Abstract
Coral bleaching has been defined as a general phenomenon, whereby reef corals turn visibly pale because of the loss of their symbiotic dinoflagellates and/or algal pigments during periods of exposure to elevated seawater temperatures. During the summer of 1997, seawater temperatures in the Florida Keys remained at or above 30°C for more than 6 weeks, and extensive coral bleaching was observed. Bleached colonies of the dominant Caribbean reef-building species, Montastrea faveolata and Montastrea franksi, were sampled over a depth gradient from 1 to 17 m during this period of elevated temperature and contained lower densities of symbiotic dinoflagellates in deeper corals than seen in previous “nonbleaching” years. Fluorescence analysis by pulse-amplitude modulation fluorometry revealed severe damage to photosystem II (PSII) in remaining symbionts within the corals, with greater damage indicated at deeper depths. Dinoflagellates with the greatest loss in PSII activity also showed a significant decline in the D1 reaction center protein of PSII, as measured by immunoblot analysis. Laboratory experiments on the temperature-sensitive species Montastrea annularis, as well as temperature-sensitive and temperature-tolerant cultured symbiotic dinoflagellates, confirmed the temperature-dependent loss of PSII activity and concomitant decrease in D1 reaction center protein seen in symbionts collected from corals naturally bleached on the reef. In addition, variation in PSII repair was detected, indicating that perturbation of PSII protein turnover rates during photoinhibition at elevated temperatures underlies the physiological collapse of symbionts in corals susceptible to heat-induced bleaching.
Global episodes of coral bleaching, where reef-building corals lose their endosymbiotic dinoflagellates and/or algal pigments during summertime elevation of seawater temperature, are recurring with increasing frequency and severity (1, 2). Previous studies have shown that symbiotic dinoflagellates maintained in culture and within the host are susceptible to thermal stress (3–5). Likewise, photosynthetically active radiation (PAR) and UV radiation may act in concert with elevated temperatures to elicit a bleaching response (6, 7), yet the underlying biochemical causes for these phenomena remain obscure. Numerous components of the photosynthetic pathway are known to be susceptible to damage by elevated temperature, especially at points within photosystem II (PSII). These include the oxygen-evolving complex (8, 9), the reaction center (10), as well as connectivity between the light-harvesting complex and the reaction center of PSII (11). In plants and green algae, thermal perturbation can predispose the photosynthetic apparatus to damage by PAR, thus inducing a state of photoinhibition (12). The primary target of photoinhibitory damage at the PSII reaction center is the D1 protein (13), which normally exhibits high rates of turnover involving light-dependent inactivation and degradation coupled with de novo synthesis and insertion of the protein into the thylakoid membrane (14, 15). Because thermal stress may disrupt thylakoid membrane stability and, therefore, proper D1 turnover, we hypothesized that such a mechanism may occur within symbiotic dinoflagellates during coral bleaching.
Seawater temperatures rose to levels above 30°C in southern Florida for several weeks during the summer of 1997. Many species of coral were reported to begin bleaching throughout the National Marine Sanctuary in the Florida Keys, and at that time we determined the extent to which symbiotic dinoflagellates within naturally bleaching corals exhibit damage to PSII by monitoring chlorophyll fluorescence and D1 protein content. Furthermore, corals were exposed to experimental thermal stress in the laboratory to ascertain whether the pattern of PSII damage observed was similar to that seen in the field and to document the role that light plays in exacerbating these mechanisms of PSII inactivation. Lastly, cultured symbiotic dinoflagellates with high and low levels of thermal tolerance were exposed to elevated temperature to compare patterns of PSII dysfunction as well as photoprotection. Results for bleached corals on the reef, in addition to corals and cultured symbiotic dinoflagellates heat-stressed in the laboratory, indicate that dysfunction of PSII reaction centers by disrupted turnover rates of the D1 protein could account for episodes of natural summertime bleaching.
MATERIALS AND METHODS
Naturally Bleached Corals.
Two species of predominant Caribbean reef-building coral, Montastrea faveolata and Montastrea franksi, were sampled from depths ranging from 1 to 17 m on Carysfort Reef within the Key Largo National Marine Sanctuary in late August 1997. At approximately 7:30 a.m., colonies of M. faveolata and the deeper species, M. franksi, both showing loss of color, were collected from a depth of 1–2, 3–6, and 9 m and 9, 12, and 17 m, respectively. In addition, 10 random colonies of M. franksi were collected from 12 m that showed a mottled bleaching pattern, where some areas of the colony appeared to be darker than other areas on the same coral. Corals were transported to the laboratory and immediately dark-acclimated for 45 min before fluorescence analysis by pulse-amplitude modulation fluorometry (PAM) by using methods described previously (5). After fluorescence analysis, coral tissue was removed with a Water Pic, and the symbiotic dinoflagellates were isolated by centrifugation. Dinoflagellate number was assessed by replicate hemacytometer counts (n = 8), and chlorophyll a was determined by spectrophotometry after extraction in 90% acetone (16). The remaining algae from samples of M. franksi from 12 and 17 m were pelleted and immediately frozen and stored at −70°C until further protein analysis. In mid-November 1997, when there were no visible signs of bleaching on the reef, replicate colonies of M. franksi again were sampled from a depth of 17 m and analyzed as described above.
