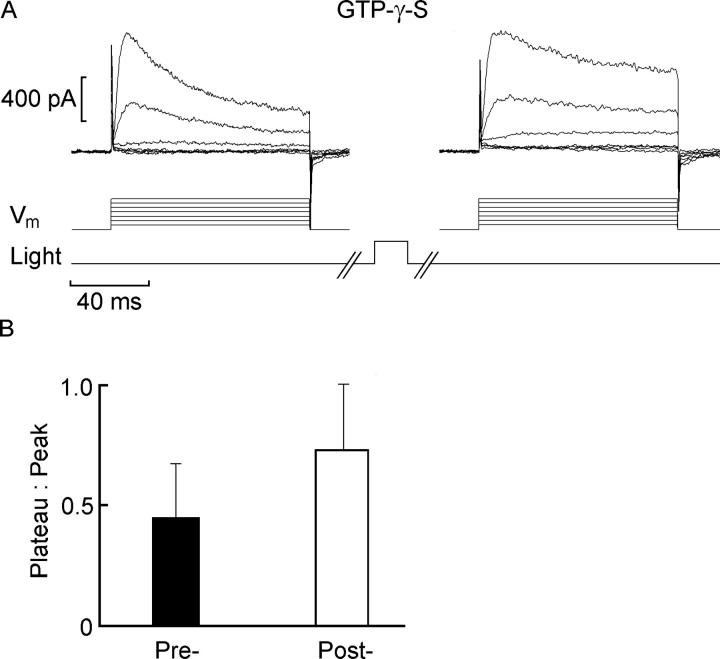
The poorly hydrolyzable GTP analogue, GTP-γ-S, is known to interfere with G protein shutoff, so that stimulation of the corresponding receptor produces a persistent activation of the signaling cascade. In the case of ciliary photoreceptor cells, we had previously demonstrated that after intracellular dialysis with GTP-γ-S, brief light stimulation evokes a response that does not deactivate normally; instead, a sustained photocurrent remains long after the flash (Gomez and Nasi, 2000a). Fig. 2 A shows the effects of 100 μM GTP-γ-S on voltage-activated outward currents: the first family of traces, recorded after several minutes of dialysis in the dark, exhibits the characteristic inactivating time course. Subsequently, the photoreceptor was stimulated with a single 500-ms flash of light (7 × 1014 photons·s−1·cm−2). After a 3-min period of dark adaptation, the voltage clamp protocol was repeated, but the outward currents had a noticeably attenuated falling phase. The data from a total of six cells are summarized in the bar graph in Fig. 2 B: the values of the plateau:peak ratio measured at −10 mV depolarization were 0.45 ± 0.22 (SD) for the initial voltage stimulation, and 0.73 ± 0.27 after brief light exposure and subsequent dark adaptation. A t test for paired measurements confirmed statistical significance of the difference (P < 0.001).
Figure 2.
GTP-γ-S induces a light-triggered persistent modulation of voltage-activated current kinetics. (A) Depolarizing voltage-clamp steps delivered at 10-mV increments from a holding potential of −80 mV in the dark, in a photoreceptor subjected to intracellular perfusion with 100 μM GTP-γ-S via the patch electrode. The first family of traces (left) was recorded after several minutes of dialysis in the dark. The cell was then briefly illuminated (500 ms; light monitor trace not drawn to scale) and then dark adapted again for 3 min. The voltage stimulation series was repeated (right), producing outward currents that exhibited a noticeably reduced decay. (B) Pooled values of the plateau:peak ratio measured with a depolarizing step to −10 mV depolarization in the dark, in GTP-γ-S–treated photoreceptors before and after exposure to a brief light stimulation (n = 6). Error bars denote standard deviation.
