Abstract
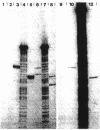
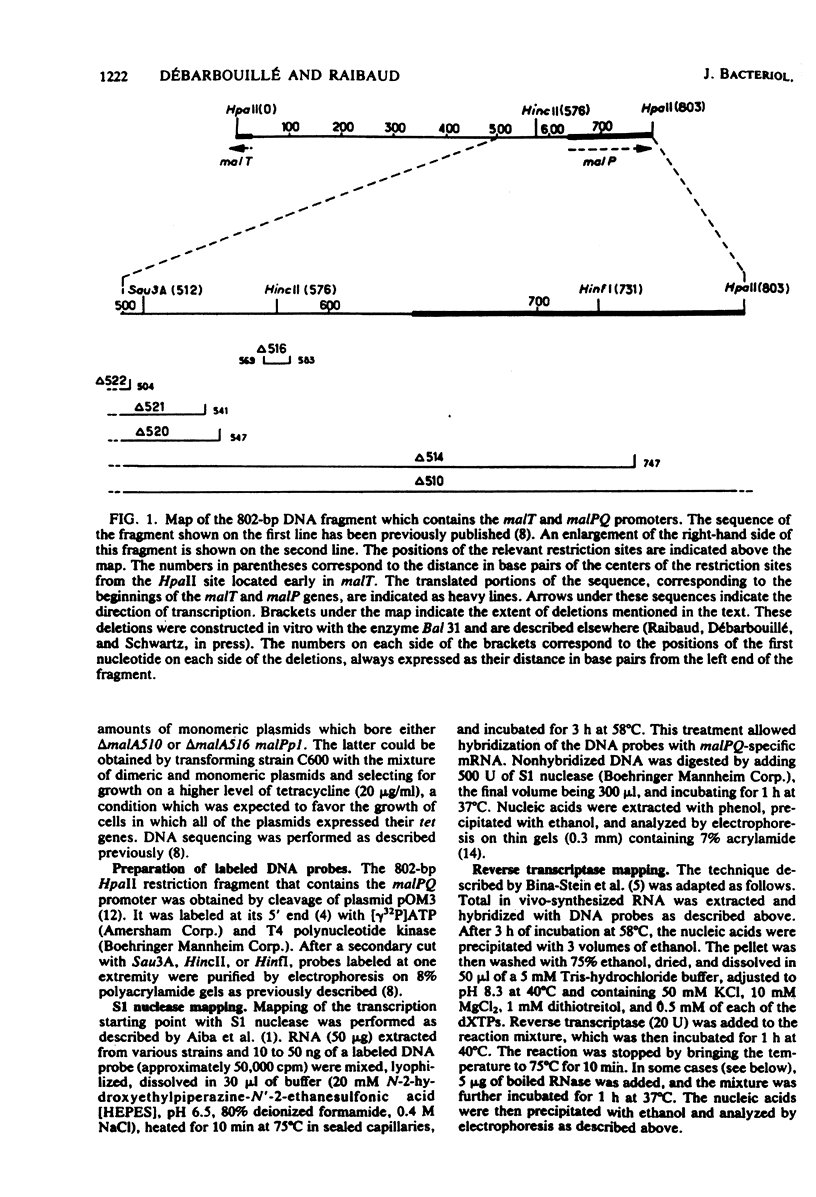
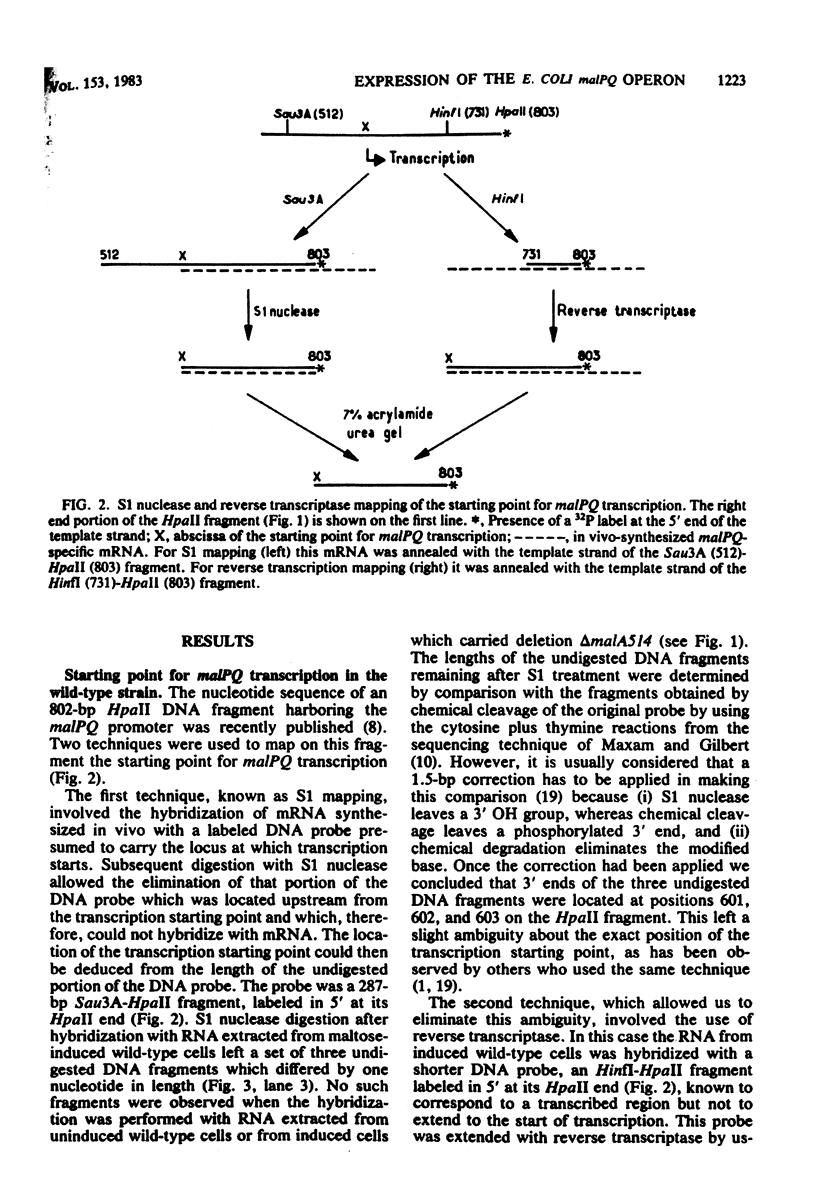
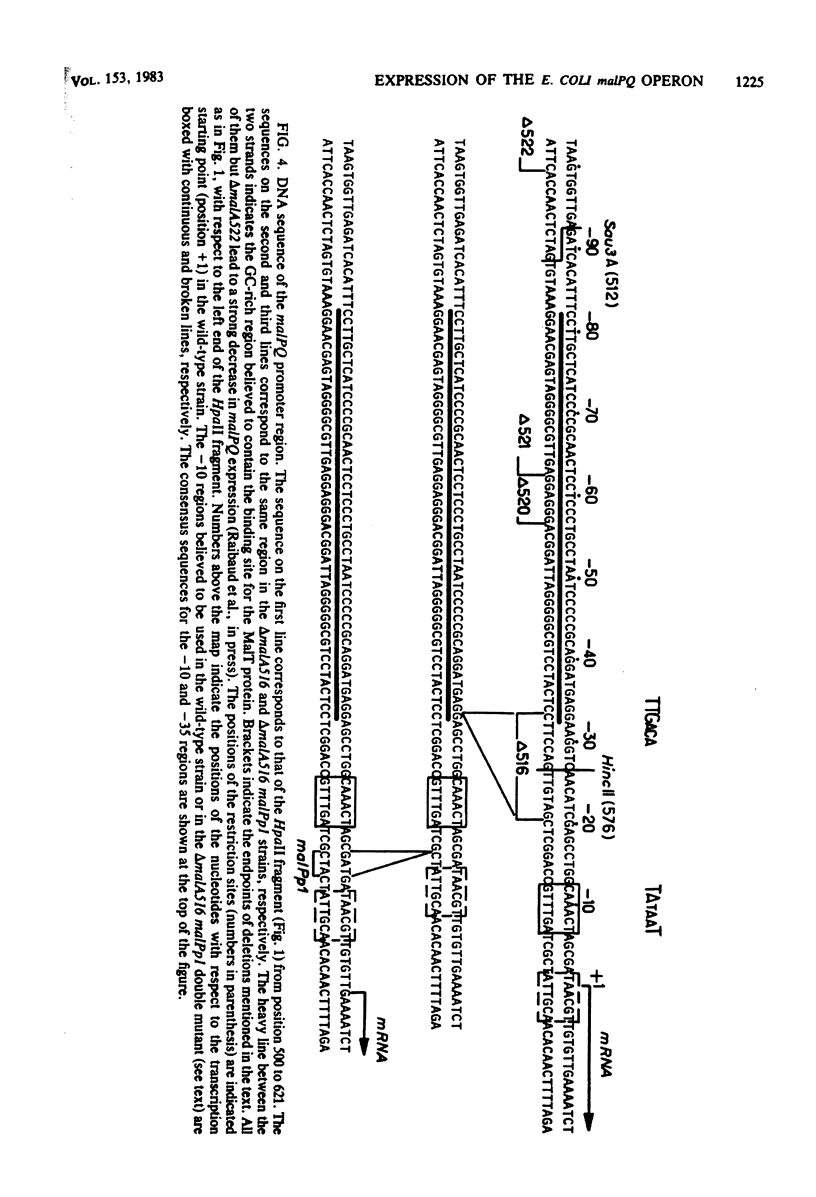
The malPQ operon, one of the three operons of the maltose regulon, is positively controlled by the product of gene malT. The starting point for malPQ transcription was deduced from experiments which involved a hybridization of in vivo-synthesized malPQ mRNA with adequate DNA probes, followed either by a digestion of nonhybridized DNA (S1 nuclease mapping) or by an extension of the hybridized probe (reverse transcriptase mapping). In the wild-type strain, this starting point was 37 nucleotides upstream from the initiation codon for malP. This analysis was also performed on a double mutant which contained both a 13-base