Experimentally Bleached Corals.
Replicate colonies (n = 4) of Montastrea annularis were collected from 13 m at South Perry Reef off Lee Stocking Island, Bahamas, in March 1998. Three pieces of each replicate colony were sampled, each being a complete column of living tissue with a discernible top and side relative to the down-welling irradiance. Corals were transported back to the Caribbean Marine Research Center on Lee Stocking Island and maintained in an outdoor, covered seawater table for 4 hr before experimentation. Experiments were carried out in open-topped glass aquaria with a continuous supply of seawater (200 ml⋅min−1) maintained at either 25 (ambient) or 31.5°C (±0.1°C) with a recirculating immersion heater. All corals were exposed to natural sunlight that was filtered by screening to achieve the near-maximal irradiance at 13 m under cloudless conditions (780 μmol quanta m−2⋅s−1). One complete column of each colony was placed in the experimental (31.5°C) aquarium, another was placed in the control (25°C) aquarium, and the third sample was processed immediately as described above. At 8, 24, and 48 hr, each piece of coral was removed from the aquarium and dark-acclimated for 30 min before fluorescence analysis of the top and side of the colony. Chlorophyll fluorescence of the treatment corals also was monitored at approximately 5 hr after sunset to ascertain whether recovery of PSII may have occurred later in the day. After 48 hr, dinoflagellates were isolated from all corals for cell counts and chlorophyll a and protein analyses.
Cultured Dinoflagellates.
Dinoflagellates from the Montastrea sp. complex have yet to be isolated and maintained successfully in culture. However, we have identified significant differences in the thermal tolerance of cultured Symbiodinium spp. isolated from the giant clam Tridacna gigas and the Caribbean coral Oculina diffusa. Cultured dinoflagellates from these hosts were maintained at log-phase growth in ASP-8A seawater medium on a 14:10 light/dark cycle with fluorescent bulbs providing 90 μmol quanta m−2⋅s−1 at 26 and 32°C for 1 week. Samples were removed every 24 hr for the first 120 hr of the experiment, followed by sampling at 168 hr. Photosynthetic efficiency was monitored by chlorophyll fluorescence at each time point. Before fluorescence analysis, cells were dark-acclimated for 30 min and subsequently filtered onto glass-fiber filters before measuring fluorescence. For D1 protein content analysis, replicate cultures (n = 3) were maintained in 1-liter flasks at 26 and 32°C for 7 and 10 days, and samples for chlorophyll fluorescence analysis were taken as well. Steady-state photosynthesis during fluorescence measurements was induced by 90 μmol quanta m−2⋅s−1 white light, which was provided by an external fiber-optic light source, and photochemical (qP) and nonphotochemical (qN) quenching of chlorophyll fluorescence were analyzed with corrections for any quenching of the Fo signal as detailed previously (17).
A comparative analysis of D1 protein turnover rate by pulse–chase labeling was not possible because of large differences in the quantity and rate of label incorporation (using [35S]SO42− or methionine) between the different dinoflagellate isolates maintained in culture. D1 turnover was measured indirectly by fluorescence analysis in the presence of the chloroplast protein synthesis inhibitor lincomycin. Cells were grown for 48 hr at 26 and 32°C with lincomycin (200 μg⋅ml−1). No adverse effects in photosynthetic efficiency were noted during short-term exposure (0–120 min) to lincomycin. Preliminary studies also were performed by using chloramphenicol or lincomycin with immunoblot analysis to ensure the loss of the D1 protein in the presence of these inhibitors.
Preparation of Thylakoid Membranes.
Cells were thawed in breaking buffer (50 mM Hepes⋅HCl, pH 7.5/50 mM MgCl2/1 mM PMSF/5 mM ɛ-amino-n-caproic acid/1 mM benzamidine) and immediately ground in liquid nitrogen with a motor-driven Teflon/glass-tissue grinder. The homogenate was centrifuged for 5 min at 500 × g at 4°C to remove unbroken cells and debris, and the supernatant was centrifuged for 30 min at 25,000 × g at 4°C to pellet the thylakoid membranes. Thylakoids were resuspended in 150 mM Tris⋅HCl, pH 8.0/60 mM DTT/5 mM ɛ-amino-n-caproic acid/1 mM benzamidine by brief sonication. The membranes then were subsampled for chlorophyll content by spectrophotometry and then stored at −70°C. Before electrophoresis, membrane samples were extracted in acetone (80%), followed by centrifugation at 12,000 × g at 4°C, and rinsed with ethanol. After brief sonication, the membranes were solubilized in 2% lithium dodecyl sulfate and 12% sucrose, boiled for 1 min, and centrifuged at 12,000 × g for 10 min before loading onto gels. Thylakoid proteins were resolved by using SDS/PAGE on 15% resolving minigels loaded on an equivalent chlorophyll basis. After electrophoresis, membrane proteins were transferred to nitrocellulose (20 μm) and immunochemical detection was performed with polyclonal antibodies specific to the 32-kDa D1 reaction-center protein of PSII derived from the D1 protein of spinach. Color development was performed by using goat anti-rabbit alkaline phosphatase-conjugated antibodies. A series of samples of known chlorophyll concentration also was analyzed in tandem to ensure a linear response in detection. Immune cross-reactions were quantified by scanning densitometry using nih image (version 1.60; National Institutes of Health, Rockville, MD).
