Abstract
Gardens of fungus-growing ants (Formicidae: Attini) traditionally have been thought to be free of microbial parasites, with the fungal mutualist maintained in nearly pure “monocultures.” We conducted extensive isolations of “alien” (nonmutualistic) fungi from ant gardens of a phylogenetically representative collection of attine ants. Contrary to the long-standing assumption that gardens are maintained free of microbial pathogens and parasites, they are in fact host to specialized parasites that are only known from attine gardens and that are found in most attine nests. These specialized garden parasites, belonging to the microfungus genus Escovopsis (Ascomycota: anamorphic Hypocreales), are horizontally transmitted between colonies. Consistent with theory of virulence evolution under this mode of pathogen transmission, Escovopsis is highly virulent and has the potential for rapid devastation of ant gardens, leading to colony mortality. The specialized parasite Escovopsis is more prevalent in gardens of the more derived ant lineages than in gardens of the more “primitive” (basal) ant lineages. Because fungal cultivars of derived attine lineages are asexual clones of apparently ancient origin whereas cultivars of primitive ant lineages were domesticated relatively recently from free-living sexual stocks, the increased virulence of pathogens associated with ancient asexual cultivars suggests an evolutionary cost to cultivar clonality, perhaps resulting from slower evolutionary rates of cultivars in the coevolutionary race with their pathogens.
Fungus gardening by ants originated ≈50 million years ago (1). The subsequent evolutionary history of this obligatory mutualism has resulted in complex associations among the ants and their fungal symbionts. The attines comprise ≈200 described species in 12 genera that are obligately dependent on fungiculture for food. Although attine ants derived from a single ancestor (2, 3), extant species cultivate multiple, phylogenetically distant lineages of fungi, most of them belonging to the family Lepiotaceae (Agaricales: Basidiomycota) (4, 5). The fungal cultivars, serving as the primary food source for the ants, are carefully manured by the ants with plant substrate, insect frass, or seeds. Foundress queens propagate the fungus clonally by carrying inocula in their mouths during their nuptial flight to establish new colonies (6, 7). Although at least some “lower” attines (phylogenetically basal lineages) propagate cultivars that were recently domesticated from free-living populations of Lepiotaceae (5), the “higher” attines (a derived monophyletic clade that includes the leaf-cutting ants) are thought to propagate ancient clones, likely several million years old (4).
Among the attines, ants in the two genera of leaf-cutting ants (Acromyrmex and Atta) exhibit the most complex fungicultural systems. Fungiculture among Atta supports colony populations in the millions of workers and explains why leafcutters are the dominant herbivore in the Neotropics (8). Colonies of Atta spp. can survive for extended periods, often living for 8–10 years or more after initial nest founding (9). Foundress queens of these species establish new colonies by digging chambers in the soil, expelling the fungal pellet that they bring from their natal nest, and initiating the cultivation of their own gardens, which are started by using fecal material provided by the queen. Nest-founding occurs within a claustral chamber that remains closed until the first brood is reared, at which point the new workers begin foraging for leaf material outside the initial chamber.
Fungus-growing ants are thought capable of cultivating their fungus in axenic (single species) “monocultures,” despite continuous exposure to the competitively advantaged microbes already present in the vegetative material that is added to the garden. The ability of the ants to maintain the fungus garden in monocultures was first proposed by Möller (10) over a century ago in the first mycological study of attine gardens. Further support for this hypothesis has been derived from later observations that ants weed out “alien” microbes, produce antimicrobial chemicals, and increase the competitive advantage of the mutualist through application of proteolytic enzymes (11–14). Although these behaviors are important for successful fungal cultivation, there is no empirical evidence indicating that they result in pure garden cultures. The few studies sampling microbes from attine-gardens have been unable to identify the presence of microbes capable of circumventing the defenses of the ants and thus persisting in the garden as specialized parasites (15–17). These studies were limited in scope, with extremely small sample sizes (1, 3, and 10 nests, respectively), included only one or two attine species, and were primarily conducted on colonies maintained under laboratory conditions for months before sampling (15–17).
