Abstract
Semiconductor nanocrystals (NCs) are increasingly being used as photoluminescen markers in biological imaging. Their brightness, large Stokes shift, and high photostability compared to organic fluorophores permit the exploration of biological phenomena at the single-molecule scale with superior temporal resolution and spatial precision. NCs have predominantly been used as extracellular markers for tagging and tracking membrane proteins. Successful internalization and intracellular labelling with NCs have been demonstrated for both fixed immunolabelled and live cells. However, the precise localization and subcellular compartment labelled are less clear. Generally, live cell studies are limited by the requirement of fairly invasive protocols for loading NCs and the relatively large size of NCs compared to the cellular machinery, along with the subsequent sequestration of NCs in endosomal/lysosomal compartments. For long-period observation the potential cytotoxicity of cytoplasmically loaded NCs must be evaluated. This review focuses on the challenges of intracellular uses of NCs.
1. INTRODUCTION
Semiconductor nanocrystals (NCs) “quantum dots” are increasingly being used in a wide range of biomedical applications, from cell biology to medical diagnostics. They have a core diameter of 2–10 nm and significantly larger hydrodynamic diameter, making them suitable as large yet relatively biocompatible markers, and have remarkable photophysical properties related to quantum confinement effects [1]. Their superior brightness, higher photostability, and narrower spectral emission compared to conventional organic fluorophores have progressively lead biophysicists to adopt them as a new tool for single-molecule imaging, in vitro and in vivo. NCs have become an alternative for organic fluorophores and complementary tool of fluorescent proteins in single-molecule fluorescence and whole-cell labelling assays.
In this review, we focus on the intracellular applications of semiconductor nanocrystals in biological imaging. We first discuss their unique optical properties, we then introduce some considerations on their surface chemistry and we explore in the following sections the different possible strategies to deliver NC inside the cell and to specifically target them to a protein of interest. Finally, we report on recent applications of NCs in whole animal imaging in vivo and address the risk of potential cytotoxicity.
2. CHEMICAL AND OPTICAL PROPERTIES
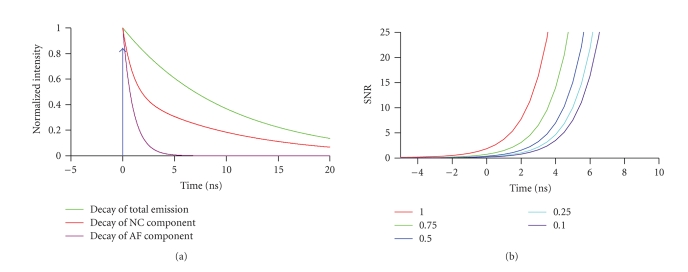
NCs are inorganic particles of 200 to 1000 atoms. NC cores are commonly synthesized from group II-VI (e.g., CdSe, CdS, ZnSe, and CdTe) and III-V (e.g., InAs, InP, and PbS) semiconductor materials. For any energy exceeding the band gap, which depends on the core diameter, absorption of a photon generates an electron-hole pair, which on recombination results in the emission of a less-energetic photon. Due to their broad absorption spectra, NCs can efficiently be excited with a multitude of laser lines. Variations in the particle composition and size result in different band-gap energies and hence NCs different photoluminescent (PL) emission, ranging from the near UV to the IR (400–1350 nm) [2]. NCs have narrow and symmetric photoluminescence (PL) emission peaks with typical full widths at half maximum (FWHM) of 25–35 nm [3] that facilitate multicolour imaging by allowing efficient single-colour excitation whilst minimizing emission cross-talk [4], see [5] for a critical discussion. Unlike with organic dyes, the PL emission arises from the radiative recombination of an exciton. For NCs, relaxation to the ground state takes nanoseconds, about one order of magnitude longer than singlet-singlet electronic transitions in organic fluorophores. The slow PL decay makes NCs attractive sources for time-gated imaging, which can be used to reduce the relative contribution of cellular autofluorescence to the total collected signal [6]. Figure 1 graphs the evolution of the collected fraction of long-lived NC emission, relative to that of the short-lived autofluorescence for different time gates Δt at a fixed lifetime ratio of 1:10. Larger gates are required to attain the same suppression of background for increasing levels of autofluorescence. For intensity-based detection NCs benefit from their large brightness (ɛ ) which results from a 10-to-100 time larger molar extinction coefficients (– M−1cm1) than organic dyes [7, 8] at comparable quantum yield . Finally, due to their significantly higher photostability than organic fluorophores, NCs are attractive for long-period observation (LPO). The resistance to photobleaching results from the deposition of an additional semiconductor shell (e.g., ZnS or CdSe) having a larger band gap than the core. The result is the confinement of the excitons to the core. However, NCs are not completely inert to prolonged illumination. The photophysical properties facilitate LPO at the single-NC level, a particularly interesting property in single-particle tracking (SPT) applications [9], tracing cell lineage [10], and live animal imaging [11], that all combine the demand for imaging small numbers of fluorophores over extended observation periods.
Figure 1.
Time-gated acquisition of nanocrystal photoluminescence suppresses short-lived autofluorescence, [6]. (a) Schematic representation of the relative timing of the laser pulse (instantaneous, blue), along with the normalized decays of autofluorescence (AF, purple, τ = 1 nanosecond), NC photoluminescence (NC, green, τ = 10 nanoseconds), and their sum (red), respectively. (b) Background rejection versus gate time. SNR is the ratio of the integrated signal of the NC divided by the integrated signal of the AF. The numbers/colors represent 5 different ratios /. To obtain the same SNR at a higher level of AF, a larger time gate is required. The shift in time is relative to the center of a sigmoidal function 1/(1 + exp (−T/t)) that describes detector gating. We assumed a detector on response (10–90%), T = 4.4 nanoseconds. Thus, at Δt = 0 detection efficiency is 50%.
