Abstract
Background
A major cornerstone of evolutionary biology theory is the explanation of the emergence of cooperation in communities of selfish individuals. There is an unexplained tendency in the plant and animal world – with examples from alpine plants, worms, fish, mole-rats, monkeys and humans – for cooperation to flourish where the environment is more adverse (harsher) or more unpredictable.
Results
Using mathematical arguments and computer simulations we show that in more adverse environments individuals perceive their resources to be more unpredictable, and that this unpredictability favours cooperation. First we show analytically that in a more adverse environment the individual experiences greater perceived uncertainty. Second we show through a simulation study that more perceived uncertainty implies higher level of cooperation in communities of selfish individuals.
Conclusion
This study captures the essential features of the natural examples: the positive impact of resource adversity or uncertainty on cooperation. These newly discovered connections between environmental adversity, uncertainty and cooperation help to explain the emergence and evolution of cooperation in animal and human societies.
Background
The drive to understand the emergence of cooperation – actions of benefit to both actor and recipient – in communities of selfish individuals has generated a large body of theoretical and empirical research in recent decades [1-14]. This research focuses on the dynamics of interactions between individuals and pays relatively little attention to the effects of the environment. However, evidence is growing, in many taxa, that as the adversity (harshness) and uncertainty of the environment increase cooperation is enhanced and we present a model here that attempts to explain this phenomenon as an adaptive facultative response favoured by selection.
An organism's environment is more adverse if some quality such as resources, physical structure, climate, competitors, parasites or predators changes in such a way as to decrease darwinian fitness. Environmental adversity is species-specific, e.g. high temperature may be adverse for some organisms, but not for thermophilic bacteria. As an example of the uncertainty or unpredictability of the environment [15], feeding in a patchy area, where some places are rich in food and others barren, results in greater uncertainty of nutritional status compared to foraging where food is distributed homogeneously. Uncertainty can be measured as the variance of a distribution of environmental quality, and adversity as the mean [16]. Both adversity and uncertainty have been conceptualised as aspects of environmental 'risk' [17].
At many levels of life, from plants to human societies, cooperation thrives in conditions where the environment is most adverse. Plants at lower temperatures and higher altitudes, where abiotic stress is high, compete less and cooperate more with their neighbours [18]; nematodes Caenorhabditis elegans aggregate in response to stressors [19]; animals form more cohesive or larger groups, with consequent greater mutualistic benefits under greater predation risk [20-23]; mole-rats, a highly social species, delay dispersion more in arid than in mesic habitats [24]; human in-group solidarity is greatest when the group is under threat or in a harsh environment [25-28].
In humans there is also evidence for enhanced cooperation where the environment is more uncertain. This holds for common pool resource groups, such as fisheries [29], for communal sharing of hunted meat in various societies [30], and for sharing in laboratory experiments [31]. Examples of enhanced cooperation under adversity and uncertainty are discussed further by Andras & Lazarus [32].
We present a model to explain this increase in cooperation under conditions of adversity and uncertainty. The model has two parts. First, we show that adversity increases the organism's uncertainty in its resource level, uncertainty being measured as subjectively perceived resource variance (sub-section 2.1). We then show that resource uncertainty increases cooperation, using a multi-agent simulation (sub-section 2.2).
Results and Discussion
Adversity and uncertainty: analytical results
We consider the influence of adversity on the minimal amount of a given resource that the individual finds acceptable for survival. We assume that the marginal fitness benefit of resource amounts decreases with the amount of resources the organism already has. For example, eating an extra food unit adds less to the fitness of a well fed animal than to the fitness of a hungry animal [32]. We also assume that the cost of acquiring resources increases monotonically with the amount of resources acquired. For example capturing a large prey takes more energy than capturing a small prey (see also Fig 1 legend for general argument). For the sake of simplicity we assume that the cost function is linear (although we note that in practice this may be depart from linearity in the sense that acquiring a certain biomass of resources in the form of five small prey items may be more expensive than acquiring the same biomass in the form of a single large prey item).