Although several studies identified adventitious extraneous microbes that are brought into the gardens on vegetative foraging material (16, 17), the presence of specialized parasites has neither been established nor adequately studied. The longstanding assumption that ant fungal gardens are free of significant pathogenic pressure is surprising because it contradicts some fundamental theories of the evolution of parasitism. Specifically, adaptation by parasites to genetically homogenous hosts is thought to be a selective force maintaining genetic diversity and hence sexual reproduction, typically referred to as the Red Queen hypothesis for the evolution of sexuality (18, 19). In fact, the devastating impact of parasites on human-cultivated monocultures is used as partial evidence in support of this theory (cf. refs. 20–22). To resolve the conflict between the theoretical prediction that parasites should exist in the clonal attine fungicultural systems and the widespread yet untested belief that ants maintain their gardens free of parasites, we conducted an extensive examination of fungal parasitism of gardens of attine ants.
MATERIALS AND METHODS
Sampling of Attine Gardens.
To assess the diversity and abundance of nonmutualistic filamentous fungi in attine ant gardens, we surveyed gardens tended by diverse attine lineages in central Panama from 1996 to 1998. Colonies were collected from the canal region of central Panama, including Ancon Hill, Naos Island, Gamboa, Soberanía Park, Pipeline Road, and Fort Sherman Military Reservation. In total, the gardens of 201 colonies from 8 attine genera, representing the known phylogenetic diversity of the attines, were sampled for fungal parasites. Nests were excavated as carefully as possible to ensure minimal disruption to the garden. Colonies were collected and subsequently maintained in sterile plastic containers with multichamber systems: an inner chamber for the garden and a larger surrounding chamber as a foraging area. The ants were permitted to stabilize their garden for 3–5 days after collection before samples were taken.
Sampling of nonmutualistic fungi was conducted by placing small individual pieces of garden material (≈3 mm3) on nutrient agar. In 1996, 20 pieces per garden were isolated, and 10 or 12 pieces per garden were isolated in 1997 and 1998. Pieces were selected from throughout the garden and were placed on potato dextrose agar medium with antibacterial antibiotics (Penicillin-G and Streptomycin sulfate) under aseptic conditions. The pieces were monitored daily for the growth of nonmutualistic filamentous fungi; if arising from the inocula, these then were isolated into pure culture. Ants were not given forage material before isolations, minimizing the possibility of obtaining fungi present only as inoculum from recently added garden substrate.
Because the microbes isolated from attine gardens may represent inactive propagules (which may overgrow the mutualist only on nutrient agar) rather than in situ microbial activity, the long-term and consistent presence of nonmutualistic fungi in attine fungal gardens was examined. Representative species of different attines were maintained in the laboratory in the Botany Department of the University of Toronto to resample for persistent, nonmutualistic fungi including 10 colonies each of Apterostigma cf. pilosum, Cyphomyrmex longiscapus, Trachymyrmex cf. zeteki, Acromyrmex octopsinosus, Atta colombica, and Atta cephalotes. Colonies were maintained in the laboratory for a 10-month period. After initial sampling in the field, each colony was resampled every 4–8 weeks. Substrate material provided in the laboratory to ants as forage was confirmed to be free from the common nonmutualistic fungi obtained in the initial field sampling. Air-sampling of spores of these fungi was conducted near ant colonies in Gamboa by using a standard Biotest-Reuter centrifugal air-sampler (Biotest AG, Breieich, Germany). To reduce the possibility of infections spreading between colonies in the laboratory, instruments were sterilized before each new colony was fed or cleaned. Also, moat, mineral-oil, and physical barriers were used to prevent the ingress spread of mites, which potentially could vector fungal propagules between nests. Reisolation of a fungus in the laboratory over an extended period of time was interpreted as evidence of chronic presence and growth within a garden.
Pathogenicity and Virulence of Garden Contaminants.
