The oxylipin jasmonic acid (JA) and its metabolites, collectively known as jasmonates, are important plant signaling molecules that mediate biotic and abiotic stress responses as well as aspects of growth and development. Although it is well known that JA regulates transcription, the mechanism of this regulation has been largely unknown. Recently, this situation changed dramatically with the discovery of a novel family of transcriptional regulators called jasmonate ZIM-domain (JAZ) proteins. Three groups independently identified members of this family in Arabidopsis and showed that they function as repressors of JA-regulated transcription. Furthermore, genetic, molecular, and biochemical studies indicate that the JAZ proteins are degraded by the ubiquitin protein ligase SCFCOI1 in response to JA. In addition, one group showed that JAZ proteins interact with the positive regulator of JA signaling, MYC2. These data support an important new model of jasmonate signaling and response. In this essay, we summarize this recent work and discuss some remaining questions concerning JA signal transduction.
THE ROLE OF SCFCOI1 IN JASMONATE SIGNALING
Coronatine is a phytotoxin produced by the plant pathogen Pseudomonas syringae that is structurally related to methyl jasmonate (MeJA) and produces very similar effects when applied to plants (Feys et al., 1994). The Arabidopsis coronatine-insensitive1 (coi1) mutant was isolated in a forward genetic screen exploiting coronatine's inhibitory effect on root elongation (Feys et al., 1994). This mutation confers insensitivity to coronatine and MeJA, as well as male sterility (Feys et al., 1994). Subsequent studies demonstrated that coi1 mutants are completely deficient in every aspect of jasmonate response (Xie et al., 1998), indicating that COI1 has an essential function in JA signaling. Ultimately, it was demonstrated that COI1 encodes an F-box protein closely related to the auxin receptor/F-box protein TIR1 (Xie et al., 1998). This finding suggested that COI1 might act as part of an SCF (Skp/Cullin/F-box) E3 ubiquitin ligase to mediate jasmonate signaling (Xie et al., 1998). Indeed, COI1 was shown to associate with components of SCF complexes, including ASK1, ASK2, CUL1, and RBX1 (Xu et al., 2002). Further support for the importance of the ubiquitin proteasome pathway in JA signaling came from observations that mutations in genes required for SCF function, such as AXR1, CUL1, and JAI4/SGT1b, result in reduced jasmonate responses (Devoto et al., 2002; Xu et al., 2002; Gray et al., 2003; Lorenzo et al., 2004; Lorenzo and Solano, 2005; Ren et al., 2005). Based on these data, it was hypothesized that SCFCOI1 targets proteins for degradation by the 26S proteasome to promote jasmonate-mediated transcriptional changes.
JASMONATE TRANSCIPTIONAL RESPONSES
Large-scale transcriptional profiling has identified a number of COI1-dependent jasmonate-regulated genes in Arabidopsis (Devoto et al., 2005; Jung et al., 2007). Moreover, a number of transcription factors that control these changes have been identified, including ERF1, WRKY70, ORA47, ORA59, and MYC2 (reviewed in Wasternack, 2007). Other recent work has demonstrated that WRKY18 (Xu et al., 2006) and two previously uncharacterized transcription factors, At1g74930 and At3g53600, which belong to the AP2/EREBP and C2H2 families, respectively (Wang et al., 2007), are positive regulators of JA responses to wounding and are dependent on COI1. The most well-characterized transcription factor in jasmonate signaling to date is MYC2, which was identified in two independent genetic screens from the mutants methyl-jasmonate resistant1 and jasmonate-insensitive1 (Berger et al., 1996; Lorenzo et al., 2004). MYC2 positively regulates genes involved in wounding responses but negatively regulates genes involved in pathogen defense (Lorenzo et al., 2004). Interestingly, ERF1 also differentially regulates these two branches of the JA signaling pathway but with the opposite effect of MYC2 (Lorenzo et al., 2003, 2004). Several studies have demonstrated that MYC2 preferentially binds to G-box or G-box–related hexamers in the promoters of target genes (Abe et al., 1997; de Pater et al., 1997; Yadav et al., 2005; Chini et al., 2007; Dombrecht et al., 2007). A number of genes encoding transcription factors involved in JA-mediated transcriptional regulation have these G-box motifs in their 5′ regulatory sequences, including MYC2 and ERF1 (Dombrecht et al., 2007).
