Abstract
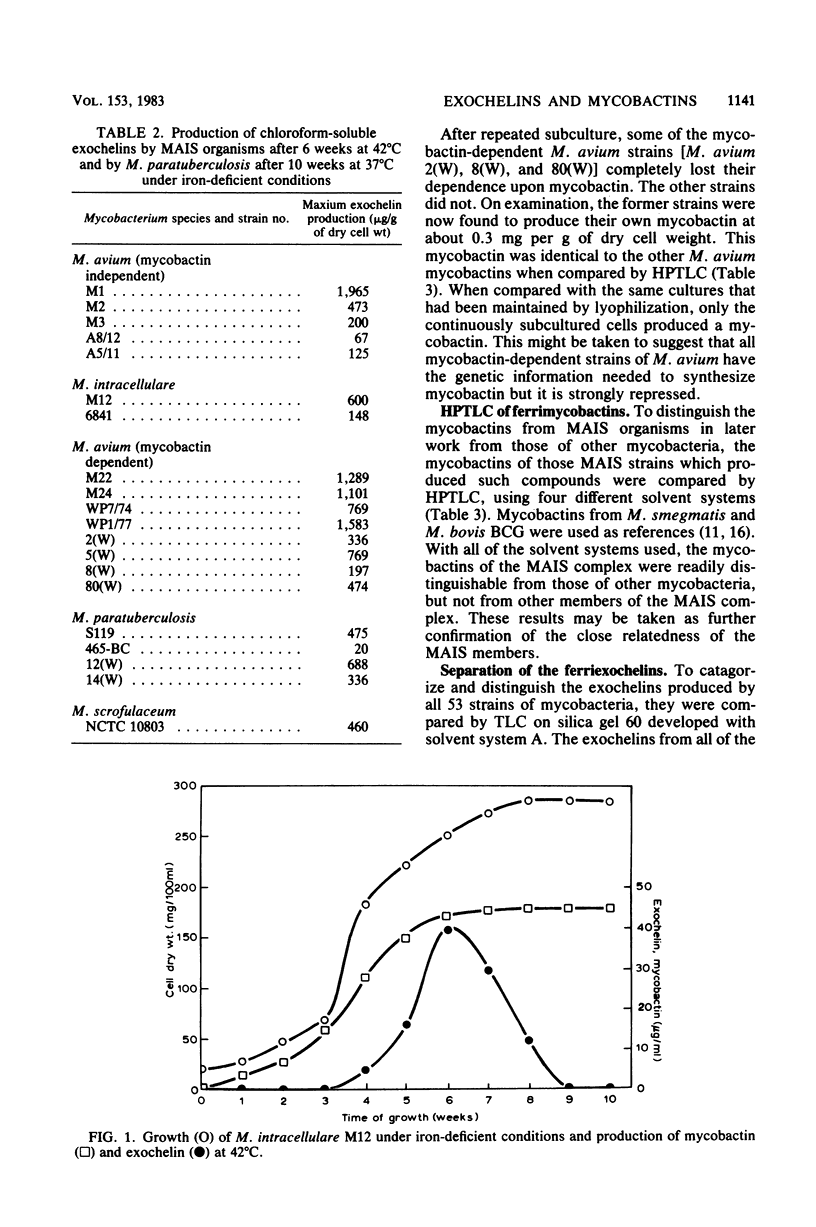
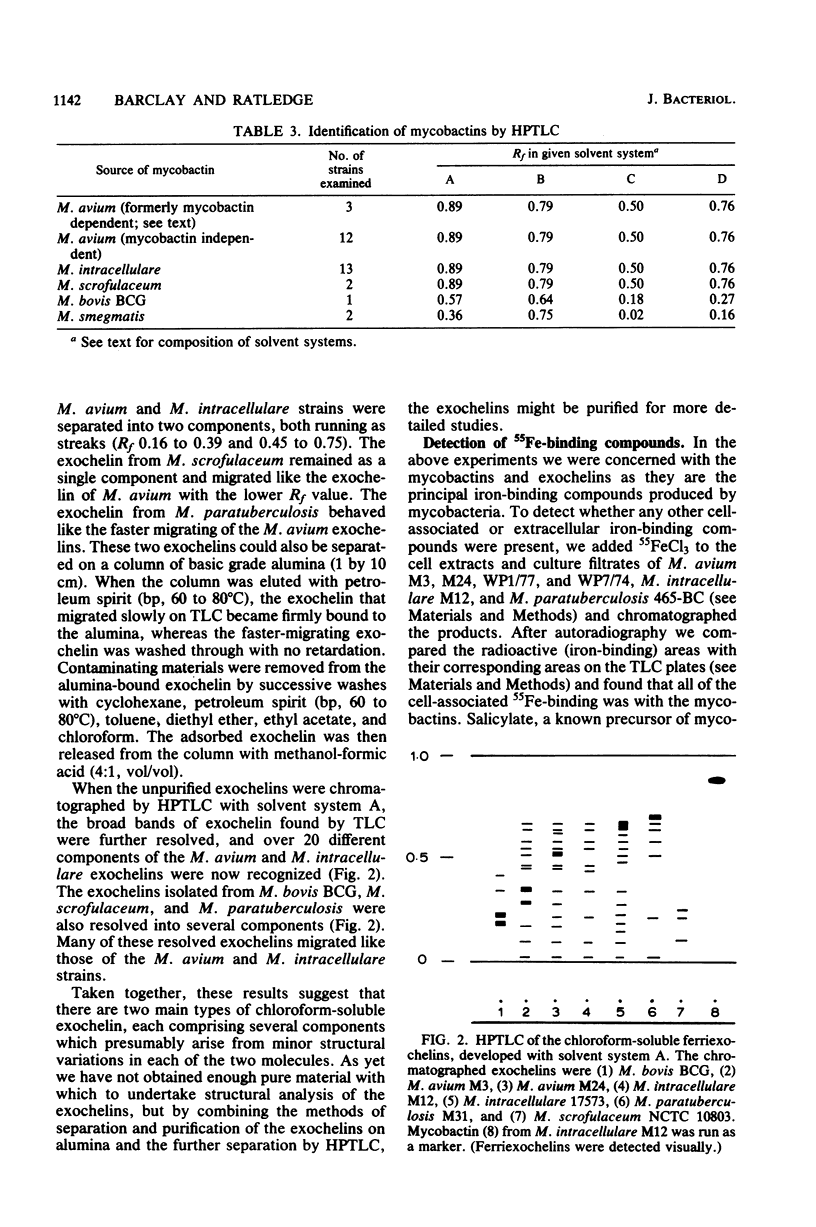
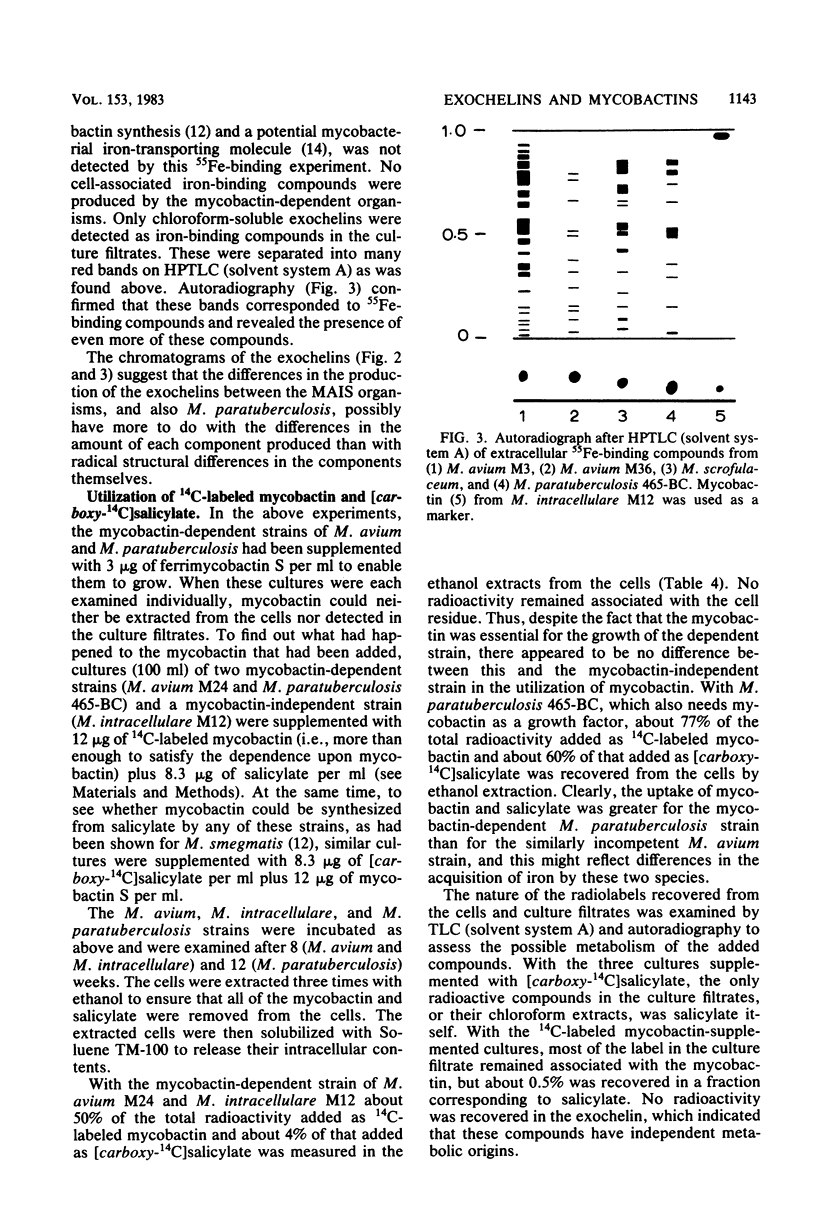
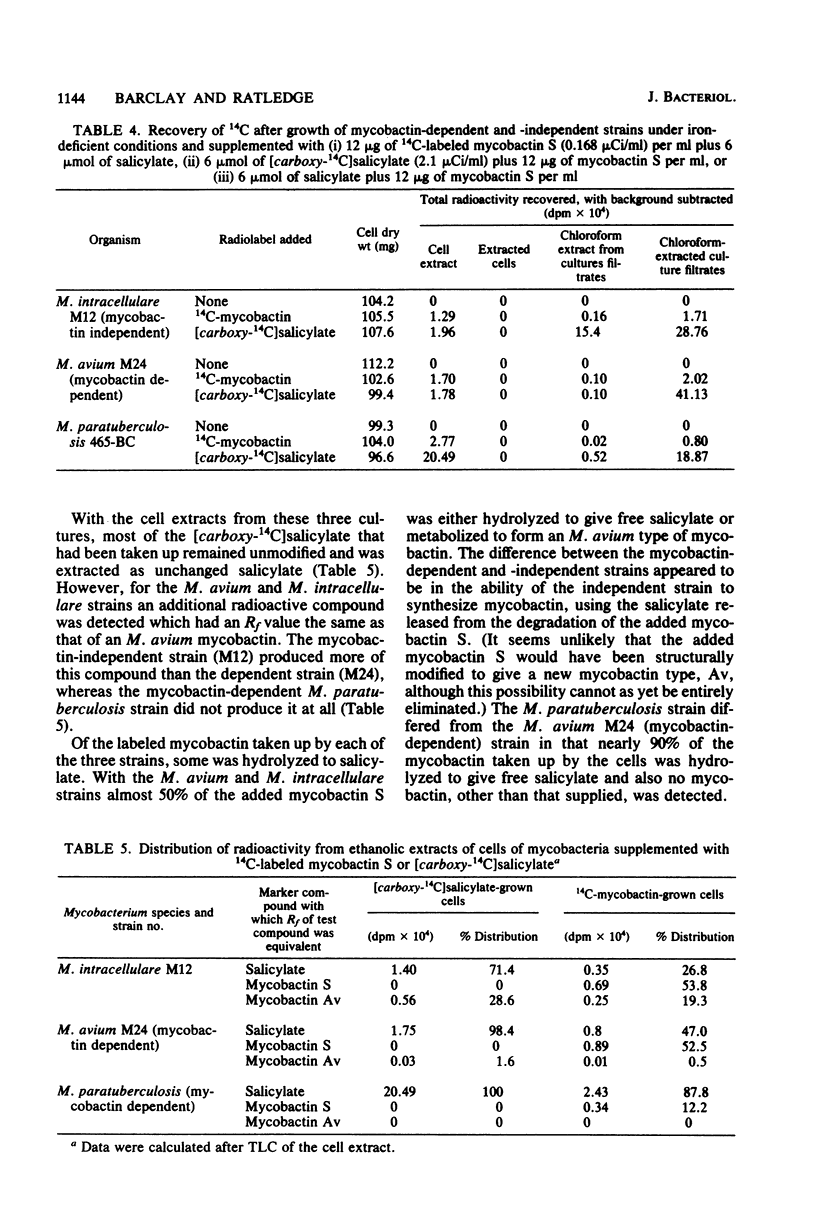
Fifty-three strains of M. avium and related species all produced one or more exochelins, the extracellular iron-binding compounds of the mycobacteria, when grown iron deficiently. Only those strains which could grow without the addition of mycobactin (i.e., mycobactin independent) produced mycobactin, the intracellular iron-binding compound of the mycobacteria. Exochelins varied from 20 to 2,000 micrograms per g of cell dry weight; mycobactins were between 1 and 10 mg per g of cell dry weight. M. paratuberculosis (13 strains) and 13 strains of M. avium, both species dependent upon mycobactin for growth, failed to produce spectrophotometrically detectable amounts of mycobactin (less than 0.2 microgram per g of cell dry weight), although mycobactin could be recognized in one strain of M. avium grown with an additional supply of salicylate and examined by a radiolabeling technique. On repeated subculture three of the mycobactin-dependent strains of M. avium, but none of those of M. paratuberculosis, lost their mycobactin dependence and on reexamination were found to