RESULTS
Naturally Bleached Corals.
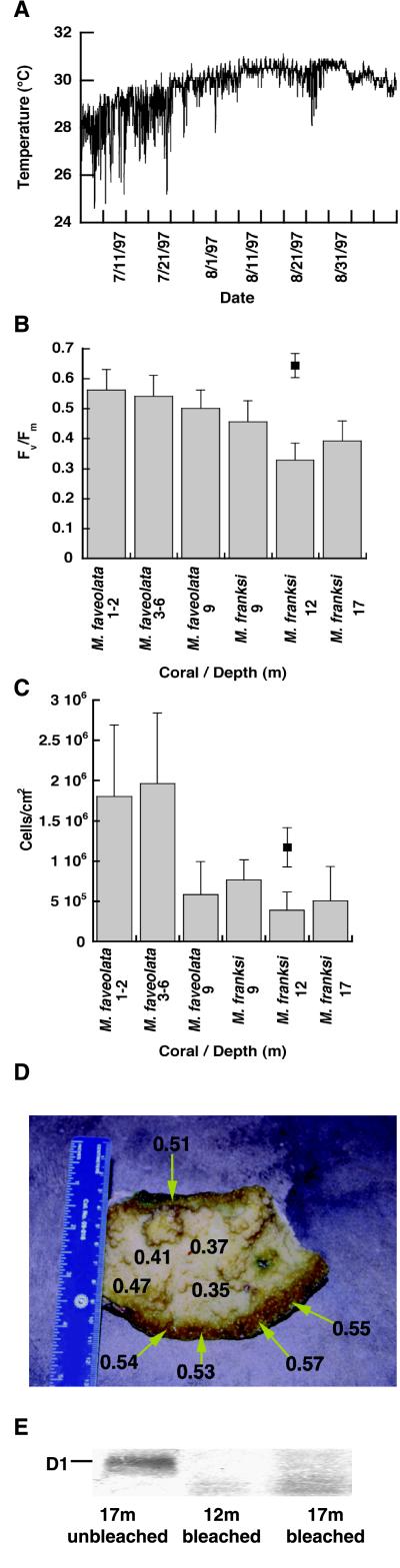
Seawater temperatures within the National Marine Sanctuary off Key Largo during the summer of 1997 increased to more than 30°C by the end of July, and the temperature remained at this level throughout the entire month of August (Fig. 1A). A qualitative survey of M. faveolata and M. franksi indicated that the extent of bleaching (the number of discolored colonies and the amount of bleaching within those colonies) increased with increasing depth between 1 and 17 m. Fluorescence analysis using PAM fluorometry showed a significant decline in photosynthetic efficiency at increasing depths (Fig. 1B). Dinoflagellates within M. franksi from 12 m had a significantly lower PSII quantum yield (Fv/Fm) as compared with the Fv/Fm values for samples of M. faveolata from all depths and M. franksi from 9 m (P < 0.05; ANOVA), whereas there was no significant difference between the Fv/Fm values recorded from M. franksi from 12 and 17 m (Fig. 1B). Deeper colonies of M. franksi (17 m) also showed significantly lower values for Fv/Fm (P < 0.05) than all colonies of M. faveolata (Fig. 1B). Likewise, Fv/Fm values, recorded from the same species at the same site at 12 m 1 year earlier were significantly higher than those sampled during the bleaching event (Fig. 1B, single point).
Figure 1.
Photoinhibition and algal loss during a natural episode of coral bleaching. (A) Seawater temperature from 20 m at Conch Reef
Densities of symbiotic dinoflagellates indicated a higher loss of symbionts with depth. M. faveolata from 9 m and M. franksi from all depths (9, 12, and 17 m) contained significantly fewer symbionts than M. faveolata collected from the shallow 1- to 2- and 3- to 6-m locations (Fig. 1C), whereas there were no significant differences between algal densities of M. franksi at all depths. One year before the 1997 bleaching event, these same species of corals at 12-m depth on the same reef contained about twice the density of symbionts (1.29 × 106 cells⋅cm−2 for M. faveolata and 1.17 × 106 cells⋅cm−2 for M. franksi; P < 0.01; ANOVA for both species). However, there were no significant differences in the level of chlorophyll a per cell when comparing corals from the summer of 1997 with the previous year at the same time and site (data not shown).