To determine whether Escovopsis, the most commonly isolated nonmutualistic fungus in the above-mentioned sampling (see Results), is a parasite of attine gardens, the virulence of fungi in this genus was investigated by (i) monitoring for colonies that have been over-grown by the fungus in the laboratory and field; (ii) excluding large garden fragments from tending ants to determine whether, in the absence of the garden-tending, Escovopsis erupts and manages to over-grow the fragment; and (iii) directly inoculating colonies with Escovopsis and monitoring these intentionally infected gardens.
We infected fungal gardens with Escovopsis in the laboratory by using young colonies of A. colombica, a common species of leaf-cutting ant in the canal region of Panama. Incipient colonies were collected in Gamboa, Republic of Panama, 6–8 weeks after the mating flight and were confirmed to be free from Escovopsis. Colonies were inoculated by spraying a suspension of fungal spores in sterile distilled water directly onto the garden with an atomizer. Colonies (garden volumes of ≈60–75 ml) were incolutated with Escovopsis at ≈300–500,000 spores per colony. We sprayed another set of clean A. colombica gardens with ≈400–600,000 spores of Trichoderma, an aggressive, necrotrophic fungal parasite that is common in tropical soil (23). Because Trichoderma is a ubiquitous and aggressive fungus, out-competing Escovopsis in Petri dish bioassays (C.R.C., unpublished results), our spray treatment of Trichoderma controls for the effect of unnaturally high inoculation by aggressive fungi. Control colonies were sham treated with sterile water. Colonies were provided ad libitum access to forage material, and garden health was monitored twice daily. To complete Koch’s postulates, the “gold standard” in demonstrating pathogenicity (24), we attempted reisolation of Escovopsis and Trichoderma after signs of garden deterioration were observed or at the completion of the study period.
Transmission of Escovopsis.
The transmission method of Escovopsis, horizontal (infection spread between established colonies) or vertical (infection passed from natal nests to newly established colonies), was examined by using foundress queens and incipient nests of A. colombica in Gamboa, Panama in 1998. Foundress queens (gynes) were collected during the A. colombica mating flight that occurred on June 23, 1998. The fungal pellet was dissected from the infrabuccal pocket of 38 gynes and was sampled for presence of Escovopsis. In addition, eight gynes, collected during the mating flight, were sampled after allowing them to establish gardens in the laboratory. To determine whether Escovopsis contamination occurred after fungal pellets were spit-up under field conditions but before foraging for vegetation began by the first workers, 22 incipient colonies were marked and excavated during the claustral period (3–4 weeks after nest initiation, a time-span sufficient for a garden to become established but during which lateral transfer cannot occur because queens plug the nest entrance and do not leave the garden chamber). Gardens of these claustral nests were sampled for Escovopsis. Incipient colonies (n = 45) that had begun foraging, identifiable by the emergence of a small turret surrounding the nest entrance, also were excavated, and their gardens sampled. Finally, 1- or 2-year-old colonies were sampled for Escovopsis to establish a base-line infection rate for young A. colombica colonies in this population.
Phylogenetic Comparison.
Our extensive and representative sampling of alien fungi from fungal gardens of the ants, in conjunction with the recently published phylogeny of the Attini (2, 3), permits a comparative examination of parasitism in this tribe. This analysis was based on sampling from 182 attine colonies and a total of 2,480 pieces of fungal garden. Some of the ant species and their corresponding genera sampled for nonmutualistic fungi were of low abundance, so insufficient sample sizes were obtained to warrant inclusion (i.e., Myrmicocrypta sp., n = 5; Sericomyrmex sp., n = 6), whereas others were extremely difficult to collect without significant disturbance (i.e., Mycocepurus spp., n = 8). Thus, the comparative examination was concentrated on collections from five attine genera: Apterostigma (n = 30), Cyphomyrmex (n = 35), Trachymyrmex (n = 39), Acromyrmex (n = 29), and Atta (n = 49). Several species were studied for each genus to ensure more representative sampling, as follows: Apterostigma auriculatum, Apt. cf. pilosum sp. 1, Apt. cf. pilosum sp. 2, C. longiscapus, Cyphomyrex costatus, T. cf. zeteki, Trachymyrex cornetzi, Trachymyrex bugnioni, Ac. octospinosus, Acromyrex echinatior (25), Atta colombica, and Atta cephalotes. Both the proportion of pieces contaminated with nonmutualistic fungi (a measure of the prevalence of potential parasites) and the prevalence of Escovopsis as a portion of total garden contamination were compared across the five genera.