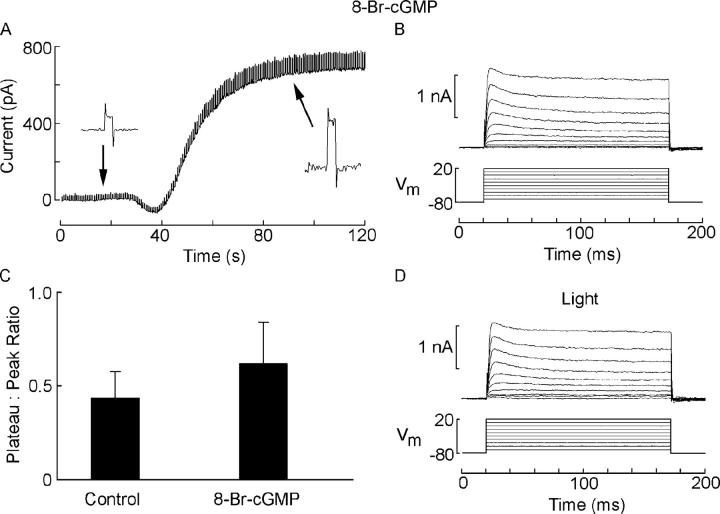
We next assessed the consequence of stimulation with cGMP analogues. As shown in Fig. 3 A, intracellular dialysis with 50 μM 8-Br-cGMP evoked an outward current, which was accompanied by an increase in membrane conductance; previous work had established that the properties of this conductance coincide with those of the light-sensitive conductance (Gomez and Nasi, 1995, 1997). In Fig. 3 B, the same cell was stimulated with depolarizing voltage steps delivered at 10-mV increments from a holding potential of −80 mV, in the dark; outward currents exhibited a much attenuated falling phase (compare with Fig. 1 A). The average extent of the decay of the current in control and in 8-Br-cGMP–treated photoreceptors is compared in Fig. 3 C, for a fixed depolarization to −10 mV. The mean values obtained were 0.45 ± 0.13 (n = 17) and 0.69 ± 0.22 (n = 11), respectively; a t test for independent samples established that the difference was highly significant (P < 0.001). Moreover, as illustrated in Fig. 3 D, the 8-Br-cGMP–treated photoreceptor lost its susceptibility to any further modulation by bright sustained illumination (7 × 1014 photons·cm−2·s−1) (plateau:peak ratio at −10 mV = 0.71 ± 0.24, P > 0.35, t test for paired measurements). Internal perfusion with 200 μM 8-Br-cAMP did not antagonize the falling phase of the depolarization-activated current (unpublished data; n = 3), thus indicating that such modulatory effect has a similar nucleotide specificity as the activation of the light-sensitive conductance (Gomez and Nasi, 1995).
Figure 3.
cGMP analogues mimic the light-induced attenuation of the falling phase of IK. (A) Effect of intracellular perfusion of 50 μM 8-Br-cGMP via the patch pipette. The recording was started immediately after establishing the whole-cell clamp configuration. The holding potential was −30 mV, and a repetitive rectangular perturbation (10 mV, 10 Hz) was applied to monitor the input resistance of the cell. (Insets) Expanded segments of recording, to illustrate the increase in membrane conductance that accompanied the development of the outward current evoked by the cGMP analogue. (B) Outward currents elicited by voltage steps from −80 to 20 mV in the dark, after the cGMP-dependent current stabilized. The outward currents exhibit only a modest decay. (C) Comparison of the plateau:peak ratio for voltage steps to −10 mV in control photoreceptors dialyzed with standard intracellular solution and cells treated with 50 μM 8-Br-cGMP. Error bars indicate standard deviation. (D) Repetition of the voltage steps in the presence of steady light, same cell as in A and B.
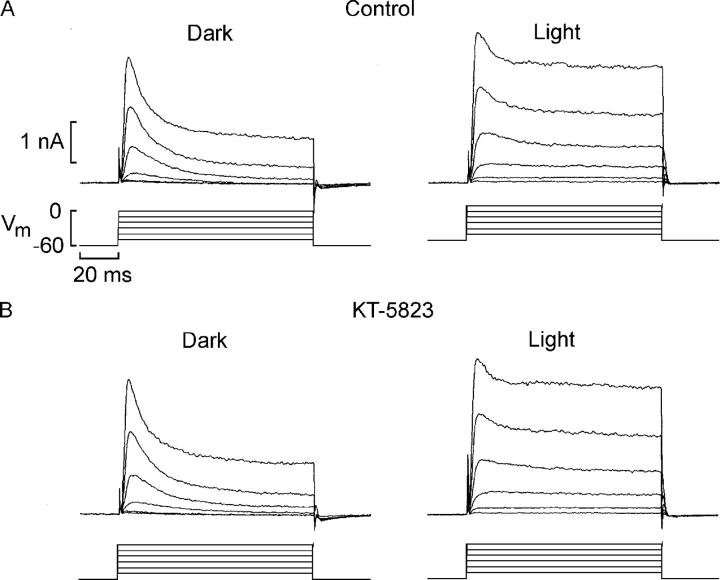
We assessed whether the action of cGMP analogs is likely to be direct, or may implicate additional effectors; channel phosphorylation by a cGMP-dependent protein kinase (PKG) is a plausible mechanism by which illumination could exert its modulatory influence on voltage-activated currents. To test this possibility, we examined the effect of a potent (K1/2 0.23 μM) and selective membrane-permeable PKG inhibitor, KT-5823. The top panels in Fig. 4 illustrate the photostimulation-induced reduction of the decay of IK in a photoreceptor under control conditions, measured in the dark (left) and during illumination (right). The same cell was subsequently locally superfused with 3 μM KT-5823 via a puffer pipette, and the voltage clamp protocol was repeated after 4 min of continuous application of the drug. The time course of the outward current recorded in the dark was completely unaffected by the PKG antagonist, as was the ability of light (3.47 × 1014 photons·cm−2·s−1) to attenuate the decay of IK. The outcome of this test is therefore not consistent with a downstream phosphorylation step, and suggests instead a direct effect of cGMP.