Figure 1.
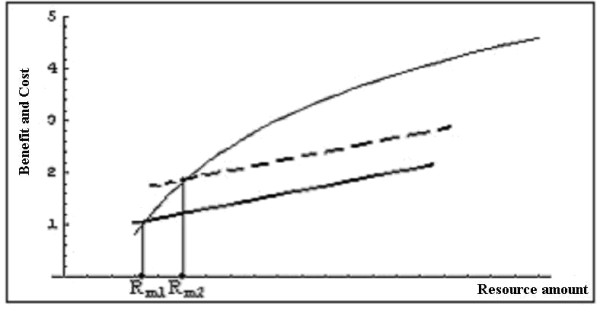
The minimal acceptable level of resources. The incremental fitness cost (assumed to be straight lines for simplicity) and benefit (curved line) are shown as a function of the resource amount gained, in a less adverse (continuous straight line) and a more adverse (segmented straight line) environment (the slope of the two lines is the same). Cost increases with resource amount since, optimally, more investment (cost) is made when the return (benefit) is greater. The resource amounts Rm1 and Rm2 are assumed to be the minimal acceptable levels of resources in the two environments.
The cost of foraging for resources is assumed to increase with adversity. For example, energy lost to foraging will be more costly in colder environments, and time spent foraging (and not available for anti-predator vigilance) will be more costly in environments with greater predation risk. We are interested in finding the minimal level of a particular resource that it is profitable to exploit, considering resource costs imposed by the environment (i.e. the minimal amount for which the benefits of acquiring the resource are larger than the costs of acquiring the resource). We term this level of resource the minimal acceptable level of resource. Note that we are not looking for the optimal level of resources, i.e., where the marginal benefit equals the marginal cost.
We denote by D(R) the probability density function of the distribution of resources (R – the amount of resources, R ≥ 0) in an individual's environment (i.e. is the probability that the amount of resources in a resource item or in a resource location is between the values R1 and R2). Let us assume that Rm1 and Rm2 are the minimum acceptable level of resource in a less adverse (1) and a more adverse (2) environment with the same resource distribution; we then get Rm1 <Rm2 (Figure 1). In other words, the minimum acceptable level of resources is greater in the more adverse environment. This prediction is supported by field studies of foraging in rodents, in which individuals select more profitable foods under environmental conditions – e.g. open habitat and moonlit nights – in which there is a greater risk from nocturnal predators [33-36]. (Animals under immediate predation risk, where anti-predator behaviour competes directly with foraging, trade-off the two concurrent demands and reduce selectivity in feeding in order to attend to the threat of predation [37-39], but this phenomenon is not relevant to the long-term response to adversity – and when prey are not under immediate threat of predation – with which we are concerned here. This distinction can explain the contrasting results of the two sets of studies.)
The minimum acceptable cut-off point means that all resources below that limit are considered by the organism to be equivalent to zero, accessing them being unprofitable. This allows us to derive a relationship between adversity and subjective resource uncertainty (i.e., the variance of the resource distribution after ignoring the resources below the minimum acceptable cut-off point – for example the variance of the distribution of sizes of acceptable food items, which are sufficiently big to be above the minimum acceptable cut-off point). Writing the formulae for the subjective variances of resources in the two environments we get:
where , are the respective means of the distributions of subjective acceptable resources. After algebraic manipulations we get
We assume that the minimum acceptable cut-off point Rm is such that the acceptable part of the resource distribution includes more than half of the full distribution, i.e., . It can then be shown that
where is the mean value of the full (not truncated) distribution of resources. Consequently, if Rm1 and Rm2 are such that the minimum acceptable cut-off point is less than the mean value of the full resource distribution, and Rm1 <Rm2 then
and consequently
if Rm1 ≤ R ≤ Rm2.