RESULTS
Sampling of Attine Gardens.
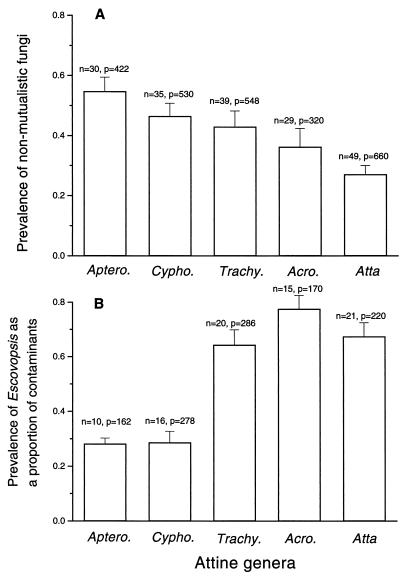
Attine gardens are occupied by numerous filamentous fungi aside from the mutualist. Of the 2,480 individual pieces of attine fungal garden sampled over the three years of this study, 39.7% of the pieces yielded nonmutualistic fungi, totaling 984 fungal cultures. Specifically, contamination ranged from a high of 54.8% of pieces from gardens of Apterostigma spp. colonies to a low of 27% from Atta spp. gardens (Fig. 1A). The fungal genus Escovopsis was second only to the mutualist in abundance. Escovopsis was the major taxon of nonmutualistic fungi isolated within infected colonies, ranging from 28.1% to 77.5% of contaminants, depending on the ant genus (Fig. 1B). This genus accounted for 256 of the 984 contaminants obtained from these samples (26.0%), was isolated from all eight attine genera examined, and infected a minimum of 33.3–51.5% of the ant colonies sampled (Table 1). Using morphological characters, these 256 isolates of Escovopsis appear to comprise at least eight distinct undescribed species (C.R.C., unpublished results).
Figure 1.
Proportion of garden pieces isolated that contained nonmutualistic filamentous fungi and the proportion of nonmutualistic fungi belonging to the genus Escovopsis. The attine genera are positioned from the most basal genus to the most derived genus (see refs. 2 and 3). (Error bars represent standard errors, n = number of colonies sampled, p = total number of pieces sampled.)
Table 1.
Proportion of attine colonies parasitized by Escovopsis from sampling in Panama, 1996–1998
| Attine genus | Number sampled | Gardens infected with Escovopsis |
|---|---|---|
| Apterostigma | 30 | 33.0% |
| Cyphomyrmex | 35 | 45.7% |
| Trachymyrmex | 39 | 51.1% |
| Acromyrmex | 29 | 51.4% |
| Atta | 49 | 42.9% |
Colonies of Apt. cf. pilosum sp. 1 (n = 3), C. longiscapus (n = 4), T. cf. zeteki (n = 5), Ac. octospinosus (n = 3), Atta colombica (n = 4), and Atta cephalotes (n = 3) naturally colonized by Escovopsis showed persistent infection after maintenance in the laboratory for 6, 3, 8, 10, 10, and 10 months, respectively. Escovopsis was not isolated in air-samples near nests in the field and laboratory or from forage material provided to the ants, so the observation of a long-term sustained presence indicates proliferation (growth) of this fungus within gardens.
Pathogenicity and Virulence of Garden Contaminants.
