Abstract
Pseudomonas aeruginosa PAO mutants defective in the transport systems for branched-chain amino acids were isolated and characterized. Two mutations in strains selected for trifluoroleucine resistance, braA300 and braB307, were mapped in the met-9020-dcu-9108 and the nar-9011-puuC10 region, respectively. The mutation loci in strains selected for azaleucine resistance, braC310 and bra-311 through bra-314, were all located near the fla genes, with an order of region I fla-bra-region II fla. Strains with braA300 showed a marked reduction in the high-affinity branched-chain amino acid transport system (LIV-I) and a considerable decrease in the lower-affinity system (LIV-II). Strains with braB307 were found to be defective in the LIV-II system. Strains selected for azaleucine resistance were all defective only in the LIV-I system and fell into three phenotypically distinct classes. Strains with braC310 produced a binding protein for leucine, isoleucine, valine, alanine, and threonine (LIVAT-BP) altered in binding ability, indicating that the braC gene is the structural one for the LIVAT-BP. Strains with bra-311 or bra-312 showed a complete loss of production of the LIVAT-BP. Strains with bra-313 or bra-314 produced normal levels of functional LIVAT-BP, suggesting that these mutations are located in a gene(s) other than braC.
Full text
PDF




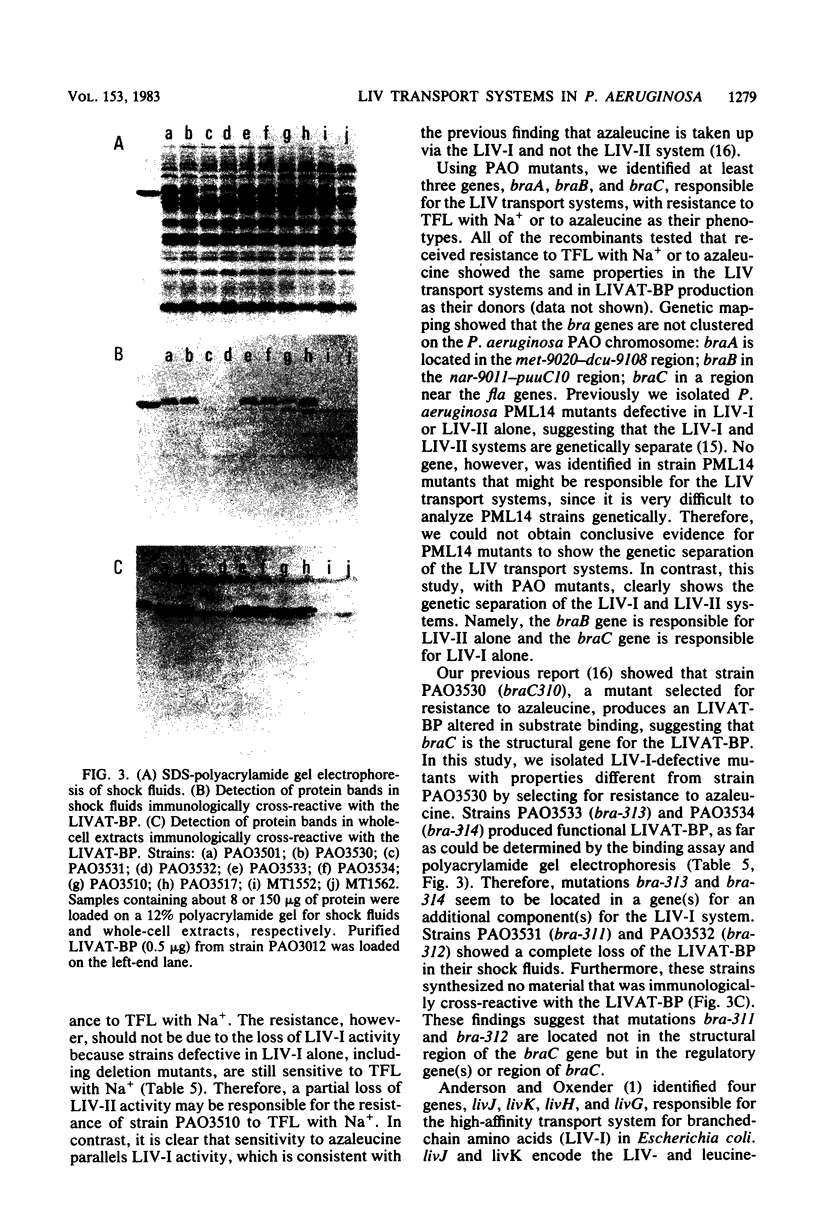


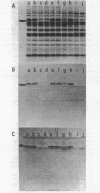
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Oxender D. L. Escherichia coli transport mutants lacking binding protein and other components of the branched-chain amino acid transport systems. J Bacteriol. 1977 Apr;130(1):384–392. doi: 10.1128/jb.130.1.384-392.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Quay S. C., Oxender D. L. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli K-12. J Bacteriol. 1976 Apr;126(1):80–90. doi: 10.1128/jb.126.1.80-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Bennett P. M., Grinsted J., Choi C. L., Richmond M. H. Characterisation of Tn501, a transposon determining resistance to mercuric ions. Mol Gen Genet. 1978 Feb 7;159(1):101–106. doi: 10.1007/BF00401753. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., FARGIE B. Fertility factors and genetic linkage in Pseudomonas aeruginosa. J Bacteriol. 1960 Sep;80:362–368. doi: 10.1128/jb.80.3.362-367.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Isolation and characterization of an R' plasmid in Pseudomonas aeruginosa. J Bacteriol. 1978 Mar;133(3):1078–1082. doi: 10.1128/jb.133.3.1078-1082.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Mutational separation of transport systems for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1982 Aug;151(2):620–628. doi: 10.1128/jb.151.2.620-628.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Sodium-dependent transport of L-leucine in membrane vesicles prepared from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):73–81. doi: 10.1128/jb.137.1.73-81.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T., Nishio K. Isolation and characterization of a Pseudomonas aeruginosa PAO mutant defective in the structural gene for the LIVAT-binding protein. J Bacteriol. 1982 Aug;151(2):729–736. doi: 10.1128/jb.151.2.729-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T. Transport systems for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1979 Sep;139(3):705–712. doi: 10.1128/jb.139.3.705-712.