IDENTIFICATION OF THE JAZ PROTEINS
Three groups independently identified a family of proteins in Arabidopsis that function as repressors in the JA signaling pathway using either genetic or transcript profiling approaches. These proteins are known as JAZ proteins because their transcripts increase in abundance upon jasmonate treatment and they share a 28–amino acid ZIM domain of unknown function (Chini et al., 2007; Thines et al., 2007). In the study by Thines et al. (2007), eight members of the JAZ family were identified as rapid JA-responsive genes in microarray experiments that involved JA treatment of the jasmonate synthesis mutant opr3. Database searches identified five additional genes that encoded related proteins, bringing the total number of family members to 12 (Thines et al., 2007). In a separate study, Chini et al. (2007) characterized the mutant jasmonate-insensitive3-1 (jai3-1) they had identified in a forward genetic screen. Positional cloning of the jai3-1 mutation identified a base change in an intron acceptor site of the gene now known as JAZ3. The jai3-1 mutation results in the production of a truncated protein that causes a jasmonate-insensitive phenotype and impaired transcriptional responses to jasmonate (Chini et al., 2007). Finally, Yan et al. (2007) selected candidate JA signaling genes from microarray experiments and made transgenic plants overexpressing these candidate genes. One of the transgenic lines, overexpressing a predicted alternatively spliced transcript from a gene they called Jasmonate-Associated1 (JAS1), reduced MeJA sensitivity in root elongation assays. Using the nomenclature of Chini et al. (2007) and Thines et al. (2007), JAS1 is also known as JAZ10.
JAZ PROTEINS ARE SUBSTRATES OF SCFCOI1
Working with different members of this protein family, all three groups found that expression of truncated proteins lacking all or part of a conserved domain in the C terminus reduced plant sensitivity to jasmonate, whereas expression of the full-length proteins had little effect (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007). Subsequent studies found that full-length JAZ1, JAZ6, and JAI3/JAZ3 proteins are degraded following jasmonate treatment in a proteasome-dependent manner (Chini et al., 2007; Thines et al., 2007). However, these same proteins were stabilized in the coi1-1 background, implicating SCFCOI1 in a JAZ degradation pathway (Chini et al., 2007; Thines et al., 2007). Truncated versions of JAZ1, JAZ6, and JAZ3, lacking their respective C-terminal domains, were resistant to jasmonate-induced degradation (Chini et al., 2007; Thines et al., 2007). Moreover, expression of the truncated version of JAI3/JAZ3, either in transient expression assays or in the jai3-1 background, blocked the degradation of full-length JAZ3, JAZ1, and JAZ9 (Chini et al., 2007). These results suggest that the dominant jasmonate-insensitive phenotype of plants expressing truncated JAZ proteins is due to stabilization not only of the truncated protein but additional JAZ family members as well. The data are consistent with a model in which the JAZ proteins are repressors of jasmonate signaling. Furthermore, mutants harboring null T-DNA insertion mutations in JAZ2, JAZ5, JAZ7, and JAZ9 failed to exhibit jasmonate-related phenotypes, suggesting that these genes function redundantly (Thines et al., 2007). Interestingly, RNA interference lines that knocked down expression of JAS1/JAZ10 exhibited hypersensitivity when treated with MeJA compared with wild-type plants (Yan et al., 2007). Perhaps the JAS1/JAZ10 RNA interference construct targets additional JAZ genes leading to the jasmonate phenotype.
