Abstract
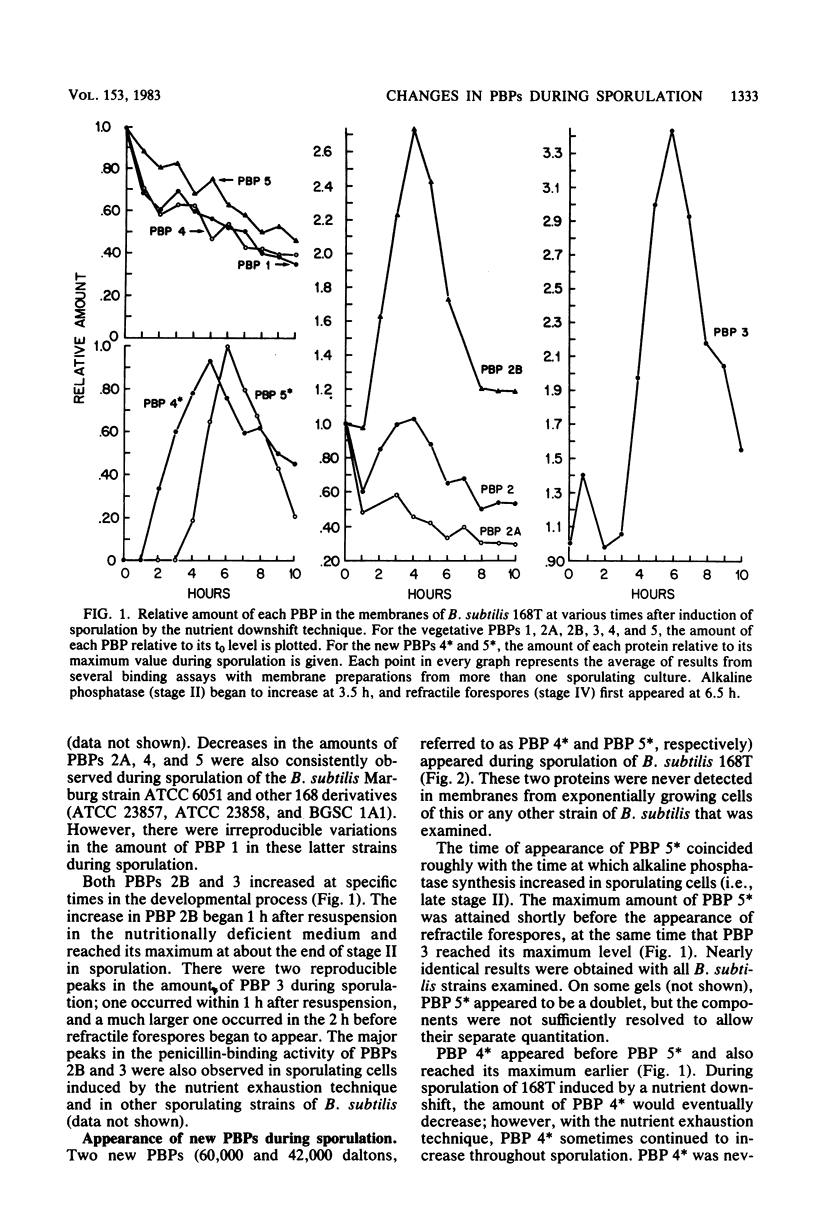
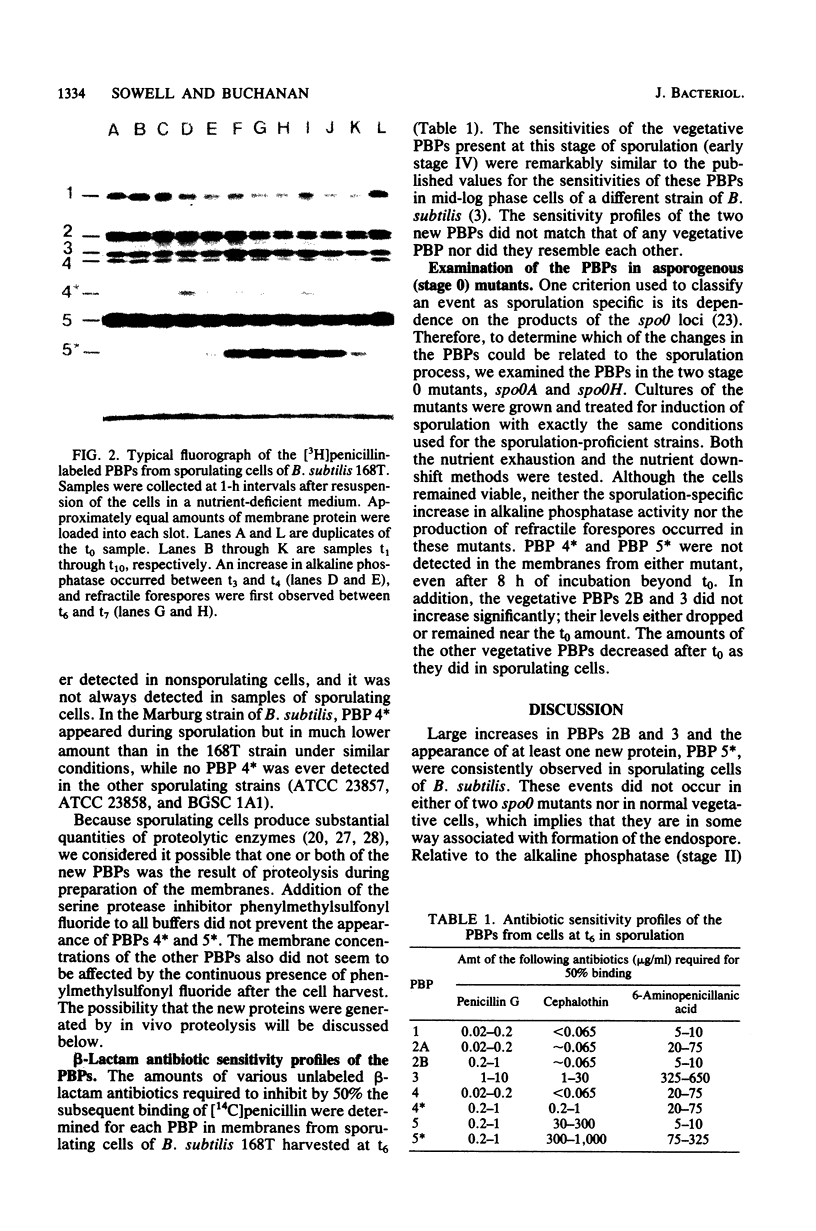
The penicillin-binding proteins (PBPs) of Bacillus subtilis were examined in samples collected at various times from sporulating cultures and compared with the PBPs in a presporulation sample. Large increases in vegetative PBPs 2B and 3 and the appearance of at least one new PBP (42,000 daltons) occurred at reproducible times during sporulation. In some strains a second new PBP (60,000 daltons) was also produced. By comparing the PBP activities in sporulating cells and two spo0 mutants we have classified these changes as sporulation-related events rather than the consequences of stationary-phase aging. The other vegetative PBPs (PBPs 1, 2A, 4, and 5) decreased during sporulation, but not in sufficient amount or at the appropriate time to account for the appearance of the new proteins. A possible connection between specific PBP changes and the penicillin-sensitive stages of sporulation is suggested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar R. A., Blumberg P. M., Strominger J. L. Penicillin binding components in Bacillus subtilis during sporulation. J Bacteriol. 1974 Feb;117(2):924–925. doi: 10.1128/jb.117.2.924-925.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Amano K., Hackstadt T., Perry L., Caldwell H. D. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982 Jul;151(1):420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchanan C. E. Altered membrane proteins in a minicell-producing mutant of Bacillus subtilis. J Bacteriol. 1979 Jul;139(1):305–307. doi: 10.1128/jb.139.1.305-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer B. N., Mandelstam J. Production and possible function of serine protease during sporulation of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):406–410. doi: 10.1128/jb.121.2.406-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer B. N. Requirement for peptidoglycan synthesis during sporulation of Bacillus subtilis. J Bacteriol. 1979 Dec;140(3):786–797. doi: 10.1128/jb.140.3.786-797.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering J. L., Bott K. F. Differential amino acid requirements for sporulation in Bacillus subtilis. J Bacteriol. 1972 Oct;112(1):345–355. doi: 10.1128/jb.112.1.345-355.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins A. D., Slepecky R. A. Antibiotic inhibition of the septation stage in sporulation of Bacillus megaterium. J Bacteriol. 