Beyond their established function as molecular markers, NCs are increasingly being used for FRET-based biosensing (see [12] for review). NCs are both a scaffold and central donor for exciting multiple organic acceptor fluorophores in these inorganic/organic hybrid FRET sensors [13–16]. Also, NCs are attractive FRET donors because, through selecting the appropriate size, they can be dialed into almost arbitrary acceptors. The large overlap integrals between donor emission and acceptor absorbance allow for larger FRET efficiencies or transfer over larger donor/acceptor distances. Due to the broad absorption bands and narrow-band emission, one can chose excitation wavelengths minimizing direct acceptor excitation and minimal bleed-through of donor fluorescence into the FRET detection channel.
At the single-NC level, the radiative recombination of the exciton can temporarily be prevented despite ongoing excitation, resulting in intermittent PL emission, known as “blinking” [17]. Blinking results from the stabilization of the exciton at the NC surface and is associated with surface defects. Dark states reduce the duty cycle, complicate the interpretation of intensity-based measurements, and prompt the elaboration of specific algorithms for quantitative SPT [18].
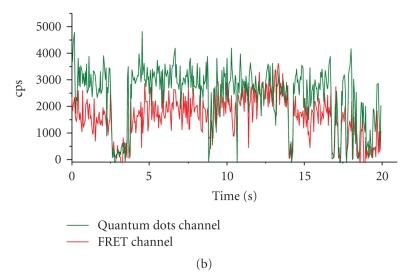
However, blinking can be turned to an advantage in as much as it allows the identification of single NCs and the detection of single-pair FRET (spFRET, Figure 2(a)), as shown on panel (b) between a QD565STV NC donor and an AlexaBiotin organic fluorophore acceptor (Yakovlev, Luccardini, and others' personal observations). Blinking of neighboring NCs can also be used for ultrahigh resolution studies beyond the classical resolution limit [19] and allows the emission of single particle to be isolated from the crowd. NC detection is not restricted on detecting PL. Their electron density and crystal structure provide sufficient contrast in transmission electron microscopy (EM) [9, 20]. Their use in EM is an additional advantage over labelling samples with conventional dyes that need to be photoconverted or require the addition of electron-dense material to generate contrast on EM images. However, the contrast obtained with NCs is lower than when using Au nanoparticles for immunolabelling.
Figure 2.
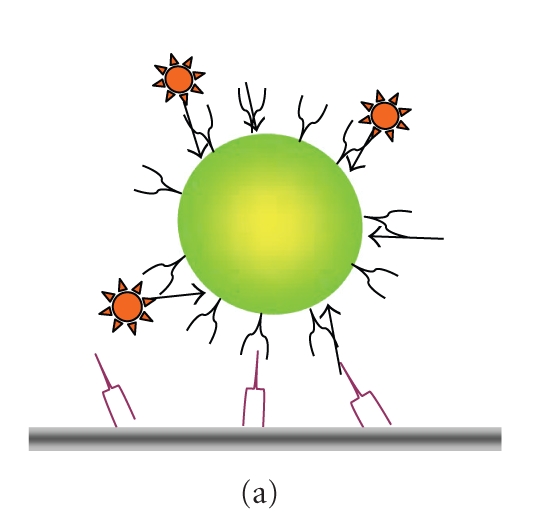
Use of blinking to detect single-particle fluorescence resonance energy transfer (spFRET). (a) Schematic representation of the donor/acceptor geometry consisting of a central QD565-ITK/STV donor (green) and biotinylated Alexa594 acceptor (red). NCs were immobilized on glass slides using a biotin-antibody linker. (b) Time-resolved traces of PL intensity simultaneously observed in the donor (D565/20 nm) and FRET channel (D655/40 nm) upon donor 440-nm excitation. The green-emitting NC donor transfers its energy to multiple orange-red fluorescing acceptors. Donor bleed through and acceptor direct excitation are negligible, and contribute less than 0.5% each to the total signal, respectively. Note the concomitant blinking in both channels, indicating no energy transfer when the quantum dot donor is in an OFF state, a hallmark of spFRET [21]. cps = counts per second.
3. NANOCRYSTAL SURFACE CHEMISTRY
Successful cell biological applications of semiconductor NCs had to await the development of reliable protocols for synthesizing water-soluble and colloidally stable nanoparticles. To be of use in cellular imaging, NCs need to be first rendered water-soluble and nonaggregating and then functionalized to be specifically targeted to a molecule of interest. They should also be stable and ideally have a long shelf life as well as to allow for experiment series under reproducible conditions. The time needed to develop potent solubilization and functionalization strategies justifies the time elapsed after the first proposition of NCs as biological probes [3, 22] and their wider use by the biological community which is only beginning. NCs are synthesised in organic solvents and are subsequently coated with a hydrophobic shell of surfactant trioctyl phospine oxide (TOPO) to maintain the particles monodispersed in organic solvents. Their water solubility is obtained by capping the NC surface with an additional hydrophilic coating layer. Among the many solubilization strategies that have been designed the most efficient, in terms of colloidal stability and biocompatibility, is at present the amphiphilic polymer coating [23–25]. Particle aggregation can further be reduced through the addition of a polyethylene glycol (PEG) layer, which also minimizes nonspecific interactions [20, 26, 27]. Taken together, the improvements in understanding NC surface chemistry and hence controlling their colloidal properties have prompted an ever increasing number of studies using colloidal semiconductor NCs as PL markers in cell biological applications (see, e.g., [28, 29] for review).