It follows from (3) and (7) that
| V2 – V1 > 0. |
Thus in a more adverse environment the individual experiences higher subjective variance (and therefore greater uncertainty) in its resources (Figure 2) (see also Appendix 1). The above discussion applies to the case when environmental adversity is measured in terms of an environmental quality (e.g., temperature, predation risk) different from the quality (usually a resource) in terms of which we measure the increased subjective environmental uncertainty. In this case the objective distributions of the second quality, resources, are the same in both environments. If the same environmental quality is used to measure both adversity and uncertainty we arrive at the same conclusion.
Figure 2.
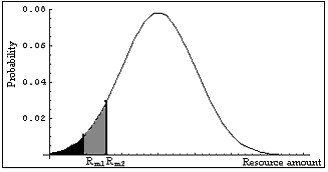
The distribution of resources in two environments having the same objective resource distribution. The resource amounts Rm1 and Rm2 are the minimal acceptable amounts of resources in the two environments. The shaded areas are the parts of the distributions ignored by animals living in the two environments. The larger the ignored area the higher is the subjective variance of the distribution, with the condition that the ignored area is smaller than half of the area below the curve of the distribution.
We have been dealing so far with subjective uncertainties that are naturally difficult to measure. For several reasons it would be preferable to deal with objective measures of uncertainty. First, it is objective measures that are employed in the following model. Second, as outlined above, we also wish to understand the possible direct effects of environmental uncertainty – as well as adversity – in enhancing cooperation. Last, if our conclusions are to be tested, objective measures of uncertainty will probably be required. Now, if the objective uncertainties of two environments differ, while the adversity (i.e., the mean value) of each is the same, then their consequent subjective uncertainties, as defined by a common minimal acceptable cut-off point, will differ in the same direction. This is because the proportion of the distribution that is unacceptable (and thus equated with zero) increases with objective variance. So, objectively more uncertain environments with the same mean expectations are also subjectively more uncertain, and objective uncertainty can stand proxy for subjective uncertainty in the model that follows.
Uncertainty and cooperation: an agent-based simulation
We have shown how increased adversity leads to increased subjective uncertainty, as measured by subjective variance. We have also shown that subjective and objective variance will be positively correlated. We now go on to show that an increase in objective variance in resources enhances cooperation. It will therefore follow that an increase in the subjective variance of resources will also enhance cooperation. Finally, since adversity causes an increase in subjective uncertainty we can conclude that adversity will favour cooperation.
Using a Prisoner's Dilemma type game theory model we built an agent-based simulation [40] to study the dynamics of the level of cooperation in relation to resource uncertainty. Our agents are generalised organisms that own resources (R) that they spend on living costs and use to generate new resources for the future. If the agent has less resource than the amount of living costs the agent dies. The agents live in a continuous two-dimensional world (i.e. unlimited flat continuous space, not a grid), each having a position (x, y) and change location by random movements, i.e. (xnew, ynew) = (x, y) + (ξx, ξy), where ξx, ξy are small random numbers. The agents have an inclination toward cooperation or competition, expressed as p the probability of cooperation of the agent with another agent. If p < 0.5 they are more likely to compete than to cooperate. They select their behaviour for each interaction in a probabilistic manner biased by their inclination. This is done by choosing a random number q from a uniform distribution over [0,1]; if q <p they cooperate, otherwise they compete.
Objective resource uncertainty was implemented as the variance of the resource distribution that is used to determine the new amounts of resources of the agents. In each time unit (the time is discrete in our simulation), each agent randomly chooses an interaction partner from its neighbourhood and the partners decide whether to cooperate or compete. The neighbourhood of an agent consists of the ten closest agents, where closeness is measured in terms of spatial distance between agents. The new resource amounts for the agents are determined by taking a sample from a normal resource distribution N(, σX. The mean value is determined by the amount of owned resources according to the payoff table shown in Table 1. The variance σX is the objective resource uncertainty characterising the environment. Varying the value of σX allows us to investigate how the level of cooperation responds to the environmental uncertainty.
Table 1.
