Abstract
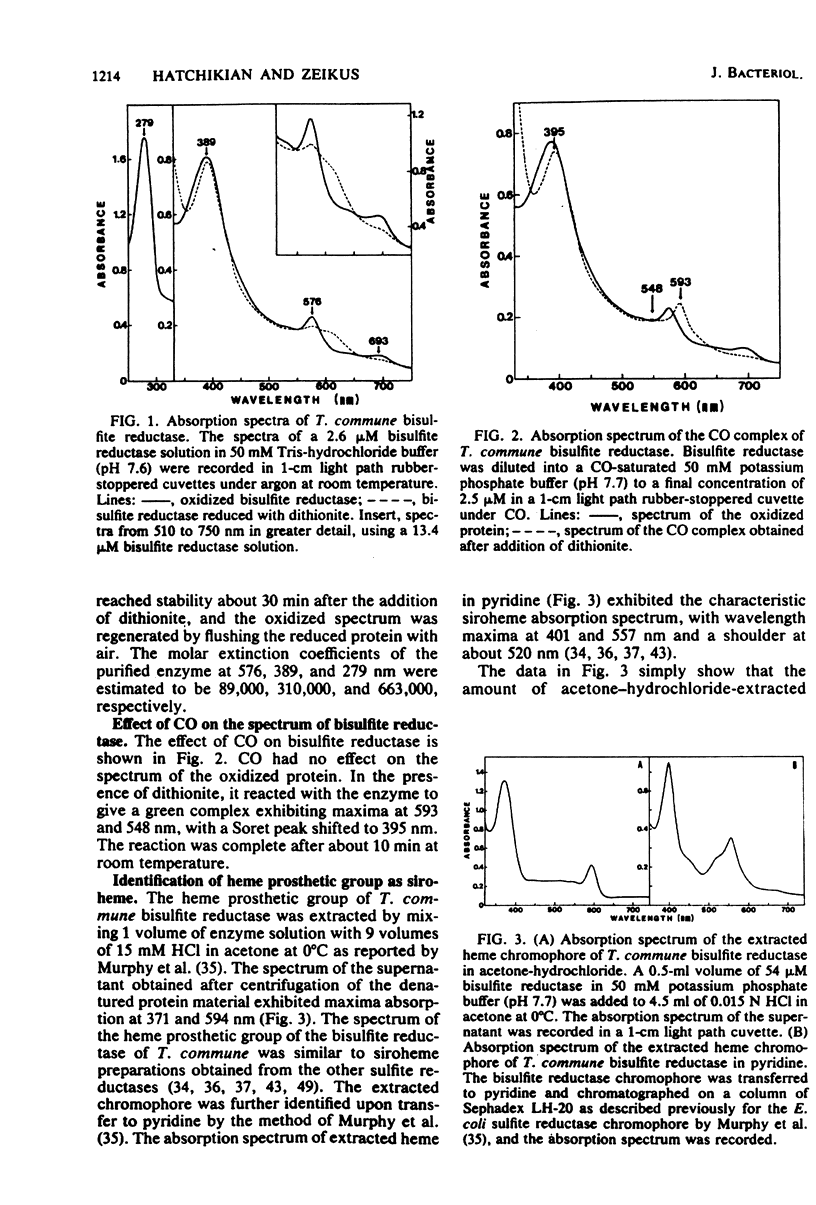
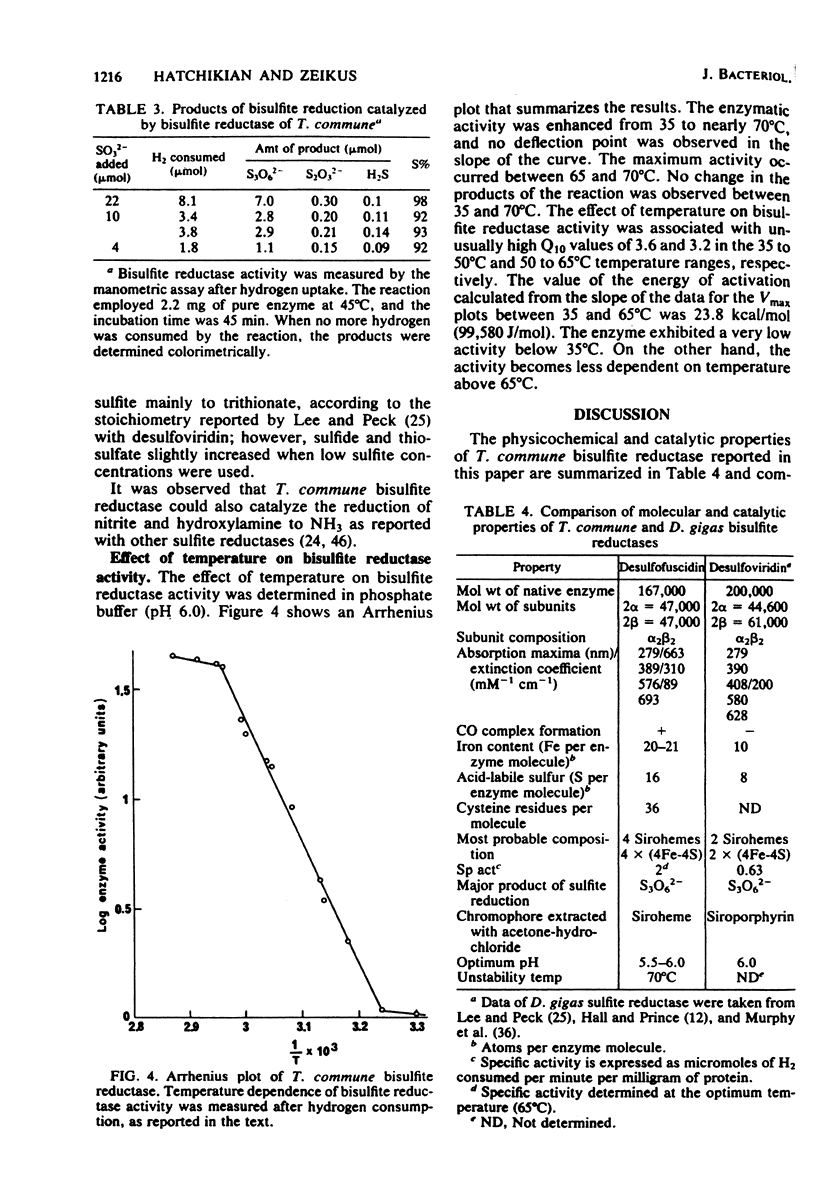
A new type of dissimilatory bisulfite reductase, desulfofuscidin, was isolated from the nonsporeforming thermophilic sulfate-reducing microorganism Thermodesulfobacterium commune. The molecular weight of the enzyme was estimated at 167,000 by sedimentation equilibrium, and the protein was pure by both disc electrophoresis and ultracentrifugation. The bisulfite reductase was a tetramer and had two types of subunits with an α2β2 structure and an individual molecular weight of 47,000. The enzyme exhibited absorption maxima at 576, 389, and 279 nm, with a weak band at 693 nm. Upon the addition of dithionite, the absorption maxima at 576 and 693 nm were weakened, and a new band appeared at 605 nm. The protein reacted with CO in the presence of dithionite to give a complex with absorption peaks at 593, 548, and 395 nm. The extinction coefficients of the purified enzyme at 576, 389, and 279 nm were 89,000, 310,000, and 663,000 M−1 cm−1, respectively. Siroheme was detected as the prosthetic group. The protein contains 20 to 21 nonheme iron atoms and 16 to 17 acid-labile sulfur groups per molecule. The data suggest the presence of four sirohemes and probably four (4Fe-4S) centers per molecule by comparison with desulfoviridin, the dissimilatory sulfite reductase from Desulfovibrio species. The protein contains 36 cysteine residues and is high in acidic and aromatic amino acids. The N-terminal amino acids of the α and β subunits were threonine and serine, respectively. With reduced methyl viologen as electron donor, the major product of sulfite reduction was trithionate, and the pH optimum for activity was 6.0. The enzyme was stable to 70°C and denatured rapidly above this temperature. The dependence of T. commune bisulfite reductase activity on temperature was linear between 35 and 65°C, and the Q10 values observed were above 3. The presence of this new type of dissimilatory bisulfite reductase in T. commune is discussed in terms of taxonomic significance.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi J. M., Chan M., Adams V. Observations on the bisulfite reductase (P582) isolated from Desulfotomaculum nigrificans. J Bacteriol. 1974 Oct;120(1):240–244. doi: 10.1128/jb.120.1.240-244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biebl H., Pfennig Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch Microbiol. 1977 Feb 4;112(1):115–117. doi: 10.1007/BF00446664. [DOI] [PubMed] [Google Scholar]