The easier accessibility of extracellular epitopes of cellular membrane antigens readily motivates the increasing number of studies using NCs instead of organic-fluorophore conjugated antibodies as extracellular markers in immunofluorescence [9, 30, 31]. Different linkers have been used for functionalizing NCs, including streptavidin [32–34], receptor ligands [35, 36], peptides [37], as well as secondary [38] or primary antibodies [39]. The popularity of NCs for studying molecular migration comes, at least in part, from the fact that NCs often offer a viable compromise between the desired stability and the tolerable degree of invasiveness. On the one hand, they are clearly more stable than small organic fluorophores that in turn exert less influence on the bound ligand. On the other hand, over tags offering a comparable long-term stability, such as the much bigger (and hence invasive) fluorescent nanobeads or light-scattering gold particles [40], through their smaller size, so that NCs are less prone to reduce ligand mobility and access to the binding site.
Despite their obvious advantage for extracellular labelling, four main difficulties are encountered when using NCs for intracellular labelling of cytoplasmic constituents in live cells. First, to deliver NCs into the cell, the plasma membrane has to be made transiently permeable for these nanoscale (but in a cellular context yet relatively large) objects, while maintaining the cell intact and viable [41]. Second, as NCs are also unspecifically taken up, probably by a process similar to pinocytosis, any specific uptake has to dominate over these nonspecific uptake mechanisms to ensure a specific labelling. Pinocytosis occurs in all types of cells, leading to pinosomes which can be bigger than 1 m (macropinocytosis). Because their size, macropinosomes provide an efficient route for nonselective endocytosis of solute macromolecules, and hence NCs in solution. Third, once the NCs have penetrated the cell, they must stay monodispersed and reach their molecular target through diffusion or transport. However, nanometric hard particles are frequently recognized as exogenous objects and are engulfed in endo-/lysosomal compartments. Finally, even in the case of a successful cytoplasmic loading, the main obstacle remains the difficulty in addressing NCs to their specific target sites and in removing the unbound NC fraction from the cytoplasm.
4. CROSSING THE PLASMA MEMBRANE
Whole-cell labelling has been demonstrated with biocompatible, but nonfunctionalized (bare) NCs. The addition of NCs to the extracellular medium leads to their spontaneous uptake [28, 42]. Not only specialized macrophages and fibroblasts but also many cells internalize both extracellular particles and fluid via phagocytosis and pinocytosis, respectively. Virtually all cells are able to take up NCs via endocytic mechanisms. This uptake leads to endodomes that are much bigger than the NCs itself (macropynosomes > 1 m, clathrin coated pits nm, caveolae nm, and clathrin- and caveolin-independent endocytosi nm [43]). However, these tracks often lead to aggregations of NCs crowded in intracellular compartments (recognized by the absence of blinking). Thus, additional and more specific loading techniques are required for specific NC loading.
Microinjection is a simple tool for loading monodispersed NCs into the cytoplasm [10, 36]. Dubertret and coworkers injected NCs into Xenopus laevis oocytes and traced the cell lineage throughout embryonic development. Single-cell electroporation [44] potentially is another technique for loading charged NCs into individual cells, but its efficiency critically depends on the size and charge of NCs (Luccardini and Yakovlev unpublished observations). However, similar to patch clamping or microinjection, it is time-consuming techniques; and more efficient techniques are desirable when the loading of larger cell populations is required.
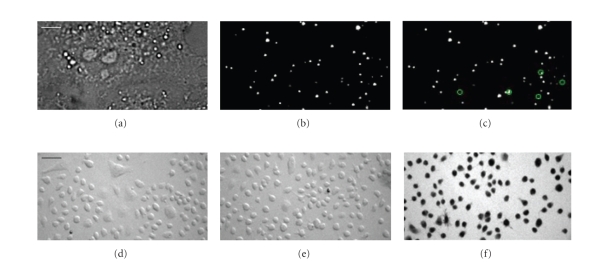
Bulk electroporation of cell suspensions allows the parallel delivery of NCs into thousands of cells, but has been reported to go along with NC aggregation [36, 45]. This technique probably traps NCs on the plasma membrane where they are endocytoted during the time that is required for the cells to settle on the cover glass before imaging (Luccardini and Yakovlev, personal observations). Thus, the osmotic lysis of pinosomes (Figure 3, upper panel) provides a simple and convenient method to efficiently load monodispersed NCs into many cells simultaneously, under identical conditions. During loading, the cell morphology did not change and plasma membrane integrity and cell viability were not affected through the osmotic shock and inclusion of NCs (Figure 3, lower panel). This technique enabled, for example, the loading of NCs to track single kinesin motors in live cells [46]. Chemical methods to deliver NCs to the cytoplasm include the use of cationic polymers [36, 45, 47] and cationic lipids [10, 48]. After liposome formation, NCs penetrate the plasma membrane, but accumulation in endosomal compartments is frequently observed [36, 39, 49]. Also, liposome-loaded NCs have been found in late endosomes/lysosomes [50], and in keeping with this observation, tend to concentrate in regions close to the nucleus [10]. Overcoming NC sequestration, encapsulation of NCs in a PEG-grafted polyethylenimine coat has been reported to permit their escape from endosomal compartments [51]. Another possibility for NCs delivery into the cytosol is their conjugation to specific peptide sequences [52, 53], similar to what has been used for the delivery of magnetic nanoparticles [54]. Although this is a particularly interesting and active area of research, and NC translocation to the cytoplasm [55, 56] and specific labelling of intracellular organelles such as mitochondria [36, 57] or the nucleus [36, 45] have been published, the true impact of these studies can only be evaluated with a careful study of the three-dimensional (3-D) intracellular localization of the NCs, for example, combining specific immunostaining and quantitative 3D imaging [35, 58], and careful colocalization analysis [5]. Finally, the conjugation of NCs to membrane-permeable toxins like botulinum toxin should represent an attractive strategy to deliver NC into the cytoplasm, although further work needs to confirm these initial observations.