The pay-off matrix for the cooperation/competition game
| Cooperate | Compete | |
| Cooperate | α · f(R1), f(R2) + Δ | |
| Compete | f(R1) + Δ, α · f(R2) | f(R1), f(R2) |
Entries indicate the mean of the payoff distribution to the row player followed by the column player. R1 and R2 are the amount of resources owned by the row and column player respectively, and Δ = [f(R1 + R2) - f(R1) - f(R2)]+ (i.e., it takes only the positive values of the expression in brackets and it is zero if the value of the expression is negative). The function f is a diminishing return function, and R1 and R2 are typically in the range where 2f(x) ≤ f(2x), and 0 <α < 1.
The agents produce offspring asexually at the end of their lifetime (unless they die because of lack of resources to cover living costs) who inherit their parent's inclination toward cooperation with some small random change (i.e., poffspring = pparent + ξ, ξ ∈ [-ε, ε ], and ε is a small number, e.g., ε = 0.025). The number of offspring depends on the amount of resources of the agent according to the equation
where is the mean and σR is the variance of the owned resources in the population of agents, and α, β, n0 are parameters. The offspring share equally the resources of their parent. The offspring start their life from their parent's last location with minor random changes, implying that the offspring of each agent will be closely packed at the beginning. The cluster of offspring diffuses with time, as the offspring make their random movements.
We simulated the evolution of 20 agent populations at each of three levels of environmental uncertainty. Each population started with around 1500 individuals (1500 ± a small random element) and the simulation ran for 1000 time units, the agents' mean lifetime being 60 time units. The inclination toward cooperation of the agents was set randomly according to a uniform distribution over [0,1]. We calculated for each simulation, for each time unit, the proportion of agents cooperating in cooperation-cooperation and cooperation-competition interactions. We focused on the proportion of agents participating in cooperation-cooperation interactions, which corresponds to c2, where c is the proportion of cooperating agents in the population. We found that as the objective resource uncertainty level increases, the stable level of cooperation in surviving populations also increases (Figure 3). We note that in the simulations the likelihood of cooperation is increased among clustered kin agents, which is due to the fact that the offspring of an agent start their life as a cluster of agents situated close to their parent's earlier location. However, as the agents follow their diffusion movement (a random walk) the clustering of kin agents is reduced.
Figure 3.
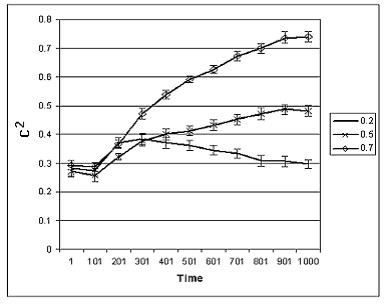
The evolution of the proportion of cooperating agents. The graphs show the mean (± SD) values of c2 (the proportion of agents participating in cooperation-cooperation interactions, where c is the proportion of cooperating agents in the population) in environments with various levels of uncertainty (see the numbers in the box).
The results of our simulation study show that a high level of objective resource uncertainty induces a high level of cooperation in agent populations. We have no analytical results that would explain our simulation results unambiguously. One possibility is that the payoff matrices of games played deviate from the Prisoner's Dilemma matrix, and this causes more cooperation as environmental risk increases [41]. Analysing our payoff matrix (see Table 1) we can exclude this possibility. Our payoff matrix is constructed such that in all cases the Prisoner's Dilemma inequalities are satisfied, i.e., payoff(cheater) > payoff (cooperation) > payoff(joint non-cooperation) > payoff (sucker), and payoff(cooperation) > [(payoff(cheater) + payoff(sucker)]/2, if Δ>0, and we have equalities instead of inequalities if Δ = 0, except the case of payoff(joint non-cooperation) > payoff (sucker), which is always satisfied. The payoff generating function f is set such that in most cases Δ>0, although the proportion of cases with Δ = 0 increases as environmental risk increases.