- Bryant F., Ljungdahl L. G. Characterization of an alcohol, dehydrogenase from Thermoanaerobacter ethanolicus active with ethanol and secondary alcohols. Biochem Biophys Res Commun. 1981 May 29;100(2):793–799. doi: 10.1016/s0006-291x(81)80244-6. [DOI] [PubMed] [Google Scholar]
- Chambers L. A., Trudinger P. A. Are thiosulfate and trithionate intermediates in dissimilatory sulfate reduction? J Bacteriol. 1975 Jul;123(1):36–40. doi: 10.1128/jb.123.1.36-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Akagi J. M. Bisulfite reductase of Desulfovibrio vulgaris: explanation for product formation. J Bacteriol. 1977 Oct;132(1):139–143. doi: 10.1128/jb.132.1.139-143.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Akagi J. M. Dissimilatory reduction of bisulfite by Desulfovibrio vulgaris. J Bacteriol. 1978 Dec;136(3):916–923. doi: 10.1128/jb.136.3.916-923.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Akagi J. M. Product analysis of bisulfite reductase activity isolated from Desulfovibrio vulgaris. J Bacteriol. 1976 May;126(2):733–738. doi: 10.1128/jb.126.2.733-738.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchikian E. C. Purification and properties of thiosulfate reductase from Desulfovibrio gigas. Arch Microbiol. 1975 Nov 7;105(3):249–256. doi: 10.1007/BF00447143. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Seki Y., Ishimoto M. Biochemical studies on sulfate-ruducing bacteria. 8. Sulfite reductase from Desulfovibrio vulgaris--mechanism of trithionate, thiosulfate, and sulfide formation and enzymatic properties. J Biochem. 1974 Mar;75(3):519–529. doi: 10.1093/oxfordjournals.jbchem.a130420. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Lamed R. J., Zeikus J. G. Novel NADP-linked alcohol--aldehyde/ketone oxidoreductase in thermophilic ethanologenic bacteria. Biochem J. 1981 Apr 1;195(1):183–190. doi: 10.1042/bj1950183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. P., LeGall J., Peck H. D., Jr Isolation of assimilatroy- and dissimilatory-type sulfite reductases from Desulfovibrio vulgaris. J Bacteriol. 1973 Aug;115(2):529–542. doi: 10.1128/jb.115.2.529-542.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. P., Peck H. D., Jr Purification of the enzyme reducing bisulfite to trithionate from Desulfovibrio gigas and its identification as desulfoviridin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):583–589. doi: 10.1016/0006-291x(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Lee J. P., Yi C. S., LeGall J., Peck H. D., Jr Isolation of a new pigment, desulforubidin, from Desulfovibrio desulfuricans (Norway strain) and its role in sulfite reduction. J Bacteriol. 