1969 Mar;97(3):1513–1515. doi: 10.1128/jb.97.3.1513-1515.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe G., Yu W., Strominger J. L. Penicillin-binding proteins in Bacillus subtilis mutants. Antimicrob Agents Chemother. 1982 Jun;21(6):979–983. doi: 10.1128/aac.21.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S., Fukuda A., Okada Y. The penicillin-binding proteins of Caulobacter crescentus. J Biochem. 1980 Jan;87(1):363–366. doi: 10.1093/oxfordjournals.jbchem.a132749. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence P. J. Penicillin: reversible inhibition of forespore septum development in Bacillus megaterium cells. Antimicrob Agents Chemother. 1974 Dec;6(6):815–820. doi: 10.1128/aac.6.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. J., Rogolsky M., Hanh V. T. Binding of radioactive benzylpenicillin to sporulating Bacillus cultures: chemistry and fluctuations in specific binding capacity. J Bacteriol. 1971 Nov;108(2):662–667. doi: 10.1128/jb.108.2.662-667.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Tipper D. J. Cell wall polymers of Bacillus sphaericus: activities of enzymes involved in peptidoglycan precursor synthesis during sporulation. J Bacteriol. 1974 Oct;120(1):342–354. doi: 10.1128/jb.120.1.342-354.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Tipper D. J. Transcriptional control of peptidoglycan precursor synthesis during sporulation in Bacillus sphaericus. J Bacteriol. 1976 Feb;125(2):565–574. doi: 10.1128/jb.125.2.565-574.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Linnett P. E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976 Apr;126(1):213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINTER V. SPORES OF MICROORGANISMS. XIV. LATE STAGES OF INTRASPORANGIAL DEVELOPMENT OF BACTERIAL SPORES: THEIR SENSITIVITY TO ANTIBIOTICS. Folia Microbiol (Praha) 1964 Mar;18:58–72. doi: 10.1007/BF02868786. [DOI] [PubMed] [Google Scholar]
- VINTER V. Spores of microorganisms. Penicillin-induced destruction of sporulating cells of Bacillus cereus. Experientia. 1962 Sep 15;18:409–410. doi: 10.1007/BF02151489. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972 Apr 11;11(8):1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci U S A. 1969 Oct;64(2):528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M., Mandelstam J. Early events during bacterial endospore formation. Adv Microb Physiol. 1979;20:103-62, 321-3. doi: 10.1016/s0065-2911(08)60207-6. [DOI] [PubMed] [Google Scholar]