Figure 4.
Effect of a cGMP-dependent protein kinase inhibitor on modulation by light of the voltage-dependent K current. (A) Outward current elicited by membrane depolarization in the dark and in the presence of steady light, under control conditions. The characteristic photostimulation-induced reduction of the falling phase of IK in a photoreceptor is observed. (B) The same cell was subsequently locally superfused with 3 μM KT-5823, a potent and selective PKG inhibitor, and subjected to the same regime of voltage and light stimulation. The ability of light to attenuate the decay of IK was unaffected.
The next goal was to test in a systematic way the conjecture of the identity of the IL and IK. Important clues can be obtained by comparing the effect of pharmacological blockers; the hypothesis that identifies the activation of the photocurrent as the removal of steady-state inactivation of the voltage-dependent current makes the explicit prediction that an antagonist effective on the latter should also suppress the light-evoked current. The converse proposition, though, is not necessarily valid (see below). The Shaker-type K channel antagonist 4-AP is also a powerful blocker of the light response of ciliary photoreceptors (Gomez and Nasi, 1994b), and a key argument in support of the contention that IL is the same as IK was the demonstration that the latter was also blocked by 2 mM 4-AP (Shimatani and Katagiri, 1995). Those effects proved fully reproducible in isolated Pecten ciliary photoreceptors: superfusion with 2 mM 4-AP markedly suppressed the depolarization-elicited transient outward current (average block 70 ± 10% for a step from −60 to 0 mV, n = 4), as well as the plateau (62 ± 10%; unpublished data).
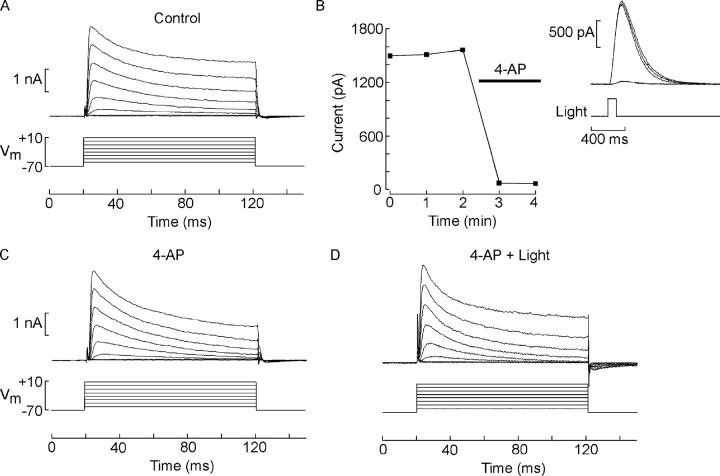
Although this observation is qualitatively in agreement with the working hypothesis, the single dose used (2 mM) is much too high to permit a useful comparison of the susceptibility of the two currents to this antagonist, since in a previous study we had reported that 4-AP suppression of the light response occurs in the micromolar range (Gomez and Nasi, 1994b). We therefore reexamined the effects of the drug at a concentration range more relevant to its action on light responsiveness, to ascertain whether the dose dependency of 4-AP effects is similar on both currents. Fig. 5 A shows a family of voltage-activated currents measured in the dark; the chamber was then superfused with 100 μM 4-AP (Fig. 5 B) while a flash of light (1.7 × 1014 photons·cm−2·s−1) was repeatedly presented; the drug caused swift suppression of the photocurrent to near undetectable levels. However, administration of voltage steps during exposure to 4-AP (Fig. 5 C) revealed that the peak outward current was completely unaffected, and only a reduction in the plateau phase of the current is visible (n = 5). Similar effects were obtained upon further reducing the 4-AP concentration to 20 μM (n = 4) or to 10 μM (n = 3). The discrepancy, by over two orders of magnitude, of the 4-AP concentrations required to suppress IK vs. those effective on IL poses a significant hurdle for the working hypothesis, if 4-AP antagonism derived from a direct occlusion of the permeation pore of the channels, which are hypothesized to be the same. The notion could be rescued, however, if at these low concentrations 4-AP acted allosterically, preventing the removal of inactivation. In such case, the activation of IK could occur normally, but, according to the model, the photocurrent would not. This interpretation may also be in line with the observation that, in cells exposed to 4-AP, light (2.76 × 1014 photons·cm−2·s−1) failed to antagonize the falling phase of the voltage-activated current, as illustrated in Fig. 5 D. Therefore, the differential effects of 4-AP do not rule out the possibility that IK and IL may be comprised by the same set of ion channels.