produce their own mycobactin at 0.3 mg per g of cell dry weight. It is concluded that mycobactin biosynthesis is probably strongly repressed in the mycobactin-dependent strains rather than being a genetic deletion. The exochelins, when examined by high-pressure thin-layer chromatography were revealed as being multiples of similar compounds, with up to 20 individual iron-binding compounds being recognizable with some strains. It is argued that the exochelins represent the single most important means of iron acquisition in mycobacteria growing in vitro and in vivo, and their elaboration by the fastidious M. paratuberculosis and related species explains how these organisms are able to grow in vivo in the absence of an external supply of mycobactin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kochan I., Pellis N. R., Golden C. A. Mechanism of Tuberculostasis in Mammalian Serum III. Neutralization of Serum Tuberculostasis by Mycobactin. Infect Immun. 1971 Apr;3(4):553–558. doi: 10.1128/iai.3.4.553-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON N. E. CIRCUMVENTION OF THE MYCOBACTIN REQUIREMENT OF MYCOBACTERIUM PARATUBERCULOSIS. J Bacteriol. 1965 Mar;89:762–767. doi: 10.1128/jb.89.3.762-767.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macham L. P., Ratledge C., Nocton J. C. Extracellular iron acquisition by mycobacteria: role of the exochelins and evidence against the participation of mycobactin. Infect Immun. 1975 Dec;12(6):1242–1251. doi: 10.1128/iai.12.6.1242-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. R., McDiarmid A., Collins P., Brown A. The dependence of some strains of Mycobacterium avium on mycobactin for initial and subsequent growth. J Med Microbiol. 1978 Feb;11(1):53–57. doi: 10.1099/00222615-11-1-53. [DOI] [PubMed] [Google Scholar]
- Merkal R. S., Curran B. J. Growth and metabolic characteristics of Mycobacterium paratuberculosis. Appl Microbiol. 1974 Aug;28(2):276–279. doi: 10.1128/am.28.2.276-279.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger A. J., Ratledge C. Iron transport in Mycobacterium smegmatis: Uptake of iron from ferric citrate. J Bacteriol. 1982 Jan;149(1):131–135. doi: 10.1128/jb.149.1.131-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Hall M. J. Influence of metal ions on the formation of mycobactin and salicylic acid in Mycobacterium smegmatis grown in static culture. J Bacteriol. 