Samples of M. franksi that had a mottled bleaching appearance showed markedly higher Fv/Fm ratios in regions that appeared darker, compared with lighter-colored areas of the coral that had the lowest values of variable fluorescence (Fig. 1D). Immunoblot analysis of thylakoid proteins from dinoflagellates of bleached M. franksi at 12 and 17 m revealed severe depletion and apparent fragmentation of the D1 protein (Fig. 1E), whereas D1 content and photosynthetic efficiency were restored to normal levels in symbionts isolated from the same species and depth collected 3 months after the bleaching event.
Experimental Bleaching.
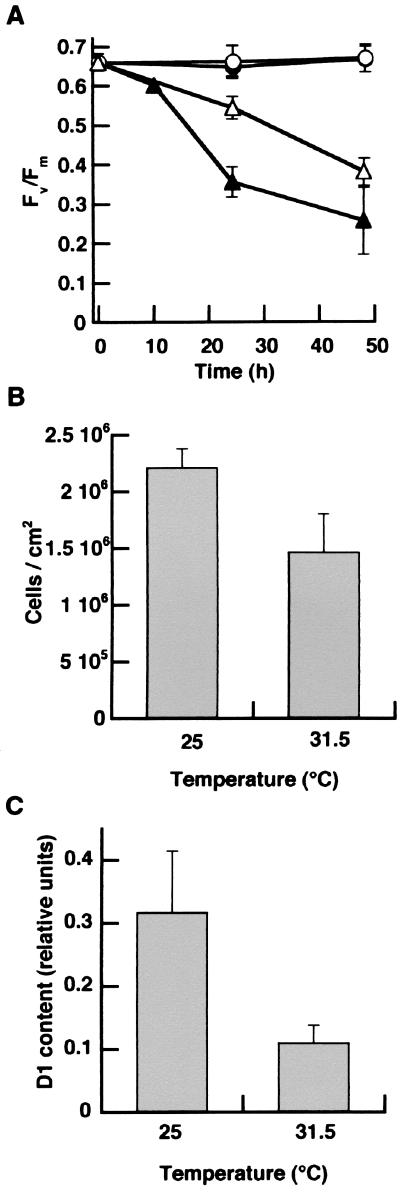
Loss in PSII function recorded from corals removed from the reef in August 1997 may have been due to a combination of elevated temperature and increased irradiance because of a sustained period of calm weather before and during the bleaching event, as has been noted during other episodes of coral bleaching (18). To test for the effects of temperature and light on symbiotic dinoflagellates and confirm the effects on PSII seen in the field, unbleached colonies of M. annularis were exposed to 31.5°C for 48 hr in a controlled outdoor-laboratory setting.
There was a progressive loss in PSII function in dinoflagellates within M. annularis exposed to 31.5°C for more than 7 hr as measured by PAM fluorometry (Fig. 2A). At 24 hr, there was a significant effect for both temperature and colony location (top vs. side), because the Fv/Fm values recorded from the top of the 31.5°C treatments were significantly lower than values obtained from measurements of the sides of the same colonies as well as any location on the control corals (P < 0.0001; ANOVA, 31.5°C top vs. side; P ≤ 0.001, 31.5°C top vs. 25°C top and side locations) (Fig. 2A). This loss in PSII activity was indicative of photodamage, because the temperature-treated corals showed no recovery in the Fv/Fm 5 hr after sunset (not shown). By 24 hr, measurements taken from the sides of the experimental corals also had significantly lower PSII quantum yields as compared with the control corals (P = 0.001 and 0.0006; ANOVA for 25°C top and side, respectively). There was no significant change in the PSII quantum yield for dinoflagellates within the control colonies throughout the experiment (Fig. 2A). Densities of symbiotic dinoflagellates in heat-stressed experimental corals decreased by about one-third by the end of the experiment compared with the controls (P = 0.001; ANOVA) (Fig. 2B). However, no significant change in chlorophyll a concentration per cell was detected (not shown). Most importantly, there was a significant loss of D1 (≈60%) in the dinoflagellates isolated from the 31.5°C-treated corals compared with control corals at 25°C ( P < 0.0001; Student’s t test) (Fig. 2C).
Figure 2.
Experimentally induced bleaching and photoinhibition of symbiotic dinoflagellates within M. annularis. (A) Quantum yield of PSII charge separation recorded from the top (●, ▴) and side (○, ▵) of replicate (n = 4) colonies of M. annularis maintained at 25 (circles) and 31.5°C (triangles), respectively. (B) Density of symbiotic dinoflagellates within M. annularis after 48 hr of exposure to 25 and 31.5°C. (C) Net content of the D1 protein from dinoflagellates within M. annularis at the end of the 48-hr treatment. (Bars = SD.) from July 1 to September 14, 1997. (B) Quantum yield of PSII charge separation (Fv/Fm) for dinoflagellates within colonies of M. faveolata and M. franksi (n = 6–10/depth); ■, values collected from six colonies of M. franksi at the same location 1 year before the bleaching event. (C) Density of dinoflagellates within M. faveolata and M. franksi; ■, mean density of dinoflagellates from six colonies of M. franksi at the same location 1 year before the bleaching event. (D) Multiple values of Fv/Fm taken from a partially bleached colony of M. franksi from 12 m. (E) Immunoblot of the D1 protein for dinoflagellates isolated from unbleached M. franksi collected at 12 m in November of 1997 (lane 1) and bleached M. franksi collected in August of 1997 at 12 and 17 m (lanes 2 and 3) during a natural episode of coral bleaching. (Bars = SD.)
