Abstract
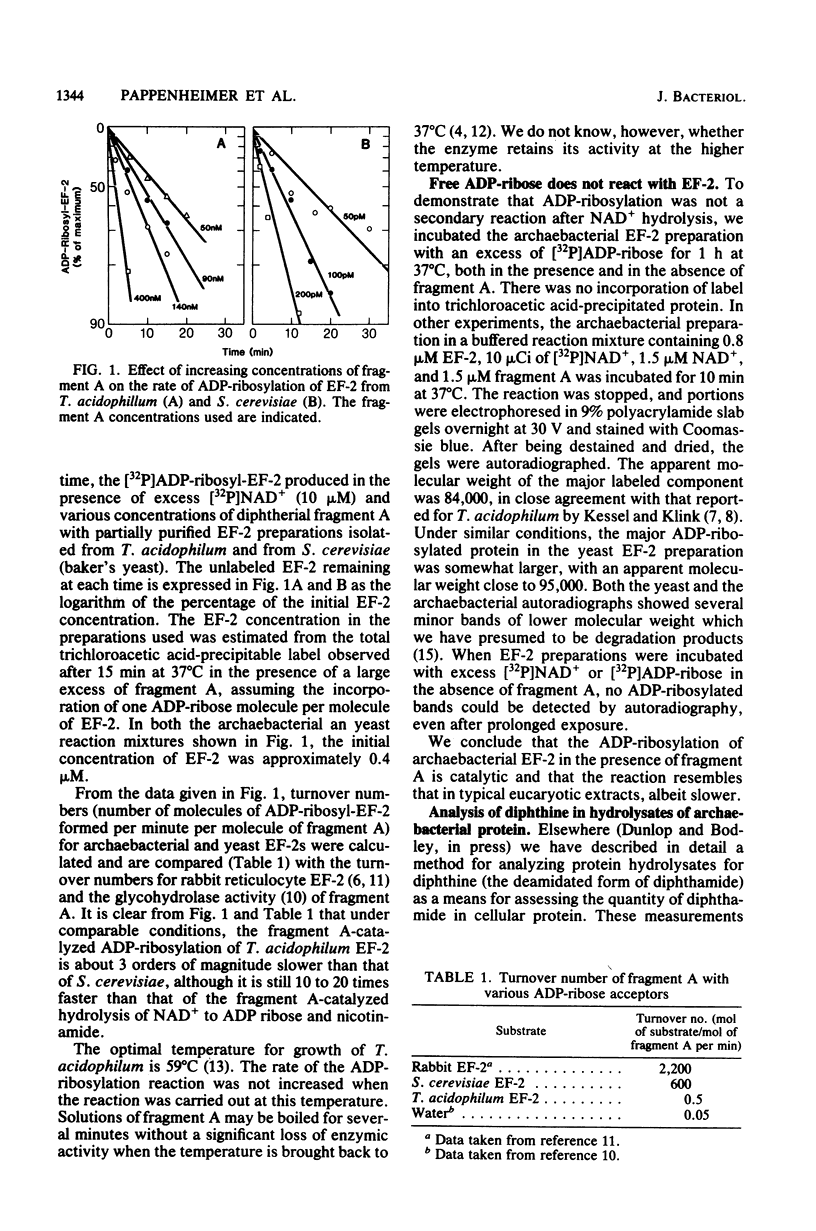
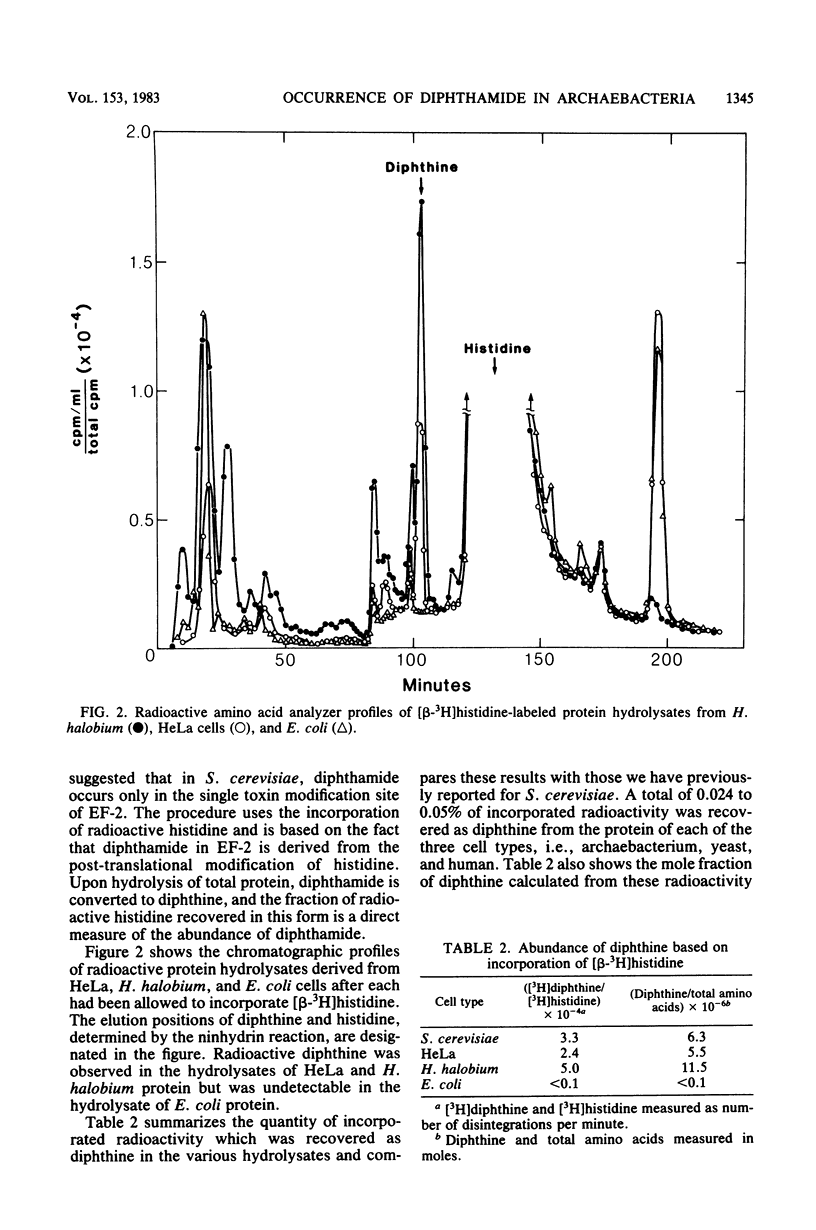
We examined the nature of the diphtheria toxin fragment A recognition site in the protein synthesis translocating factor present in cell-free preparations from the archaebacteria Thermoplasma acidophilum and Halobacterium halobium. In agreement with earlier work (M. Kessel and F. Klink, Nature (London) 287:250-251, 1980), we found that extracts from these organisms contain a protein factor which is a substrate for the ADP-ribosylation reaction catalyzed by diphtheria toxin fragment A. However, the rate of the reaction was approximately 1,000 times slower than that typically observed with eucaryotic elongation factor 2. We also demonstrated the presence of diphthine (the deamidated form of diphthamide, i.e., 2-[3-carboxyamide-3-(trimethylammonio)propyl]histidine) in acid hydrolysates of H. halobium protein in amounts comparable to those found in hydrolysates of similar preparations from eucaryotic cells (Saccharomyces cerevisiae and HeLa). Diphthine could not be detected in hydrolysates of protein from the eubacterium Escherichia coli. Whereas both archaebacterial and eucaryotic elongation factors contain diphthamide, they differ importantly in other respects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boquet P., Silverman M. S., Pappenheimer A. M., Jr, Vernon W. B. Binding of triton X-100 to diphtheria toxin, crossreacting material 45, and their fragments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4449–4453. doi: 10.1073/pnas.73.12.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Bodley J. W. Primary structure at the site in beef and wheat elongation factor 2 of ADP-ribosylation by diphtheria toxin. FEBS Lett. 1979 Jul 15;103(2):253–255. doi: 10.1016/0014-5793(79)81339-3. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Klink F. Archaebacterial elongation factor is ADP-ribosylated by diphtheria toxin. Nature. 1980 Sep 18;287(5779):250–251. doi: 10.1038/287250a0. [DOI] [PubMed] [Google Scholar]