1971 Oct;108(1):314–319. doi: 10.1128/jb.108.1.314-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Hall M. J. Uptake of salicylic acid into mycobactin S by growing cells of Mycobacterium smegmatis. FEBS Lett. 1970 Oct;10(5):309–312. doi: 10.1016/0014-5793(70)80460-4. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Macham L. P., Brown K. A., Marshall B. J. Iron transport in Mycobacterium smegmatis: a restricted role for salicylic acid in the extracellular environment. Biochim Biophys Acta. 1974 Nov 4;372(1):39–51. doi: 10.1016/0304-4165(74)90071-3. [DOI] [PubMed] [Google Scholar]
- Ratledge C., Marshall B. J. Iron transport in Mycobacterium smegmatis: the role of mycobactin. Biochim Biophys Acta. 1972 Aug 18;279(1):58–74. doi: 10.1016/0304-4165(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Snow G. A. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev. 1970 Jun;34(2):99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. C., Ratledge C. Iron transport in mycobacterium smegmatis: uptake of iron from Ferriexochelin. J Gen Microbiol. 1979 Jan;110(1):193–202. doi: 10.1099/00221287-110-1-193. [DOI] [PubMed] [Google Scholar]
- Stephenson M. C., Ratledge C. Specificity of exochelins for iron transport in three species of mycobacteria. J Gen Microbiol. 1980 Feb;116(2):521–523. doi: 10.1099/00221287-116-2-521. [DOI] [PubMed] [Google Scholar]