Figure 5.
Effects of superfusion with 100 μM 4-AP on the current evoked by membrane depolarization. (A) Control family of traces in response to 100-ms voltage steps at 10-mV increments, from a holding voltage of −70 mV. (B) Time course of the peak amplitude of the photocurrent evoked by a flash of fixed intensity in ASW and during administration of 100 μM 4-AP by local superfusion via a puffer pipette (indicated by the horizontal bar); holding potential −30 mV. Same cell as in A. (Inset) Raw traces of the light-evoked currents. (C) Repetition of the depolarizing voltage steps during superfusion with 100 μM 4-AP. At this concentration, the reduction in the peak of IK is negligible, and only a modest depression of the plateau level is observed. (D) Voltage steps administered to the same cell during steady illumination in the presence of the antagonist.
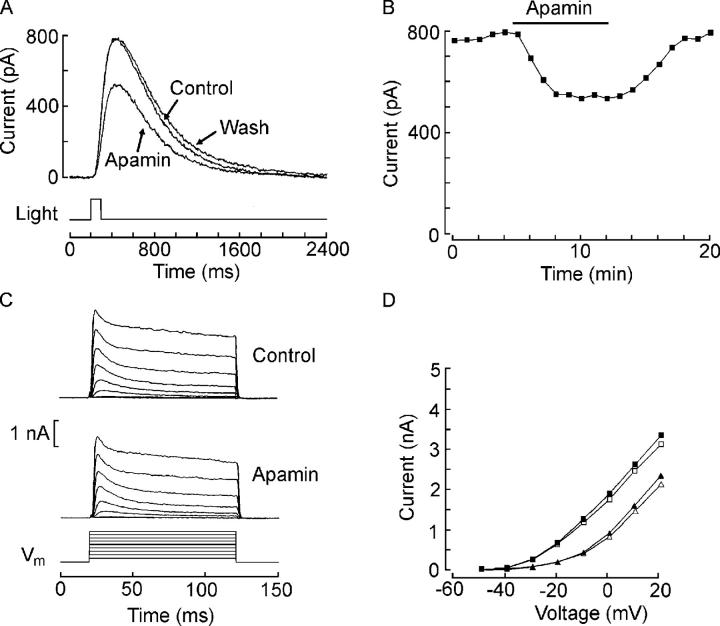
A differential effect of a channel antagonist would be immediately informative if the blockade affected only the transient voltage-dependent K current, irrespective of mechanism, without compromising the photocurrent, because in such case the notion that IL stems from removal of IK inactivation would become untenable. We screened a wide variety of toxins known to target different types of voltage-gated K channels; the high specificity of action, at least in some cases, and blocking affinities that can reach into the nanomolar range made this class of antagonists particularly appealing. The list included agitoxin 2, dendrotoxin-K, and margatoxin, especially promising because they affect some transient voltage-gated K channels (of the Kv 1 family); in addition, we examined the effects of apamin, BDS-I, BDS-II, charybdotoxin, E4031, and tertiapin. All proved inert on both voltage- and light-evoked currents, except for apamin, a toxin known for its powerful effects on small-conductance Ca-activated K+ channels. As shown in Fig. 6 A, local superfusion with 100 nM apamin reversibly depressed the current evoked by light flashes of constant intensity (1.1 × 1014 photons·cm−2·s−1). The changes in the peak amplitude of the light response before, during, and after application of apamin are plotted as a function of time in Fig. 6 B. By contrast, the toxin was completely inert on the voltage-activated currents. Fig. 6 C shows families of traces in ASW and during application of the same concentration of apamin. The I–V relations for the peak and the plateau amplitude of the depolarization-activated current in the two conditions are plotted in Fig. 6 D. Inhibition by apamin, therefore, is qualitatively akin to that of 4-AP, though more modest, and thus provides no additional insight. It is interesting to note that an apamin-sensitive, cGMP-dependent K channel has been previously described (Mule et al., 1999).
Figure 6.
Apamin selectively reduces the light response. (A) Superimposed responses to flashes of constant intensity administered in ASW, during local superfusion with 100 nM apamin, and after washing the toxin. (B) Time course of the effects of apamin. The peak amplitude of the photocurrent is plotted as a function of time, as the cell was stimulated with one flash every minute. (C) Families of outward currents evoked in the dark in ASW and during application of apamin. (D) I–V plot for the peak (squares) and plateau (triangles) IK in control conditions (filled symbols) and in the presence of apamin (open symbols).