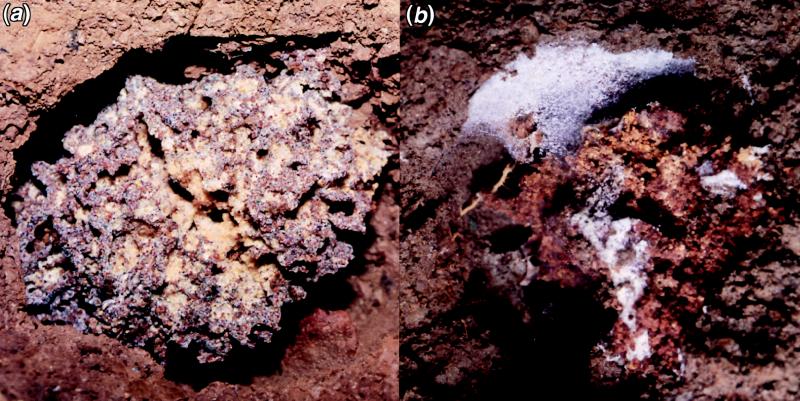
Escovopsis can be a virulent pathogen of attine fungal gardens. The fungus has been observed to overgrow gardens, even in the presence of the ants, both in the field (see Fig. 2) and in the laboratory (also see refs. 9 and 24). Maintaining garden material in the absence of ants (from infected colonies) almost invariably leads to rapid (1–2 days) Escovopsis overgrowth. We observed this in all Escovopsis-infected garden material maintained in isolation from tending ants of the three higher attines, T. cf. zeteki (n = 10), Ac. octospinosus (n = 6), and Atta colombica (n = 10). Contrary to anecdotal suggestions in the literature that removal of tending ants leads to rapid over-growth of the garden by multiple different contaminants (cf. refs. 9 and 16), gardens with endemic Escovopsis were only overgrown by this fungus. In addition, colonies judged free of Escovopsis were overgrown by other fungi only 7–14 days after the removal of the tending ants. After intentionally infecting 16 A. colombica gardens with spores of Escovopsis, gardens of 6 colonies collapsed and were lost within 72 hours (see Fig. 2 for a collapsed, Escovopsis-infected Trachymyrmex garden), and 3 of the remaining 10 colonies lost their gardens within 9, 13, and 17 days, respectively. None of the colonies treated with Trichoderma or sterile water showed signs of garden loss or even noticeable signs of garden stress. Escovopsis was reisolated from experimentally infected colonies; however, attempts to reisolate Trichoderma were unsuccessful.
Figure 2.
(a) A healthy Trachymrymex sp. garden. (b) A completely devastated Trachymyrmex sp. garden overgrown by the parasite Escovopsis.
Transmission of Escovopsis.
Escovopsis is not vertically transmitted in A. colombica. New queens were found to carry axenic inocula of the mutualist during mating flights, all incipient gardens were free of Escovopsis during the caustral phase of colony foundation, and only a small percentage of nests (6.6%) contained Escovopsis shortly after the commencement of foraging (Table 2). In contrast, Escovopsis was isolated from almost 60% of the 1- to 2-year-old colonies of A. colombica in the same population. Thus, transmission of Escovopsis appears to be exclusively horizontal.
Table 2.
Isolation of Escovopsis spp. from different life history stages of A. colombica
| A. colombica life history stage | Number sampled | Gardens infected with Escovopsis |
|---|---|---|
| Foundress queens: From infrabuccal pocket | 38 | 0% |
| Foundress queens: Gardens established in the laboratory | 8 | 0% |
| Incipient colonies: Before commencement of foraging | 22 | 0% |
| Incipient colonies: After commencement of foraging | 45 | 6.6% |
| Established colonies: 1– to 2–year-old colonies | 24 | 58.3% |
Phylogenetic Comparison.
Two distinct evolutionary trends emerged from our phylogenetic comparison of nonmutualistic fungi in attine gardens. First, total prevalence of nonmutualistic fungi decreases across the phylogeny from the basal genera to the more derived genera (Fig. 1A). Secondly, there is a greater prevalence of Escovopsis within colonies of the more derived attines, even though overall general contamination (with microfungi of any kind) decreases in these species in comparison to the more basal attines (Fig. 1B).