Figure 3.
Evaluation of cytoplasmic nanoparticle loading in live cells by osmotic lysis of pinosomes. COS-7 cells were incubated in hypertonic solution (10 minutes, 37°C, Invitrogen I-14402) for pinocytic loading of QD565ITK nanocrystals (NCs, Quantum dot corporation). Shifting to hypotonic culture medium caused the osmotic lysis of the internalized pinosomes and release of NCs into the cytoplasm. (a) Bright-field image at × 100 magnification. Scale bar for (a) to (c): 4 m. (b)–(c) Epifluorescence images from a time-resolved image stack of the same cell. Green circles identify individual NCs that intermittently changed from ON to OFF state (blinking) between frame 250 (b) and 253 (c). Cell viability following loading was tested using the trypan blue exclusion assay at low magnification, × 10. Osmotic shock without (d) and with 1 nM QD565ITK nanocrystals in the extracellular fluid (e) did not compromise cell viability. (f) In contrast, adding ethanol reliably killed cells as reported by the dark trypan blue labelling. Scale bar for (d) to (f): 40 m.
In summary, while many different strategies of NC loading have been explored and some of them to produce a monodispersed cytoplasmic labelling at least in the cell types studied, the absence of rigorous criteria for successful cytoplasmic loading and the lack of appropriate controls along with the often uncritical and overoptimistic interpretation of intracellular fluorescent puncta make it hard to be directly extrapolated from the published literature on the own experiment. In principle, if NCs are localized in the cytoplasm rather than sequestered in some intracellular compartment, they should be evenly distributed in and randomly diffused throughout the accessible volume; in contrast, many images rather show localized distributions and heterogeneous clusters of different sizes and brightnesses. A definite proof needs SPT and the analysis of single molecule fluorescence. Blinking and consistent diffusion coefficients will clarify if particles are monodispersed and trapped or they can diffuse freely. As yet, it seems safe to say that the uptake and internalization of nanoscale particles into cells has not been completely understood and probably varies both from cell type to cell type. Also, it depends on the surface chemistry of the nanoparticles. Additionally, purification steps could play a crucial role; for example, in determining the concentration of excess ligands in solution.
5. REACHING SPECIFIC INTRACELLULAR TARGETS
Site-specific labelling of intracellular proteins is far more difficult than extracellular target recognition, since the cytoplasm constitutes a crowded molecular environment, containing a plethora of proteins, nucleic acids, and other molecules. So as to achieve specificity in intracellular targeting, tagging strategies rely on specific target recognition (reviewed in [12, 59]). Another requirement for LPO imaging is that the chemical bond linking the cytoplasmic target and the label chosen for its detection is stable over the experiment time.
It is in response to this need that the Tsien laboratory (University of California, Calif, USA) introduced genetically encoded fluorescent proteins in cell biology (reviewed in [60]). An alternative strategy uses self-labelling protein tags. The introduction of a small protein tag or of a unique combination of amino acids on the target protein allows their interaction with a specific fluorophore-bearing substrate, here an NC. Examples of self-labelling protein tags are biarsenical compounds [61, 62], SNAP tag [63], and Halo tag [64]. These approaches are helpful for developing new NC functionalization strategies for specific intracellular targeting.
6. WHOLE ANIMAL IMAGING, IN VIVO
Compared with applications to subcellular imaging in cell biology, NC-based whole-animal imaging has developed very fast [65]. Due to their long-wavelength emission, brightness, and long-term photostability, NCs are ideal probes for sensitive in vivoimaging in deep tissues of small animals or imaging superficial tissue layers of larger species [11]. The possibility of synthesizing NCs emitting in the infrared wavelengths minimizes scattering, optimizes depth penetration and allows discrimination against collagen autofluorescence and thus should permit ultradeep imaging of “optically thick” tissue [66, 67], provided that cytotoxicity is not an issue (see Section 7).
One of the first live-animal applications of NCs was the selective labelling of tumor vasculature in mice by using PEGylated NCs coated with specific peptidic sequences against vascular markers. In 2002 Åkerman et al. [26] showed in histological staining that after intravenous NC injection, functionalized NCs can be addressed to specific blood vessels. A high level of PEG substitution on top of the functionalization of the NCs reduced their uptake into the endothelial reticulum. One year later, Larson et al. were able to image by multiphoton microscopy NCs through the skin of live mice, in capillaries embedded 100 m in tissue [4]. Ballou et al. demonstrated the importance of long-chain PEG (5 kDa) coating for increasing the duration of NCs circulating in the blood flow of mice [20]. They were able to detect NCs by noninvasive whole body fluorescent imaging, upto four months after injection. The same report also showed that NCs deposit in liver, skin, and bone marrow in a surface-coating dependent manner and that polymer- and PEG-coated (upto 3,400 Da MW) NCs are cleared from the blood after injection. Gao et al. developed polymer-coated NCs functionalized with a monoclonal antibody directed against prostate cancer cells as a cell-specific marker [68]. After NC injection in mice, transplanted with human cancer prostate cells, they succeeded in specifically detecting and imaging the tumor site. However, as their NCs emitted in the visible spectrum, the authors used spectral unmixing algorithms to detect the NC signal in the presence of autofluorescence. Along these lines, Kim et al. [11] intradermally injected near-infrared-emitting NCs in mice and pigs and imaged sentinel lymph nodes (SNL) one cm deep in tissue. This work enables for the first time SNL mapping and cancer surgery under image guidance. Metastatic tumor cell extravasations were monitored in mice by intravenous injection of cells labelled with NC, which were examined by fluorescence emission spectroscopy [47]. More recently, Stroh et al. combined NCs and multiphoton intravital microscopy to distinguish in mice tumor vessels from perivascular cells and extracellular matrix [48]. With this approach, they also investigated the ability of NC-loaded silica beads (100–500 nm diameter) to access the tumor and monitored the trafficking of the precursor cells, a promising technique for cancer prevention and treatment.