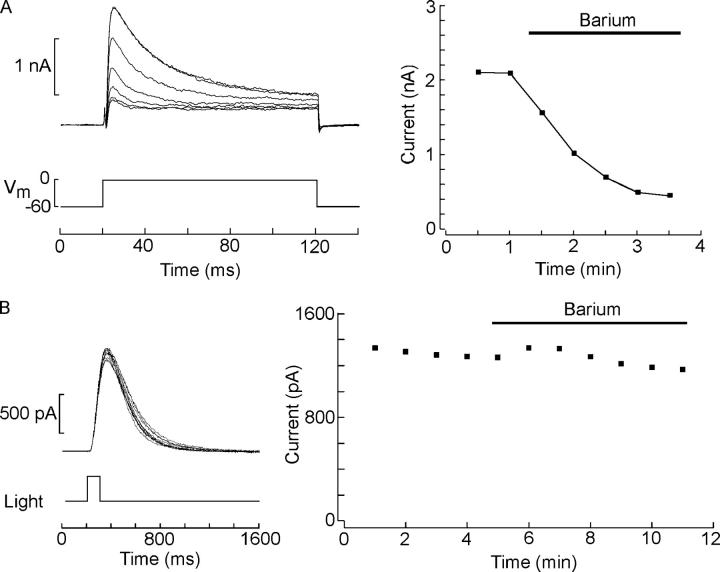
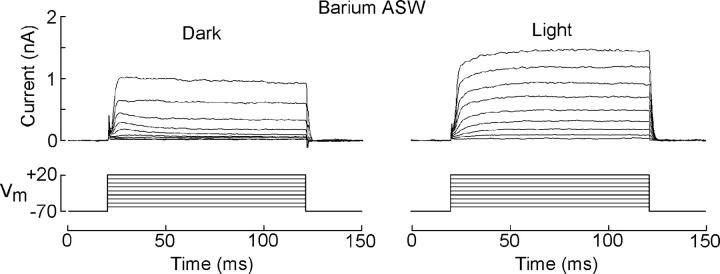
Having failed to identify suitable toxin blockers of the voltage-gated transient K current, we turned to inorganic blockers, even though their affinity is typically orders of magnitude lower and their action is seldom as selective. Barium is known to occlude the permeation pore in a variety of voltage-dependent potassium channels, including delayed rectifiers (Eaton and Brodwick, 1980), calcium-activated maxi-K channels (Miller et al., 1987), inward rectifiers (Hagiwara et al., 1978), and transient K channels such as Shaker (Hurst et al., 1995). Fig. 7 A shows the outward currents evoked by repetitive depolarizing 100-ms voltage steps to 0 mV, administered every 30 s. Upon switching to Ba-ASW (replacing Ca on an equimolar basis) the transient outward current was virtually abolished; the sustained component of the voltage-activated outward current appears to be less susceptible, and was depressed by ∼40%. The changes in peak amplitude of the current are plotted on the right (n = 6). By contrast, no detectable effect of replacing calcium with barium was observed on the amplitude of the response elicited by a repetitive flash of fixed intensity, as shown by Fig. 7 B (n = 5). These results unambiguously indicate that a completely normal photocurrent can be obtained under conditions that suppress the transient outward current, and thus strongly argue against the possibility that the light-dependent current may arise from the removal of steady-state inactivation of the voltage-dependent conductance, and against the notion that the two currents share a common permeation pathway. Finally, we also evaluated the effect of illumination in the time course of the depolarization-evoked outward currents in the presence of barium. Fig. 8 shows two families of depolarization-activated currents measured in the same cell superfused with 10 mM Ba, in the dark and during steady light. Clearly, light was still capable of modulating the kinetics of the currents, but under these conditions, the time course changed from nearly rectangular in the dark (owing to the Ba-induced suppression of the transient phase), to a sluggish outward relaxation reaching a larger plateau when the photoreceptor was illuminated (n = 3). In conclusion, there appears to be little doubt that the light-induced loss of decay kinetics in depolarization-activated current is not a reflection of removal of inactivation, but rather the development of an outward current that compensates for the inactivation of IK.
Figure 7.
Effects of extracellular Ba2+ on the voltage- and the light-activated currents. (A) A repetitive depolarizing step to 0 mV was administered every 30 s. After two control stimuli in ASW, the solution was switched to 10 mM Ba2+ (replacing Ca2+ on an equimolar basis); the transient outward current was gradually eliminated, and the sustained component suffered a significant reduction. On the right, the peak amplitude of the current is plotted as a function of time. (B) Response to a repetitive 100-ms flash of fixed intensity delivered every minute. No significant changes were observed upon switching to Ba-ASW. The amplitude of the photocurrent during the course of the experiment is plotted on the right.