Genetic data suggest that the JAZ proteins serve as substrates for SCFCOI1. To test this idea further, both the Browse and Solano groups examined the ability of JAZ1 and JAI3/JAZ3 to interact with COI1 using in vitro pull-down assays, and both groups demonstrated an interaction (Chini et al., 2007; Thines et al., 2007). This interaction was confirmed for JAZ1 using yeast two-hybrid (Y2H) assays (Thines et al., 2007). Interestingly, Chini et al. (2007), demonstrated that it is the N-terminal half of JAI3/JAZ3 that interacts with COI1. This is somewhat surprising given that C-terminal domain deletions stabilize these proteins.
An important issue in JA signaling is the chemical nature of the signal. Although exogenous JA elicits a response, earlier data strongly suggest that it is not the active signaling molecule. The JASMONIC ACID RESISTANT1 (JAR1) protein is an adenylating enzyme that catalyzes the conjugation of JA to amino acids such as l-isoleucine (JA-Ile) (Staswick and Tiryaki, 2004). Because jar1 mutants are resistant to the inhibitory effects of MeJA on growth, conjugated forms of JA are predicted to be the active molecules (Staswick et al., 1992). Indeed, the COI1–JAZ1 protein interaction in Y2H assays was promoted by the inclusion of JA-Ile but not of JA alone, MeJA, or another precursor called 12-oxo-phytodienoic acid (Thines et al., 2007). In vitro pull-down assays confirmed the Y2H results and demonstrated that a JA-Leu derivative could promote the COI1–JAZ1 interaction but was at least 50-fold less effective than JA-Ile, whereas JA-Phe and JA-Trp had no activity (Thines et al., 2007).
JAZ PROTEINS LINK SCFCOI1 WITH TRANSCRIPTIONAL ACTIVATION
The JAZ proteins do not have an obvious DNA binding domain, suggesting that their effects on transcription could be indirect (Chini et al., 2007). Pull-down and Y2H assays indicate that the C-terminal domain of JAI3/JAZ3 interacts with sequences at the N terminus of MYC2 (Chini et al., 2007). Therefore, it is possible that JAI3/JAZ3 directly blocks MYC2 function. JAI3/JAZ3 degradation via SCFCOI1 in response to JA would permit MYC2 to activate or repress downstream target genes in JA signaling pathways. Interestingly, several of the JAZ genes are themselves upregulated in response to jasmonate or are constitutively expressed in untreated plants expressing MYC2 from the cauliflower mosaic virus 35S promoter (Chini et al., 2007). These findings indicate that a negative feedback mechanism may limit the response after the initial jasmonate perception.
CONCLUSIONS AND FUTURE STUDIES
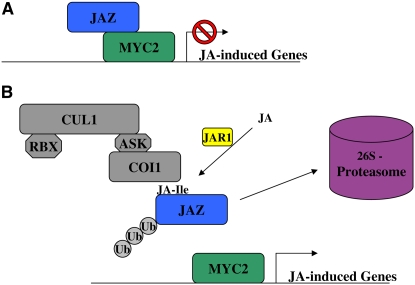
The discovery of the JAZ proteins and the elucidation of their role in JA signaling are exciting and important because it links several active areas of research into a coherent signaling pathway (Figure 1). The data presented in these recent aricles imply that JAZ proteins bind and repress the transcription factors that modulate transcription of JA-responsive genes. In summary, JAR1 conjugates JA to JA-Ile, which in turn promotes the interaction between SCFCOI1 and the JAZ repressors. This interaction results in the degradation of the JAZ proteins and subsequent derepression of transcription factors, such as MYC2. It is striking how similar the JA signaling pathway is to the auxin signaling pathway established only a few years ago with the discovery that SCFTIR1 serves as an auxin receptor (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005). Indeed, COI1 is the closest known relative to the TIR1/AFB family of F-box proteins (Dharmasiri et al., 2005b), and based on this similarity, it has been suggested that COI1 may be a JA receptor (Parry and Estelle, 2006; Tan et al., 2007). Now, with the identification of COI1 substrates and the recent determination of the structure of TIR1, it should be possible to test this possibility directly. However, even with the establishment of COI1 as a potential site of JA perception, some fascinating questions remain. For example, what constitutes the JA receptor? Does JA-Ile bind COI1, JAZs proteins, or are both proteins required to form the receptor? How many ligands derived from JA promote the interaction of COI1 and JAZ proteins and is there any specificity with respect to different JA conjugates and members of the JAZ family? Coronatine is a phytotoxin from the plant pathogen Pseudomonas with structural similarity to JA-Ile. Does it directly promote COI1–JAZ interactions, and how many other pathogens produce molecules that can directly activate JA responses through this signaling mechanism?