1973 Jul;115(1):453–455. doi: 10.1128/jb.115.1.453-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. L., DerVartanian D. V., Peck H. D., Jr On the redox properties of three bisulfite reductases from the sulfate-reducing bacteria. Biochem Biophys Res Commun. 1979 Dec 14;91(3):962–970. doi: 10.1016/0006-291x(79)91973-9. [DOI] [PubMed] [Google Scholar]
- MILLER J. D., SALEH A. M. A SULPHATE-REDUCING BACTERIUM CONTAINING CYTOCHROME C3 BUT LACKING DESULFOVIRIDIN. J Gen Microbiol. 1964 Dec;37:419–423. doi: 10.1099/00221287-37-3-419. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Abels R., Platenkamp R. The production of dithionite and SO2 - by chemical reaction of (BI) sulphite with methyl viologen semiquinone. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1397–1403. doi: 10.1016/s0006-291x(77)80134-4. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., DerVartanian D. V., Lee J. P., LeGall J., Peck H. D., Jr An iron tetrahydroporphyrin prosthetic group common to both assimilatory and dissimilatory sulfite reductases. Biochem Biophys Res Commun. 1973 Sep 5;54(1):82–88. doi: 10.1016/0006-291x(73)90891-7. [DOI] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., Rosenthal D. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. II. Identification of a new class of heme prosthetic group: an iron-tetrahydroporphyrin (isobacteriochlorin type) with eight carboxylic acid groups. J Biol Chem. 1973 Apr 25;248(8):2801–2814. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Tove S. R., Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedel M., Trüper H. G. Purification of Thiobacillus denitrificans siroheme sulfite reductase and investigation of some molecular and catalytic properties. Biochim Biophys Acta. 1979 Jun 6;568(2):454–466. doi: 10.1016/0005-2744(79)90314-0. [DOI] [PubMed] [Google Scholar]
- Seki Y., Kobayashi K., Ishimoto M. Biochemical studies on sulfate-reducing bacteria. XV. Separation and comparison of two forms of desulfoviridin. J Biochem. 1979 Mar;85(3):705–711. [PubMed] [Google Scholar]
- Siegel L. M., Murphy M. J., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. I. The Escherichia coli hemoflavoprotein: molecular parameters and prosthetic groups. J Biol Chem. 1973 Jan 10;248(1):251–264. [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A., Sato R. Studies on yeast sulfite reductase. I. Purification and characterization. Biochim Biophys Acta. 1968 Apr 2;153(3):555–575. doi: 10.1016/0005-2728(68)90185-0. [DOI] [PubMed] [Google Scholar]