Figure 8.
A slowly developing outward current is unmasked by barium. A photoreceptor was tested with depolarizing voltage steps while exposed to 10 mM Ba2+; under these conditions, the outward current virtually lacked the transient component. Repetition of the procedure during steady illumination revealed a sluggish additional outward component.
DISCUSSION
cAMP and cGMP have long been implicated in the gating of a variety of ion channels that subserve disparate functions, such as visual transduction (Fesenko et al., 1985; Yau and Baylor, 1989), olfactory transduction (Nakamura and Gold, 1987), and chemotaxis (Galindo et al., 2000). Cyclic nucleotide-activated channels are genetically related to the family of voltage-gated K channels (Kaupp, 1991; Guy et al., 1991; Yau, 1994), and even possess an S4 domain, the putative voltage sensor, although their gating is only weakly voltage dependent. It is therefore plausible that in the course of the evolutionary modifications that may have given rise to the family of CNGCs, some structural elements implicated in regulating the flow of ions in their ancestral voltage-dependent cousins may have been retained as the chemically operated gate. In the case of ciliary visual receptors, an explicit proposal was that the photoresponse reflects light-induced removal of the steady-state inactivation in the transient depolarization-activated K conductance (Shimatani and Katagiri, 1995). Our observations confirmed and extended the basic phenomenology, demonstrating that direct chemical manipulations of the light-transduction cascade at the level of both the G protein and the messenger, cGMP, mimic the effect of light on the decay of IK. Generation of the photocurrent and modulation of voltage-triggered currents can thus be ascribed to a common internal messenger system. To test the hypothesis that underlying conductances are the same, it would be tempting to simply ascertain whether they are additive or occlusive. Unfortunately, even if the permeation pathway was indeed the same, the predictions would be inextricably linked to the assumptions one makes about the gating scheme: if light removed steady-state inactivation, as in the original proposal, the population of channels available for activation by photostimulation would be the same that had been opened by voltage and had undergone inactivation; therefore the larger the transient voltage-activated current, the larger the photocurrent that could be subsequently evoked. By contrast, if light and voltage acted on the activation gate, then opening a certain fraction of the channels by either stimulus would decrease (by the same amount) the fraction of channels susceptible to be opened using the other stimulus, i.e., the interaction would be occlusive. This model dependency prompted us to use an alternative strategy to test the working hypothesis.
Support for the notion of a shared ionic pathway can be garnered by demonstrating similar inhibitory effects of pharmacological agents on the light-dependent and the voltage-dependent currents. The observed blockade of both conductances by millimolar concentrations of 4-AP is in line with that reasoning; moreover, we have observed a similar, although more modest, effect with l-cis-diltiazem (unpublished data). Nonetheless, these results are not conclusive; by contrast, differential effects on the two currents could be strongly indicative of separate underlying conductance systems, provided that certain requirements are met. Micromolar concentrations of 4-AP, a range far closer to the previously reported IC50 for the light response (Gomez and Nasi, 1994b), nearly abolished the light response without any detectable antagonism on the transient voltage-dependent current. The interpretation of such an effect, however, depends on the mechanism of block. Should the antagonism entail occlusion of the permeation pore, then clearly it would become untenable to propose that the two currents flow through the same ion channels. The mechanisms of block of 4-AP are complex and varied; there is some general consensus that its main mode of action involves permeation into the cytoplasm in its uncharged form, and subsequent blockage from the intracellular side in its charged form (Kirsch and Narahashi, 1983; Howe and Ritchie, 1991; Choquet and Korn, 1992). However, the effectiveness of 4-AP blockade varies dramatically and exhibits opposite state dependencies across different K channel types, suggesting that various targets may be implicated, including gating (Kirsch and Drewe, 1993; McCormack et al., 1994). It is thus conceivable that 4-AP at low concentration could impair the proposed mechanism of removal of steady-state inactivation, without hampering ionic conduction. An ad hoc scenario, for example, would be some interference with the action of cGMP. The working hypothesis is therefore not necessarily invalidated by the observed effect of micromolar 4-AP, or the qualitatively similar action of apamin. On the other hand, a differential effect in the opposite direction (namely, antagonism of the transient voltage-gated K current without interference with the photocurrent) would be far less ambiguous. Our observations with barium are of that nature, and strongly argue against the idea that the mechanism of the light response consists in the control by light of an inactivation gate in a voltage-sensitive K conductance. There are additional considerations that are problematic for this hypothesis. The proposed mechanism predicts a strong outward rectification of the photocurrent, because at negative potentials, the probability of opening the voltage-dependent channels is low and hence removal of inactivation would not be relevant. Although such nonlinear I–V relation has indeed been described (Gomez and Nasi, 1994a), it was shown to arise from voltage-dependent block by extracellular divalent cations (Gomez and Nasi, 1994b; Nasi and Gomez, 1999); in fact, in Ca- and Mg-free media, conduction becomes ohmic, and sizable photocurrents are measured as negative as −110 mV (i.e., >70 mV more negative than the activation threshold for IK), which cannot simply reflect a shift in surface potential.