Cultured Dinoflagellates.
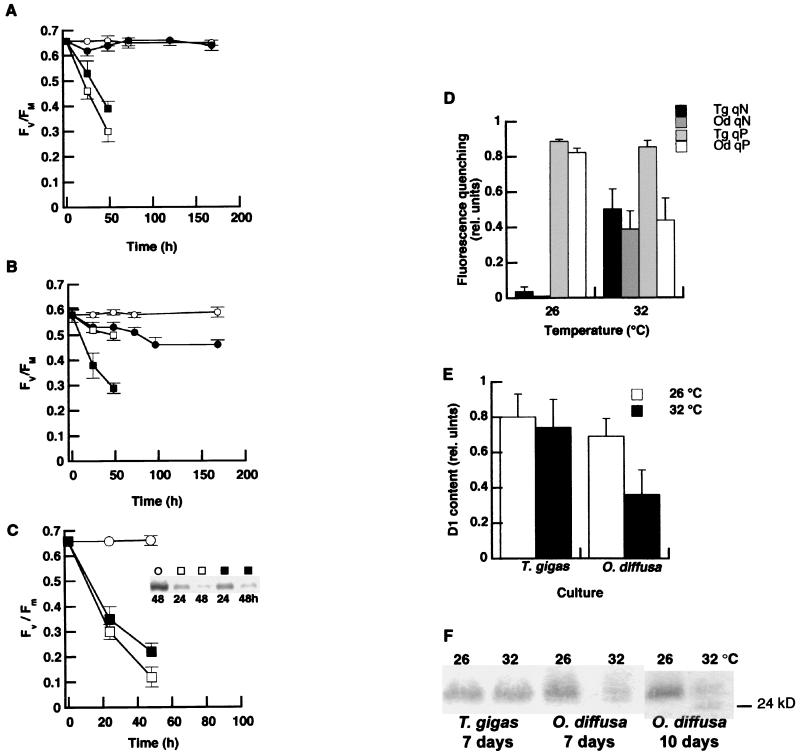
Cultured dinoflagellates from T. gigas showed resistance to elevated temperature stress because PSII function remained stable throughout the exposure period (Fig. 3A, circles). In contrast, a significant decline in photosynthetic efficiency occurred in the dinoflagellates from O. diffusa exposed to the same heat stress (Fig. 3B). Preliminary experiments with chloroplast protein synthesis inhibitors indicated a progressive loss in the amount of D1 protein in the time frame used for fluorescence analysis (0–48 hr) at 26 and 32°C (see Fig. 3C). When de novo synthesis of D1 was blocked by lincomycin, there was a marked loss in PSII function as measured by PAM fluorometry at both 26°C and 32°C in both cultures (Fig. 3 A and B). When chloroplast protein synthesis was blocked in the dinoflagellates from O. diffusa, there was a sharp decline in PSII function (Fv/Fm) at 32°C, but a much slower decrease at 26°C (Fig. 3B). However, the dinoflagellates from T. gigas showed the opposite pattern, with a slightly faster decline in PSII function occurring at 26°C (Fig. 3A).
Figure 3.
Photoprotection and thermally induced photoinhibition in cultured symbiotic dinoflagellates. (A) The quantum yield of PSII without (○, ●) and with (□, ■) the addition of lincomycin for Symbiodinium sp. from the giant clam T. gigas grown at 26 (open symbols) and 32°C (closed symbols) (n = 6 for each temperature). (B) The quantum yield of PSII for Symbiodinium sp. isolated from the Caribbean coral O. diffusa grown at 26 and 32°C with and without the addition of lincomycin (symbols same as for A). (C) Relationship between PSII efficiency and the amount of D1 protein (Inset) in dinoflagellates from T. gigas grown at 26 (□) and 32°C (■) with chloramphenicol and at 26°C (○) without the inhibitor (n = 3). Similar results were found with lincomycin, as well. (D) qN and qP of chlorophyll fluorescence for Symbiodinium sp. from T. gigas and O. diffusa grown at 26 and 32°C for 7 days. (E) Net content of the D1 protein within the dinoflagellates after 7 days of exposure to 26 and 32°C (n = 3). (F) Representative immunoblots of the D1 protein from Symbiodinium sp. from T. gigas grown at 26 and 32°C for 7 days and Symbiodinium sp. from O. diffusa grown at 26 and 32°C for 7 and 10 days. (Bars = SD.)