In our view the phenomenological explanation is that populations of agents survive in an uncertain environment only if the experienced individual uncertainties are around a steady-state level. At the steady-state level of experienced uncertainty the population maintains its size without major variations. By cooperation agents share individually perceived uncertainties, reducing their actual experienced uncertainty (similar to the reduction of risk on joining an insurance scheme). Higher environmental uncertainty implies that more cooperation is needed to reduce experienced individual uncertainties of agents in order to keep these uncertainties around the steady-state level. According to this argument, higher environmental uncertainty implies the presence of more cooperation in surviving populations, which is in good agreement with our simulation findings. Some research works suggest that above random level segregation of cooperating and non-cooperating agents is a key mechanism in the development of populations with high level of cooperation [14,42-44]. In our case, as we noted above, the offspring of reproducing agents form originally a cluster around the earlier location of their parent, and later this cluster diffuses as the agent follow their random walk. This indicates that possibly the segregation mechanism contributes to some extent in our simulation to the emergence of agent populations that have sufficient level of cooperation that makes the experienced uncertainty of agents stay around the steady-state level.
Conclusion
The simulation study captures the essential features of the natural examples: the positive impact of resource adversity or uncertainty on cooperation. As our simulation is based on a very limited set of assumptions, the results are likely to be of broad applicability and may explain why cooperation flourishes in risky environments, whether risk is conceptualised as adversity, objective environmental variance or subjective environmental uncertainty [17]. Elsewhere we demonstrate another way in which adversity and uncertainty may enhance cooperation [32].
Social psychological influences of perceived threat on group solidarity in humans [26-28] may seem removed from conclusions concerning cooperative behaviour, and outside the explanatory scope of models based on fitness considerations. However, human cooperative tendencies, and the associated prosocial cognitions and affective states, evolved in the small groups of early human societies [32,45] – or earlier – where social responses to environmental adversity [27] and uncertainty [31] will have impacted directly on fitness. We should therefore expect to find our predicted relationships between perceived risk and behaviours broadly classifiable as cooperative in contemporary societies, as long as these contemporary perceptions and behaviours are recognized and mediated by the putative evolved cognitive mechanisms.
Methods
The methods are described in the main body of this paper (Results and discussion section).
Appendix
We show, using an alternative route, that the subjective variance of resources increases monotonically with the increase of environmental adversity if the acceptable part of the resource distribution includes more than half of the full distribution, i.e.,
where Rm is the minimum acceptable cut-off point.
Define as the subjective mean of the resource distribution:
where δ0 is a Dirac-delta function centred on 0 such that .
Writing the subjective variance as a function of Rm we get
We calculate the derivative of the subjective variance with respect to the minimum cut-off point Rm
If condition (A1) is satisfied it can be shown that
So, we can conclude that if condition (A1) is satisfied then
This means that, given the condition (A1) being satisfied, the variance is an increasing function of the minimum cut-off point value, which increases with the adversity of the environment. This implies that the subjective resource variance of a more adverse environment is larger than the subjective variance of a less adverse environment, if the acceptable part of the resource distribution includes more than half of the full resource distribution.
Authors' contributions
PA derived the analytical results, developed the simulation environment and ran the simulations, and drafted the versions of the paper. JL provided the review and organization of the background and contributed with critical review and discussion of draft versions. GR contributed with critical review and discussion of draft versions. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are grateful to Miles Hewstone and Ian Vine for discussion and for providing references on the social psychology of cooperation and risk.
Contributor Information
Peter Andras, Email: peter.andras@ncl.ac.uk.
John Lazarus, Email: j.lazarus@ncl.ac.uk.
Gilbert Roberts, Email: gilbert.roberts@ncl.ac.uk.