DISCUSSION
The mutualism between fungus-growing ants and their fungi is parasitized by a fungus in the hyphomycete genus Escovopsis. The parasitic nature of this fungus is revealed by two findings: First, Escovopsis is a common, prolific, and persistent microbe in the gardens of attine ants; second, inoculations of healthy garden with Escovopsis spores revealed the pathogenicity of Escovopsis. Specifically, experimentally infected gardens showed high morbidity and mortality, establishing that this pathogen has the potential to be a virulent parasite of the mutualism. As speculated by Seifert et al. (26), the genus Escovopsis is specialized on fungal gardens of attine ants, as it has not been isolated from any other habitats (see refs. 26 and 27), and it was not obtained in air samples or samples of forage material in this study. Escovopsis is consistently present in gardens of fungus-growing ants that span the breadth of the attine phylogeny, having been isolated from all eight genera examined in this study. In addition, this fungus occurs throughout the range of the attines, having been isolated from attine ant gardens from Brazil, Ecuador, Guyana, Panama, Texas, and Trinidad (refs. 26 and 27; C.R.C., unpublished results). Although only two species of Escovopsis have been previously described (each was isolated on one occasion from a single attine garden; see ref. 27), the specific habitat of this fungus and the limited scope of previous mycological studies of attine gardens has apparently resulted in an underestimation of the species diversity of the genus.
The exact mechanisms of pathogenicity by Escovopsis remain unclear. Preliminary evidence suggests that Escovopsis may be a mycoparasite, obtaining nutrients only from the mycelium of the ant-cultivated fungus but not from the vegetative material of the garden. In both gardens with workers removed and in bioassays challenging Escovopsis against the fungal mutualist, Escovopsis was observed to overgrow the fungal mutualist quickly and completely, leaving no apparent microscopic evidence of the mutualist fungus. This suggests that Escovopsis excretes lytic enzymes and/or toxins, a common trait among necrotrophic mycoparasitic fungi (23), thus supporting a mycoparasitic interpretation. Alternatively, it is possible that Escovopsis is a highly evolved “weed” that parasitizes this mutualism by competitively inhibiting the fungal mutualist after colonizing the vegetative garden substrate. Further work should attempt to differentiate between the “mycoparasitic” and the “weed-parasitic” mechanisms of Escovopsis pathogenicity.
Microbes are ubiquitous on and within the vegetative material added to the fungal garden of attine ants. These fungi and bacteria, including competitive saprotrophs and aggressive necrotrophs, are apparently suppressed by the ants through their continuous garden-tending activities (12–14) and the antimicrobial defenses of the mutualist fungi (28, 29). Thus, these “generalist” microbes, which by definition have not evolved specializations to circumvent the generalized behavioral and chemical defenses of the ants, do not persist, proliferate, or, indeed, impact the garden. They are functionally equivalent to ungerminated seeds in human agricultural soils, and, as such, their presence as passive occupants does not refute the near axenic monoculture hypothesis. Unlike these generalist microbes, however, Escovopsis is a specialist that apparently has evolved specializations to circumvent the defenses of the ants. It readily proliferates and persists in the colonies, with a high potential for devastating attine ant gardens, despite careful tending by the ants. Therefore, the documented chronic presence and growth of Escovopsis shows that fungal gardens of attine ants are not maintained in near axenic monocultures.
In addition to the identification of a specialized and virulent parasite of the garden, our phylogenetic comparative examination suggests an evolutionary cost of long-term clonality of cultivars in terms of increased severity of infections by specialist parasites. In the only well studied analogous system, human agriculture, large and genetically homogeneous cultivars are extremely susceptible to devastating epidemics, which at times have shaped human history and culture [e.g., the Irish potato famine (30–33)]. Such susceptibility to pathogens is thought to be a result of the cultivars’ limited genetic diversity, which facilitates rapid specialization, attack, and spread of diseases. Such pathogen adaptation can occur extremely quickly; for example, blue mold has been observed to devastate homogeneous crops of newly introduced “resistant” tobacco cultivars in <3 years (31).