Chlorophyll fluorescence patterns under moderate irradiance conditions (90 μmol quanta m−2⋅s−1) indicated significant increases in the dissipation of PSII excitation energy as qN rose in both cultures (P < 0.001; ANOVA) (Fig. 3D). However, the temperature-sensitive dinoflagellates from O. diffusa also showed a significant decline in qP of chlorophyll fluorescence at 32°C compared with 26°C (P < 0.001; ANOVA), implying a decrease in the efficiency of energy utilization. After 7 days of exposure to 32°C, the net content of the D1 protein within this alga declined significantly (P < 0.01; Student’s t test) (Fig. 3E). No such effect was seen for similarly treated dinoflagellates from T. gigas; D1 content and qP remained the same at both temperatures. Furthermore, by the 10th day of exposure to 32°C, cultures of O. diffusa dinoflagellates yielded immunoblots containing a second protein band of approximately 24 kDa, suggesting fragmentation of D1 (Fig. 3F).
DISCUSSION
This study provides direct evidence of irreversible damage to photosystem II in heat-stressed symbiotic dinoflagellates within corals during a natural bleaching event. The dysfunction of PSII correlates with the loss of the PSII reaction-center protein, D1. Controlled laboratory experiments with intact corals, as well as cultured symbiotic dinoflagellates, corroborates our findings with naturally bleached corals. Moreover, dinoflagellates of bleaching-resistant corals have significantly greater capacity for maintenance of PSII at elevated temperatures than do those of thermal-sensitive corals (5).
Mechanisms for protection from chronic photoinhibition include enhanced turnover rates of D1 (19, 20), the dissipation of excess excitation energy away from the PSII reaction center by xanthophyll cycling (21), and down-regulation of antenna (22, 23) or inactivation of intact reaction centers (24). Without such protection, the PSII reaction center is the primary weak point in the biochemical photosynthetic pathway (13). Results from our in vitro experiments with cultured symbiotic dinoflagellates show that D1 turnover is rapid at both 26 and 32°C in the symbionts from T. gigas, but D1 protein turnover rates in the symbionts from O. diffusa must increase dramatically during periods of thermal stress (compare Fig. 3 A with B), indicating that the loss of PSII function is due to the rate of D1 degradation being greater than that of its resynthesis at high temperature. In addition, our analyses of cultured dinoflagellates obviate contribution of the host in this thermal response. A number of studies with plants and algae have shown a loss in the D1 protein under conditions of high light (13, 25). However, there has been far less work investigating the effects of thermal stress on D1. Heat-induced damage commonly occurs at photosystem II (9, 10), which correlates to a significant loss in the D1 content (10, 26). Recent investigations into the turnover of the reaction center of PSII in plants have provided strong evidence for a dominant mechanism of D1 turnover that occurs under low- and high-light conditions (27). Though photoinhibition may occur under low light, damage is not detected because of a highly efficient mechanism of D1 repair (19).
It is important to note that there was an increase in qN in both cultured symbiotic dinoflagellates used in our laboratory experiments. Enhanced qN may be indicative of the development of photoprotective processes that can be related to the epoxidation state of the carotenoid pool (23, 28, 29). However, the temperature-sensitive dinoflagellates from O. diffusa also showed a significant decline in qP of chlorophyll fluorescence, thereby reflecting a loss in the capacity to utilize excitation energy through photochemistry. These results suggest that the majority of photoprotection at high temperature is originating from changes at the light-harvesting complex of these symbionts rather than from down-regulation of inactive, intact reaction centers and that, depending on the dinoflagellate, such photoprotective pathways may not allow for complete avoidance of PSII disruption. For instance, prolonged exposure of the symbiont from O. diffusa to 32°C resulted in fragmentation of the D1 protein (Fig. 3F). A similar banding pattern has been noted in photoinhibited terrestrial plants in vivo (30) and in vitro (31) and has been interpreted as a breakdown product of the D1 protein proceeding photoinhibition via a donor-side pathway. The donor-side pathway is initiated by the loss in function of the oxygen-evolving complex (32), supporting interpretations of previous bleaching studies with the cultured dinoflagellate Symbiodinium microadriaticum (3) as well as freshly isolated dinoflagellates from a number of Caribbean corals (7), showing a marked reduction in gross oxygen production after brief periods of elevated temperature stress.
Fluorescence analysis of naturally bleached corals that appeared mottled indicated that these patterns of bleaching can be explained by differential photosynthetic efficiency of the symbionts at the specific sites in the coral colony. Rowan et al. (18) compared bleaching patterns of M. annularis and M. faveolata and suggested that bleaching at the tops and sides of colonies may be due to differential tolerances to light and elevated temperature of different taxa of dinoflagellates residing in particular portions of the coral. One interpretation of our results showing greater stress to deeper M. franksi is that this coral contains a monomorphic assemblage of symbionts belonging to clade “C” (33), purported by Rowan et al. (18) to be the most sensitive taxon under “slight increases of temperature and irradiance.” The thermally tolerant dinoflagellate of T. gigas conforms to clade “A,” whereas the thermally sensitive dinoflagellate from O. diffusa belongs to clade “B” (T. Lajeunesse, personal communication). Likewise, S. microadriaticum from Cassiopeia xamachana is thermally sensitive (ref. 3; personal observation) and belongs to clade “A” (34). Therefore, there is no clear correlation between the phylotype (clade) of these algae and their physiological tolerance to elevated temperature.