References
- Andras P, Lazarus J, Roberts G, Lynden SJ. Uncertainty and cooperation: analytical results and a simulated agent society. JASSS – Journal of Artificial Societies and Social Simulation. 2006;9:7. [Google Scholar]
- Axelrod R., Hamilton, WD The evolution of cooperation. Science. 1981;211:1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- Colman AM. Cooperation, psychological game theory, and limitations of rationality in social interaction. Behav Brain Sci. 2003;26:139–153. doi: 10.1017/s0140525x03000050. [DOI] [PubMed] [Google Scholar]
- Dugatkin LA. Cooperation Among Animals. An Evolutionary Perspective. New York , Oxford University Press; 1997. [Google Scholar]
- Fehr E., Rockenbach, B Detrimental effects of sanctions on human altruism. Nature. 2003;422:137–140. doi: 10.1038/nature01474. [DOI] [PubMed] [Google Scholar]
- Fehr E., Fischbacher, U The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- Lazarus J. Let’s cooperate to understand cooperation. Behav Brain Sci. 2003;26:169–170. [Google Scholar]
- Milinski M., Semmann, D, Krambeck, HJ Reputation helps solve the ‘tragedy of the commons’. Nature. 2002;415:424–426. doi: 10.1038/415424a. [DOI] [PubMed] [Google Scholar]
- Nowak MA., Sigmund, K Evolution of indirect reciprocity by image scoring. Nature. 1998;393:573–577. doi: 10.1038/31225. [DOI] [PubMed] [Google Scholar]
- Roberts G., Sherratt, TN Development of cooperative relationships through increasing investment. Nature. 1998;394:175–179. doi: 10.1038/28160. [DOI] [PubMed] [Google Scholar]
- Rockenbach E., Milinski, M The efficient interaction of indirect reciprocity and costly punishment. Nature. 2006;444:718–723. doi: 10.1038/nature05229. [DOI] [PubMed] [Google Scholar]
- Trivers RL. The evolution of reciprocal altruism. Review of Biology. 1971;46:35–57. [Google Scholar]
- Wilkinson GS. Reciprocal food sharing in the vampire bat. Nature. 1984. pp. 181–184.
- Pepper JW., Smuts, B.B. The evolution of cooperation in an ecological context: an agent-based model. In: Kohler TGG, editor. Dynamics in human and primate societies: agent-based modelling of social and spatial processes. New York , Oxford University Press; 2000. pp. 45–76. [Google Scholar]
- Gigerenzer G. Reckoning With Risk. London , Penguin; 2002. [Google Scholar]
- Andras P., Roberts, G, Lazarus, J . Environmental risk, cooperation and communication complexity. In: Alonso EKDKD, editor. Adaptive Agents and Multi-Agent Systems. Berlin , Springer-Verlag; 2003. pp. 49–65. [Google Scholar]
- Daly M., Wilson, M Two special issues on risk. Evolution and Human Behavior. 2002;23:1–2. [Google Scholar]
- Callaway RM., Brooker, RW, Choler, P, Kikvidze, Z, Lortie, CJ, Michalet, R, Paolini, L, Pugnaire, FI, Newingham, B, Aschehoug, ET, Armas, C, Kikodze, D, & Cook, BJ Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–847. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- De Bono M., Tobin, DM, Davis, MW, Avery L, Bargmann CI Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. Primate Social Systems. London , Croom Helm; 1988. [Google Scholar]
- Farr JA. The role of predation in the evolution of social behavior of natural populations of the guppy. Poecilia reticulata (Pisces: Poeciliidae). . Evolution and Human Behavior. 1975;29:151–158. doi: 10.1111/j.1558-5646.1975.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Seghers BH. Schooling behaviour in the guppy (Poecilia reticulata): an evolutionary response to predation. Evolution. 1974;28:486–489. doi: 10.1111/j.1558-5646.1974.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Spieler M. Risk of predation affects aggregation size: a study with tadpoles of Phrynomantis microps (Anura: Microhylidae) Animal Behaviour. 2003;65:179–184. [Google Scholar]