Figure 1.
Model for Jasmonate Response in Arabidopsis.
(A) MYC2 (green) binds to G-box motifs adjacent to JA-regulated genes. JAZ proteins (blue) repress MYC2 activity through a direct interaction between the C terminus of JAZ and the N terminus of MYC2.
(B) JA is conjugated to l-Ile by JAR1 (yellow). JA-Ile promotes the interaction of JAZ proteins with SCFCOI1 (gray), leading to polyubiquitination (Ub) and subsequent degradation by the 26S proteasome (pink). Degradation of the JAZ repressors frees MYC2 to mediate JA-responsive transcriptional changes.
To date, Aux/IAA proteins are the only known substrates of SCFTIR1/AFB complexes, but it is possible that there are others. Are the JAZ proteins the only substrates SCFCOI1? Histone deacetylases can negatively regulate transcription, have been shown to interact with COI1 in planta (Devoto et al., 2002), and have been implicated in JA signaling (Zhou et al., 2005). Could they also be substrates for COI1? On the other side of the signaling equation, how many transcription factors can the JAZ repressors bind and is there any specificity for repressor-transcription factor binding? As these questions and others are addressed in the future, we can expect new insights into the mechanism of JA signaling and its importance in various aspects of plant growth and survival.
Acknowledgments
Research in the authors' lab is supported by grants from the National Institutes of Health, the National Science Foundation, and the Department of Energy to M.E.
References
- Abe, H., Yamaguchi-Shinozaki, K., Urao, T., Iwasaki, T., Hosokawa, D., and Shinozaki, K. (1997). Role of arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, S., Bell, E., and Mullet, J.E. (1996). Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiol. 111 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini, A., Fonseca, S., Fernandez, G., Adie, B., Chico, J.M., Lorenzo, O., Garcia-Casado, G., Lopez-Vidriero, I., Lozano, F.M., Ponce, M.R., Micol, J.L., and Solano, R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448 666–671. [DOI] [PubMed] [Google Scholar]
- de Pater, S., Pham, K., Memelink, J., and Kijne, J. (1997). RAP-1 is an Arabidopsis MYC-like R protein homologue that binds to G-box sequence motifs. Plant Mol. Biol. 34 169–174. [DOI] [PubMed] [Google Scholar]
- Devoto, A., Ellis, C., Magusin, A., Chang, H.S., Chilcott, C., Zhu, T., and Turner, J.G. (2005). Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 58 497–513. [DOI] [PubMed] [Google Scholar]
- Devoto, A., Nieto-Rostro, M., Xie, D., Ellis, C., Harmston, R., Patrick, E., Davis, J., Sherratt, L., Coleman, M., and Turner, J.G. (2002). COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 32 457–466. [DOI] [PubMed] [Google Scholar]
- Dharmasiri, N., Dharmasiri, S., and Estelle, M. (2005. a). The F-box protein TIR1 is an auxin receptor. Nature 435 441–445. [DOI] [PubMed] [Google Scholar]
- Dharmasiri, N., Dharmasiri, S., Weijers, D., Lechner, E., Yamada, M., Hobbie, L., Ehrismann, J.S., Jurgens, G., and Estelle, M. (2005. b). Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9 109–119. [DOI] [PubMed] [Google Scholar]
- Dombrecht, B., Xue, G.P., Sprague, S.J., Kirkegaard, J.A., Ross, J.J., Reid, J.B., Fitt, G.P., Sewelam, N., Schenk, P.M., Manners, J.M., and Kazan, K. (2007). MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19 2225–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B., Benedetti, C.E., Penfold, C.N., and Turner, J.G. (1994). Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, W.M., Muskett, P.R., Chuang, H.W., and Parker, J.E. (2003). Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, C., Lyou, S.H., Yeu, S., Kim, M.A., Rhee, S., Kim, M., Lee, J.S., Choi, Y.D., and Cheong, J.J. (2007). Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana. Plant Cell Rep. 26 1053–1063. [DOI] [PubMed] [Google Scholar]