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landick R., Anderson J. J., Mayo M. M., Gunsalus R. P., Mavromara P., Daniels C. J., Oxender D. L. Regulation of high-affinity leucine transport in Escherichia coli. J Supramol Struct. 1980;14(4):527–537. doi: 10.1002/jss.400140410. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Nakazawa T., Ohta S., Terawaki Y. Chromosomal locations of catA, pobA, dcu and chu genes in Pseudomonas aeruginosa. Genet Res. 1981 Dec;38(3):251–266. doi: 10.1017/s0016672300020590. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Ohta S., Kobayashi R., Terawaki Y. Chromosomal location of genes participating in the degradation of purines in Pseudomonas aeruginosa. Mol Gen Genet. 1978 Nov 29;167(2):165–176. doi: 10.1007/BF00266910. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T. FP5 factor, an undescribed sex factor of Pseudomonas aeruginosa. Jpn J Microbiol. 1973 Sep;17(5):409–417. doi: 10.1111/j.1348-0421.1973.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Morgan A. F. Transduction of Pseudomonas aeruginosa with a mutant of bacteriophage E79. J Bacteriol. 1979 Jul;139(1):137–140. doi: 10.1128/jb.139.1.137-140.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Selker E., Yanofsky C. Structural and functional analysis of cloned DNA containing genes responsible for branched-chain amino acid transport in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1412–1416. doi: 10.1073/pnas.77.3.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Yanofsky C. Amino-terminal sequence and processing of the precursor of the leucine-specific binding protein, and evidence for conformational differences between the precursor and the mature form. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2005–2009. doi: 10.1073/pnas.77.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S. Bactericidal activity of the tail of Pseudomonas aeruginosa bacteriophage PS17. J Virol. 1979 Dec;32(3):958–967. doi: 10.1128/jvi.32.3.958-967.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisich V. A., Holloway B. W. A mutant sex factor of Pseudomonas aeruginosa. Genet Res. 1972 Feb;19(1):91–108. doi: 10.1017/s0016672300014294. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Iino T. Ordering of the flagellar genes in Pseudomonas aeruginosa by insertions of mercury transposon Tn501. J Bacteriol. 1983 Feb;153(2):1008–1017. doi: 10.1128/jb.153.2.1008-1017.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Oguchi T., Iino T. Analysis of flagellar genes in Pseudomonas aeruginosa by use of Rfla plasmids and conjugations. J Bacteriol. 1981 Sep;147(3):1008–1014. doi: 10.1128/jb.147.3.1008-1014.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato I., Anraku Y. Genetic and biochemical studies of transport systems for branched-chain amino acids in Escherichia coli K-12: isolation and properties of mutants defective in leucine-repressible transport activities. J Bacteriol. 1980 Oct;144(1):36–44. doi: 10.1128/jb.144.1.36-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato I., Ohki M., Anraku Y. Genetic and biochemical studies of transport systems for branched-chain amino acids in Escherichia coli. J Bacteriol. 1979 Apr;138(1):24–32. doi: 10.1128/jb.138.1.24-32.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]