So et al. designed recently “self-illuminating” NC conjugates permitting in vivo imaging without an external light source; instead, luciferase on the NC surface transfers its excitation to the NC core in a Bioluminescence resonance energy transfer (BRET) assay [69]. Intramuscular or subcutaneous injection in mice of 5 pmol of polymer-coated NCs conjugated to the Renilla reniformis luciferase was enough to image a BRET emanating from 3 mm depth tissue, after coelenterazine injection for activation. We note that this study is one of the few applications that used NCs as acceptors rather than donors.
7. CYTOTOXICITY
As NCs are increasingly being used as biological photoluminescent probes, in both acute cell assays and chronic, in the entire animal, in vivo, it is important to evaluate if they represent a specific risk of toxicity for the organism under study.
Although probably not classically termed cytotoxicity in a strict sense, one obvious problem resulting from the nanoscopic size of nanoparticles is that NCs can directly affect the biological system under study by impairing the mobility, interaction, binding, or other biological action of the ligand molecule to which they are attached. Hence, any study using NC-conjugated biomolecules must exclude the inhibition of the enzyme, receptor, motor, or other by the NC.
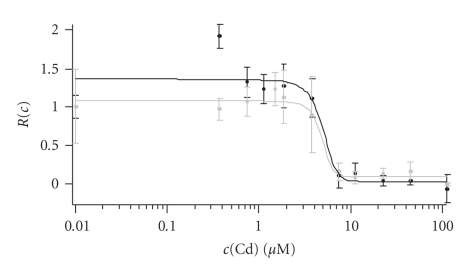
Concerns against the use of semiconductor NCs for cell biological applications go well beyond arguments of steric hindrance. It is well known that Cd2+ can be released from the CdSe core after oxydative attack (corrosion) [70]. Bare CdSe NCs are particularly harmful in this regard [36, 71], limiting their utility for direct-injection strategies. Additional shells (ZnS) and capping (silanization) can reduce Cd2+ leakage, and further purification steps can remove already released Cd2+ [71–73]. In our hands, a supplementary purification step prior to loading NC reduces the toxic action of NCs [74], as measured by a resazurin or cell adhesion assay (Figure 4). Nevertheless, it is important to bear in mind that despite sporadic claims of nanoparticles being indiscriminately harmless [57], there is a general consensus that NC are toxic and that their toxicity depends on their concentration, precise chemical composition, the particle size, colloidal stability, as well as solubilization and functionalization groups. Also, CdSe particles are generally more toxic inside the cell than extracellularly, in line with the known action of Cd2+ by inhibiting protein synthesis, carbohydrate metabolism and—with time— by its accumulation in kidney and liver [75]. At the same time, the undisputable cytotoxic action of Cd nanoparticles has not precluded acute staining experiments of cells, because the concentration of NCs can be always kept low enough to prevent immediate cytotoxic damage within the experimental time window, but still high enough for enough fluorescence [4, 20, 39, 45, 47, 50]. However, because of the ligand desorption over time, a simple ligand exchange functionalization is not effective to durably prevent intracellular NC degradation. Since as much as NCs are intrinsically colloidally unstable and cytotoxic for cells [76], the specific kind of coating is essential for at least retarding the cytotoxic effect [29, 36, 73]. PEG coating can reduce the unspecific uptake of NCs, it reduces their toxic effect for extracellular application at the same initial concentration [29, 71].
Figure 4.
Experimental evaluation of cytotoxicity of polymer-coated CdSe nanoparticles. Toxicity to NIH-3T3 fibroblasts of a NC-containing solution was estimated after 48-hour application. Dose-response curve for cell viability was measured with a Resazurin assay [74]. Reazurin is a nonfluorescent dye that is metabolized in functional mitochondria and converted into resorufin which fluoresces red. Black and grey curves show two experiments (n = 8 measurements each). Normalized survival R(c) after application of a Cd2+ concentrationc(Cd) was estimated from the change in absorbance of the converted dye measured at 600 nm. Changes in R became significant at c (Cd) around 3–5 μM, whereby c (Cd) refers to the concentration of Cd2+ on the surface of the CdSe nanoparticles, which accounts for the different toxicity of different-size NCs. This concentration of Cd2+ corresponds to a concentration of CdSe nanoparticles of 50-70 nm. Similar data was obtained with a cell adhesion assay [71] (not shown). In contrast for free cobalt ions (Co2+), cytotoxic effects became significant at around 50 M (data not shown).
For biomedical applications as well as chronic animal experiments, the major healthcare concern of NC labelling is related to the leakage of Cd2+ into the organism, even at low dose. Extracellular application of CdSe particles already presents a cytotoxic risk because Cd2+ does not only block Ca2+ ion channels (like Co2+ as well, which is released from magnetic NCs) but also it permeates through the channel and enters the cytoplasm. We note that the absence of a visible effect, often based on the detection of cell morphology changes and cell viability assays does not exclude a cumulative poisoning of the organism which first impairs the metabolism of the cells, without being immediately noxious. Interestingly, a similar debate has long haunted the evaluation of nonlinear photodamage caused by two-photon fluorescence excitation, where the introduction of rigorous physiologically relevant criteria based on microscopic observables like the kinetics of Ca2+ transients [77, 78] has ended the futile discussion.