Previous work has shown that light plays a key role in the breakdown of photosynthesis during bleaching (5–7, 35). Exposure to down-welling irradiance exacerbates the stress to the symbionts within M. annularis. Nonetheless, dinoflagellates on the sides of the coral showed a significant decline in PSII efficiency as well (Fig. 2A), providing evidence that, at environmentally relevant, elevated temperatures, high PAR is not a prerequisite for photosystem damage per se. In fact, fluorescence-induction kinetics with cultured Symbiodinium kawagutii exposed to elevated temperatures showed that thermal perturbation can lead to stress at the PSII reaction center whereas the algae are maintained in complete darkness (35). Likewise, we have shown previously that variable fluorescence also declines in corals that are exposed to elevated temperatures (32–36°C) in the dark (5). Thus, these findings also indicate that damage to the dark reactions of photosynthesis is not a prerequisite for PSII dysfunction at temperatures of 31–34°C (cf. ref. 36).
Corals sampled during bleaching events have been reported to exhibit both decreased chlorophyll a per algal cell and algal cell density (37, 38) or a decrease in density of algal cells exclusively (39). Our results follow the latter trend, with both naturally bleached M. franksi and M. faveolata showing significant decreases in algal density but no change in cellular chlorophyll a. Moreover, deeper corals showed higher levels of damage and a greater loss of symbionts compared with shallow corals. One explanation for this pattern is that the symbionts of shallow-water corals could be genotypically distinct in regard to PSII maintenance at elevated temperatures. In addition, shallow-water corals normally harbor greater densities of symbiotic dinoflagellates than their deeper counterparts (personal observation), presumably enabling some protection by self-shading and requiring a longer period of exposure to show signs of stress to PSII.
Thermal perturbation of photosynthesis can occur at one or more levels, any of which would lead to an imbalance in the light energy supplied and that which can be used for electron transport and carbon assimilation (40, 41). Consequently, thermal stress could alter the redox state of the intersystem electron transport chain in symbiotic dinoflagellates, resulting in an increase in the excitation pressure placed on PSII to initiate its breakdown. Iglesias-Prieto et al. (3) hypothesized that such a loss in photosynthesis could lead to the disruption in transfer of photosynthate to the coral host, thereby leading to the eventual expulsion of the algal cell. Previous results have shown a sequential loss in photosynthesis occurring before a reduction in algal density (5, 7). However, some symbiotic dinoflagellates are tolerant to thermal stress, and identifying the mechanisms by which PSII damage is averted is important for understanding how some corals and other algal/invertebrate associations appear to be unaffected during a bleaching event. Although a definitive sequence of events leading to the loss of D1 is not known, our results strongly suggest that thermal stress does have a primary effect on enhanced PSII reaction-center destabilization outpacing its repair, and these are pivotal physiological alterations in symbiotic dinoflagellates that underlie coral bleaching.
Acknowledgments
We thank the staff of the Caribbean Marine Research Center, University of North Carolina Wilmington–National Undersea Research Center in Key Largo, and the Florida Keys National Marine Sanctuary for logistical support. Funding was provided by the National Science Foundation to M.E.W. and W.K.F., the National Oceanic and Atmospheric Administration National Undersea Research Program to W.K.F., and the Department of Energy to G.W.S.