An evolutionary cost of long-term cultivar clonality among fungus-growing ants is supported by our finding of an increased prevalence of Escovopsis within gardens of the more derived genera, possibly because of evolution toward increased virulence by Escovopsis. The increased prevalence of Escovopsis within the more derived attine genera suggests that the long clonal history of these fungal cultivars, perhaps as long as 23 million years (4), makes them more susceptible to loosing the “arms race” with parasites (18, 19). By contrast, lower attines routinely acquire new fungal cultivars from free-living, sexual populations (5), leading to greater genetic diversity in the fungal mutualist population. This may account for the apparent lower susceptibility to parasitism of the less derived attine lineages.
Biologists have long been fascinated not only by the farming life-history of the attines and the conspicuous foraging trails of the leaf-cutting ants, but also by the interesting evolutionary trends represented by extant assemblages of ants. There are distinct phylogenetic trends in the attines for increasing worker size, worker polymorphism, and colony size (8). This has led to the hypothesis that there has been an evolutionary improvement in fungal cultivation across the attine ants (8, 34). Our finding of decreasing abundance of nonmutualistic generalist fungi across the phylogeny of the fungus-growing ants is empirical evidence supporting the hypothesized improved fungal cultivation across the attine phylogeny.
Because leaf-cutting ants and their fungi are obligately mutually dependent, the success of this highly evolved mutualism depends on the maintenance of stable cultures. This predicts that successful maintenance of gardens in fungus-growing ants involves a continual struggle to suppress the specialized fungal parasites Escovopsis. The mechanism of this suppression has recently been identified in the form of a third mutualist associated with both higher and lower attine ants, an actinomycete that produces antibiotics that specifically target and inhibit the growth of Escovopsis (35). Thus, it appears that, through chemical intervention, attine ants are able to cultivate genetically homogeneous fungal gardens. Further studies on the interaction of garden parasites should provide additional insight into the evolution of this fascinating mutualism. Moreover, the discovery of a highly virulent parasite of the ant gardens of the attines may open new avenues for the biological control of this devastating agricultural pest in the New World tropics.
Acknowledgments
We thank the Smithsonian Tropical Research Institute and Autoridad Nacional del Ambiente of the Republic of Panama for facilitating the research and granting collecting permits. For valuable logistical support we are grateful to I. Ahmad, N. Alasti-Faridani, G. de Alba, S. Barrett, E. Bermingham, J. Bot, A. Caballero, J. Hunt, L. Ketch, M. Leone, G. Maggiori, S. Margaritescu, T. Myles, T. Schultz, K. Seifert, and D. Wedin. We are especially thankful to K. Boomsma, G. Currie, A. Herre, H. Herz, S. Rand, S. Rehner, J. Scott, N. Straus, B. Wcislo, and B. Wong for invaluable comments on this study and/or manuscript. This work was supported by Smithsonian and Natural Sciences and Engineering Research Council (Canada) predoctoral awards to C.R.C., a Smithsonian post-doctoral fellowship and National Science Foundation Grant DEB-9707209 to U.G.M., and a Natural Sciences and Engineering Research Council (Canada) grant to D.M.