In conclusion, more work is needed to critically evaluate the cytotoxicity of NCs, both upon short- and long-term exposure. To better understand the deleterious action of different NCs on the organism under study, standardized samples, experimental conditions, cells, and assays would be a great leap forward and pave the ground for biomedical applications that would additionally benefit from a tight collaboration with toxicologists.
8. CONCLUSIONS
In this review, we focus on nanocrystal applications in vivo, both in cell biology and medical diagnostics, and on the potential toxicity of NCs for biological imaging. The advances in understanding NC colloidal properties together with the ability of developing stable surface chemistries has brought about a large choice of functionalization strategies which now offer to biologists a versatile tool kit for many applications that rely on fluorescence and electron microscopy. The main advantages of NCs over conventional organic fluorophores are the possibility to detect easily single molecules, mostly derived from their superior brightness and the long-term photostability; the spectral tunability and narrow-band emission; and, going along with these, the ease of NC use in multicolour fluorescence. However, nanoparticles are not a cure-all. Particularly Cd-based NCs are potentially cytotoxic, and the modulation of their optical properties (e.g., their intrinsic fluorescence intermittency) through their local chemical environment (see, e.g., [79]) needs to be considered in each application.
ACKNOWLEDGMENTS
Work in laboratories related to this article was partially funded by the French Ministry of Research and Technology (Action Concertée Incitative (ACI) Nanosciences), by the GIP-ANR Nanoscience Program (PRA-PNANO-051 “NanoFRET”), and the European Union (NanoInteract to W. J. Parak). C. Luccardini initially recipient of a postdoctoral research fellowship financed by the European Union. (Long-Period Observation of Single Biomolecules by Novel Non-Invasive Fluorescence Lifetime Imaging Nanoscopy (FLIN), STRP NMP4-CT-2004-013880 to M. Oheim) is now a Fondation pour la Recherche Médicale (FRM) fellow and A. Yakovlev is a postdoctoral fellow of the Centre National de la Recherche (CNRS to A. Feltz). S. Gaillard acknowledges support from the GIP-ANR. Marcel van ‘t Hoff’s work is financed by a Marie-Curie Research-training Grant (From FLIM to FLIN, FP6-2005-019481 to M. Oheim). C. Luccardini, A. Yakovlev, S. Gaillard, J.-M. Mallet, W. J. Parak, A. Feltz, and M. Oheim are members of the “NanoFRET” consortium, supported by the Groupement d’Intérêt Publique-Agence Nationale de la Recherche, Programme Nanosciences et Nanotechnologies (GIP-ANR PNANO).
References
- 1.Alivisatos P. Colloidal quantum dots. From scaling laws to biological applications. Pure and Applied Chemistry. 2000;72(1-2):3–9. [Google Scholar]
- 2.Kim S, Fisher B, Eisler H-J, Bawendi M. Type-II quantum dots: CdTe/CdSe(core/shell) and CdSe/ZnTe(core/shell) heterostructures. Journal of the American Chemical Society. 2003;125(38):11466–11467. doi: 10.1021/ja0361749. [DOI] [PubMed] [Google Scholar]
- 3.Chan WCW, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281(5385):2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 4.Larson DR, Zipfel WR, Williams RM, et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300(5624):1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 5.Oheim M, Li D. Quantitative co-localisation imaging: concepts, measurements, and pitfalls. In: Frischknecht F, Pawley J, Shorte S, editors. Imaging Cellular and Molecular Biological Function. Berlin, Germany: Springer; 2007. pp. 115–153. [Google Scholar]
- 6.Dahan M, Laurence T, Pinaud F, et al. Time-gated biological imaging by use of colloidal quantum dots. Optics Letters. 2001;26(11):825–827. doi: 10.1364/ol.26.000825. [DOI] [PubMed] [Google Scholar]
- 7.Chan WCW, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Current Opinion in Biotechnology. 2002;13(1):40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 8.Yu WW, Qu L, Guo W, Peng X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chemistry of Materials. 2003;15(14):2854–2860. [Google Scholar]
- 9.Dahan M, Lèvi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302(5644):442–445. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 10.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298(5599):1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnology. 2004;22(1):93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapsford KE, Berti L, Medintz IL. Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations. Angewandte Chemie International Edition. 2006;45(28):4562–4589. doi: 10.1002/anie.200503873. [DOI] [PubMed] [Google Scholar]
- 13.Clapp AR, Medintz IL, Mauro JM, Fisher BR, Bawendi MG, Mattoussi H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. Journal of the American Chemical Society. 2004;126(1):301–310. doi: 10.1021/ja037088b. [DOI] [PubMed] [Google Scholar]
- 14.Clapp AR, Medintz IL, Tetsuo Uyeda H, et al. Quantum dot-based multiplexed fluorescence resonance energy transfer. Journal of the American Chemical Society. 2005;127(51):18212–18221. doi: 10.1021/ja054630i. [DOI] [PubMed] [Google Scholar]
- 15.Medintz IL, Clapp AR, Brunel FM, et al. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nature Materials. 2006;5(7):581–589. doi: 10.1038/nmat1676. [DOI] [PubMed] [Google Scholar]
- 16.Medintz IL, Konnert JH, Clapp AR, et al. A fluorescence resonance energy transfer-derived structure of a quantum dot-protein bioconjugate nanoassembly. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9612–9617. doi: 10.1073/pnas.0403343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nirmal M, Dabbousi BO, Bawendi MG, et al. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature. 1996;383(6603):802–804. [Google Scholar]