ABBREVIATIONS
- PSII
photosystem II
- PAR
photosynthetically active radiation
- PAM
pulse-amplitude modulation fluorometry
- Fv/Fm
quantum yield of photosystem II
- qN
nonphotochemical quenching of chlorophyll fluorescence
- qP
photochemical quenching of chlorophyll fluorescence
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Glynn P W. Coral Reefs. 1993;12:1–17. [Google Scholar]
- 2.Brown B E. Coral Reefs. 1997;16:s129–s138. [Google Scholar]
- 3.Iglesias-Prieto R, Matta J L, Robins W A, Trench R K. Proc Natl Acad Sci USA. 1992;89:10302–10305. doi: 10.1073/pnas.89.21.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jokiel P L, Coles S L. Coral Reefs. 1990;8:155–162. [Google Scholar]
- 5.Warner M E, Fitt W K, Schmidt G W. Plant Cell Environ. 1996;19:291–299. [Google Scholar]
- 6.Lesser M P, Stochaj W R, Tapley D W, Shick J M. Coral Reefs. 1990;8:225–232. [Google Scholar]
- 7.Fitt W K, Warner M E. Biol Bull. 1995;189:298–307. doi: 10.2307/1542147. [DOI] [PubMed] [Google Scholar]
- 8.Becker G B, Norman J, Moholt-Siebert M. In: Current Research in Photosynthesis. Baltscheffsky M, editor. IV. Dordrecht, The Netherlands: Kluwer; 1990. pp. 705–708. [Google Scholar]
- 9.Havaux M. Plant Sci. 1993;94:19–33. [Google Scholar]
- 10.Heckathorn S A, Coleman J S, Hallberg R L. Photosynthetica. 1997;34:13–20. [Google Scholar]
- 11.Schreiber U, Armond P A. Biochim Biophys Acta. 1978;502:138–151. doi: 10.1016/0005-2728(78)90138-x. [DOI] [PubMed] [Google Scholar]
- 12.Al-Khatib K, Paulsen G M. Plant Physiol. 1989;90:1041–1048. doi: 10.1104/pp.90.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohad I, Keren N, Zer H, Gong H, Mor T S, Gal A, Tal S, Domovich Y. In: Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field. Baker N R, Bowyer J R, editors. Oxford: BIOS Scientific; 1994. pp. 161–173. [Google Scholar]
- 14.Aidir N, Shochat S, Ohad I. J Biol Chem. 1990;265:12563–12568. [PubMed] [Google Scholar]
- 15.Matto A, Hoffman-Falk H, Marder J, Edelman M. Proc Natl Acad Sci USA. 1984;81:1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffery S W, Humphrey G F. Biochem Physiol Pflanzen. 1975;167:191–194. [Google Scholar]
- 17.van Kooten O, Snel J F H. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- 18.Rowan R, Knowlton N, Baker A, Jara J. Nature (London) 1997;388:265–269. doi: 10.1038/40843. [DOI] [PubMed] [Google Scholar]
- 19.Anderson J M, Park Y-I, Chow W S. Physiol Plant. 1997;100:214–233. [Google Scholar]
- 20.Öquist G, Anderson J M, McCaffery S, Chow W S. Planta. 1992;188:422–431. doi: 10.1007/BF00192810. [DOI] [PubMed] [Google Scholar]
- 21.Brown, B. E., Ambarsari, I., Warner, M. E., Fitt, W. K., Dunne, R. P., Gibb, S. W. & Cummings, D. G. (1999) Coral Reefs, in press.
- 22.Horton P, Ruban A V, Walters R G. Annu Rev Plant Physiol Plant Mol Biol. 1997;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 23.Demmig-Adams B, Adams W W I. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- 24.Schnettger B, Critchley C, Santore U J, Graf M, Krause G H. Plant Cell Environ. 1994;17:55–64. [Google Scholar]
- 25.Baroli I, Melis A. Planta. 1996;198:640–646. doi: 10.1007/BF00262653. [DOI] [PubMed] [Google Scholar]
- 26.Michalski B, Wettern M. J Plant Physiol. 1994;144:424–426. [Google Scholar]
- 27.Tyystjärvi T, Mulo P, Mäenpää P, Aro E-M. Photosynth Res. 1996;47:111–120. doi: 10.1007/BF00016174. [DOI] [PubMed] [Google Scholar]
- 28.Ambarsari I, Brown B E, Barlow R G, Britton G, Cummings D. Mar Ecol Prog Ser. 1997;159:303–307. [Google Scholar]
- 29.Young A J, Frank H A. J Photochem Photobiol. 1996;36:3–15. doi: 10.1016/S1011-1344(96)07397-6. [DOI] [PubMed] [Google Scholar]
- 30.Kettunen R, Tyystjärvi E, Aro E-M. Plant Physiol. 1996;111:1183–1190. doi: 10.1104/pp.111.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbato R, Friso G, Giacometti G M. Photosynthetica. 1993;28:165–183. [Google Scholar]
- 32.Telfer A, Barber J. In: Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field. Baker N R, Bowyer J R, editors. Oxford: BIOS Scientific; 1994. pp. 25–45. [Google Scholar]
- 33.Rowan R, Knowlton N. Proc Natl Acad Sci USA. 1995;92:2850–2853. doi: 10.1073/pnas.92.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowan R, Powers D A. Science. 1991;251:1348–1351. doi: 10.1126/science.251.4999.1348. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias-Prieto R. Proc 8th Int Coral Reef Symp (Panama) 1996;2:1313–1318. [Google Scholar]
- 36.Jones R J, Hoegh-Guldberg O, Larkum A W D, Schreiber U. Plant Cell Environ. 1998;21:1219–1230. [Google Scholar]
- 37.Kleppel G S, Dodge R E, Reese C J. Limnol Oceanogr. 1989;34:1331–1335. [Google Scholar]
- 38.Porter J W, Fitt W K, Spero H J, Rogers C S. Proc Natl Acad Sci USA. 1989;86:9342–9346. doi: 10.1073/pnas.86.23.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones R J. Mar Ecol Prog Ser. 1997;158:51–59. [Google Scholar]
- 40.Huner N P A, Maxwell D P, Gray G R, Savitch L V, Krol M, Ivanov A G, Falk S. Physiol Plant. 1996;98:358–364. [Google Scholar]
- 41.Maxwell D P, Falk S, Trick C G, Huner N P A. Plant Physiol. 1994;105:535–543. doi: 10.1104/pp.105.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]