pair deletion and a 3-base pair insertion in the promoter region. This double mutant expressed the malPQ operon exactly as the wild-type strain did, in a maltose-inducible manner. In this strain, the starting point for malPQ transcription was shifted 11 nucleotides downstream from the wild-type location. An analysis of these results suggests that (i) the binding site for the malT product is located upstream from the region which is severely altered in the double mutant, i.e., upstream from position -31; and (ii) the 30-base pair sequence which precedes the transcription starting point contains very few positions which are essential for promoter activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Restriction map of the Escherichia coli malA region and identification of the malT product. J Bacteriol. 1980 Aug;143(2):761–771. doi: 10.1128/jb.143.2.761-771.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeissner U., Court D., McKenney K., Rosenberg M. Positively activated transcription of lambda integrase gene initiates with UTP in vivo. Nature. 1981 Jul 9;292(5819):173–175. doi: 10.1038/292173a0. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Sur l'existence chez Escherichia coli K 12 d'une régulation commune à la biosynthèse des récepteurs du bactériophage et au métabolisme du maltose. Ann Inst Pasteur (Paris) 1967 Nov;113(5):685–704. [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Youderian P., Bouvier S., Susskind M. M. Sequence determinants of promoter activity. Cell. 1982 Oct;30(3):843–853. doi: 10.1016/0092-8674(82)90289-6. [DOI] [PubMed] [Google Scholar]