- 18.Bonneau S, Dahan M, Cohen LD. Single quantum dot tracking based on perceptual grouping using minimal paths in a spatiotemporal volume. IEEE Transactions on Image Processing. 2005;14(9):1384–1395. doi: 10.1109/tip.2005.852794. [DOI] [PubMed] [Google Scholar]
- 19.Gordon MP, Ha T, Selvin PR. Single-molecule high-resolution imaging with photobleaching. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6462–6465. doi: 10.1073/pnas.0401638101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjugate Chemistry. 2004;15(1):79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Argüelles MT, Yakovlev A, Sperling RA, et al. Synthesis and characterization of polymer-coated quantum dots with integrated acceptor dyes as FRET-based nanoprobes. Nano Letters. 2007;7(9):2613–2617. doi: 10.1021/nl070971d. [DOI] [PubMed] [Google Scholar]
- 22.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281(5385):2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 23.Gao X, Chung LW, Nie S. Quantum dots for in vivo molecular and cellular imaging. Methods in Molecular Biology. 2007;374:135–146. doi: 10.1385/1-59745-369-2:135. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrino T, Parak WJ, Boudreau R, et al. Quantum dot-based cell motility assay. Differentiation. 2003;71(9-10):542–548. doi: 10.1111/j.1432-0436.2003.07109006.x. [DOI] [PubMed] [Google Scholar]
- 25.Luccardini C, Tribet C, Vial F, Marchi-Artzner V, Dahan M. Size, charge, and interactions with giant lipid vesicles of quantum dots coated with an amphiphilic macromolecule. Langmuir. 2006;22(5):2304–2310. doi: 10.1021/la052704y. [DOI] [PubMed] [Google Scholar]
- 26.Åkerman ME, Chan WCW, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling RA, Pellegrino T, Li JK, Chang WH, Parak WJ. Electrophoretic separation of nanoparticles with a discrete number of functional groups. Advanced Functional Materials. 2006;16(7):943–948. [Google Scholar]
- 28.Parak WJ, Pellegrino T, Plank C. Labelling of cells with quantum dots. Nanotechnology. 2005;16(2):R9–R25. doi: 10.1088/0957-4484/16/2/R01. [DOI] [PubMed] [Google Scholar]
- 29.Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R. Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small. 2006;2(12):1412–1417. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal SJ, Tomlinson I, Adkins EM, et al. Targeting cell surface receptors with ligand-conjugated nanocrystals. Journal of the American Chemical Society. 2002;124(17):4586–4594. doi: 10.1021/ja003486s. [DOI] [PubMed] [Google Scholar]
- 31.Howarth M, Takao K, Hayashi Y, Ting AY. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(21):7583–7588. doi: 10.1073/pnas.0503125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nature Biotechnology. 2003;21(1):41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 33.Bouzigues C, Levi S, Triller A, Dahan M. Single quantum dot tracking of membrane receptors. Methods in Molecular Biology. 2007;374:81–92. doi: 10.1385/1-59745-369-2:81. [DOI] [PubMed] [Google Scholar]
- 34.Lidke DS, Nagy P, Jovin TM, Arndt-Jovin DJ. Biotin-ligand complexes with streptavidin quantum dots for in vivo cell labeling of membrane receptors . Methods in Molecular Biology. 2007;374:69–80. doi: 10.1385/1-59745-369-2:69. [DOI] [PubMed] [Google Scholar]
- 35.Lidke DS, Nagy P, Heintzmann R, et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nature Biotechnology. 2004;22(2):198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- 36.Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Letters. 2004;4(1):11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinaud F, King D, Moore H-P, Weiss S. Bioactivation and cell targeting of semiconductor CdSe/ZnS nanocrystals with phytochelatin-related peptides. Journal of the American Chemical Society. 2004;126(19):6115–6123. doi: 10.1021/ja031691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrensperger M-V, Hanus C, Vannier C, Triller A, Dahan M. Multiple association states between glycine receptors and gephyrin identified by SPT analysis. Biophysical Journal. 2007;92(10):3706–3718. doi: 10.1529/biophysj.106.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nature Biotechnology. 2003;21(1):47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 40.Cognet L, Tardin C, Boyer D, Choquett D, Tamarat P, Lounis B. Single metallic nanoparticle imaging for protein detection in cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(20):11350–11355. doi: 10.1073/pnas.1534635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Letters. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 42.Jaiswal JK, Goldman ER, Mattoussi H, Simon SM. Use of quantum dots for live cell imaging. Nature Methods. 2004;1(1):73–78. doi: 10.1038/nmeth1004-73. [DOI] [PubMed] [Google Scholar]
- 43.Parton RG, Simons K. The multiple faces of caveolae. Nature Reviews Molecular Cell Biology. 2007;8(3):185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 44.Rathenberg J, Nevian T, Witzemann V. High-efficiency transfection of individual neurons using modified electrophysiology techniques. Journal of Neuroscience Methods. 2003;126(1):91–98. doi: 10.1016/s0165-0270(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 45.Chen F, Gerion D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Letters. 2004;4(10):1827–1832. [Google Scholar]
- 46.Courty S, Luccardini C, Bellaiche Y, Cappello G, Dahan M. Tracking individual kinesin motors in living cells using single quantum-dot imaging. Nano Letters. 2006;6(7):1491–1495. doi: 10.1021/nl060921t. [DOI] [PubMed] [Google Scholar]
- 47.Voura EB, Jaiswal JK, Mattoussi H, Simon SM. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature Medicine. 2004;10(9):993–998. doi: 10.1038/nm1096. [DOI] [PubMed] [Google Scholar]
- 48.Stroh M, Zimmer JP, Duda DG, et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nature Medicine. 2005;11(6):678–682. doi: 10.1038/nm1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parak WJ, Boudreau R, Le Gros M, et al. Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Advanced Materials. 2002;14(12):882–885. [Google Scholar]