- Kepinski, S., and Leyser, O. (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435 446–451. [DOI] [PubMed] [Google Scholar]
- Lorenzo, O., Chico, J.M., Sanchez-Serrano, J.J., and Solano, R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo, O., Piqueras, R., Sanchez-Serrano, J.J., and Solano, R. (2003). ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo, O., and Solano, R. (2005). Molecular players regulating the jasmonate signalling network. Curr. Opin. Plant Biol. 8 532–540. [DOI] [PubMed] [Google Scholar]
- Parry, G., and Estelle, M. (2006). Auxin receptors: A new role for F-box proteins. Curr. Opin. Cell Biol. 18 152–156. [DOI] [PubMed] [Google Scholar]
- Ren, C., Pan, J., Peng, W., Genschik, P., Hobbie, L., Hellmann, H., Estelle, M., Gao, B., Peng, J., Sun, C., and Xie, D. (2005). Point mutations in Arabidopsis Cullin1 reveal its essential role in jasmonate response. Plant J. 42 514–524. [DOI] [PubMed] [Google Scholar]
- Staswick, P.E., Su, W., and Howell, S.H. (1992). Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 89 6837–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick, P.E., and Tiryaki, I. (2004). The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, X., Calderon-Villalobos, L.I., Sharon, M., Zheng, C., Robinson, C.V., Estelle, M., and Zheng, N. (2007). Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446 640–645. [DOI] [PubMed] [Google Scholar]
- Thines, B., Katsir, L., Melotto, M., Niu, Y., Mandaokar, A., Liu, G., Nomura, K., He, S.Y., Howe, G.A., and Browse, J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448 661–665. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Cao, G., Wang, X., Miao, J., Liu, X., Chen, Z., Qu, L.J., and Gu, H. (2007). Identification and characterization of COI1-dependent transcription factor genes involved in JA-mediated response to wounding in Arabidopsis plants. Plant Cell Rep. 27 125–135. [DOI] [PubMed] [Google Scholar]
- Wasternack, C. (2007). Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. (Lond.) 100 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, D.X., Feys, B.F., James, S., Nieto-Rostro, M., and Turner, J.G. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091–1094. [DOI] [PubMed] [Google Scholar]
- Xu, L., Liu, F., Lechner, E., Genschik, P., Crosby, W.L., Ma, H., Peng, W., Huang, D., and Xie, D. (2002). The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14 1919–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., Chen, C., Fan, B., and Chen, Z. (2006). Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 18 1310–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, V., Mallappa, C., Gangappa, S.N., Bhatia, S., and Chattopadhyay, S. (2005). A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light-mediated photomorphogenic growth. Plant Cell 17 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y., Stolz, S., Chetelat, A., Reymond, P., Pagni, M., Dubugnon, L., and Farmer, E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19 2470–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., Zhang, L., Duan, J., Miki, B., and Wu, K. (2005). HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 17 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]