The question remains of the nature of the striking differences in the voltage step–elicited currents measured in the dark or in the presence of light. Clearly the phenomenon parallels the activation of the transduction cascade, and represents the gradual increase of a conductance separate from IK. We have previously reported that when the membrane potential is stepped abruptly during illumination, the current through light-dependent channels shows a distinct relaxation: with depolarizing steps, the conductance shows a time-dependent increase, while it decreases with hyperpolarizing steps. Such relaxations were shown to arise from the equilibration of voltage-dependent blockade by divalent cations (Nasi and Gomez, 1999). The time constant of this process was measured in the range of 15–20 ms, becoming faster with depolarization. These values are quite similar to those of the inactivation kinetics of the depolarization-activated K current and have a similar voltage dependency; the amplitudes were also in the same range. In the presence of steady illumination, therefore, the falling phase of the voltage-gated current would be compensated by the growing current through the photoconductance. A perfect match between the two processes, which would be quite coincidental, is not required to give the appearance of loss of inactivation, as long as the τ of the inactivation is not much shorter than that of the compensating raising current. Otherwise, an inflection would appear on the trace, as we have occasionally observed in the presence of light. Our results therefore refute the intriguing hypothesis on the gating mechanism of the light-sensitive conductance in ciliary photoreceptors; nonetheless, future investigations might still reveal that these light-controlled channels, while being distinct from the voltage-dependent K conductances expressed in the same cells, employ some common elements as mechanisms to regulate the ionic flux.
Acknowledgments
Supported by National Institutes of Health grant EY07559.
David C. Gadsby served as editor.
Abbreviations used in this paper: 4-AP, 4-aminopyridine; ASW, artificial sea water; 8-Br-cGMP, 8-bromo cyclic guanosine monophosphate; CNGC, cyclic nucleotide–gated channel; GTP-γ-S, guanosine 5′-O-[3-thiotriphosphate].
References
- Armstrong, C.M. 1971. Interactions of tetraethylammonium ion derivatives with the potassium channel of giant axons. J. Gen. Physiol. 58:413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C.M., F. Bezanilla, and E. Rojas. 1973. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J. Gen. Physiol. 62:375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, V.C., E. Evans, and M.F. Land. 1967. The fine structure of the eye of the mollusk Pecten maximus. Z. Zellforsch. Mikrosk. Anat. 76:295–312. [PubMed] [Google Scholar]
- Choquet, D., and H. Korn. 1992. Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J. Gen. Physiol. 99:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, D.C., and M.S. Brodwick. 1980. Effects of barium on the potassium conductance of squid axon. J. Gen. Physiol. 75:727–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko, E.E., S.S. Kolesnikov, and A.L. Lyubarsky. 1985. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 313:310–313. [DOI] [PubMed] [Google Scholar]
- Galindo, B.E., C. Beltran, E.J. Cragoe, and A. Darszon. 2000. Participation of a K+ channel modulated directly by cGMP in the speract-induced signaling cascade of Strongylocentrotus purpuratus sea urchin sperm. Dev. Biol. 221:285–294. [DOI] [PubMed] [Google Scholar]
- Gomez, M., and E. Nasi. 1994. a. The light-sensitive conductance of hyperpolarizing invertebrate photoreceptors: a patch-clamp study. J. Gen. Physiol. 103:939–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M., and E. Nasi. 1994. b. Blockage of the light-sensitive conductance in hyperpolarizing photoreceptors of the scallop. Effects of tetraethylammonium and 4-aminopyridine. J. Gen. Physiol. 104:487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M., and E. Nasi. 1995. Activation of light-dependent potassium channels in ciliary invertebrate photoreceptors involves cGMP but not the IP3/Ca cascade. Neuron. 15:607–618. [DOI] [PubMed] [Google Scholar]
- Gomez, M., and E. Nasi. 1997. Antagonists of the cGMP-gated conductance of vertebrate rods block the photocurrent in scallop ciliary photoreceptors. J. Physiol. 500:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M., and E. Nasi. 2000. a. Light transduction in invertebrate hyperpolarizing photoreceptors: possible involvement of a Go-regulated guanylate cyclase. J. Neurosci. 20:5254–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, M., and E. Nasi. 2000. b. On the gating mechanisms of the light-dependent conductance in Pecten hyperpolarizing photoreceptors. Biophys. J. 78:480A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman, A.L.F., J.C. Woolum, and M.C. Cornwall. 1982. Selectivity of the Ca2+-activated and light-dependent K+ channels for monovalent cations. Biophys. J. 38:319–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy, H.R., S.R. Durell, J. Warmke, R. Drysdale, and B. Ganetzky. 1991. Similarities in amino acid sequences of Drosophila eag and cyclic nucleotide-gated channels. Science. 254:730. [DOI] [PubMed] [Google Scholar]