- 50.Hanaki K-I, Momo A, Oku T, et al. Semiconductor quantum dot/albumin complex is a long-life and highly photostable endosome marker. Biochemical and Biophysical Research Communications. 2003;302(3):496–501. doi: 10.1016/s0006-291x(03)00211-0. [DOI] [PubMed] [Google Scholar]
- 51.Duan H, Nie S. Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. Journal of the American Chemical Society. 2007;129(11):3333–3338. doi: 10.1021/ja068158s. [DOI] [PubMed] [Google Scholar]
- 52.Lagerholm BC. Peptide-mediated intracellular delivery of quantum dots. Methods in Molecular Biology. 2007;374:105–112. doi: 10.1385/1-59745-369-2:105. [DOI] [PubMed] [Google Scholar]
- 53.Iyer G, Pinaud F, Tsay J, et al. Peptide coated quantum dots for biological applications. IEEE Transactions on Nanobioscience. 2006;5(4):231–238. doi: 10.1109/tnb.2006.886563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewin M, Carlesso N, Tung C-H, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature Biotechnology. 2000;18(4):410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 55.Mattheakis LC, Dias JM, Choi Y-J, et al. Optical coding of mammalian cells using semiconductor quantum dots. Analytical Biochemistry. 2004;327(2):200–208. doi: 10.1016/j.ab.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 56.Delehanty JB, Medintz IL, Pons T, Brunel FM, Dawson PE, Mattoussi H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjugate Chemistry. 2006;17(4):920–927. doi: 10.1021/bc060044i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoshino A, Fujioka K, Oku T, et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Letters. 2004;4(11):2163–2169. [Google Scholar]
- 58.Funnell WRJ, Maysinger D. Three-dimensional reconstruction of cell nuclei, internalized quantum dots and sites of lipid peroxidation. Journal of Nanobiotechnology. 2006;4:1–10. doi: 10.1186/1477-3155-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen I, Ting AY. Site-specific labeling of proteins with small molecules in live cells. Current Opinion in Biotechnology. 2005;16(1):35–40. doi: 10.1016/j.copbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Lippincott-Schwartz J, Patterson GH. Development and use of fluorescent protein markers in living cells. Science. 2003;300(5616):87–91. doi: 10.1126/science.1082520. [DOI] [PubMed] [Google Scholar]
- 61.Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281(5374):269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 62.Adams SR, Campbell RE, Gross LA, et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. Journal of the American Chemical Society. 2002;124(21):6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 63.Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nature Biotechnology. 2003;21(1):86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, So M-K, Loening AM, Yao H, Gambhir SS, Rao J. HaloTag protein-mediated site-specific conjugation of bioluminescent proteins to quantum dots. Angewandte Chemie International Edition. 2006;45(30):4936–4940. doi: 10.1002/anie.200601197. [DOI] [PubMed] [Google Scholar]
- 65.Fischer HC, Liu L, Pang KS, Chan WCW. Pharmacokinetics of nanoscale quantum dots: in vivo distribution, sequestration, and clearance in the rat. Advanced Functional Materials. 2006;16(10):1299–1305. [Google Scholar]
- 66.Oheim M, Beaurepaire E, Chaigneau E, Mertz J, Charpak S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. Journal of Neuroscience Methods. 2001;111(1):29–37. doi: 10.1016/s0165-0270(01)00438-1. [DOI] [PubMed] [Google Scholar]
- 67.Theer P, Denk W. On the fundamental imaging-depth limit in two-photon microscopy. Journal of the Optical Society of America A. 2006;23(12):3139–3149. doi: 10.1364/josaa.23.003139. [DOI] [PubMed] [Google Scholar]
- 68.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology. 2004;22(8):969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 69.So M-K, Xu C, Loening AM, Gambhir SS, Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nature Biotechnology. 2006;24(3):339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 70.Zaitseva N, Manna L, Leon F, Gerion D, Saw C. Precipitation of se crystals from solutions of CdSe nanocrystals. Advanced Materials. 2005;17:1321–1324. doi: 10.1002/adma.200401597. [DOI] [PubMed] [Google Scholar]
- 71.Kirchner C, Liedl T, Kudera S, et al. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Letters. 2005;5(2):331–338. doi: 10.1021/nl047996m. [DOI] [PubMed] [Google Scholar]
- 72.Selvan ST, Tan TT, Ying JY. Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Advanced Materials. 2005;17(13):1620–1625. [Google Scholar]
- 73.Zhang T, Stilwell JL, Gerion D, et al. Cellular effect of high doses of silica-coated quantum dot profiled with high throughput gene expression analysis and high content cellomics measurements. Nano Letters. 2006;6(4):800–808. doi: 10.1021/nl0603350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 2000;267(17):5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 75.Nath R, Prasad R, Palinal VK, Chopra RK. Molecular basis of cadmium toxicity. Progress in Food and Nutrition Science. 1984;8(1-2):109–163. [PubMed] [Google Scholar]
- 76.Lovrić J, Bazzi HS, Cuie Y, Fortin GRA, Winnik FM, Maysinger D. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. Journal of Molecular Medicine. 2005;83(5):377–385. doi: 10.1007/s00109-004-0629-x. [DOI] [PubMed] [Google Scholar]
- 77.Koester HJ, Baur D, Uhl R, Hell SW. fluorescence imaging with pico- and femtosecond two-photon excitation: signal and photodamage. Biophysical Journal. 1999;77(4):2226–2236. doi: 10.1016/S0006-3495(99)77063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hopt A, Neher E. Highly nonlinear photodamage in two-photon fluorescence microscopy. Biophysical Journal. 2001;80(4):2029–2036. doi: 10.1016/S0006-3495(01)76173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hohng S, Ha T. Near-complete suppression of quantum dot blinking in ambient conditions. Journal of the American Chemical Society. 2004;126(5):1324–1325. doi: 10.1021/ja039686w. [DOI] [PubMed] [Google Scholar]