- Spinks AC, Jarvis JUM, Bennett NC. Comparative patterns of philopatry and dispersal in two common mole-rat populations: implications for the evolution of mole-rat sociality. Journal of Animal Ecology. 2000;69:224–234. [Google Scholar]
- Goody E. The learning of prosocial behaviour in small-scale egalitarian societies: an anthropological view. In: Hinde RAGJ, editor. Cooperation and Prosocial Behaviour. Cambridge , Cambridge University Press; 1991. pp. 106–128. [Google Scholar]
- Hogg MA. The social psychology of group cohesiveness. From attraction to social identity. New York , Harvester Wheatsheaf; 1992. [Google Scholar]
- Hewstone M., Rubin, M, Willis, H Intergroup bias. Annual Review of Psychology. 2002;53:575–604. doi: 10.1146/annurev.psych.53.100901.135109. [DOI] [PubMed] [Google Scholar]
- Levine RA., Campbell, DT . Ethnocentrism: Theories of conflict, ethnic attitudes and group behavior. New York , John Wiley; 1972. [Google Scholar]
- Ostrom E. Governing the Commons: The Evolution of Institutions for Collective Action. Cambridge , Cambridge University Press; 1990. [Google Scholar]
- Gurven M. To give and not to give: The behavioral ecology of human food transfers. Behavioral and Brain Sciences. 2004;27:543–583. [Google Scholar]
- Kameda T, Takezawa M, Tindale RS, Smith CM. Social sharing and risk reduction – Exploring a computational algorithm for the psychology of windfall gains. Evolution and Human Behavior. 2002;23:11–33. [Google Scholar]
- Andras P., Lazarus, J . Cooperation, risk and the evolution of teamwork. In: Gold N, editor. Teamwork: Multi-Disciplinary Perspectives. Basingstoke , Palgrave Macmillan; 2005. pp. 56–77. [Google Scholar]
- Bowers MA. Seed removal experiments on desert rodents: the microhabitat by moonlight effect. Journal of Mammalogy. 1988;69:201–204. [Google Scholar]
- Bowers MA. Exploitation of seed aggregates by Merriam's kangaroo rat: harvesting rates and predatory risk. Ecology. 1990;71:2334–2344. [Google Scholar]
- Hay ME, Fuller PJ. Seed escape from heteromyid rodents: the importance of microhabitat and seed preference. Ecology. 1981;62:1395–1399. [Google Scholar]
- Leaver LA., Daly, M Effect of predation risk on selectivity in heteromyid rodents. Behavioural Processes. 2003;64:71–75. doi: 10.1016/s0376-6357(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Huntingford FA, Thorpe JE. Predation risk impairs diet selection in juvenile salmon. Animal Behaviour. 1987;35:931–933. [Google Scholar]
- Milinski M, Heller R. Influence of a predator on the optimal foraging behaviour of sticklebacks (Gasterosteus aculeatus L.) Nature. 1978;275:642–644. [Google Scholar]
- Ibrahim AA, Huntingford FA. Laboratory and field studies of the effect of predation risk on foraging in three-spined sticklebacks (Gasterosteus aculeatus) Behaviour. 1989;109:46–57. [Google Scholar]
- Axelrod R. The Complexity Of Cooperation: Agent-Based Models Of Competition And Collaboration. Princeton , Princeton University Press; 1997. [Google Scholar]
- Johnson DDP., Stopka, P, Bell, J Individual variation evades the Prisoner’s Dilemma. BMC Evolutionary Biology. 2002;2:15. doi: 10.1186/1471-2148-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitteldorf J., Wilson, D.S. Population viscosity and the evolution of altruism. Journal of Theoretical Biology. 2000;204:481–496. doi: 10.1006/jtbi.2000.2007. [DOI] [PubMed] [Google Scholar]
- Pepper JW. Simple models of assortment through environmental feedback. Artificial Life. 2007;13:1–9. doi: 10.1162/artl.2007.13.1.1. [DOI] [PubMed] [Google Scholar]
- Pepper JW, Smuts BB. A mechanism for the evolution of altruism among nonkin: Positive assortment through environmental feedback. American Naturalist. 2002;160:205–213. doi: 10.1086/341018. [DOI] [PubMed] [Google Scholar]
- Caporael LR, Dawes RM, Orbell JM, van de Kragt AJC. Selfishness examined: Cooperation in the absence of egoistic incentives. Behav Brain Sci. 1989;12:683–739. [Google Scholar]