- Hagiwara, S., S. Miyazaki, W. Moody, and J. Patlak. 1978. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J. Physiol. 279:167–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren, M., K.S. Shin, and G. Yellen. 1998. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 21:617–621. [DOI] [PubMed] [Google Scholar]
- Hoshi, T., W.N. Zagotta, and R.W. Aldrich. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538. [DOI] [PubMed] [Google Scholar]
- Howe, J.R., and J.M. Ritchie. 1991. On the active form of 4-aminopyridine: block of K+ current in rabbit Schwann cells. J. Physiol. 433:183–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, R.S., R. Latorre, L. Toro, and E. Stefani. 1995. External barium block of Shaker potassium channels: evidence for two binding sites. J. Gen. Physiol. 106:1069–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Ruta, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Kaupp, B.U. 1991. The cyclic nucleotide-gated channels of vertebrate photoreceptors and olfactory epithelium. Trends Neurosci. 14:150–157. [DOI] [PubMed] [Google Scholar]
- Kirsch, G.E., and T. Narahashi. 1983. Site of action and active form of aminopyridines in squid axon membranes. J. Pharmacol. Exp. Ther. 226:174–179. [PubMed] [Google Scholar]
- Kirsch, G.E., and J.A. Drewe. 1993. Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J. Gen. Physiol. 102:797–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, D., A. Terakita, T. Ishikawa, Y. Tsukahara, A. Maeda, and Y. Shichida. 1997. A novel Go-mediated phototransduction cascade in scallop visual cells. J. Biol. Chem. 272:22979–22982. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M.E. Jurman, and G. Yellen. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M. Holmgren, M.E. Jurman, and G. Yellen. 1997. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 19:175–184. [DOI] [PubMed] [Google Scholar]
- McCormack, K., W.J. Joiner, and S.H. Heinemann. 1994. A characterization of the activating structural rearrangements in voltage-dependent Shaker K+ channels. Neuron. 12:301–315. [DOI] [PubMed] [Google Scholar]
- McReynolds, J.S., and A.L.F. Gorman. 1970. Photoreceptor potentials of opposite polarity in the eye of the scallop, Pecten irradians. J. Gen. Physiol. 56:376–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W.H. 1958. Derivatives of cilia in the distal sense cells of the retina of Pecten. J. Biophys. Biochem. Cytol. 4:227–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- wMiller, C., R. Latorre, and I. Reisin. 1987. Coupling of voltage-dependent gating and Ba++ block in the high-conductance, Ca++-activated K+ channel. J. Gen. Physiol. 90:427–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mpitsos, G.J. 1973. Physiology of vision in the mollusk Lima scabra. J. Neurophysiol. 36:371–383. [DOI] [PubMed] [Google Scholar]
- Mule, F., S. D'Angelo, and R. Serio. 1999. Tonic inhibitory action by nitric oxide on spontaneous mechanical activity in rat proximal colon. Involvement of cyclic GMP and apamin-sensitive K+ channels. Br. J. Pharmacol. 127:514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, T., and T.H. Gold. 1987. A cyclic-nucleotide-gated conductance in olfactory receptor cilia. Nature. 325:442–444. [DOI] [PubMed] [Google Scholar]
- Nasi, E. 1991. Two light-dependent conductances in the membrane of Lima photoreceptor cells. J. Gen. Physiol. 97:55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi, E., and M. Gomez. 1999. Divalent cation interactions with light-dependent K channels: kinetics of voltage-dependent block and requirement for an open pore. J. Gen. Physiol. 114:653–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimatani, Y., and Y. Katagiri. 1995. Light removes inactivation of the A-type potassium channels in scallop hyperpolarizing photoreceptors. J. Neurosci. 15:6489–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, K.J. 2004. Towards a structural view of gating in potassium channels. Nat. Rev. Neurosci. 5:905–916. [DOI] [PubMed] [Google Scholar]
- Yau, K.-W. 1994. Cyclic-nucleotide-gated channels: an expanding new family of ion channels. Proc. Natl. Acad. Sci. USA. 91:3481–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau, K.-W., and D.A. Baylor. 1989. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu. Rev. Neurosci. 12:289–327. [DOI] [PubMed] [Google Scholar]