- Kessel M., Klink F. Two elongation factors from the extremely halophilic archaebacterium Halobacterium cutirubrum. Assay systems and purification at high salt concentrations. Eur J Biochem. 1981 Mar;114(3):481–486. doi: 10.1111/j.1432-1033.1981.tb05170.x. [DOI] [PubMed] [Google Scholar]
- Kun E., Chang A. C., Sharma M. L., Ferro A. M., Nitecki D. Covalent modification of proteins by metabolites of NAD+. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3131–3135. doi: 10.1073/pnas.73.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S., Carroll S. F., Bernard P. D., Collier R. J. Ligand interactions of diphtheria toxin. I. Binding and hydrolysis of NAD. J Biol Chem. 1980 Dec 25;255(24):12011–12015. [PubMed] [Google Scholar]
- Moynihan M. R., Pappenheimer A. M., Jr Kinetics of adenosinediphosphoribosylation of elongation factor 2 in cells exposed to diphtheria toxin. Infect Immun. 1981 May;32(2):575–582. doi: 10.1128/iai.32.2.575-582.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Searcy D. G., Stein D. B., Searcy K. B. A mycoplasma-like archaebacterium possibly related to the nucleus and cytoplasms of eukaryotic cells. Ann N Y Acad Sci. 1981;361:312–324. doi: 10.1111/j.1749-6632.1981.tb46527.x. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Barrowclough B., Bodley J. W. Recognition of elongation factor 2 by diphtheria toxin is not solely defined by the presence of diphthamide. FEBS Lett. 1980 Oct 20;120(1):4–6. doi: 10.1016/0014-5793(80)81032-5. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Howard J. B., Bodley J. W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. Isolation and properties of the novel ribosyl-amino acid and its hydrolysis products. J Biol Chem. 1980 Nov 25;255(22):10717–10720. [PubMed] [Google Scholar]
- Van Ness B. G., Howard J. B., Bodley J. W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem. 1980 Nov 25;255(22):10710–10716. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]