References
- 1.Wilson E O. The Insect Societies. Cambridge, MA: Belknap; 1971. [Google Scholar]
- 2.Schultz T R, Meier R A. Syst Entomol. 1995;20:337–370. [Google Scholar]
- 3.Wetterer J K, Schultz T R, Meier R. Mol Phylogenet Evol. 1998;9:42–47. doi: 10.1006/mpev.1997.0466. [DOI] [PubMed] [Google Scholar]
- 4.Chapela I H, Rehner S A, Schultz T R, Mueller U G. Science. 1994;266:1691–1694. doi: 10.1126/science.266.5191.1691. [DOI] [PubMed] [Google Scholar]
- 5.Mueller U G, Rehner S A, Schultz T R. Science. 1998;281:2034–2038. doi: 10.1126/science.281.5385.2034. [DOI] [PubMed] [Google Scholar]
- 6.von Ihering H. Zool Anzeig. 1898;21:238–245. [Google Scholar]
- 7.Huber J. Biologisches Centralblatt. 1905;25:606–619. [Google Scholar]
- 8.Hölldobler B, Wilson E O. The Ants. Cambridge, MA: Belknap; 1990. [Google Scholar]
- 9.Weber N. Science. 1966;121:587–604. doi: 10.1126/science.153.3736.587. [DOI] [PubMed] [Google Scholar]
- 10.Möller A F W. Die Pilzgärten einiger südamerikanischer Ameisen. Jena, Germany: Gustav Fischer; 1893. [Google Scholar]
- 11.Weber N A. Anat Rec. 1957;128:638. [Google Scholar]
- 12.Schildknecht H, Koob U. Angew Chem Int Ed Engl. 1970;9:173. doi: 10.1002/anie.197001731. [DOI] [PubMed] [Google Scholar]
- 13.Martin M. Invertebrate-Microbial Interactions. Ithaca, NY: Cornell Univ. Press; 1987. [Google Scholar]
- 14.Bass M, Cherrett J M. Ecol Entomol. 1994;19:215–220. [Google Scholar]
- 15.Craven S E, Dix M D, Michaels G E. Science. 1970;169:184–186. doi: 10.1126/science.169.3941.184. [DOI] [PubMed] [Google Scholar]
- 16.Fisher P J, Stradling D J, Sutton B C, Petrini L E. Mycol Res. 1996;100:541–546. [Google Scholar]
- 17.Carreiro S C, Pagnocca F C, Bueno O C, Bacci M, Jr, Hebling M J A, da Silva O A. Antonie Leeuwenhoek. 1997;71:243–248. doi: 10.1023/a:1000182108648. [DOI] [PubMed] [Google Scholar]
- 18.Jaenike J. Evol Theory. 1978;3:191–194. [Google Scholar]
- 19.Hamilton W D. Oikos. 1980;35:282–290. [Google Scholar]
- 20.Bremermann H J. In: The Evolution of Sex and Its Consequences. Stearns S C, editor. Boston: Birkhauser; 1987. pp. 135–162. [Google Scholar]
- 21.Seger J, Hamilton W D. In: The Evolution of Sex. Michod R E, Levin B R, editors. Sunderland, MA: Sinauer; 1988. pp. 176–193. [Google Scholar]
- 22.Ebert D, Hamilton W D. Trends Ecol Evol. 1996;11:79–82. doi: 10.1016/0169-5347(96)81047-0. [DOI] [PubMed] [Google Scholar]
- 23.Dix N J, Webster J. Fungal Ecology. London: Chapman & Hall; 1995. [Google Scholar]
- 24.Agrios G N. Plant Pathology. San Diego: Academic; 1988. [Google Scholar]
- 25.Schultz T R, Bekkevold D, Boomsma J J. Insects Sociaux. 1998;45:457–472. [Google Scholar]
- 26.Seifert K A, Samson R A, Chapela I H. Mycologia. 1995;87:407–413. [Google Scholar]
- 27.Muchovej J J, Della Lucia T M C. Mycotaxon. 1990;37:191–195. [Google Scholar]
- 28.Hervey A, Nair M S R. Mycologia. 1979;71:1064–1066. [PubMed] [Google Scholar]
- 29.Wang Y, Mueller U G, Clardy J. Chem Ecol. 1999;25:935–941. [Google Scholar]
- 30.Cowling E B. In: Plant Disease: An Advanced Treatise. Horsfall J G, Cowling E B, editors. II. New York: Academic; 1978. pp. 361–381. [Google Scholar]
- 31.Lucas G B. Science. 1980;210:147–153. doi: 10.1126/science.210.4466.147. [DOI] [PubMed] [Google Scholar]
- 32.Barrett J A. In: Genetic Consequences of Man-Made Change. Bishop J A, Cook L M, editors. London: Academic; 1981. pp. 209–248. [Google Scholar]
- 33.Brown J K M. Trends Microbiol. 1994;2:470–475. doi: 10.1016/0966-842x(94)90650-5. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler W M. Ants: Their Structure Development, and Behavior. New York: Columbia Univ. Press; 1910. [Google Scholar]
- 35.Currie C R, Scott J A, Summberbell R C, Malloch D. Nature (London) 1999;398:701–704. [Google Scholar]